Case
In early March 2020, a 53-year-old man was admitted to King’s College Hospital (KCH). He presented with a 2-week history of a dry cough, followed by fevers. He had a long flight 9 weeks prior to admission. His presentation to the emergency department was prompted by feeling increasingly breathless; his cough was occasionally productive of yellow sputum with streaks of haemoptysis. He had no medical history. His temperature was 38°C with peripheral oxygen saturation level of 92% on room air, respiratory rate was 28 breaths per minute but he was haemodynamically stable with blood pressure 128/75 mm Hg and heart rate 98 beats per minute.
A chest X-ray on arrival showed bilateral infiltrates with denser consolidation in the right lower and upper zone. An arterial blood gas showed type 1 respiratory failure with pH 7.52, PaCO2 3.87 kPA, PaO2 8.45 kPa and lymphopenia 0.86×109/L. ECG demonstrated sinus rhythm.
The patient was suspected to have viral pneumonia—likely COVID-19. He was given oxygen to target saturations 94%–98%, covered for bacterial infection as per local community-acquired pneumonia protocol and given venous thromboembolism (VTE) prophylaxis in the form of enoxaparin 40 mg once daily. Viral RT-PCR (reverse transcription polymerase chain reaction) for COVID-19 from naso-oropharyngeal and oropharyngeal swabs on both day 1 and day 2 of admission were negative. Urinary pneumococcal and legionella antigens were also negative. His D dimer was 2560 ng/mL (normal <500 ng/mL) and serial troponin Ts were 3 and 7 ng/L (normal <14 ng/L).
Given his ongoing oxygen requirement and negative viral swab, a CTPA (computed tomography pulmonary angiogram) was performed. This showed acute bilateral pulmonary emboli (PE) with evidence of pulmonary infarcts and background changes suggestive of infective aetiology or acute respiratory distress syndrome (ARDS). A third swab for viral RT-PCR on day 3 confirmed COVID-19 infection. The patient was switched to oral edoxaban after 5 days of treatment dose enoxaparin and was discharged off oxygen 5 days after presentation.
Since this early case of COVID-19 in the UK, in our practice, based in London, we have observed numerous PE events in patients with COVID-19 infection. We found this striking given the difficulty in identifying concomitant PE in this patient group based on the clinical history and serological tests.
We have summarised the demographics and clinical findings of the first 10 patients admitted to KCH with COVID-19 pneumonia and coexistent PE. The patient summarised above is case G.
The demographics, clinical and imaging findings of 10 patients identified with PE and COVID-19 infection in March 2020 presenting to our institution are detailed in table 1. Sample images are shown in figure 1. All scans were retrospectively reviewed by an experienced chest radiologist.
Table 1.
Summary of 10 cases of COVID-19 pneumonia with pulmonary embolism identified on CT angiography
| Case | Age | Sex | Presenting history | Chest radiograph | Relevant comorbid conditions | D-dimer (ng/mL) | Troponin (ng/L) |
Indication for CTPA | HIghest level of PE on CTPA | Right heart strain on CT | CT evidence of infarcts | Management of VTE | Ventilatory support | Evidence of DVT on USS |
| A | 56 | Male | 7 days cough, fever | Bilateral infiltrates | T2DM | >8000 | 8 | Chest pain | Subsegmental | No | No | DOAC | High flow oxygen | No |
| B | 64 | Male | 9 days cough, SOB | Bilateral infiltrates | HTN, CKD | >8000 | – | Syncopal episode | Main | Yes | Yes | LMWH | High flow oxygen | No |
| C | 57 | Female | 15 days fever, cough SOB | Clear | – | >8000 | 309 | >3 weeks of SOB | Main | Yes | Yes | Thrombolysis | High flow oxygen | No |
| D | 71 | Male | 7 days lethargy, fever, cough | Bilateral infiltrates | – | >8000 | 406 | Persistent high P/F ratio | Lobar | Yes | Yes | LMWH | High flow oxygen | No |
| E | 66 | Male | 9 days cough, fever, SOB | Right upper lobe and left lower zone consolidation | – | 4990 | 13 | Persistent high P/F ratio | Segmental | Yes | No | LMWH | High flow oxygen | Yes |
| F | 62 | Male | 10 days SOB, diarrhoea | Bilateral infiltrates | – | >8000 | 37 | Chest pain | Segmental | Yes | No | LMWH | Intubated and Ventilated | No |
| G | 53 | Male | 13 days cough, fever, SOB | Bilateral consolidative change | – | 2560 | 7 | Pleuritic chest pain. Persistent high P/F ratio | Lobar | Yes | Yes | DOAC | High flow oxygen | No |
| H | 71 | Male | 13 days fever, coryza | Unilateral linear atelectasis | T2DM | 2490 | 177 | Syncopal episode | Main | Yes | No | LMWH | High flow oxygen | No |
| I | 63 | Male | 7 days SOB, cough, fever | Bilateral infiltrates | T2DM, HTN, IHD | >8000 | 21 | Clinical evidence of DVT, raised D-dimer | Main | Yes | No | LMWH | High flow oxygen | Yes |
| J | 75 | Female | Inpatient - 2 days of SOB and increasing oxygen requirement | Bilateral infiltrates | Bladder cancer with ureteric obstruction, COPD | >8000 | 74 | Staging CT scan | Subsegmental | No | No | Heparin infusion | High flow oxygen | Yes |
DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; HTN, hypertension; IHD, ischaemic heart disease; LMWH, low-molecular-weight heparin; P/F ratio, ratio of arterial oxygen partial pressure to fractional inspired oxygen; SOB, shortness of breath; T2DM, type 2 diabetes; USS, ultrasound.
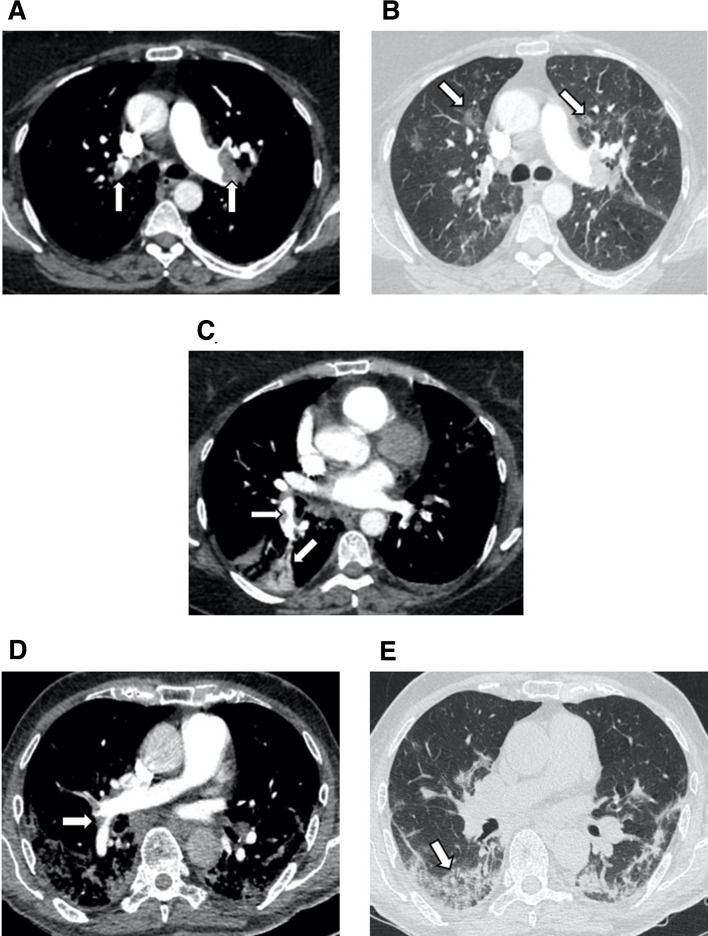
Figure 1.
(A-C) show sample axial CT images of a CTPA in patient C with moderate COVID-19 pneumonia as demonstrated by bilateral round peripheral and bronchocentric ground glass opacities (arrowed). There are extensive bilateral main and lobar pulmonary emboli (PEs) (arrowed) with a wedge shaped infarct in the right lower lobe (arrowed). (D, E) show axial CT images of CTPA (computed tomography pulmonary angiogram) in patient D with severe COVID-19 pneumonia as demonstrated by peripheral ground glass opacification and consolidation (arrowed) with perilobular pattern (arrow). There are segmental PEs (arrowed).
Discussion
Though the relationship between SARS-CoV2 infection and predisposition to pulmonary embolism is poorly described, there is a strong association between VTE and viral pneumonia. In a critically ill ARDS cohort with Influenza A H1N1 infection, the incidence of pulmonary embolus was 29% compared with 8.5% in those without H1N1.1 These data suggest a mechanism of clot formation beyond critical illness that may be related to viral damage.
Coagulopathy is a common feature of severe COVID-19 infection, the most striking feature an elevated level of D dimer, a fibrin degradation product. Unlike disseminated intravascular coagulation, patients are not typically thrombocytopenic nor have evidence of severe clotting time derangement; this would suggest a process of localised thrombin generation and fibrinolysis. In part, this may be secondary to the over-expression of the cytokine interleukin-6 (IL-6) which is produced by multiple cell types, primarily immune cells but also non-immune tissue such as vascular endothelium. It has both pro and anti-inflammatory properties and plays a key role in the immune response to viral pneumonia. In H1N1 infection, IL-6 expression may be advantageous by protecting neutrophils from virus-induced death in the lung and promoting neutrophil-mediated viral clearance.2 Conversely, in COVID-19, IL-6 appears central to an overzealous immune response with levels nearly threefold higher in patients with COVID-19 infection complicated by ARDS vs non-complicated disease.3 At the time of writing, it is suggested that an IL-6-mediated pulmonary hyperinflammatory process may extend into the adjacent microcirculation resulting in severe local vascular dysfunction including micro-thrombosis and haemorrhage with a resultant lung-centric pulmonary intravascular coagulopathy.4
This notion is supported by examination of pathology specimens from COVID-19-infected patients which also demonstrate evidence of pulmonary vascular changes with vessel wall thickening, lumen stenosis, occlusion and microthrombosis formation.5 However, it is unclear if a similar process results in ‘in situ’ pulmonary embolus formation. In clinical practice, retrospective data from China suggest reduced mortality in patients treated with low molecular weight heparin6 but this is yet to be prospectively evaluated.
We have identified 10 cases of coexisting PE and COVID-19; in general, these were identified with symptoms highly suggestive of PE and a very elevated D dimer. It is likely that there are many more cases of PE that have been missed due to the similarities in presentation between PE and COVID-19 pneumonia. These cases highlight a diagnostic challenge in severe COVID-19 infection, and it is not yet clear whether associated PE affects outcome in these patients. Patients typically present to hospital with an elevated alveolar-arterial oxygen gradient and bilateral infiltrates on chest radiography. D-dimer levels are measured for prognostication, but due to the limited specificity of the assay, it is easy to overlook concomitant VTE in this patient group.
Until large prospectively collected data are available, based on our experiences to date, we suggest a lower threshold to perform CT angiography in patients COVID-19 pneumonia, particularly in those with a prolonged history of illness and immobility before admission, grossly elevated D-dimer, lack of improvement or clinical deterioration with increasing oxygen requirement.
Footnotes
Twitter: @d33pan, @jimstanp
Contributors: KA and PS are joint first authors contributing equally to this manuscript. All authors contributed to the authorship of this manuscript and verify its accuracy.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Obi AT, Tignanelli CJ, Jacobs BN, et al. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord 2019;7:317–24. 10.1016/j.jvsv.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 2. Dienz O, Rud JG, Eaton SM, et al. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol 2012;5:258–66. 10.1038/mi.2012.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao X, Zhang B, Li P, et al. Incidence clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. medRxiv 2020. [Google Scholar]
- 4. Sharif K, Bridgewood C. Interleukin-6 use in COVID-19 pneumonia related macrophage activation syndrome. [DOI] [PMC free article] [PubMed]
- 5. Luo W, Yu H, Gou J, et al. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19). Pathology & Pathobiology 2020:2020020407. [Google Scholar]
- 6. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–9. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]