Abstract
Background
Six melancholic features (MFs) of the Hamilton Depression Rating Scale (HAM-D6) represent the construct of melancholia along a continuum of severity (from least to most severe: depressed mood, work and activities, somatic symptoms, psychic anxiety, guilty feelings, psychomotor retardation). We aimed to evaluate the association between these MFs and inflammatory cytokines (IC) in the blood.
Methods
Each IC [interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin 2 (IL-2), IL-4, IL-6, IL-10, and IL-17] was associated with the HAM-D6 MFs of 139 severely depressed inpatients, using multiple linear regressions adjusted for covariates. Levels were compared with those of 100 healthy controls.
Results
Depressed mood was associated with higher levels of IL-4 (β = 0.167; p = 0.041). Psychic anxiety: lower IL-17 levels (β = –0.173; p = 0.039). Guilt feelings: lower IL-2 levels (β = −0.168; p = 0.041) Psychomotor retardation: higher IL-6 levels (β = 0.195; p = 0.017). Depressed patients’ TNF-α, INF-γ, and IL-4 levels were not significantly different from controls. Depressed patients’ IL-2, IL-6, IL-10, and IL-17 levels were higher than those of controls (p <0.001).
Conclusion
Less severe MFs (depressed mood, psychic anxiety, and guilt feelings) were associated with an anti-inflammatory pattern (higher IL-4, lower IL-17 and lower IL-2, respectively). The presence of the most severe MF, psychomotor retardation, was associated with a higher pro-inflammatory response (higher IL-6).
Keywords: cytokines, inflammation, major depressive disorder, melancholia, psychomotor performance
Introduction
Major depressive disorder (MDD) is one of the leading causes of disability, and is among the most prevalent of psychiatric disorders worldwide.1,2 Several clinical trials, such as the STAR*D trial, showed that about one-third of MDD patients did not achieve remission after multiple trials with different antidepressants; additionally, most antidepressant drugs act, at least in part, by increasing monoaminergic transmission.3 Hence, in the search for novel therapeutic targets to treat MDD, there is growing interest on the role of inflammation and the immune system in MDD pathogenesis.4,5
There is significant pre-clinical and clinical evidence that the immune system contributes to the pathological expression of MDD. It has been consistently shown that higher levels of pro- and anti-inflammatory cytokines are associated with MDD.3,6–10 Other evidence supports the idea that depressive symptoms can be induced by pro-inflammatory cytokines, for example, the interferon (IFN) treatment among patients affected by the hepatitis C virus.11 Furthermore, inflammatory somatic disorders, such as psoriasis, inflammatory bowel disease, and rheumatoid arthritis, are highly comorbid with MDD, and recent large randomized trials revealed that treatments that targeted inflammatory cytokines were associated with greater reductions in depressive symptoms than other treatments.12,13 Recent meta-analyses of randomized clinical trials showed that anti-cytokine treatment was superior to placebo for depressive symptoms among patients with chronic inflammatory conditions.14,15 Finally, it has been proposed that the relationship between MDD and the immune system can be attributed to the hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis as a result of anomalous feedback inhibition from endogenous glucocorticoids.16
Most studies that evaluated pro- and anti-inflammatory cytokines focused on comparing their levels between patients and controls, not testing which MDD signs and symptoms were implicated in the immune dysregulation.17 Cumulative meta-analysis showed that the most consistent findings of cytokine changes in patients with MDD were higher levels of interleukin (IL)-6, and C-reactive protein compared with controls.18 Another meta-analysis of 82 studies showed that patients with MDD have higher levels (compared with controls) of IL-6, IL-2, IL-10, IL-13, IL-18, tumor necrosis factor (TNF) alpha (-α), C-C chemokine ligand 2, the IL-1 receptor antagonist, and the soluble TNF receptor 2.19 The same study showed that the same patients had lower levels of IFN gamma (-γ).19 Nevertheless, a growing body of evidence suggests that the individual Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) criteria for MDD have different risk factors and genetic backgrounds.20–22 In addition, although the melancholic features of depression are associated with genetic and biological determinants and abnormalities in the HPA axis,23–25 few studies have evaluated changes in inflammatory cytokines among patients with those signs and symptoms.26,27 Also, those studies were restricted to small sample sizes, and, as far as we know, no study aimed to test specifically which melancholic features were associated with higher or lower cytokine levels in a more homogeneous subgroup of patients and using a melancholia rating scale. One study, however, aimed to test the association between the Hamilton Depression Rating Scale (HDRS-17) and the Beck Depression Inventory (BDI-II) with some ILs, TNF-α, and IFN-γ in a sample of 30 inpatients and outpatients and found that a somatic-affective factor of BDI-II (loss of pleasure, interest, energy, or interest in sex; agitation; indecisiveness; changes in sleeping pattern or appetite; irritability; concentration difficulty; fatigue) were negatively associated with all the cytokines that were tested.17
Regarding the clinical evaluation of melancholic depression, the specifiers of melancholia seem too non-specific in the DSM-5. These identifiers do not enable practitioners to evaluate distinct depression subtypes;28 even the DSM-5 criteria for MDD can result in 1482 different presentations of the same syndrome.29 In contrast, six items of the HDRS-17, (HAM-D6) were proposed to measure the melancholic features that were traditionally correlated with biological substrates.30–33 The six items of the HAM-D6 are depressed mood, feelings of guilt, impairment of work and activities, psychomotor retardation, general somatic symptoms, and psychic anxiety.33 A recent systematic review showed that the HAM-D6 is superior to the HDRS-17 in detecting symptomatic changes of depression after biological treatments.34 Furthermore, we could show, through the item-response theory, that these six items could represent a homogeneous construct (i.e. melancholic depression) and that these features could be ordered by their severity.35 According to the item-response theory (a statistical model derived from educational sciences), signs and symptoms of a rating scale could be compared with a school test, where there are easier and more difficult questions. The most difficult questions (more severe symptoms) would discriminate better the ones with better performance (more depressed patients) than the others.35 Hence, only the most severe patients would be the more likely to score higher in the most severe signs and symptoms. We showed that depressed mood and impaired work and activities are the least severe melancholic symptoms, followed by somatic symptoms and psychic anxiety in the middle, and guilt feelings and psychomotor retardation at the top. Since the most severe signs and symptoms may be only present in the most severe cases, we believe that they may be more representative of a biological substrate of depression. For example, depressed mood was computed as the less severe symptom of MDD, probably because it is a ‘filter symptom’, since MDD patients have to present with depressed mood or anhedonia to fulfill the DSM-5 diagnostic criteria.35,36 However, only the presence of depressed mood may not be representative of a biological substrate, since multiple presentations of MDD are possible even though all patients present with depressed mood. So, a homogeneous continuum of signs-and-symptoms severity may address this issue. A previous study also showed that the most severe feature of the HAM-D6, psychomotor retardation, is associated with lower brain-derived neurotrophic factor (BDNF) levels.37
Aims of the study
The objective of this study was to evaluate the association between melancholic features, measured through the melancholic subscale of the HDRS (six items), and cytokine levels, more specifically, TNF-α, IFN- γ, IL-2, IL-4, IL-6, IL-10, and IL-17. Our primary hypothesis was that higher levels of the pro-inflammatory cytokines (TNF-α, IFN-γ, IL-2, IL-6, and IL-17) and lower levels of anti-inflammatory cytokines (IL-4 and IL-10) would be associated with the more severe features of melancholia.
Material and methods
We conducted a cross-sectional study with a healthy control group. Inpatients were recruited from the Hospital de Clínicas de Porto Alegre, located in southern Brazil, between May 2011 and October 2013. The Hospital de Clínicas de Porto Alegre is a university-associated general tertiary care hospital and is a psychiatry referral center in its region of Brazil. Its psychiatry unit is located within the general hospital and serves patients from the public health system as well as those with health insurance plans.
For this study, the included patients were inpatients ⩾18 years of age who were diagnosed with MDD by the DSM-IV criteria using the Mini International Neuropsychiatric Interview (MINI) on the day of the admission.38 Structured interviews were performed by one member of a team of psychiatrists who were not involved in the patients’ care. Both patients with unipolar depression and patients in the depressive state of bipolar disorder were included in the analysis. We excluded patients with: hospital stays <7 days; drug or alcohol addiction or dependence as the main diagnosis; pregnancy or breastfeeding; acute or chronic infectious, autoimmune, neoplastic, or endocrine disease; or the occurrence of myocardial infarction, or other major cardiovascular disorders in the last 6 months.
After the diagnosis of MDD, the symptomatology of MDD was evaluated through the HDRS-17,39 HAM-D6 (melancholic subscale of HDRS-17), and Clinical Global Impression (CGI) scale. The items 1, 2, 7, 8, 10, and 13 of HDRS-17 compose the HAM-D6, that is, depressed mood, guilt feelings, work and activities, psychomotor retardation, psychic anxiety, and somatic symptoms.36 The items 1, 2, 7, 8 and 10 are rated from 0 to 4, while item 13 is rated from 0 to 2. The sum of scores can provide cut-points of severity according to CGI evaluated by experienced psychiatrists: 0–4: no depression; 5–6: doubtful; 7–8: mild depression; 9–11: moderate depression; 12–22: severe depression.36 Other demographic and clinical variables were also collected: years of study (continuous variable; it represents the amount of years that the patient stayed in formal education); smoking; use of alcohol; previous suicide attempt; past manic or hypomanic episode; antidepressant use (all of them were yes/no variable); ethnicity and marriage status.
Also, we collected venous blood samples (10 ml) by venipuncture into an anti-coagulant-free tube. The samples were centrifuged at 4000g for 10 min and serum was collected and stored at −80°C. Serum cytokine concentrations were determined by flow cytometry using the BD™ cytometric bead array T-helper cell 1 (Th1)/Th2/Th17 Human Cytokine Kit (BD Biosciences, San Diego, CA, USA). This kit allows the discrimination of the anti-inflammatory cytokines IL-4 and IL-10, and the pro-inflammatory cytokines, IL-2, IL-6, TNF-α, IFN-γ, and IL-17. We used a FACSCalibur flow cytometer (BD Biosciences) for sample processing and data analyses. The results were generated in graphical and tabular formats using the FCAP Array™ cytometric bead array analysis software (BD Biosciences).
One hundred healthy blood donors were invited to participate as a healthy control group. A psychiatrist not affiliated with the hematologic center conducted the same structured interviews using MINI and, if the subjects met the diagnostic criteria for any psychiatric disorder, they were excluded from the study. Controls were also excluded if they were using any psychiatric medications at the time of the interview. Inflammatory marker measurements were conducted after screening the participants’ medical histories, physical examinations, and laboratory tests in the Brazilian hematologic center of the Federal University of Rio Grande do Sul.
Both patients and controls signed a written informed consent form before entering the study. The Comissão Científica e Comitê de Ética em Pesquisa from the Hospital de Clínicas de Porto Alegre approved the study (approval number 10-0265).
Statistical analyses were performed using the SPSS® version 24.0 software package (IBM Corporation, Armonk, NY, USA). Patients’ demographic data were described using means and standard deviations (for normal data), medians and interquartile ranges (IQRs; for non-normal data), or frequencies. The normality of the data was assessed via the Shapiro–Wilk normality test. Bivariate Spearman correlations or Mann–Whitney U tests evaluated the association of potential confounders with cytokine levels and entered the multivariate analysis if p >0.2. We performed multiple linear regressions with each cytokine as the outcome variable, and the HAM-D6 variables as predictors corrected by covariates among patients with MDD. The variables considered as potential confounders were sex, age (in years), marital status, years of study (in years), ethnicity, body mass index (BMI), bipolar versus unipolar depression, current use of tobacco, use of alcohol, use of antidepressants, use of antipsychotics, use of anticonvulsants, or use of lithium. All drugs or substance use were binary variables (yes/no). Cytokine values were normalized using a logarithmic transformation. We used the backward method, regarding the HAM-D6 variables, and covariates were not excluded from the models. We stopped performing models when either all predictors (HAM-D6) had a p value <0.05 or no variables were statistically significant. We checked the assumptions of the regression models by the visual inspection of the residual plots and Shapiro–Wilk normality tests of the residuals.
The levels of the patients’ inflammatory cytokines were compared with those of the controls. As the variables did not fulfill the normality assumptions, we compared the groups using the Mann–Whitney U test. All data were considered significant (two tailed) at p <0.05.
Results
A total of 139 MDD inpatients were included in the analysis. The patients and controls’ demographic data are presented in Table 1. Most of the patients were women with at least one previous psychiatric hospitalization and at least one previous suicide attempt. Most patients had a baseline CGI of 5 or more (markedly ill) and a mean HDRS-17 score of 22.57 (±6.74).
Table 1.
Demographic and clinical information among severely depressed inpatients and healthy controls.
| Patients (n = 139) | Controls (n = 100) | p value | |
|---|---|---|---|
| Age, median (IQR) | 43 (33–55) | 31 (25–42) | <0.001 |
| Sex | |||
| Female, n (%) | 81 (58.3) | 44 (44) | 0.03 |
| Ethnicity | |||
| White, n (%) | 115 (82.7) | 83 (83) | 0.9 |
| Non-White, n (%) | 24 (17.3) | 17 (17) | |
| Marriage status, n (%) | |||
| Single | 45 (32.4) | 42 (42) | <0.001 |
| In a relationship/married | 59 (42.4) | 53 (53) | |
| Separated | 27 (19.4) | 5 (5) | |
| Widowed | 8 (5.8) | 0 (0) | |
| Years of study, median (IQR) | 10 (6–11) | 11 (11–15) | <0.001 |
| Smoking, n (%) | 41 (34.3) | 18 (24.7) | 0.04 |
| Use of alcohol, n (%) | 24 (17.3) | 75 (75) | <0.001 |
| Number of previous psychiatric admissions, median (IQR) | 1 (0–3.25) | – | |
| Reported previous suicide attempts, n (%) | 90 (64.7) | – | |
| Past manic or hypomanic episode, n (%) | 48 (34.5) | – | |
| HDRS-17, mean (±SD) | 22.57 (±6.74) | – | |
| HAM-D6, mean (±SD) | 10.82 (3.63) | – | |
| CGI, median (IQR) | 5 (4–5) | – | |
| Antidepressant Use, n (%) | 53 (38.8) | – | |
| SSRI, n (%) | 50 (23.74) | ||
| SNRI, n (%) | 5 (3.60) | ||
| TCA, n (%) | 12 (8.63) | ||
| Other, n (%) | 6 (4.32) | ||
| Antipsychotic use, n (%) | 62 (44.60) | – | |
| First generation | 36 (25.90) | ||
| Second generation | 48 (34.53) | ||
| Anticonvulsants use, n (%) | 25 (17.98) | – | |
| Carbamazepine | 5 (2.00) | ||
| Lamotrigine | 2 (1.43) | ||
| Valproate | 19 (13.67) | ||
| Lithium use, n (%) | 25 (17.98) | – | |
CGI, Clinical Global Impression; HDRS-17, Hamilton Depression Rating Scale 17; HAM-D6, six items of the HDRS-17; IQR, interquartile range; SD, standard deviation; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitors; TCA, tricyclic antidepressant.
The patients’ and controls’ inflammatory cytokines levels are shown in Table 2. The levels of TNF-α, IFN-γ, and IL-4 among patients were not statistically significantly different from those of controls; however, the levels of IL-2, IL-6, IL-10, and IL-17 were higher among patients than controls.
Table 2.
Comparison of inflammatory cytokines between patients and controls.
| Patients (n = 139) | Controls (n = 100) | p value | |
|---|---|---|---|
| TNF-α pg/ml, median (IQR) | 1.91 (1.47–2.30) | 1.8 (1.4–2.15) | 0.15 |
| IFN-γ pg/ml, median (IQR) | 1.41 (1.1–1.77) | 1.52 (1.17–1.79) | 0.22 |
| IL-2 pg/ml, median (IQR) | 1.03 (0.84–1.25) | 0.81 (0.67–1) | <0.001 * |
| IL-4 pg/ml, median (IQR) | 0.51 (0.4–0.68) | 0.48 (0.37–0.59) | 0.09 |
| IL-6 pg/ml, median (IQR) | 2.73 (1.34–6.18) | 0.69 (0.48–1.19) | <0.001 * |
| IL-10 pg/ml, median (IQR) | 0.89 (0.67–1.16) | 0.52 (0.42–0.7) | <0.001 * |
| IL-17 pg/ml, median (IQR) | 54.28 (35.89–71.56) | 26.93 (20.7–45.34) | <0.001 * |
p <0.05 in the Mann–Whitney U test.
IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
We found no significant association between the potential confounders in bipolar versus unipolar depression, current use of tobacco, use of alcohol, use of antidepressants, use of antipsychotics with any cytokine. However, the following confounders remained significant (p >0.2), which we used as covariates: sex, BMI and use of anticonvulsants for IFN; age, BMI and years of study for IL-10; age, BMI, years of study, marital status and use of lithium for IL-6; use of anticonvulsant for IL-4; age, sex and use of anticonvulsant for IL-2; age and years of study for IL-17. Hence, these variables remained in the final model of the multivariate analysis. Overall severity of depression measured by HAM-D6 was not associated with any of the studied cytokines, either on simple non-parametric correlations or on adjusted models for covariates (Supplemental material). Results of initial and final multivariate models regarding association between individual melancholic features and cytokines are presented in Table 3.
Table 3.
Multivariate models associating melancholic features of depression and cytokines adjusted for confounders among depressed patients.
| Cytokine | Initial model | Final model | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | β | B (SE) | p value | Variables | β | B (SE) | p value | |
| INF-γ | Sex | −0.12 | −0.15 (0.11) | 0.16 | Sex | −0.09 | −0.12 (0.11) | 0.26 |
| BMI | −0.15 | −0.02 (0.01) | 0.07 | BMI | −0.15 | −0.02 (0.01) | 0.08 | |
| Use of anticonvulsant | 0.30 | 0.54 (0.15) | <0.001 | Use of anticonvulsant | 0.26 | 0.46 (0.14) | 0.002 | |
| Depressed mood | 0.18 | 0.09 (0.05) | 0.08 | |||||
| Work and activities | 0.08 | 0.05 (0.05) | 0.36 | |||||
| Somatic symptoms | −0.12 | −0.11 (0.09) | 0.20 | |||||
| Psychic anxiety | 0.14 | 0.09 (0.06) | 0.13 | |||||
| Guilt feelings | −0.08 | −0.04 (0.05) | 0.40 | |||||
| Psychomotor retardation | −0.08 | −0.06 (0.07) | 0.36 | |||||
| TNF-α | Depressed mood | 0.06 | 0.03 (0.05) | 0.58 | – | |||
| Work and activities | −0.04 | −0.02 (0.04) | 0.70 | |||||
| Somatic symptoms | 0.07 | 0.05 (0.08) | 0.50 | |||||
| Psychic anxiety | −0.05 | −0.02 (0.05) | 0.62 | |||||
| Guilt feelings | −0.15 | −0.07 (0.05) | 0.12 | |||||
| Psychomotor retardation | −0.12 | −0.07 (0.06) | 0.20 | |||||
| IL-10 | Age | 0.11 | 0.01 (0.01) | 0.27 | Age | 0.10 | 0.01 (0.01) | 0.31 |
| BMI | 0.12 | 0.02 (0.01) | 0.22 | BMI | 0.14 | 0.02 (0.01) | 0.12 | |
| Years of study | −0.002 | 0.00 (0.02) | 0.98 | Years of study | −0.003 | −0.001 (0.02) | 0.97 | |
| Depressed mood | 0.17 | 0.10 (0.07) | 0.16 | |||||
| Work and activities | 0.08 | 0.05 (0.07) | 0.46 | |||||
| Somatic symptoms | −0.10 | −0.11 (0.11) | 0.34 | |||||
| Psychic anxiety | −0.01 | −0.01 (0.08) | 0.95 | |||||
| Guilt feelings | −0.11 | −0.08 (0.07) | 0.28 | |||||
| Psychomotor retardation | 0.06 | 0.05 (0.09) | 0.58 | |||||
| IL-6 | Age | 0.36 | 0.03 (0.01) | <0.001 | Age | 0.36 | −1.48 (0.77) | <0.001 |
| BMI | 0.14 | 0.03 (0.02) | 0.09 | BMI | 0.15 | 0.03 (0.02) | 0.12 | |
| Use of lithium | 0.06 | 0.20 (0.33) | 0.79 | Use of lithium | 0.07 | 0.23 (0.32) | 0.47 | |
| Years of study | −0.10 | −0.03 (0.03) | 0.30 | Years of study | −0.10 | −0.03 (0.03) | 0.26 | |
| Marriage status Single |
Ref. | Marriage status Single |
Ref. | |||||
| In a relationship/married | −0.19 | −0.47 (0.26) | 0.07 | In a relationship/married | −0.18 | −0.43 (0.26) | 0.09 | |
| Separated | −0.11 | −0.38 (0.39) | 0.33 | Separated | −0.10 | −0.38 (0.38) | 0.33 | |
| Widowed | 0.10 | 0.71 (0.69) | 0.30 | Widowed | 0.10 | 0.73 (0.67) | 0.28 | |
| Depressed mood | 0.13 | 0.13 (0.11) | 0.23 | Psychomotor retardation | 0.21 | 0.30 (0.12) | 0.02 | |
| Work and activities | 0.05 | 0.05 (0.10) | 0.63 | |||||
| Somatic symptoms | −0.02 | −0.04 (0.17) | 0.82 | |||||
| Psychic anxiety | −0.07 | −0.09 (0.12) | 0.42 | |||||
| Guilt feelings | −0.03 | −0.03 (0.10) | 0.74 | |||||
| Psychomotor retardation | 0.16 | 0.22 (0.13) | 0.09 | |||||
| IL-4 | Use of anticonvulsant | 0.33 | 0.62 (0.16) | <0.001 | Use of anticonvulsant | 0.3 | 0.57 (0.05) | <0.001 |
| Depressed mood | 0.18 | 0.10 (0.06) | 0.08 | Depressed mood | 0.17 | 0.09 (0.05) | 0.04 | |
| Work and activities | −0.006 | −0.01 (0.05) | 0.95 | |||||
| Somatic symptoms | 0.10 | 0.10 (0.09) | 0.30 | |||||
| Psychic anxiety | 0.08 | 0.06 (0.06) | 0.34 | |||||
| Guilt feelings | −0.15 | −0.09 (0.06) | 0.09 | |||||
| Psychomotor retardation | −0.03 | −0.02 (0.07) | 0.77 | |||||
| IL-2 | Age | 0.07 | 0.01 (0.01) | 0.39 | Age | 0.08 | 0.01 (0.01) | 0.33 |
| Sex | −0.13 | −0.13 (0.09) | 0.13 | Sex | −0.1 | −0.11 (0.08) | 0.21 | |
| Use of anticonvulsant | 0.32 | 0.45 (0.12) | <0.001 | Use of anticonvulsant | 0.31 | 0.43 (0.11) | <0.001 | |
| Depressed mood | 0.05 | 0.02 (0.04) | 0.60 | Guilt feelings | −0.17 | −0.08 (0.04) | 0.04 | |
| Work and activities | −0.04 | −0.02 (0.04) | 0.63 | |||||
| Somatic symptoms | −0.10 | −0.07 (0.07) | 0.30 | |||||
| Psychic anxiety | 0.08 | 0.04 (0.05) | 0.35 | |||||
| Guilt feelings | −0.16 | −0.08 (0.04) | 0.07 | |||||
| Psychomotor retardation | −0.08 | −0.05 (0.05) | 0.39 | |||||
| IL-17 | Age | 0.19 | 0.01 (0.01) | 0.05 | Age | 0.17 | 0.01 (0.01) | 0.07 |
| Years of study | −0.05 | −0.01 (0.01) | 0.59 | Years of study | −0.05 | −0.01 (0.01) | 0.57 | |
| Depressed mood | 0.02 | 0.01 (0.06) | 0.83 | Psychic anxiety | −0.17 | −0.11 (0.05) | 0.04 | |
| Work and activities | −0.02 | −0.01 (0.06) | 0.86 | |||||
| Somatic symptoms | 0.01 | 0.01 (0.10) | 0.93 | |||||
| Psychic anxiety | −0.16 | −0.10 (0.07) | 0.13 | |||||
| Guilt feelings | −0.04 | −0.02 (0.06) | 0.68 | |||||
| Psychomotor retardation | 0.10 | 0.08 (0.08) | 0.30 | |||||
BMI, body mass index; IL, interleukin; IFN, interferon; Ref., reference; SE, standard error; TNF, tumor necrosis factor.
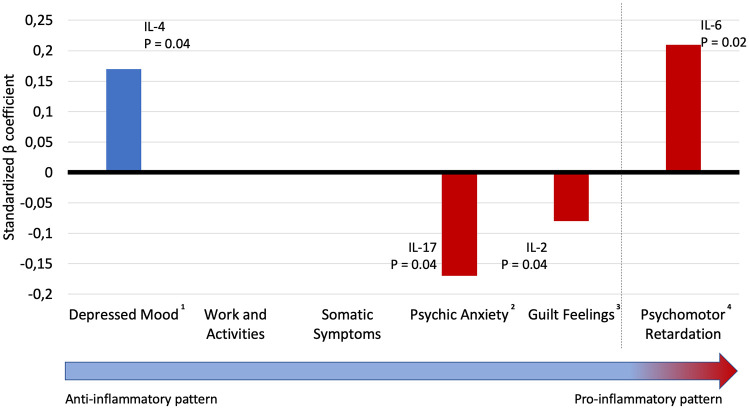
Figure 1 shows the correlations between the cytokines and the melancholic features of depression, ordered by severity, and adjusted for significant confounders. Depressed mood was positively associated with higher IL-4 levels (β = 0.17; p = 0.041). Psychic anxiety was associated with lower levels of IL-17 (β = –0.173; p = 0.039). Guilt feelings were associated with lower levels of IL-2 (β = 0.168; p = 0.041). The presence of psychomotor retardation was positively associated with IL-6 (β = 0.195; p = 0.017). No other models had significant associations between melancholic features and cytokines.
Figure 1.
Correlations between inflammatory cytokines and melancholic features of depression adjusted for confounders.
Blue bars represent an anti-inflammatory cytokine and the red bar represents a pro-inflammatory cytokine.
1Adjusted for use of anticonvulsants.
2Adjusted for age.
3Adjusted for sex, age and use of anticonvulsant.
4Adjusted for use of age, body mass index and use of lithium.
IL, interleukin.
Discussion
To our knowledge, this is the first study that aims to evaluate the association between the melancholic features of depression and inflammatory cytokines in a sample of severely depressed inpatients. Previously, we have shown that the melancholic features measured by the HAM-D6 are a homogeneous group of signs and symptoms and that can be ordered by their severity.35,36 In the present study, the main finding was that less severe melancholic symptoms (depressed mood, psychic anxiety, and guilt feelings) were associated with an anti-inflammatory response (higher levels of IL-4 and lower levels of IL-17 and IL-2). In contrast, the most severe melancholic feature, psychomotor retardation, was associated a pro-inflammatory profile, that is, higher levels of IL-6.
The finding that depressed mood, psychic anxiety, and guilt feelings were positively associated with IL-4 negatively associated with IL-17 and IL-2, respectively, suggests that an anti-inflammatory response is present in the neurobiology of MDD, but only for the less severe melancholic symptoms. This can be explained by the theory that there is an imbalance between the cytokines produced by Th1 lymphocytes (INF-γ, TNF, IL-2; pro-inflammatory) and Th2 lymphocytes (IL-4, IL-10; anti-inflammatory) among patients with MDD.40 These changes, in which there is a preponderance of Th2 over Th1, could be also mediated by elevated cortisol levels in the initial phases of MDD.41 Therefore, one possible explanation for our findings is that there is an anti-inflammatory response in the early stages of MDD (perhaps mediated through high cortisol levels due to stress), but that it becomes a high pro-inflammatory response among patients in the most severe stages (i.e. those who present with psychomotor retardation). However, we should note that, although higher levels of IL-4 were present among patients with less severe symptoms, patients’ IL-4 levels were not different from controls. In contrast, although patients with MDD presented with higher levels of IL-10 than controls, we found no specific melancholic feature that was responsible for this increase. Indeed, even other clinical and demographic data that we elected as potential confounders remained significant in the multivariate model for this cytokine. The fact that an anti-inflammatory cytokine was positively associated with melancholic features suggests that cytokines are not necessarily ‘pro-depressive’, but may be related to a specific regulation of signs and symptoms of depression.17 On the other hand, the fact that we found no variable that could explain higher levels of IL-10 suggests that there remain unknown variables responsible for immune dysregulation among patients with MDD.
Also, we found that psychomotor retardation was associated with higher levels of IL-6. Firstly, there is robust evidence that this clinical sign is a specific feature of melancholia and may reflect its underlying pathophysiology.42–44 Further, recent studies showed that the presence of psychomotor retardation is associated with lower levels of BDNF37 and that higher levels of inflammatory cytokines (such as IL-6 and IL-10) are associated with decreased psychomotor speed.45 Regarding IL-6, it is the most consistently elevated interleukin in MDD; IL-6 levels among MDD patients are higher than those of controls.3,9,10 In addition, there is evidence that associates higher levels of IL-6 and the severity of depressive symptoms among patients with MDD with melancholic features.27 IL-6 typically acts together with TNF-α and is usually secreted by T cells and macrophages.4 Also, IL-6 seems to induce the repression of BDNF, implicating IL-6 in the development of MDD.46 Thus, our findings corroborate the literature in that psychomotor retardation may be a clinical feature that represents an underlying neurobiological mechanism in the pathogenesis and maintenance of MDD.47
Our study has some limitations. First, all severely depressed inpatients were included in the analysis, without any specific criteria for selecting the melancholic subtype of depression. This decision was made based on the rationale that, although not all severe depression is necessarily melancholic, we could find more melancholic features in a sample of depressed inpatients than in other settings, even as proposed by the DSM-5.48 Further, there is no highly specific classification system for detecting melancholia. However, by using this strategy, we may have created a conservative bias against our hypothesis. Therefore, the inclusion of non-melancholic patients in the analysis could decrease the strength of the association between our variables and explain the small magnitude of the effect found in our study. Second, our main hypothesis was to evaluate whether cytokines were associated with individual melancholic signs and symptoms, in order that our control group was useful for evaluating demographic differences between our sample of severely depressed inpatients and baseline levels of cytokines. However, conclusions on differences between cases and controls can hardly be drawn based on unadjusted comparisons. Future studies could elucidate this hypothesis.
Third, we decided not to use any specific correction for multiple comparisons in the statistical analysis. This is consistent with the rationale that the emphasis on null hypothesis significance testing may harm the reproducibility of the findings and does not incorporate prior information from the literature, as Bayesian analysis does.49 Recently, we also highlighted the harm that the correction for multiple statistical testing can make for psychiatric research.50 Since this is a novel and preliminary study that evaluated several inflammatory markers among six melancholic features, we decided to keep the crude p values, which would be replicated later in other samples. We suggest that further studies may incorporate our findings when reproducing the results and may compute the estimative effects more precisely. However, the possibility of alpha error among some of the associations between the inflammatory markers and melancholic features cannot be ruled out. Finally, we did not control our analysis to other potential non-measured variables, such as anti-inflammatory drugs.
Conclusion
In short, we demonstrated that there are different inflammatory patterns among the different melancholic features of MDD. This finding helps to further elucidate the pathophysiology of MDD, indicating that there is a trend toward an anti-inflammatory response among less severe symptoms (depressed mood) and a high inflammatory response in the most severe clinical feature (psychomotor retardation). We believe that, supported by previous evidence and our present findings, these findings could help practitioners to integrate biological markers and clinical features in the search for novel therapeutic agents to treat MDD.
Supplemental Material
Supplemental material, Supplement for Different cytokine patterns associate with melancholia severity among inpatients with major depressive disorder by Lucas Primo de Carvalho Alves and Neusa Sica da Rocha in Therapeutic Advances in Psychopharmacology
Footnotes
Conflict of interest statement: Lucas Primo de Carvalho Alves has provided services for Roche Químicos e Farmacêuticos SA on teaching online classes of biostatistics, not related to the present article. Neusa Sica da Rocha has no conflict to declare.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was funded by CAPES (Coordenação de Aperfeiçoamento Profissional de Nível Superior): n° 530/2010, CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FIPE/HCPA (Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre): n° 10-0265, PPG-Psiq/FAMED/UFRGS (Programa de Pós-Graduação em Psiquiatria da Faculdade de Medicina da Universidade Federal do Rio Grande do Sul), and FAPERGS (Fundo de Amparo à Pesquisa do Estado do Rio Grande do Sul). The abstract of this study was accepted for poster publishing in The Lancet Summit event, on 2018.
ORCID iD: Lucas Primo de Carvalho Alves  https://orcid.org/0000-0003-4387-224X
https://orcid.org/0000-0003-4387-224X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lucas Primo de Carvalho Alves, Federal University of Rio Grande do Sul, Dr Walter Só Jobim Avenue, 102-423 Jardim Lindoia Porto Alegre – RS, 91050-230 Brazil.
Neusa Sica da Rocha, Federal University of Rio Grande do Sul, Porto Alegre – RS, Brazil; Hospital de Clínicas de Porto Alegre, Porto Alegre – RS, Brazil; Faculdade de Medicina, Programa de Pós-Graduação em Psiquiatria e Ciências do Comportamento, Porto Alegre – RS, Brazil.
References
- 1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 2005; 62: 593. [DOI] [PubMed] [Google Scholar]
- 2. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 2013; 10: e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67: 446–457. [DOI] [PubMed] [Google Scholar]
- 4. Furtado M, Katzman MA. Examining the role of neuroinflammation in major depression. Psychiatry Res 2015; 229: 27–36. [DOI] [PubMed] [Google Scholar]
- 5. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016; 16: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenblat JD, Kakar R, Berk M, et al. Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis. Bipolar Disord 2016; 18: 89–101. [DOI] [PubMed] [Google Scholar]
- 7. Martínez-Cengotitabengoa M, Carrascón L, O’Brien J, et al. Peripheral inflammatory parameters in late-life depression: a systematic review. Int J Mol Sci 2016; 17: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Byrne ML, Whittle S, Allen NB. The role of brain structure and function in the association between inflammation and depressive symptoms. Psychosom Med 2016; 78: 389–400. [DOI] [PubMed] [Google Scholar]
- 9. Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 2013; 150: 736–744. [DOI] [PubMed] [Google Scholar]
- 10. Hiles SA., Baker AL, de Malmanche T, et al. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behav Immun 2012; 26: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 11. Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol 2002; 5: S1461145702003103. [DOI] [PubMed] [Google Scholar]
- 12. Gordon KB, Armstrong AW, Han C, et al. Anxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the phase 3 VOYAGE 2 study. J Eur Acad Dermatology Venereol 2018; 32: 1940–1949. [DOI] [PubMed] [Google Scholar]
- 13. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in psoriasis longitudinal assessment and registry (PSOLAR). J Am Acad Dermatol 2018; 78: 70–80. [DOI] [PubMed] [Google Scholar]
- 14. Kappelmann N, Lewis G, Dantzer R, et al. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry 2018; 23: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wittenberg GM, Stylianou A, Zhang Y, et al. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry. Epub ahead of print 19 August 2019. DOI: 10.1038/s41380-019-0471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev 2013; 37: 2331–2371. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt FM, Schröder T, Kirkby KC, et al. Pro- and anti-inflammatory cytokines, but not CRP, are inversely correlated with severity and symptoms of major depression. Psychiatry Res 2016; 239: 85–91. [DOI] [PubMed] [Google Scholar]
- 18. Haapakoski R, Mathieu J, Ebmeier KP, et al. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 2015; 49: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Köhler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 2017; 135: 373–387. [DOI] [PubMed] [Google Scholar]
- 20. Fried EI, Nesse RM, Zivin K, et al. Depression is more than the sum score of its parts: individual DSM symptoms have different risk factors. Psychol Med 2014; 44: 2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lux V, Kendler KS. Deconstructing major depression: a validation study of the DSM-IV symptomatic criteria. Psychol Med 2010; 40: 1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kendler KS, Aggen SH, Neale MC. Evidence for multiple genetic factors underlying DSM-IV criteria for major depression. JAMA Psychiatry 2013; 70: 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harald B, Gordon P. Meta-review of depressive subtyping models. J Affect Disord 2012; 139: 126–140. [DOI] [PubMed] [Google Scholar]
- 24. Fink M, Taylor MA. Resurrecting melancholia. Acta Psychiatr Scand 2007; 115: 14–20. [DOI] [PubMed] [Google Scholar]
- 25. Shorter E. The doctrine of the two depressions in historical perspective. Acta Psychiatr Scand 2007; 115: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rudolf S, Greggersen W, Kahl KG, et al. Elevated IL-6 levels in patients with atypical depression but not in patients with typical depression. Psychiatry Res 2014; 217: 34–38. [DOI] [PubMed] [Google Scholar]
- 27. Karlović D, Serretti A, Vrkić N, et al. Serum concentrations of CRP, IL-6, TNF-α and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res 2012; 198: 74–80. [DOI] [PubMed] [Google Scholar]
- 28. Parker G, Fink M, Shorter E, et al. Issues for DSM-5: whither melancholia? The case for its classification as a distinct mood disorder. Am J Psychiatry 2010; 167: 745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Østergaard SD, Jensen SOW, Bech P. The heterogeneity of the depressive syndrome: when numbers get serious. Acta Psychiatr Scand 2011; 124: 495–496. [DOI] [PubMed] [Google Scholar]
- 30. Østergaard SD. Do not blame the SSRIs: blame the Hamilton Depression Rating Scale. Acta Neuropsychiatr 2018; 30: 241–243. [DOI] [PubMed] [Google Scholar]
- 31. Primo de Carvalho Alves L, Sica da Rocha N. Debate on “defining Melancholia: a core mood disorder.” Bipolar Disord 2017; 19: 522–523. [DOI] [PubMed] [Google Scholar]
- 32. Østergaard SD, Bech P, Miskowiak KW. Fewer study participants needed to demonstrate superior antidepressant efficacy when using the Hamilton Melancholia subscale (HAM-D6) as outcome measure. J Affect Disord 2016; 190: 842–845. [DOI] [PubMed] [Google Scholar]
- 33. Bech P, Allerup P, Gram LF, et al. The Hamilton Depression Scale. Acta Psychiatr Scand 1981; 63: 290–299. [DOI] [PubMed] [Google Scholar]
- 34. Timmerby N, Andersen JH, Søndergaard S, et al. A systematic review of the clinimetric properties of the 6-item version of The Hamilton Depression Rating Scale (HAM-D6). Psychother Psychosom 2017; 86: 141–149. [DOI] [PubMed] [Google Scholar]
- 35. Primo de Carvalho Alves L, Pio de Almeida Fleck M, Boni A, et al. The major depressive disorder hierarchy: rasch analysis of 6 items of the Hamilton Depression Scale covering the continuum of depressive syndrome. PLoS One 2017; 12: e0170000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bech P. Clinical Psychometrics [Internet]. Chichester, West Sussex: John Wiley & Sons, Ltd, http://doi.wiley.com/10.1002/9781118511800 (2012, accessed 4 June 2015). [Google Scholar]
- 37. Primo de Carvalho Alves L, Sica da Rocha N. Lower levels of brain-derived neurotrophic factor are associated with melancholic psychomotor retardation among depressed inpatients. Bipolar Disord 2018; 20: 746–752. [DOI] [PubMed] [Google Scholar]
- 38. Lecrubier Y, Sheehan D, Hergueta T, et al. The Mini International Neuropsychiatric Interview. Eur Psychiatry 1998; 13(Suppl. 20): 198s. [DOI] [PubMed] [Google Scholar]
- 39. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Berger A. Science commentary: Th1 and Th2 responses: what are they? BMJ 2000; 321: 424–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pavon L, Sandovallopez G, Eugeniahernandez M, et al. Th2 cytokine response in major depressive disorder patients before treatment. J Neuroimmunol 2006; 172: 156–165. [DOI] [PubMed] [Google Scholar]
- 42. Parker G, Bassett D, Outhred T, et al. Defining melancholia: a core mood disorder. Bipolar Disord 2017; 19: 235–237. [DOI] [PubMed] [Google Scholar]
- 43. Parker G. Defining melancholia: the primacy of psychomotor disturbance. Acta Psychiatr Scand 2007; 115: 21–30. [DOI] [PubMed] [Google Scholar]
- 44. Parker G, McCraw S, Blanch B, et al. Discriminating melancholic and non-melancholic depression by prototypic clinical features. J Affect Disord 2013; 144: 199–207. [DOI] [PubMed] [Google Scholar]
- 45. Goldsmith DR, Haroon E, Woolwine BJ, et al. Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behav Immun 2016; 56: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharma RP, Tun N, Grayson DR. Depolarization induces downregulation of DNMT1 and DNMT3 in primary cortical cultures. Epigenetics 2008; 3: 74–80. [DOI] [PubMed] [Google Scholar]
- 47. Lamers F, Vogelzangs N, Merikangas KR, et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 2013; 18: 692–699. [DOI] [PubMed] [Google Scholar]
- 48. American Psychiatry Association. Diagnostic and statistical manual of mental disorders. 5th ed. [Internet]. American Psychiatric Publishing Inc., http://dsm.psychiatryonline.org//book.aspx?doi=10.1176/appi.books.9780890425596.893619 (2013, accessed 1 August 2014). [Google Scholar]
- 49. Lash TL. The harm done to reproducibility by the culture of null hypothesis significance testing. Am J Epidemiol 2017; 186: 627–635. [DOI] [PubMed] [Google Scholar]
- 50. Primo de Carvalho Alves L, Sica da Rocha N. The harm of adjusting for multiple statistical testing in psychiatric research. Acta Psychiatr Scand 2019; 5: 1–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplement for Different cytokine patterns associate with melancholia severity among inpatients with major depressive disorder by Lucas Primo de Carvalho Alves and Neusa Sica da Rocha in Therapeutic Advances in Psychopharmacology