Novel coronavirus (COVID-19) pneumonia has emerged as major global health threat as it continues to spread with increasing number of deaths. COVID-19 is an enveloped, single-stranded RNA coronavirus that is largely transmitted by inhalation of respiratory droplets. Most patients with COVID-19 have mild disease, but approximately 20% develop severe disease with subsequent respiratory symptoms.1 Early detection of lung involvement by COVID-19 in bronchial fluid may be important for timely therapeutic management. To date, the description of morphological changes in COVID-19 pneumonia is largely based on findings in a small number of autopsies.1 2
We present a case of a man in his 70s who was transferred to our hospital on 26 March 2020 with a hyperglycaemic crisis and increasing shortness of breath. He was known to have high blood pressure and type 2 diabetes that had been treated with linagliptin. The patient had a history of rectal cancer in 2016, which had been successfully treated by surgery, followed by chemotherapy for 1 month.
At admission, the patient was dehydrated and his blood glucose level was 30 mmol/L (normal range 3.9–5.8 mmol/L). His body temperature was normal. Within a few hours, his oxygen saturation level dropped from 93% to 50% and he was immediately admitted to the isolation ward and intubated. He was given ceftriaxone, clarithromycin and piperacillin/tazobactam (2×500 mg intravenous). CT from the lung revealed multifokal bilateral ground glass infiltrates at the periphery of the upper lobes with involvement of the right middle lobe suggesting bilateral interstitial pneumonia (figure 1).3 In addition, there were infiltrates with a positive bronchogram in both lower lobes and a pneumomediastinum, not typical for interstitial pneumonia. There were no pleural effusions.
Figure 1.
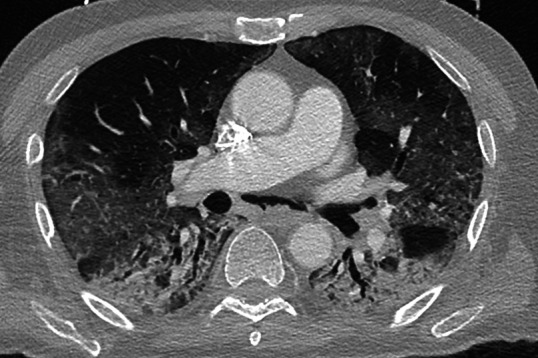
Multifocal bilateral ground glass infiltrates at the periphery of the upper lobes with involvement of the right middle lobe and consolidation in both lower lobes.
A nasopharyngeal sample was negative for Middle East Respiratory Syndrome coronavirus (MERS-CoV), coronavirus types 229E, HKU1, NL63, OC43 and SARS-CoV-2 (COVID-19), influenza viruses A, B, parainfluenza viruses 1–4, respiratory syncytial virus (RSV), metapneumovirus, rhinovirus, adenovirus, mycoplasma pneumoniae, chlamydophila pneumoniae and bordetella pertussis in the PCR analyses. On 27 March 2020, a bronchoalveolar lavage (BAL) from the posterior upper lobe of the right lung and a bronchial secretion sample were taken. Multidrug-resistant Staphylococcus aureus (MRSA) cultures in the BAL sample were positive and therefore vancomycin was added. The bronchial secretion sample was sent to the Institute of Pathology for cytopathological examination.
Cytopathology revealed multinucleated giant cells with an inflammatory background. The giant cells showed nuclear chromatin clearing with peripheral margination of the nuclear membranes (figure 2A). There were some nuclei with prominent nucleoli. The inflammatory infiltrate consisted mainly of lymphocytes, macrophages and also neutrophilic granulocytes. In the background of the giant cells, collagenous fibres could be seen (figure 2B). No hemosiderophages or other signs of alveolar haemorrhage could be identified. The giant cells corresponded to syncytial histiocytic cells as confirmed by positive CD68 expression (figure 2C). The multinucleated giant cells were seen along single large atypical pneumocytes, also with nuclear clearing (figure 2D). A diagnosis of cytomorphological changes consistent with viral infection was made. A second PCR analysis of the BAL showed infection with COVID-19.
Figure 2.
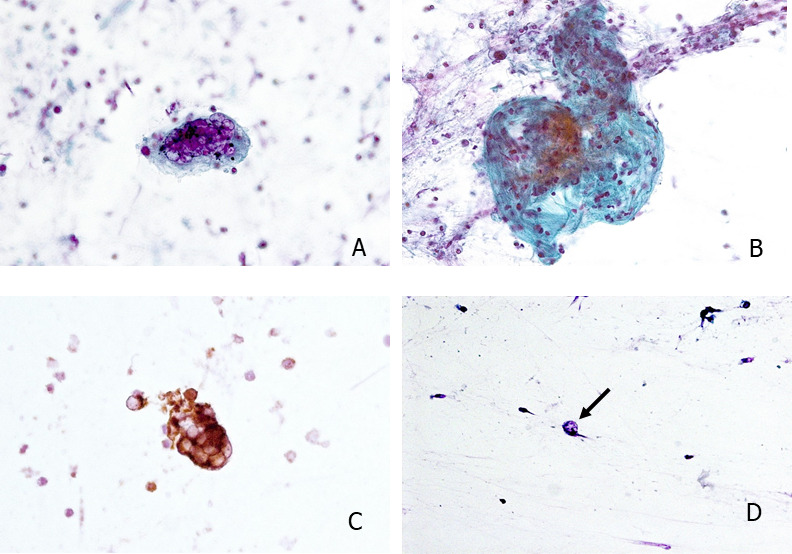
(A) Multinucleated syncytial giant cells with viropathic clearing of the nuclei and occasional prominent nucleoli. (B) Foci of collagenous fibres in the background of an inflammatory infiltrate. (C) CD68 expression in the multinucleated syncytial giant cells confirmes histiocytic origin. (D) Isolated atypical pneumocytes with enlarged nuclei and nuclear clearing (arrow).
Real time-PCR (RT-PCR) from nasopharyngeal swabs is routinely used for detection of COVID-19. The positive rate of RT-PCR for nasopharyngeal samples was reported to be 63% in early stage of COVID-19, therefore, a significant number of patients may have false negative results.4 Previous data from SARS-CoV infections showed that RNA levels first peaked in upper respiratory tract but remained higher in lower respiratory tract specimens 3 weeks after onset of illness.4 The same may be also expected with COVID-19 infections.5 6 Detection of early cytomorphological changes in the lung from bronchial fluid or BAL may, therefore, help with timely start of COVID-19 surveillance and supportive therapy.
The histopathological features described in COVID-19 infected lungs so far are very similar to those seen in previous SARS-CoV and MERS-CoV infections.7 So far they were non-specific and included oedema with or without hyaline membranes, pneumocyte hyperplasia, interstitial fibrosis, pulmonary infarction and a predominantly lymphocytic inflammation.1 Occasional viral inclusion was suspected in single cells by some authors.1 Remarkably, all authors observed multinucleated giant cells which were not further described. In MERS-CoV infections, multinucleate syncytial cells, some of them with prominent eosinophilic nucleoli, were also described.7 It was suspected that multinucleated epithelial cells, pneumocytes and bronchial submucosal gland cells are the main targets in MERS-CoV infection.7In our case, the viropathic multinucleated giant cells were of histiocytic origin. Thus, macrophages in COVID-19 infection may also be targets for COVID-19 beside pneumocytes as was described in pneumonia caused by measles,8 even though a secondary reactive non-viropathic giant cell response to the viral infection as a cause of the observed changes cannot be excluded. The prominent neutrophilic inflammation in our case may be contributed to the superinfection with MRSA.
In conclusion, the cytopathological detection of virus infected multinuclear macrophages in bronchial fluid or BAL might add substantial early information in COVID-19.
Footnotes
Handling editor: Runjan Chetty.
Contributors: All authors have contributed substantially to the manuscript justifying their authorship.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Hanley B, Lucas SB, Youd E, et al. Autopsy in suspected COVID-19 cases. J Clin Pathol 2020;73:239–42. 10.1136/jclinpath-2020-206522 [DOI] [PubMed] [Google Scholar]
- 2. Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol 2020;153:725–33. 10.1093/ajcp/aqaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus Disease-19 (COVID-19): relationship to duration of infection. Radiology 2020:200463. 10.1148/radiol.2020200463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loeffelholz MJ, Tang Y-W. Laboratory diagnosis of emerging human coronavirus infections – the state of the art. Emerg Microbes Infect 2020;9:747–56. 10.1080/22221751.2020.1745095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pan Y, Zhang D, Yang P, et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 2020;20:411–2. 10.1016/S1473-3099(20)30113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020. 10.1001/jama.2020.3786. [Epub ahead of print: 11 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J, Zheng X, Tong Q, et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol 2020;92:491–4. 10.1002/jmv.25709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allen IV, McQuaid S, Penalva R, et al. Macrophages and dendritic cells are the predominant cells infected in measles in humans. mSphere 2018;3:e00570–17. 10.1128/mSphere.00570-17 [DOI] [PMC free article] [PubMed] [Google Scholar]


