Abstract
Objective
To assess the prevalence, characteristics and prognostic value of pulmonary hypertension (PH) and right ventricular dysfunction (RVD) in hospitalised, non-intensive care unit (ICU) patients with coronavirus disease 2019 (COVID-19).
Methods
This single-centre, observational, cross-sectional study included 211 patients with COVID-19 admitted to non-ICU departments who underwent a single transthoracic echocardiography (TTE). Patients with poor acoustic window (n=11) were excluded. Clinical, imaging, laboratory and TTE findings were compared in patients with versus without PH (estimated systolic pulmonary artery pressure >35 mm Hg) and with versus without RVD (tricuspid annular plane systolic excursion <17 mm or S wave <9.5 cm/s). The primary endpoint was in-hospital death or ICU admission.
Results
A total of 200 patients were included in the final analysis (median age 62 (IQR 52–74) years, 65.5% men). The prevalence of PH and RVD was 12.0% (24/200) and 14.5% (29/200), respectively. Patients with PH were older and had a higher burden of pre-existing cardiac comorbidities and signs of more severe severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (radiological lung involvement, laboratory findings and oxygenation status) compared with those without PH. Conversely, patients with RVD had a higher burden of pre-existing cardiac comorbidities but no evidence of more severe SARS-CoV-2 infection compared with those without RVD. The presence of PH was associated with a higher rate of in-hospital death or ICU admission (41.7 vs 8.5%, p<0.001), while the presence of RVD was not (17.2 vs 11.7%, p=0.404).
Conclusions
Among hospitalised non-ICU patients with COVID-19, PH (and not RVD) was associated with signs of more severe COVID-19 and with worse in-hospital clinical outcome.
Trial registration number
Keywords: echocardiography, pulmonary vascular disease
Introduction
Coronavirus disease 2019 (COVID-19) is a global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), characterised by prominent pulmonary involvement.1 Preliminary pathological findings demonstrate lung oedema, thickening of alveolar septa, inflammatory infiltrates and vascular congestion also in the early phase of the disease.2 Similarly, chest computed tomograpy (CT) studies showed lung abnormalities in asymptomatic SARS-CoV-2-infected individuals.3 Lung parenchymal damage and altered pulmonary haemodynamics may determine pulmonary hypertension (PH) and secondary right ventricular (RV) involvement in patients with COVID-19, even in non-advanced disease stages, as a consequence of hypoxic vasoconstriction of the pulmonary circulation,4 use of positive end-expiratory pressure (PEEP) in mechanical ventilation,5 pulmonary endothelial injury,6 and local inflammatory thrombotic or thromboembolic processes.7 Of note, early studies reported myocardial injury in approximately 20%–30% of hospitalised patients with COVID-198 9; this subclinical cardiac involvement could be explained, at least in part, by PH and RV impairment. Preliminary data suggest that the actual prevalence of PH in patients with COVID-19 may be around 13%, but its prognostic role remains unclear.10 On the contrary, reduced RV longitudinal strain was found as a powerful predictor of higher mortality in patients with COVID-19.11 Hence, our aim was to describe the prevalence, characteristics and prognostic impact of PH and RV involvement in a cohort of hospitalised, non-critically ill patients with COVID-19.
Methods
Study design and study population
This single-centre, observational, cross-sectional study was conducted at a large tertiary centre (San Raffaele Scientific Institute) in Milan, Italy. Within an institutional, centralised, prospective, all-comers registry collecting clinical, laboratory, biological and imaging data on all hospitalised patients with COVID-19 (COVID-BioB), the two co-first authors designed this substudy. The COVID-BioB study was registered on ClinicalTrials.gov (NCT04318366). Patients admitted to non-intensive care unit (ICU) departments with an established diagnosis of COVID-19 (according to current WHO criteria)12 were evaluated for inclusion in the study. All COVID-19 dedicated non-ICU departments (according to our institution rearrangement13) were screened for patient enrolment. Patients were included and evaluated with a single transthoracic echocardiography (TTE) between 24 March and 29 April 2020, up to a prespecified study sample size of 200 patients with analysable TTE data. Subjects with non-adequate transthoracic acoustic window (non-analysable TTE data) were not included in the final analysis. Patients were grouped according to the presence or absence of PH and right ventricular dysfunction (RVD) at TTE assessment.
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Equipment, echocardiographic protocol and data collection
Eight authors (MP, LB, AB, VP, FC, MG, GI and AN) performed all TTEs included in this report on a voluntary basis. All physicians were provided with personal protection equipment according to current WHO standards and local institutional protocols, including FFP2 mask or equivalent, double gowns, double pair gloves and eye protection goggles.14 A dedicated echocardiographic machine was used to obtain echocardiographic data (Philips iE33, Philips Healthcare, Eindhoven, The Netherlands) in non-ICU COVID-19 departments. Images were obtained according to a prespecified acquisition protocol, specifically focusing on pulmonary haemodynamics, RV morphology and RV function according to current guidelines.15 16
Two operators simultaneously entered the patients’ room for echocardiographic examination and data collection. Measures were obtained in real time during image acquisition and noted in a case report form to limit overall exposure time. The dedicated TTE machine was cleaned and disinfected before leaving the COVID-19 departments. Images were stored in the hospital’s centralised server after TTE acquisition to allow offline postprocessing. Echocardiographic data were collected in a dedicated electronic database, along with relevant clinical, laboratory and imaging variables. Laboratory data were collected at the time of TTE (same day); if unavailable at the time of TTE, laboratory data obtained within 5 days before TTE were collected. Chest X-ray (CXR) imaging and contrast-enhanced chest CT scan (when performed according to standard clinical practice) were also analysed within the same time window.
Definitions and study endpoints
According to the latest international recommendations, RVD was defined as either tricuspid annular plane systolic excursion (TAPSE) of <17 mm or tissue Doppler imaging S wave (S’ wave) of <9.5 cm/s.15 Systolic pulmonary artery pressure (SPAP) was estimated with the following formula: SPAP=4×tricuspid regurgitation (TR) peak velocity2+right atrial pressure (RAP);16 PH was defined as SPAP >35 mm Hg. The estimation of RAP (central venous pressure) and TR grading were performed according to current guidelines.16 17
The degree of hypoxaemia at hospital admission was assessed by means of the arterial partial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2), according to established criteria.12 18 At time of TTE, the pulse oximetric saturation to fraction of inspired oxygen ratio (SpO2/FiO2) was used to estimate oxygenation status.19 A CXR severity score was used to grade the extent of radiographical lung involvement, as previously described.20
The primary outcome of interest was the composite of in-hospital all-cause mortality or ICU admission. Secondary outcomes of interest were the two individual endpoints of in-hospital all-cause mortality and ICU admission, hospital discharge, need of invasive mechanical ventilation, need of extracorporeal membrane oxygenation and sepsis.
Statistical analyses
Continuous variables are presented as medians and IQRs and were compared with the Mann-Whitney U test (non-normally distributed continuous data). Categorical variables are presented as numbers and percentages and were compared with the χ2 test or Fisher’s exact test (as appropriate). Primary and secondary outcomes of interest were compared between patients with or without PH and between patients with or without RVD. The impact of variables of interest on the primary endpoint was adjusted for relevant covariates by means of multivariable binary logistic regression. Considering the low number of events, a limited number of covariates were entered in the multivariable model (in order to avoid overfitting). Results of logistic regression are reported as adjusted OR (ORadj) and 95% CI; the Hosmer-Lemeshow (H-L) goodness-of-fit test and C-statistic were used to confirm good calibration and discrimination of the multivariable model. The composite of in-hospital all-cause mortality or ICU admission was also compared between groups using the Kaplan-Meier method (log-rank test).
All reported p values are two-sided, and a p value <0.05 was considered statistically significant.
Statistical analyses were performed using Stata version 13.0 (Stata Corp., College Station, Texas).
Results
A total of 211 patients with COVID-19 hospitalised in non-ICU departments were included in the study; TTE was not feasible in 11 patients because of unsuitable acoustic window. Hence, a total of 200 patients were assessed with TTE and included in the final analysis.
Baseline patient characteristics
Baseline clinical characteristics are reported in table 1. The median age was 62 (IQR 55–74) years, and 65.5% of the patients were men. The median time from symptom onset to hospital admission was 7 (3–10) days, and the median PaO2/FiO2 on hospital admission was 243 (132–314). At TTE evaluation, PH was observed in 24 patients (12.0%), whereas RVD was observed in 29 patients (14.5%); 8 patients (4.0%) had both PH and RVD.
Table 1.
Baseline clinical characteristics
| Overall (n=200) |
PH (n=24) |
No PH (n=176) |
P value | RVD (n=29) |
No RVD (n=171) |
P value | |
| Age (years) | 62 (55–74) | 76 (67–82) | 62 (54–72) | <0.001 | 65 (55–76) | 62 (54–74) | 0.813 |
| Male sex | 131/200 (65.5) | 12/24 (50.0) | 119/176 (67.6) | 0.089* | 20/29 (69.0) | 111/171 (64.9) | 0.671* |
| BMI (kg/m2) | 25.7 (24.2–28.8) | 24.2 (22.1–27.3) | 25.9 (24.2–29.3) | 0.059 | 27.5 (24.8–29.0) | 25.4 (23.8–28.6) | 0.068 |
| Smoking | 0.067† | 1.000† | |||||
| Prior smoking | 33/200 (16.5) | 8/24 (33.3) | 25/176 (14.2) | 5/29 (17.2) | 28/171 (16.4) | ||
| Current smoking | 8/200 (4.0) | 0/24 (0.0) | 8/176 (4.6) | 1/29 (3.5) | 7/171 (4.1) | ||
| Dyslipidaemia | 45/200 (22.5) | 2/24 (8.3) | 43/176 (24.4) | 0.057† | 3/29 (10.3) | 42/171 (24.6) | 0.099† |
| Diabetes mellitus | 0.049† | 0.228† | |||||
| Non-insulin-dependent | 28/200 (14.0) | 5/24 (20.8) | 23/176 (13.1) | 3/29 (10.3) | 25/171 (14.6) | ||
| Insulin-dependent | 9/200 (4.5) | 3/24 (12.5) | 6/176 (3.4) | 3/29 (10.3) | 6/171 (3.5) | ||
| Hypertension | 84/200 (42.0) | 16/24 (66.7) | 68/176 (38.6) | 0.009* | 11/29 (37.9) | 73/171 (42.7) | 0.631* |
| Family history of CAD | 15/200 (7.5) | 1/24 (4.2) | 14/176 (8.0) | 1.000† | 4/29 (13.8) | 11/171 (6.4) | 0.241† |
| Prior MI | 17/200 (8.5) | 4/24 (16.7) | 13/176 (7.4) | 0.129† | 7/29 (24.1) | 10/171 (5.9) | 0.005† |
| Prior PCI | 13/200 (6.5) | 1/24 (4.2) | 12/176 (6.8) | 1.000† | 3/29 (10.3) | 10/171 (5.9) | 0.408† |
| Prior CABG | 6/200 (3.0) | 2/24 (8.3) | 4/176 (2.3) | 0.153† | 5/29 (17.2) | 1/171 (0.6) | <0.001† |
| Prior valve intervention | 4/200 (2.0) | 0/24 (0.0) | 4/176 (2.3) | 1.000† | 4/29 (13.8) | 0/171 (0.0) | <0.001† |
| Prior AF or flutter | 22/200 (11.0) | 10/24 (41.7) | 12/176 (6.8) | <0.001† | 9/29 (31.0) | 13/171 (7.6) | 0.001† |
| Prior HF diagnosis | 7/200 (3.5) | 4/24 (16.7) | 3/176 (1.7) | 0.004† | 5/29 (17.2) | 2/171 (1.2) | 0.001† |
| Known cardiomyopathy | 5/200 (2.5) | 3/24 (12.5) | 2/176 (1.1) | 0.013† | 4/29 (13.8) | 1/171 (0.6) | 0.002† |
| Prior stroke or TIA | 9/200 (4.5) | 2/24 (8.3) | 7/176 (4.0) | 0.295† | 2/29 (6.9) | 7/171 (4.1) | 0.621† |
| Peripheral artery disease | 13/200 (6.5) | 3/24 (12.5) | 10/176 (5.7) | 0.193† | 2/29 (6.9) | 11/171 (6.4) | 1.000† |
| CKD stages IV–V (eGFR ≤30 mL/min/1.73 m2) | 12/200 (6.0) | 5/24 (20.8) | 7/176 (4.0) | 0.007† | 1/29 (3.5) | 11/171 (6.4) | 1.000† |
| Dialysis | 3/200 (1.5) | 1/24 (4.2) | 2/176 (1.1) | 0.320† | 0/29 (0.0) | 3/171 (1.8) | 1.000† |
| COPD | 11/200 (5.5) | 4/24 (16.7) | 7/176 (4.0) | 0.030† | 3/29 (10.3) | 8/171 (4.7) | 0.202† |
| History of cancer | 32/200 (16.0) | 4/24 (16.7) | 28/176 (15.9) | 1.000† | 4/29 (13.8) | 28/171 (16.4) | 1.000† |
| Known autoimmune disease | 9/200 (4.5) | 1/24 (4.2) | 8/176 (4.6) | 1.000† | 3/29 (10.3) | 6/171 (3.5) | 0.126† |
| Time from symptoms onset to hospital admission (days) | 7 (3–10) | 7 (3–9) | 8 (3–11) | 0.224 | 5 (3–8) | 8 (4–11) | 0.034 |
| PaO2/FiO2 on hospital admission | 243 (132–314) | 210 (85–286) | 248 (147–317) | 0.060 | 238 (153–276) | 245 (131–317) | 0.241 |
| 200 < PaO2/FiO2≤300 | 72/187 (38.5) | 11/23 (47.8) | 61/164 (37.2) | 0.193† | 15/27 (55.6) | 57/160 (35.6) | 0.169† |
| 100 < PaO2/FiO2≤200 | 33/187 (17.7) | 3/23 (13.0) | 30/164 (18.3) | 3/27 (11.1) | 30/160 (18.8) | ||
| PaO2/FiO2≤100 | 30/187 (16.0) | 6/23 (26.1) | 24/164 (14.6) | 5/27 (18.5) | 25/160 (15.6) | ||
| PaCO2 on hospital admission | 32 (29–36) | 31 (27–37) | 32 (29–36) | 0.475 | 34 (30–37) | 32 (29–36) | 0.412 |
Data are presented as n/N (%) or median (Q25–Q75).
Bold values represent significant p values.
*χ2 test.
†Fisher’s exact test.
AF, atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary angioplasty; PH, pulmonary hypertension; RVD, right ventricular dysfunction; TIA, transient ischaemic attack.
Patients with PH were older compared with patients without PH (76 (67–82) vs 62 (54–72) years, p<0.001) and had more frequently underlying comorbidities, including diabetes mellitus (p=0.049), hypertension (p=0.009), chronic kidney disease stages IV–V (p=0.007) and chronic obstructive pulmonary disease (p=0.030). Selected cardiac comorbidities were present more often in the PH group, including prior atrial fibrillation or flutter (p<0.001), prior heart failure (p=0.004) and known cardiomyopathy (p=0.013). A non-significant trend towards lower PaO2/FiO2 on hospital admission was observed in patients with PH (210 (85–286) vs 248 (147–317), p=0.060).
Patients with RVD had more frequently underlying cardiac comorbidities compared with patients without RVD, including prior myocardial infarction (p=0.005), prior coronary artery bypass graft (p<0.001), prior valve intervention (p<0.001), prior atrial fibrillation or flutter (p=0.001), prior heart failure (p=0.001) and known cardiomyopathy (p=0.002). Age and PaO2/FiO2 on hospital admission were similar between RVD and no RVD groups. Time from symptom onset to hospital admission was significantly longer in patients without RVD (p=0.034).
Radiological and laboratory findings
Table 2 depicts CXR and laboratory findings. Multiple and bilateral lung infiltrates were observed at CXR in 87.5% and 82.5% of patients, respectively.
Table 2.
CXR and laboratory findings
| Overall (n=200) |
PH (n=24) |
No PH (n=176) |
P value | RVD (n=29) |
No RVD (n=171) |
P value | |
| CXR findings | |||||||
| Multiple lung infiltrates | 175/200 (87.5) | 23/24 (95.8) | 152/176 (86.4) | 0.322† | 25/29 (86.2) | 150/171 (87.7) | 0.766† |
| Bilateral lung infiltrates | 165/200 (82.5) | 21/24 (87.5) | 144/176 (81.8) | 0.774† | 23/29 (79.3) | 142/171 (83.0) | 0.625* |
| Right lung severity score (0–4) | 1 (1–2) | 2 (2–3) | 1 (1–2) | <0.001 | 1 (1–2) | 1 (1–2) | 0.987 |
| Left lung severity score (0–4) | 1 (1–2) | 3 (2–3) | 1 (1–2) | <0.001 | 2 (1–2) | 1 (1–2) | 0.567 |
| Final severity score (0–8) | 3 (2–4) | 5 (3–7) | 3 (2–4) | <0.001 | 3 (2–5) | 3 (2–4) | 0.568 |
| Final severity score ≥4 | 77/200 (38.5) | 17/24 (70.8) | 60/176 (34.1) | 0.001* | 13/29 (44.8) | 64/171 (37.4) | 0.449* |
| Laboratory findings | |||||||
| Leucocytes (*109/L) | 7.2 (5.5–9.8) | 9.0 (7.6–12.2) | 7.0 (5.3–9.5) | 0.003 | 7.1 (5.7–8.4) | 7.2 (5.4–10.0) | 0.552 |
| Lymphocytes (*109/L) | 1.2 (0.9–1.8) | 0.9 (0.7–1.2) | 1.2 (1.0–1.8) | 0.001 | 1.2 (1.0–1.7) | 1.2 (0.9–1.8) | 0.910 |
| Haemoglobin (g/dL) | 12.3 (10.9–13.7) | 12.3 (10.5–14.0) | 12.4 (10.9–13.7) | 0.829 | 12.8 (11.4–14.4) | 12.3 (10.7–13.5) | 0.126 |
| Platelets (*109/L) | 270 (201–367) | 212 (173–294) | 282 (214–370) | 0.029 | 283 (192–394) | 269 (210–365) | 0.819 |
| Creatinine (mg/dL) | 0.9 (0.8–1.2) | 1.0 (0.8–1.7) | 0.9 (0.8–1.1) | 0.223 | 0.9 (0.8–1.1) | 0.9 (0.7–1.2) | 0.576 |
| C reactive protein (mg/L) | 38.1 (13.3–93.5) | 106.2 (17.2–147.5) | 36.3 (12.6–81.3) | 0.011 | 18 (4.0–45.9) | 42.0 (16.4–95.4) | 0.019 |
| D-dimer (µg/mL) | 1.2 (0.5–2.9) | 3.4 (0.6–6.8) | 1.1 (0.5–2.5) | 0.013 | 0.7 (0.5–1.5) | 1.3 (0.5–3.5) | 0.075 |
| Interleukin-6 (pg/mL) | 41 (21–97) | 120 (39–185) | 39 (18–84) | 0.003 | 42 (26–157) | 40 (19–96) | 0.360 |
| Ferritin (ng/mL) | 1032 (591–1638) | 1107 (538–2596) | 1027 (593–1618) | 0.941 | 1128 (526–1679) | 1019 (591–1638) | 0.863 |
| Lactate dehydrogenase (U/L) | 328 (259–452) | 419 (350–560) | 321 (254–442) | 0.003 | 376 (286–516) | 326 (255–443) | 0.161 |
| Total bilirubin (mg/L) | 0.6 (0.4–0.9) | 0.8 (0.5–1.0) | 0.6 (0.4–0.9) | 0.109 | 0.6 (0.4–1.0) | 0.6 (0.4–0.9) | 0.406 |
| Aspartate transaminase (U/L) | 43 (31–61) | 51 (32–64) | 42 (31–61) | 0.358 | 46 (32–63) | 42 (30–61) | 0.304 |
| Alanine transaminase (U/L) | 41 (27–71) | 35 (25–61) | 43 (27–73) | 0.300 | 56 (28–78) | 40 (26–67) | 0.243 |
| High-sensitivity troponin T (ng/L) | 13.6 (6.0–30.0) | 43.5 (15.3–75.5) | 12.0 (5.8–25.0) | 0.001 | 24.3 (11.4–67.1) | 12.7 (5.5–24.6) | 0.006 |
| Creatine phosphokinase (U/L) | 85 (47–139) | 105 (68–294) | 81 (47–135) | 0.062 | 123 (61–175) | 76 (44–129) | 0.010 |
| NT-proBNP (pg/mL) | 256 (89–707) | 1408 (610–2963) | 194 (85–577) | <0.001 | 625 (143–1481) | 209 (85–604) | 0.011 |
Data are presented as n/N (%) or median (Q25–Q75).
Bold values represent significant p values.
*χ2 test.
†Fisher’s exact test.
CXR, chest X-ray; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PH, pulmonary hypertension; RVD, right ventricular dysfunction.
Patients with PH had higher CXR severity score compared with patients without PH (5 [3–7] vs 3 [2–4]; p<0.001), and a higher proportion of CXR severity score of ≥4 was observed in the PH group (70.8 vs 34.1%, p=0.001). A total of 56/200 patients (28.0%) underwent contrast-enhanced chest CT scan (data not presented in tables); among these, pulmonary thromboembolism was more frequently observed among patients with PH (7/9, 77.8%) compared with patients without PH (9/47, 19.2%; p=0.001).
Compared with patients without RVD, patients with RVD had similar CXR severity score and a similar proportion of CXR severity score of ≥4. Among patients with available contrast-enhanced chest CT scan, the prevalence of pulmonary thromboembolism was not significantly different between patients with RVD (1/8, 12.5%) compared with those without RVD (15/48, 31.3%; p=0.416).
Regarding laboratory findings, patients with PH had higher leucocyte count (p=0.003), lower lymphocyte count (p=0.001) and lower platelet count (p=0.029) compared with patients without PH. The PH group showed higher levels of D-dimer (p=0.013), C reactive protein (p=0.011), interleukin-6 (p=0.003), lactate dehydrogenase (p=0.003), high-sensitivity troponin T (p=0.001) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) (p<0.001).
Compared with patients without RVD, patients with RVD had lower levels of C reactive protein (p=0.019) and higher levels of high-sensitivity troponin T (p=0.006), creatine phosphokinase (p=0.010) and NT-proBNP (p=0.011).
Echocardiographic assessment
Data on clinical setting at the time of TTE and TTE findings are reported in table 3. In the overall population, the proportion of patients with suboptimal transthoracic window (yet sufficient for TTE data analysis) was 44.0%. The median time from hospital admission to TTE was 7 (3–13) days.
Table 3.
Echocardiographic assessment
| Overall (n=200) | PH (n=24) | No PH (n=176) | P value | RVD (n=29) | No RVD (n=171) | P value | |
| Clinical setting at time of TTE | |||||||
| Window quality | 0.528* | 0.616* | |||||
| Good | 112/200 (56.0) | 12/24 (50.0) | 100/176 (56.8) | 15/29 (51.7) | 97/171 (56.7) | ||
| Sufficient/suboptimal | 88/200 (44.0) | 12/24 (50.0) | 76/176 (43.2) | 14/29 (48.3) | 74/171 (43.3) | ||
| Time from hospital admission to TTE (days) | 7 (3–13) | 8 (4–11) | 7 (3–13) | 0.901 | 8 (3–10) | 7 (3–13) | 0.862 |
| SBP (mm Hg) | 120 (107–128) | 113 (105–121) | 120 (110–130) | 0.156 | 115 (100–120) | 120 (107–130) | 0.239 |
| HR (beats/min) | 80 (70–88) | 80 (75–90) | 80 (70–88) | 0.296 | 78 (74–85) | 80 (70–88) | 0.724 |
| NIV | <0.001† | 0.905† | |||||
| Intermittent cycles (no NIV during TTE) | 38/200 (19.0) | 5/24 (20.8) | 33/176 (18.5) | 5/29 (17.2) | 33/171 (19.3) | ||
| NIV during TTE | 24/200 (12.0) | 9/24 (37.5) | 15/176 (8.5) | 4/29 (13.8) | 20/171 (11.7) | ||
| SpO2/FiO2 | 283 (167–394) | 166 (110–280) | 312 (193–448) | <0.001 | 286 (188–457) | 283 (167–354) | 0.622 |
| TTE findings | |||||||
| Basal RVEDD (mm) | 36 (32–40) | 42 (38–48) | 36 (32–39) | <0.001 | 38 (35–42) | 36 (32–40) | 0.129 |
| Mid RVEDD (mm) | 30 (26–33) | 37 (31–40) | 30 (26–32) | <0.001 | 32 (30–36) | 30 (26–33) | 0.028 |
| RV length (mm) | 65 (60–73) | 70 (60–73) | 65 (60–72) | 0.488 | 69 (61–72) | 65 (60–73) | 0.561 |
| Proximal RVOT diameter (mm) | 30 (27–34) | 30 (27–36) | 30 (27–34) | 0.893 | 30 (27–39) | 30 (27–34) | 0.364 |
| TAPSE (mm) | 22 (20–25) | 20 (17–22) | 22 (20–25) | 0.004 | 16 (15–19) | 23 (20–25) | <0.001 |
| S’ TDI (cm/s) | 13 (11–15) | 12 (9–13) | 13 (11–15) | 0.004 | 9 (8–9) | 14 (12–15) | <0.001 |
| Tricuspid regurgitation | <0.001† | 0.015† | |||||
| None/trivial | 123/200 (61.5) | 0/24 (0.0) | 123/176 (69.9) | 14/29 (48.3) | 100/171 (63.7) | ||
| Mild | 63/200 (31.5) | 12/24 (50.0) | 51/176 (29.0) | 9/29 (31.0) | 54/171 (31.6) | ||
| Moderate | 14/200 (7.0) | 12/24 (50.0) | 2/176 (1.1) | 6/29 (20.7) | 8/171 (4.7) | ||
| Severe | 0/200 (0.0) | 0/24 (0.0) | 0/176 (0.0) | 0/29 (0.0) | 0/171 (0.0) | ||
| RA area (cm2) | 14 (12–16) | 16 (13–21) | 14 (11–16) | 0.062 | 14 (11–21) | 14 (12–16) | 0.725 |
| SPAP (mm Hg) | 29 (23–33) | 42 (39–47) | 25 (22–30) | <0.001 | 30 (25–40) | 28 (23–31) | 0.064 |
| LVEF (%) | 59 (55–63) | 59 (56–65) | 59 (55–63) | 0.900 | 59 (50–63) | 59 (56–63) | 0.085 |
| CVP (mm Hg) | <0.001† | 0.006† | |||||
| 0–5 | 152/200 (76.0) | 10/24 (41.7) | 142/176 (80.7) | 17/29 (58.6) | 135/171 (79.0) | ||
| 5–10 | 38/200 (19.0) | 8/24 (33.3) | 30/176 (17.1) | 7/29 (24.1) | 31/171 (18.1) | ||
| 10–20 | 10/200 (5.0) | 6/24 (25.0) | 4/176 (2.3) | 5/29 (17.2) | 5/171 (2.9) | ||
Data are presented as n/N (%) or median (Q25–Q75).
Bold values represent significant p values.
*χ2 test.
†Fisher’s exact test.
CVP, central venous pressure; HR, heart rate; LVEF, left ventricular ejection fraction; NIV, non-invasive ventilation; PEEP, positive end-expiratory pressure; PH, pulmonary hypertension; RV, right ventricle; RVD, right ventricular dysfunction; RVEDD, right ventricle end-diastolic diameter; RVOT, right ventricle outflow tract; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; S’TDI, tissue Doppler imaging S wave; TAPSE, tricuspid annular plane systolic excursion; TTE, transthoracic echocardiography.
Patients with PH were more frequently treated with non-invasive ventilation (NIV) compared with patients without PH (intermittent cycle—no NIV during TTE 20.8 vs 18.5%, NIV during TTE 37.5 vs 8.5%; p<0.001). The SpO2/FiO2 ratio at time of TTE was significantly lower in the PH group (166 (110–280) vs 312 (193–448), p<0.001). The proportion of patients treated with NIV and SpO2/FiO2 ratio was similar between the RVD and the no RVD groups.
In terms of TTE findings, patients with PH had significantly higher basal RV end-diastolic diameter (42 (38–48) vs 36 (32–39) mm, p<0.001) and mid RV end-diastolic diameter (37 (31–40) vs 30 (26–32) mm, p<0.001), and lower TAPSE (20 (17–22) vs 22 (20–25) mm, p=0.004) and S’ wave (12 (9–13) vs 13 (11–15) cm/s, p=0.004) compared with patients without PH. The median estimated SPAP in the PH group was 42 (39–47) mm Hg. Patients with PH had higher degrees of TR (p<0.001) and estimated central venous pressure (p<0.001) compared with patients without PH. Left ventricular ejection fraction was similar between the PH and no PH groups (p=0.900).
Compared with patients without RVD, patients with RVD had higher mid RV end-diastolic diameter (32 (30–36) vs 30 (26–33) mm, p=0.028). Median TAPSE and S’ wave in the RVD group were 16 (15–19) mm and 9 (8–9) cm/s, respectively. Degrees of TR (p=0.015) and estimated central venous pressure (p=0.006) were higher in the RVD group. Left ventricular ejection fraction was not significantly different between the RVD and no RVD groups (p=0.085).
In-hospital clinical outcomes
After a median follow-up of 9 (4–14) days, 19 patients died (9.5%); 7 patients required ICU admission (3.5%); 135 patients were discharged (67.5%); and 40 patients were still hospitalised in a non-ICU department (20.0%). As shown in table 4, the rate of all-cause death or ICU admission (primary endpoint) was 12.5% (25 patients).
Table 4.
In-hospital clinical outcomes
| Overall (n=200) |
PH (n=24) |
No PH (n=176) |
P value | RVD (n=29) |
No RVD (n=171) |
P value | |
| All-cause death or ICU admission | 25/200 (12.5) | 10/24 (41.7) | 15/176 (8.5) | <0.001† | 5/29 (17.2) | 20/171 (11.7) | 0.404* |
| All-cause death | 19/200 (9.5) | 8/24 (33.3) | 11/176 (6.3) | <0.001† | 4/29 (13.8) | 15/171 (8.8) | 0.489† |
| ICU admission | 7/200 (3.5) | 2/24 (8.3) | 5/176 (2.8) | 0.199† | 1/29 (3.5) | 6/171 (3.5) | 1.000† |
| Need of invasive ventilation | 7/200 (3.5) | 2/24 (8.3) | 5/176 (2.8) | 0.199† | 1/29 (3.5) | 6/171 (3.5) | 1.000† |
| Need of ECMO | 2/200 (1.0) | 0/24 (0.0) | 2/176 (1.1) | 1.000† | 0/29 (0.0) | 2/171 (1.2) | 1.000† |
| Sepsis | 14/200 (7.0) | 1/24 (4.2) | 13/176 (7.4) | 1.000† | 1/29 (3.5) | 13/171 (7.6) | 0.697† |
| Discharge | 135/200 (67.5) | 7/24 (29.2) | 128/176 (72.7) | <0.001* | 17/29 (58.6) | 118/171 (69.0) | 0.270* |
Data are presented as n/N (%).
Bold values represent significant p values.
*χ2 test.
†Fisher’s exact test.
ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; PH, pulmonary hypertension; RVD, right ventricular dysfunction.
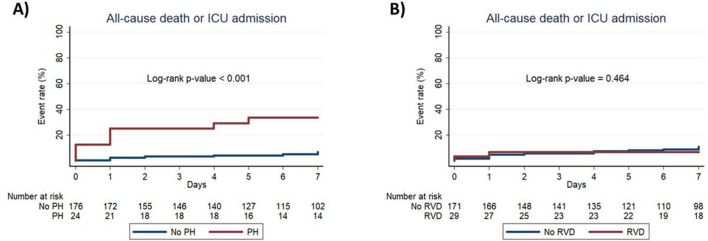
The primary endpoint was significantly higher among patients with PH compared with patients without PH (41.7 vs 8.5%, p<0.001), driven by a higher incidence of all-cause mortality in the PH group (33.3 vs 6.3%, p<0.001). The proportion of discharged patients was significantly lower in the PH group (29.2 vs 72.7%, p<0.001). Figure 1 shows the Kaplan-Meier curves for the composite of in-hospital all-cause mortality or ICU admission in patients with or without PH, confirming the significantly higher incidence in the pH group (log-rank p value of <0.001).
Figure 1.
In-hospital all-cause mortality or ICU admission in COVID-19 with or without PH and RVD. The figure shows Kaplan-Meier curves for in-hospital all-cause mortality or ICU admission in patients with COVID-19 with versus without PH (A) and with versus without RVD (B). ICU, intensive care unit; PH, pulmonary hypertension; RVD, right ventricular dysfunction.
The rate of the primary endpoint (17.2 vs 11.7%; p=0.404) and of all-cause mortality (13.8 vs 8.8%; p=0.489) were not significantly different in patients with or without RVD. The proportion of discharged patients was similar between patients with or without RVD (58.6 vs 69.0%; p=0.270). As shown in figure 1, Kaplan-Meier analysis showed no significant difference in the composite of in-hospital all-cause mortality or ICU admission between RVD and no RVD groups (log-rank p=0.464).
At multivariable logistic regression analysis (online supplementary table 1), the significant association between PH and the composite of all-cause death or ICU admission remained after adjustment for age, sex, CXR severity score ≥4, high-sensitivity troponin T and PaO2/FiO2 on hospital admission (ORadj 4.98, 95% CI 1.24 to 19.94, p=0.023; C-statistic=0.889, H-L p=0.978).
heartjnl-2020-317355supp001.pdf (62.7KB, pdf)
Discussion
The main findings of our study are:
Among hospitalised patients with COVID-19 in a non-ICU setting, the observed prevalence of PH and RVD was 12.0% and 14.5%, respectively.
Patients with PH and RVD had more frequently a history of prior cardiac comorbidities; however, only patients with PH showed signs of more severe SARS-CoV-2 infection in terms of CXR lung damage, laboratory parameters, oxygenation status and need of NIV.
The composite of all-cause mortality or ICU admission was significantly higher among patients with PH (mainly driven by a higher mortality rate), and this association remained also after adjustment for selected covariates. Conversely, RVD was not associated with unfavourable in-hospital outcomes.
The association between severe acute respiratory failure and PH has been demonstrated by several studies, mainly focusing on critically ill patients treated in ICU setting.21–24 Secondary alterations of pulmonary vascular haemodynamics during acute respiratory distress syndrome (ARDS) are multifactorial, depending on hypoxia, vascular remodelling or compression by oedema or fibrosis, increased alveolar pressure, vasoconstriction, local thrombosis or pulmonary embolism, and reduced pulmonary compliance and use of PEEP.4 5 7 25 While the advanced stages of COVID-19 are characterised by severe ARDS and need of mechanical ventilation (and the mentioned pathophysiological mechanisms could be directly translated to such scenario), whether a less-advanced SARS-CoV-2 pneumonia could determine a significant change in pulmonary vascular haemodynamics leading to PH and RV involvement was unknown. In our study, the observed prevalence of PH and RVD among hospitalised non-ICU patients with confirmed COVID-19 was 12.0% and 14.5%, respectively. Of note, the cross-sectional nature of the study determined a systematic TTE assessment of unselected patients admitted to COVID-19 dedicated departments, hence limiting selection bias and allowing quantification of the actual prevalence of PH and RV involvement. As expected, the observed figures seem lower compared with previous studies exploring PH and RV failure among ARDS patients in ICU settings.22 26 27 Although the absence of positive-pressure ventilation-mediated changes in pulmonary haemodynamics could be implicated in the observed prevalence of PH and RVD in our population, most included patients fulfilled criteria of ARDS even though outside the ICU setting (median PaO2/FiO2 243 (132–314), bilateral lung infiltrates in 82.5% of patients).12 18 Therefore, the observed findings refer to a population with moderate–severe COVID-19, without critical lung involvement (requiring ICU admission) but also without mild SARS-CoV-2 pneumonia (likely not determining hospital admission).
A history of prior cardiac comorbidities was more frequent both in patients with PH and in those with RVD; similarly, biomarkers of cardiac involvement (high-sensitivity troponin T and NT-proBNP) were higher in both PH and RVD groups. Interestingly, however, only patients with PH had signs of more severe SARS-CoV-2 infection in terms of lung involvement (higher CXR severity score), laboratory assessment (lower lymphocyte count, higher D-dimer, interleukin-6, C reactive protein and lactate dehydrogenase), oxygenation status (lower SpO2/FiO2 and at TTE time) and need of NIV therapy. It could be speculated that while the presence of prior or concomitant cardiac disorders may be implicated in the occurrence of both PH and RVD in some patients with COVID-19, PH may better capture SARS-CoV-2-related cardiopulmonary dynamic changes. This could be particularly true in a non-critically ill patient population, where the initial pneumonia-related alterations in pulmonary vascular haemodynamics may determine only modest increase in pulmonary artery pressure, not enough to cause secondary RV failure. In addition, RV impairment has been more directly related to mechanical ventilation, a factor that is missing in our non-ICU population, thus eliminating a potential pathophysiological link between non-critical SARS-CoV-2 pneumonia and RV involvement.28 Despite being readily available at bedside, TAPSE and S’ wave may have not reached an adequate sensibility to identify also milder degrees of RV involvement in this cohort. Indeed, a recent study has identified RV longitudinal strain as a powerful predictor of adverse prognosis in patients with COVID-1911; in this cohort, TAPSE (but not S’ wave) identified patients with worse outcomes applying a cut-off of 23 mm, which is higher than that recommended for RV dysfunction diagnosis.11 15
In our study, only PH (and not RVD) had a significant impact on in-hospital all-cause mortality or ICU admission, driven by a higher mortality rate. This prognostic association remained also after adjustment for selected covariates that were significantly different between PH and no PH groups or that could impact on short-term prognosis of patients with COVID-19 (age, sex, CXR severity score ≥4, high-sensitivity troponin T and PaO2/FiO2 on hospital admission).
Interestingly, concomitant pulmonary thromboembolism was more frequently observed among patients with PH (77.8% of patients with available contrast-enhanced chest CT scan). The hypothesis that the observed laboratory findings in patients with PH (ie, higher D-dimer values) and the prognostic value of PH identification on TTE may be linked to concomitant pulmonary thromboembolism is intriguing, as preliminary reports suggest a high burden of thromboembolic complications during COVID-197 29 30; this hypothesis, however, should be substantiated with further dedicated studies.
Our study had an observational nature and, therefore, had all the usual limitations associated with this design. Furthermore, no independent adjudication of clinical events was performed; study endpoints were, however, hard clinical outcomes at low risk of assessment bias. The absence of core-laboratory analysis of echocardiographic data could have impacted on the observed prevalence of PH and RVD and, hence, on subsequent study findings. Another study limitation is the lack of a control population, since a comparison with a similar cohort of patients with non-SARS-CoV-2-related pneumonia could have allowed understanding of whether the observed prevalence of PH and RVD is expected (based on age, comorbidities and presence of pneumonia) or higher than expected (because of SARS-CoV-2-specific mechanisms). Moreover, the present study focused on PH and RVD only, not exploring other potential mechanisms and types of cardiac injury during COVID-19.
Conclusions
Among hospitalised non-ICU patients with COVID-19, the prevalence of PH and RVD was 12.0% and 14.5%, respectively. Both patients with PH and RVD presented more frequently with prior cardiac comorbidities. Only PH was associated with clinical, imaging and laboratory findings of more severe COVID-19 and with worse in-hospital clinical outcomes.
Key questions.
What is already known on this subject?
COVID-19 is characterised by prominent lung injury with associated thromboembolic phenomena and signs of myocardial damage.
What might this study add?
Among hospitalised non-critically ill patients with COVID-19, pulmonary hypertension (PH) is associated with signs of more severe severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and with worse in-hospital clinical outcome.
How might this impact on clinical practice?
Identification of PH could be prognostically relevant in hospitalised patients with COVID-19 and signs of more severe SARS-CoV-2 infection.
Footnotes
Twitter: @lucabaldetti, @ABeneduceMD, @MarioGramegnaMD, @AnnalisaRugger1, @giovannilandoni, @CiceriFabio
MP and LB contributed equally.
Contributors: The co-first authors (MP and LB) designed the study, performed data analyses, had full access to all the data in the study and takes responsibility for the content of the manuscript. MP, LB, AB, FC, MG, VP, GI and AN performed data acquisition and collection. MP, LB, RF, AR, SA, GM, PGC, PS, MT, GL, FC, AMS, EA and AC contributed to data interpretation and manuscript writing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: The COVID-BioB study (prospective, all-comers registry collecting clinical, laboratory, biologic and imaging data on all hospitalized COVID-19 patients) was approved by the Hospital Ethics Committee (protocol n. 34/int/2020).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The deidentified participant dataset generated during the current study is not publicly available and is not in a repository.
References
- 1. Guan W-jie, Ni Z-yi, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med Overseas Ed 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tian S, Hu W, Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020;15:700–4. 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inui S, Fujikawa A, Jitsu M, et al. Chest CT Findings in Cases from the Cruise Ship “Diamond Princess” with Coronavirus Disease 2019 (COVID-19). Radiology 2020;2:e200110 10.1148/ryct.2020200110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sylvester JT, Shimoda LA, Aaronson PI, et al. Hypoxic pulmonary vasoconstriction. Physiol Rev 2012;92:367–520. 10.1152/physrev.00041.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jardin F, Delorme G, Hardy A, et al. Reevaluation of hemodynamic consequences of positive pressure ventilation: emphasis on cyclic right ventricular afterloading by mechanical lung inflation. Anesthesiology 1990;72:966. 10.1097/00000542-199006000-00003 [DOI] [PubMed] [Google Scholar]
- 6. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–8. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc 2020;31:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020. 10.1001/jamacardio.2020.0950. [Epub ahead of print: 25 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol 2020;311:116–21. 10.1016/j.ijcard.2020.03.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC: Cardiovascular Imaging 2020. 10.1016/j.jcmg.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Available: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 13. Zangrillo A, Beretta L, Silvani P, et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19): interim guidance. Available: https://apps.who.int/iris/handle/10665/331498
- 15. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2015;28:1–39. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 16. Bossone E, D’Andrea A, D’Alto M, D'Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. Journal of the American Society of Echocardiography 2013;26:1–14. 10.1016/j.echo.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 17. Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of echocardiography developed in collaboration with the Society for cardiovascular magnetic resonance. J Am Soc Echocardiogr 2017;30:303–71. 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 18. Ranieri VM, Rubenfeld GD, ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:252–33. [DOI] [PubMed] [Google Scholar]
- 19. Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007;132:410–7. 10.1378/chest.07-0617 [DOI] [PubMed] [Google Scholar]
- 20. Wong HYF, Lam HYS, Fong AH-T, et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology 2019:201160. 10.1148/radiol.2020201160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med 1977;296:476–80. 10.1056/NEJM197703032960903 [DOI] [PubMed] [Google Scholar]
- 22. Ñamendys-Silva SA, Santos-Martínez LE, Pulido T, et al. Pulmonary hypertension due to acute respiratory distress syndrome. Braz J Med Biol Res 2014;47:904–10. 10.1590/1414-431X20143316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Squara P, Dhainaut JF, Artigas A, et al. Hemodynamic profile in severe ARDS: results of the European collaborative ARDS study. Intensive Care Med 1998;24:1018–28. 10.1007/s001340050710 [DOI] [PubMed] [Google Scholar]
- 24. Calcaianu G, Calcaianu M, Gschwend A, et al. Hemodynamic profile of pulmonary hypertension (pH) in ARDS. Pulm Circ 2018;8:2045893217753415. 10.1177/2045893217753415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J 2003;21:720–7. 10.1183/09031936.03.00120102 [DOI] [PubMed] [Google Scholar]
- 26. Beiderlinden M, Kuehl H, Boes T, et al. Prevalence of pulmonary hypertension associated with severe acute respiratory distress syndrome: predictive value of computed tomography. Intensive Care Med 2006;32:852–7. 10.1007/s00134-006-0122-9 [DOI] [PubMed] [Google Scholar]
- 27. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med 2001;29:1551–5. 10.1097/00003246-200108000-00009 [DOI] [PubMed] [Google Scholar]
- 28. Vieillard-Baron A, Jardin F. Why protect the right ventricle in patients with acute respiratory distress syndrome? Curr Opin Crit Care 2003;9:15–21. 10.1097/00075198-200302000-00004 [DOI] [PubMed] [Google Scholar]
- 29. Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation 2020. 10.1161/CIRCULATIONAHA.120.047430. [Epub ahead of print: 24 Apr 2020]. [DOI] [PubMed] [Google Scholar]
- 30. Bikdeli B, Madhavan M, Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2020-317355supp001.pdf (62.7KB, pdf)