A recent case report described the radiological features of a suspected COVID-19 necrotising haemorrhagic encephalopathy.1 We present here a description of clinical, biological, radiological and immunological features of a COVID-19 patient case, evocative of virus-associated acute necrotising encephalopathy (ANE) possibly mediated by antibodies. Patient’s representative consent has been obtained in agreement with the journal’s policy.
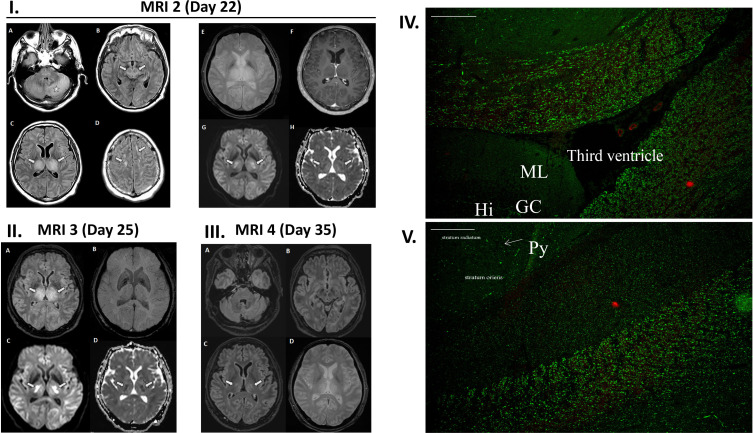
A 51-year-old man without personal or family history of neurological disease was hospitalised after 10 days of fever and cough. COVID-19 was diagnosed by reverse-transcriptase PCR on nasal swab and bilateral ground-glass opacities on thoracic CT scan. At day 12, he was admitted in intensive care unit (ICU) for non-invasive ventilation. The result of the neurological examination was normal. At day 21, while the patient had been weaned off oxygen, he became unresponsive and rapidly comatose (Glasgow Coma Scale 6: E1, V1, M4) with a disconjugated gaze. The patient was groaning and showing rhythmic movements of the right upper limb. An urgent brain MRI, including diffusion-weighted imaging (DWI) and MR angiogram, ruled out vertebrobasilar ischaemic stroke; gradient echo T2*-weighted images excluded haemorrhage and thrombus in the venous system. It revealed only subtle hyperintensities in bilateral thalami on FLAIR sequence (figure 1). Consciousness impairment required tracheal intubation. He was hyperthermic (39°C) without shock. Blood and cerebrospinal fluid (CSF) samples revealed thrombopenia and lymphopenia, mild inflammatory response (C reactive protein, ferritin and fibrinogen), CSF albumin-cytological dissociation with increased CSF IgG antibodies (91.9 mg/L, normal 10–30 mg/L) and altered blood–brain barrier integrity (CSF/serum albumin index=17.3, normal <6.5) (online supplementary material). An electroencephalogram revealed symmetrical background activity of low-voltage delta waves without spatial organisation, triphasic waves or paroxysmal activity. He showed unresponsive coma (Glasgow Coma Scale=3), pyramidal syndrome, right-sided sixth nerve palsy and no corneal reflex. A second brain contrast-enhanced MRI at day 22 revealed progressing lesions with diffuse hyperintense lesions in the thalami, cerebellum, brainstem, supratentorial grey and white matters on FLAIR images without gadolinium-enhanced lesions (figure 1).
jnnp-2020-323678supp001.pdf (20.5KB, pdf)
Figure 1.
(I–III) MRI of a 51-year-old man with acute encephalopathy. (I) MRI 1 is not shown. MRI 2 (day 22) 1 day after neuroICU admission. (A–D) Axial FLAIR images demonstrate diffuse hyperintense lesions in the cerebellum (star), brainstem (arrows on B), supratentorial grey and white matters (arrows on D), and bilateral and symmetrical lesions in the thalami (arrows on C). (E) Gradient echo T2-weighted image does not reveal any haemorrhage within thalamic lesions, and (F) post-gadolinium T1-weighted image does not show enhancement. (G) Diffusion-weighted image and (H) apparent diffusion coefficient map show mild hyperintensity in the thalami with heterogeneous and variable diffusion (arrows). (II) MRI 3 (day 25) 3 days after treatment initiation: (A) axial FLAIR demonstrates no extension of hyperintensity in both thalami (arrows) and (B) susceptibility-weighted image does not detect any haemorrhage. (C) Diffusion-weighted image and (D) apparent diffusion coefficient map now depict areas of restricted diffusion and cytotoxic oedema (arrows), a feature highly suggesting acute necrotising encephalopathy. (III) MRI 4 (day 35) 13 days after treatment initiation: (A–C) axial FLAIR images demonstrate significant reduction of hyperintensities with only residual lesions in bilateral thalami (arrows). (D) Gradient echo T2-weighted image shows no haemorrhage within the lesions. (IV, V) Indirect immunofluorescence (IF) patterns of patient’s IgG on rat hippocampal slices (×10 magnification, scale bar 100 µm). IF shows an unusual binding of IgG on specific areas in the fibre tracts, sparing the hippocampus and the cortex, (IV) around the ventricle, near the dentate gyrus and (V) close to the hippocampus Ammon’s horn. Hi, hile of the dentate gyrus; GC, granule cells of the dentate gyrus; ML, molecular layer; Py, pyramidal cells in the hippocampus Ammon’s horn.
At this time, the patient no longer had respiratory viral excretion (two negative RT-PCR at 48-hour interval on tracheal aspirations). A second CSF sampling revealed no meningitic cytological pattern and PCR results were negative for SARS-CoV-2 and other common viruses (online supplementary material). Anti-ganglioside autoantibodies were not found in patient’s serum (immunodot anti-ganglioside 5003; GA Generic Assays, Dahlewitz, Germany) and autoantibodies against myelin oligodendrocyte glycoprotein were not found in serum and CSF (cell-based assay, FA 1156-1003-50; EuroImmun, Lübeck, Germany). Anti-neuronal autoantibodies against intracellular (immunodot PNS9DIV-24; Ravo, Freiburg, Germany) and surface (cell-based assays: NMDAR, AMPAR, GABAbR, AMPA1/2, Caspr2, Lgi1, DPPX, FA 112d-1003-6; EuroImmun) neuronal antigens were negative in serum and CSF. Indirect immunofluorescence (performed by the immunological laboratory) revealed a specific and atypical IgG staining on monkey cerebellum slices (Ref 504225; Inova Diagnostic, San Diego, USA) and on rat hippocampal slices (EuroImmun) (figure 1). No specific staining was observed on other tissues (rat stomach, kidney and liver and on HeLa cells). On monkey cerebellum (data not shown), a staining around Purkinje cells, evoking basket cells, was associated with an unusual but weak staining of fibres in the inner part of the molecular layer. The granule cell layer and the rest of the molecular layer were not stained. On rat hippocampal slices, an unusual and strong staining was observed in the fibre tracts. Hippocampal structure was completely negative as well as some areas of this fibre tract adjacent to the hippocampus Ammon’s horn. However, other regions of the fibre network, as ventral hippocampal commissure, the brachium of the colliculus and the stria medullaris, seemed strongly stained (figure 1).
High-dose methylprednisolone (1 g.day−1 for 3 days) and intravenous polyvalent immunoglobulins (total dose: 2 g.kg-1 over 5 days) were initiated at day 22. A new MRI at day 25 showed stable lesions, except for a restriction in apparent diffusion coefficient in both thalami and supratentorial white matter (figure 1). Bilateral and symmetrical distribution of such lesions with a delayed onset of restricted diffusion allowed us to rule out stroke, but rather suggested an evolution towards necrosis. Neurological status improved, allowing weaning from mechanical ventilation after 5 days of treatment and discharge from ICU at day 29. The patient showed complete motor recovery. Comprehension was initially limited to simple motor commands. Verbal stereotypes and perseverations were observed at day 26. At day 35, a new MRI showed a significant improvement of previously observed brain lesions while clinical examination revealed fatigue, memory and attentional impairment.
The brain imaging abnormalities were here highly evocative of ANE, due to the bilateral distribution to brainstem, thalami, cerebellum and white matter, and FLAIR hyperintensities and restricted diffusion in bilateral thalami.2 Bilateral thalami damage is often a distinctive feature in ANE.2 Haemorrhage in these thalamic lesions is an additional MRI feature suggestive of ANE, but may be missing, without ruling out the diagnosis.3 Absence of haemorrhage in ANE seems to be associated to a better outcome.3 The appearance of diffusion restriction, evocative of necrosis on the third MRI, was missing on the first two, possibly because it was performed early. Clinical, biological and brain imaging findings may suggest an acute disseminated encephalomyelitis (ADEM). However, ADEM induces asymmetric lesions with ill-defined margins, mainly localised in periventricular regions and basal ganglia, with an hyperintense rim on DWI and a gadolinium enhancement.4 In this case, the lesions were rather well defined, symmetric and not gadolinium enhanced. Thiamine deficiency seemed unlikely as this patient was young, without risk factors and benefited from thiamine supplementation in ICU.
Respiratory virus-induced neuroimmunopathology due to a dysregulation of host immune response has been described, in particular for ANE.2 This can be induced by the cytokinic storm secondary to viral infections,2 5 a molecular mimicry between virus and neuronal antigens or a replication-induced neurotoxicity. This case does not support the latter. Even if the immunological data presented here cannot ascertain the causality of the neurological disease, neurotoxicity triggered by host immune response to COVID-19, associated with IgG targeting a neuronal antigen present in fibre tracts, is suspected here. These antibodies may target an autoantigen through molecular mimicry with the virus.2 Cell destruction, releasing large amounts of autoantigens, may also stimulate self-reactive cells and lead to self-reactive antibodies. The inflammatory storm described after COVID-19 infection could have contributed to this IgG production and blood–brain barrier opening, thus causing an ANE.2
The fulgurant evolution of lesions on MRI should alert clinicians on the interest of repeating brain imaging in case of impaired consciousness after COVID-19 infection. The remarkable efficacy of an early treatment by high-dose steroids and polyvalent immunoglobulin to stop the process should invite clinicians to consider it as soon as a central nervous system infection has been ruled out. COVID-19-mediated ANE with IgG antibodies emerging from peripheral tissues and targeting the cerebral fibre network around basal ganglia is a possible new entity that should be further studied.
Footnotes
Twitter: @louisdelamarre
Collaborators: The members of the NeuroICU Research Group are Diane Osinski, MD; Ségolène Mrozek, MD, PhD; Edouard Naboulsi, MD; Maxime Pommier, MD; Maud Prezman-Pietri, MD; Maxime Beilvert, MD; Vincent Minville, MD, PhD; and Samuel Groyer, MD.
Contributors: LD, CGo, FG, GJ, CGa and EA wrote the first draft. JD and FB ensured neuroradiological analysis and editing. GG, PD and GM-B wrote the infectiology section, analysis of literature and corrections. CB and FF performed and analysed the immunological assays. CGo and JP supervised the diagnostic process and patient neurological expert assessment. TG and LD supervised the manuscript writing and edited the final version. Other authors ensured corrections and literature analysis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
NeuroICU Research Group:
Diane Osinski, Ségolène Mrozek, Edouard Naboulsi, Maxime Pommier, Maud Prezman-Pietri, Maxime Beilvert, Vincent Minville, and Samuel Groyer
References
- 1. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu X, Wu W, Pan W, et al. Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediators Inflamm 2015;2015:1–10. 10.1155/2015/792578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong AM, Simon EM, Zimmerman RA, et al. Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. AJNR Am J Neuroradiol 2006;27:1919–23. [PMC free article] [PubMed] [Google Scholar]
- 4. Bookstaver PB, Mohorn PL, Shah A, et al. Management of viral central nervous system infections: a primer for clinicians. J Cent Nerv Syst Dis 2017;9:117957351770334. 10.1177/1179573517703342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2020-323678supp001.pdf (20.5KB, pdf)