Abstract
As a malignant hematopoietic stem cell disease, leukemia remains life-threatening due to its increasing incidence rate and mortality rate. Therefore, its early diagnosis and treatment play a very important role. In the present work, we systematically reviewed the current applications and future directions of positron emission tomography (PET) in patients with leukemia, especially 18F-FDG PET/CT. As a useful imaging approach, PET significantly contributes to the diagnosis and treatment of different types of leukemia, especially in the evaluation of extramedullary infiltration, monitoring of leukemia relapse, detection of Richter’s transformation (RT), and assessment of the inflammatory activity associated with acute graft versus host disease. Future investigations should be focused on the potential of PET/CT in the prediction of clinical outcomes in patients with leukemia and the utility of novel radiotracers.
Keywords: PET, FDG, leukemia, diagnosis, prognosis, extramedullary
Introduction
As a type of malignant clonal disease, leukemia originates from hematopoietic stem cells. A large number of cloned leukemia cells proliferate and accumulate in bone marrow and other hematopoietic tissues, leading to inhibited normal hematopoietic function and infiltration of other non-hematopoietic tissues and organs. According to the differentiation and maturity of leukemia cells and the natural course of the disease, leukemia can be broadly classified into 4 main types as follows: acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia (AML), chronic lymphoblastic leukemia (CLL) and chronic myeloblastic leukemia (CML). CLL is the most common form in elderly patients, and ALL is the most common form in pediatric patients. Other rare types include hairy cell leukemia (HCL), acute promyelocytic leukemia (APML) and T-cell prolymphocytic leukemia (T-PLL).1 Acute leukemia (AL) has become a public health concern due to its increasing incidence rate and mortality rate. Therefore, its early diagnosis and treatment greatly contribute to the prognosis of leukemia patients.
Positron emission tomography (PET) is widely applied as a molecular imaging approach, in which radiopharmaceuticals are used as tracers to graphically display the metabolism of the whole body and focal lesions to diagnose diseases. PET scans are now routinely combined with computed tomography (PET/CT) or magnetic resonance imaging (PET/MRI). The functional metabolic imaging of PET is fused with anatomical structure imaging of CT or MRI, which can not only obtain information, such as function and metabolism, but also have high spatial resolution and accuracy. Moreover, a variety of PET radiotracers have been introduced.
As the most common PET radiotracer, 18-fluorodeoxyglucose (18F-FDG) is a radio-labeled glucose analog, which can reflect the increased level of glucose consumption. The malignant tumors often have a significantly higher level of 18F-FDG uptake because of high glucose uptake and accelerated glucose metabolism.2,3 18F-FDG can also accumulate under a variety of benign and physiological conditions, leading to false-positive interpretation. In general, the 18F-FDG uptake in the benign disease and physiological conditions remains low.4,5
18F-FDG PET/CT has been widely used as a new non-invasive molecular imaging technique in diagnosing, staging, restaging and response assessment of hematologic malignancies, such as lymphoma,6,7 myeloid sarcoma8 and multiple myeloma.9 In recent years, more and more research reports have shown that 18F-FDG PET/CT also plays an important role in the diagnosis and treatment of different types of leukemia, especially AL.10 In the present work, we systematically reviewed the current applications and future directions of PET in leukemia patients, especially 18F-FDG PET/CT.
The utility of 18F-FDG PET/CT in the diagnosis of leukemia
18F-FDG PET/CT is not routinely used in the assessment of leukemia, because typical leukemia does not present with a solid tumor, and bone marrow (BM) examination remains the gold standard test of its diagnosis.
Increased uptake of 18F-FDG in BM may be a characteristic finding in leukemia patients, including diffuse and focal patterns. Alam et al.3 have revealed that 18F-FDG super BM uptake is a highly potent indicator for the BM malignant infiltration, which mostly originates from lymphoma and leukemia. Arimoto et al.11 have shown that all 9 leukemia patients have increased BM uptake of 18F-FDG on PET/CT in the vertebrae, pelvis, sternum, ribs and extremities, reflecting the increased number and elevated metabolic activity of leukemic cells in the BM. Furthermore, some patients have focal or inhomogeneous BM uptake of 18F-FDG, especially in the extremities. This may reflect the focal bone localization of leukemia or focal sites with the decreased number and reduced activity of leukemic cells due to BM necrosis. In addition, increased focal uptake of 18F-FDG in BM can detect focal bone localization of leukemia, especially in patients with relapsed leukemia.12
18F-FDG PET/CT may become a critical diagnostic tool for the early diagnosis of leukemia in patients without a remarkable abnormality in peripheral blood or patients presenting with non-specific symptoms, such as bone pain and fever. Arslan et al.13 have reported an ALL patient presenting with fever of unknown origin, who is diagnosed after 18F-FDG PET/CT showing diffuse BM involvement. Ennishi et al.14 have reported a patient with a long-term fever and diffuse bone pain. Routine hematologic and radiologic (CT and MRI) investigations do not reveal any abnormalities. 18F-FDG PET/CT shows an increased diffuse BM uptake, and the BM biopsy reveals 98% lymphoblasts. Therefore, 18F-FDG PET/CT may be a useful tool for the non-invasive assessment of patients with suspected leukemia.
However, there are some limitations when 18F-FDG PET/CT is used in the diagnosis of leukemia. The increased BM uptake is not specific for leukemia, which can also be observed in the patients with infections and those treated with granulocyte colony-stimulating factor (GCSF) or erythropoietin (EPO).15,16 In these situations, it is difficult to distinguish whether such elevation is caused by malignant disease or inflammatory response, although some articles have reported that BM malignant infiltration derived from lymphoma and leukemia generally has a markedly higher 18F-FDG uptake in the BM compared with benign etiologies.3,17 Li et al.18 have developed a 18F-FDG PET/CT radiomic analysis with machine learning model for the patients with suspicious relapsed AL, and found that high-dimensional, high-throughput radiomic features provide an objective and efficient mechanism for identifying the BM involvement, and it can complement the visual analysis to derive a more comprehensive, confident and accurate diagnosis.
The Utility of 18F-FDG PET/CT in Extramedullary AL
Clinical Features of Extramedullary Involvement of AL
Leukemia usually originates from the BM with the typical symptoms of inhibited hematopoietic function, such as anemia, bleeding and fever. Extramedullary AL refers to lesions that occur in any anatomical sites outside BM, and it is common in monocytic and myelomonocytic leukemia. Granulocytic sarcoma (GS) (also known as myeloid sarcoma or chloroma) is a rare extramedullary manifestation, which is commonly found in patients with AML but uncommonly found in myelodysplastic syndrome, CML or myeloproliferative disorders, and GS can precede the diagnosis or occur during relapse after initial treatment.19,20 Most previous studies have reported that the prevalence of extramedullary AL remains below 10%.19,21 However, some recent studies have shown that up to 30% of AML patients are accompanied by extramedullary involvement,22 and such situation has been increasingly reported in ALL patients.23 Extramedullary AML is most frequently located in the skin, while it can affect almost every part of the body.24,25 Moreover, some studies have found that testis and kidney involvement are only seen in the ALL patients.26,27
Detection of Extramedullary AL
Extramedullary infiltration of AL is not easily diagnosed, especially in the recurrent AL patients, because it often grows slowly and its clinical symptoms and laboratory tests are usually not abnormal. Patients are often diagnosed until they have intolerable symptoms, extramedullary lesions are often large or widespread, and outcomes are poor by this time. The difficult cure of leukemia and its high mortality can be largely attributed to the existence of these extramedullary lesions. Therefore, the extramedullary infiltration should be detected and identified as early as possible.
Although traditional imaging modalities, such as CT, MRI and ultrasound, have been widely used for the detection of extramedullary involvement, they have limitations since the sensitivity of these approaches is not high enough to detect the small lesions and occult lesions, and they cannot provide a panoramic picture of the whole body. Some articles have shown that 18F-FDG PET/CT may be a useful tool in detecting extramedullary AML and guiding biopsies.
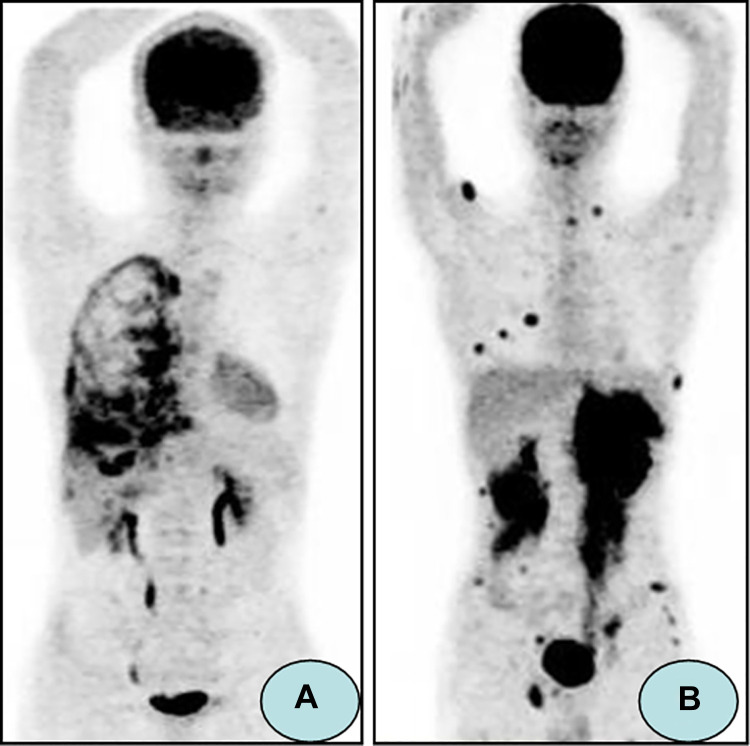
Aschoff et al.28 have compared the results of CT alone or PET alone with 18F-FDG PET/CT in 10 GS patients. The study shows that the combination of PET and CT avoids 5 false-positive manifestations compared with PET alone, as well as 13 false-negative and 1 false-positive compared with CT alone. Cribe et al.22 have reported that 18F-FDG PET/CT can reveal more than twice as many patients with extramedullary AL than found by clinical examination, and the responses of extramedullary AL detected by 18F-FDG PET/CT are concordant with the BM responses assessed by pathology examination. Zhou et al.26 have also shown that 18F-FDG PET/CT is a sensitive imaging modality (93.3%) for the detection of extramedullary AL with the intense 18F-FDG uptake of the lesions (Figure 1).
Figure 1.
18FDG-PET/CT images demonstrating extramedullary AL. (A) A 21-year-old male received allo-HSCT for ALL. 18FDG-PET/CT images revealed right pleura with diffuse intense 18F-FDG uptake, which was proved to be extramedullary AL by biopsy. (B) A 20-year-old male received allo-HSCT for ALL. 18FDG-PET/CT images showed bilateral kidneys, muscle, and subcutaneous tissue were involved by extramedullary AL (Reference: Zhou WL, et al.26).
Monitoring of Extramedullary Relapse
Leukemia relapse refers to the reappearance of leukemia cells in the peripheral blood, or the extramedullary invasion of leukemia cells, which occurs within 2 years after complete remission. It can occur intramedullary or extramedullary, or both, and intramedullary relapse is more frequently detected. Despite the high rates of initial complete remission in AL, relapse remains a major cause of failure in treatment.29 In the past, retrospective data have indicated that the rate of extramedullary relapse alone is only 0.65% in AML patients after allogeneic hematopoietic stem cell transplantation (HSCT) compared with 30% in those with combined BM and extramedullary disease.30 However, it has been reported that the incidence of extramedullary relapse is increasing, especially in the patients who have received HSCT.31,32 One study has shown that up to 20% of AML patients have extramedullary relapse after HSCT, and ALL patients have a higher recurrence rate.33
Intramedullary relapse can often easily be diagnosed by BM biopsy and blood cell examination. However, it is difficult to detect extramedullary relapse. Some articles have indicated that FDG-PET/CT can be helpful for detecting extramedullary relapse.23,34,35 FDG-PET/CT is sensitive in determining the location and metabolic activity of recurrent malignant tumors compared with other imaging modalities, and it can be helpful in managing individualized treatment of patients. Tan et al.36 have reported that a 16-year-old girl, who is diagnosed as ALL and achieves complete remission after allo-HSCT 9 years ago, is presented with a mass in the left eye through a normal visual inspection. FDG PET/CT imaging has found increased FDG uptake in an oval mass in the soft tissue between the left eyeball and the left nasal. The extramedullary relapse is confirmed by biopsy, while the BM aspiration biopsy is negative. Stolzel et al.37 have performed PET/CT for 10 patients with de novo and relapsed AML and histologically proven extramedullary disease. The scans detect the known extramedullary lesions in 9 out of 10 patients (90%). Furthermore, additional extramedullary sites are detected in 6 patients (60%). It is possible to identify known and clinically undetectable extramedullary manifestations of AML by using PET/CT.
Since most of these patients relapse within a short period of time after initiation of therapy or have refractory disease, using 18F-FDG PET/CT to detect extramedullary disease has an important impact on treatment decisions and outcomes. In order to obtain a higher survival rate and a better prognosis, more and more clinical attention has been paid to the occurrence and detection of extramedullary involvement of AL.
Staging and Response Assessment of Leukemia
18F-FDG PET/CT has been widely used in staging, restaging and response assessment of lymphoma. Likewise, for AL patients, PET/CT can also help determine the extent of the disease and assess the therapy response. It has been determined in a study consisting of 124 AL patients by Cunningham et al.38 The whole body 18F-FDG PET/CT imaging before and after treatment can evaluate whether the treatment is effective by a comparison. Rao et al.39 have reported a case of a 64-year-old man who is diagnosed with AML and subsequently has recurrent extramedullary relapses. Doma et al.40 have reported a case of a 66-year-old man diagnosed with HCL, who has an extensive pathological retroperitoneal mass and infiltration of the spleen and skeletal involvement which are highly avid on 18F-FDG PET/CT. In addition, all previous pathological sites show normal FDG uptake on 18F-FDG PET/CT after treatment. These cases demonstrate the usefulness of FDG-PET for staging and assessment of the treatment response.
Moreover, 18F-FDG PET/CT may be useful in children for relapsed/refractory leukemia. A study has reported 2 children with AML who undergo PET/CT at diagnosis as well as in remission and detected 5 extramedullary disease lesions, only 2 of which are detectable on clinical examination.41 Kaya et al.42 have found that 18F-FDG PET/CT may be a complementary imaging modality to improve the detection of subtle leukemic infiltration in children with suspected leukemia progression or recurrence after chemotherapy or allo-SCT. Four patients who have undergone allo-HSCT are observed with increased multifocal BM uptake and extramedullary leukemia on 18F-FDG PET/CT, while they show negative BM biopsies. Focal BM involvement may be missed by BM biopsy alone, while 18F-FDG PET/CT-guided targeted biopsies may overcome such problem in these patients.
The Utility of 18F-FDG PET/CT in Patients With CLL and Richter’s Syndrome (RS)
CLL is the most common type of leukemia in Western countries.43 It is generally an indolent and low-grade lymphoproliferative disease.44 RS or Richter’s transformation (RT) refers to the development of CLL to another more aggressive lymphoma or lymphoid malignancy, most commonly to diffuse large B-cell lymphoma (DLBCL).45 Transformations into PLL, Hodgkin disease and small non-cleaved cell lymphoma have also been documented.46,47 The syndrome is first described by Richter MN in 1928, who has reported a patient with CLL transformed into fatal rapidly progressing generalized lymphadenopathy and hepatosplenomegaly.48 RS is one of the main complications of CLL, which occurs in about 2.2-8% of CLL patients. RS may represent the end stage of CLL. The prognosis is poor, and the median survival duration ranges from 5 months to 8 months.49 Therefore, prompt diagnosis is necessary. However, the clinical features of RS are non-specific, such as fever, progressively enlarging lymph nodes and suggestive lab findings of elevated lactate dehydrogenase (LDH) and β-2 microglobulin levels. Previous reports have shown that high-dose 67 Ga scanning can detect RT. However, it cannot be a routine imaging test for lymphoma due to some restrictions.50,51 Conventional imaging, such as CT, can be used to evaluate lymphomatous lesions with compressive or infiltrative patterns. In the case reported by Mota et al.,52 the CT scan can detect an ocular lymphoma with RS. Papajik et al.53 have reported the case that there is no advantage in performing 18F-FDG PET/CT over CT as a surveillance tool in CLL patients, while if RS is suspected, an 18F-FDG PET/CT can be extremely beneficial in confirming the diagnosis.
18F-FDG PET/CT can detect RT of CLL to large cell lymphoma with a high sensitivity, specificity and negative predictive value. Earlier studies have reported a low degree of 18F-FDG avidity of PET in patients with CLL or small lymphocytic lymphoma (SLL), which is likely attributed to the lower mitotic activity and glucose consumption of lymphocytes.54 Conversely, a high level of 18F-FDG accumulation has been observed in RS patients. Bruzzi et al.55 have shown that the abnormal increase in the standardized uptake value (SUV) of 18F-FDG raises the suspicion for RS, especially if SUVmax ≥ 5.0, and should be considered highly suggestive of histological transformation. The overall sensitivity and specificity of 18F-FDG PET/CT for RS have been reported to be 91% and 80%, respectively. Michallet et al.56 have reported that an SUVmax > 10 seems to be the optimal threshold for distinguishing RS in CLL, and such threshold is also strongly correlated with mortality. In addition, diagnosis of RS is confirmed by biopsy of enlarging lymph node or other involved sites (Figure 2). Furthermore, for patients suspected of having RS, 18F-FDG PET/CT can also identify sites of increased 18F-FDG uptake as intensely metabolically active nodes to guide biopsy or surveillance.55 Sood et al.57 have presented a case of CLL with suggestive clinical features of RS. 18F-FDG PET/CT shows liver and lung involvement with no lymphadenopathy, which is an unusual presentation of RS.
Figure 2.
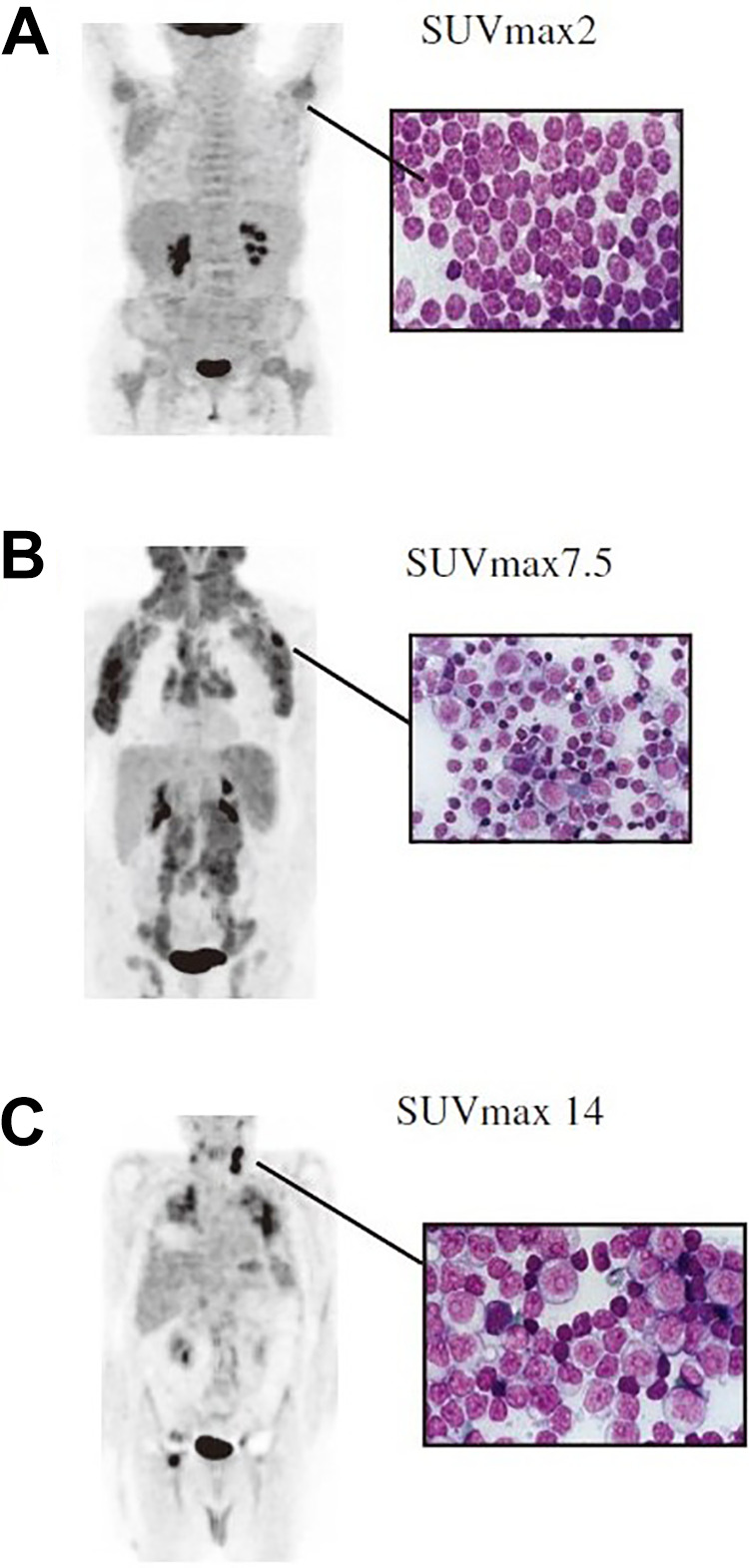
18FDG-PET/CT images demonstrating RS of CLL. (A) Patient with CLL stable disease (SUVmax: 2); (B) Patient with rapid disease progression (SUVmax: 7.5); (C) Patient with RS, which was confirmed by lymph node biopsy showing large B cells with high proliferation rate (SUVmax: 14). (Reference: Michallet, et al.56).
In addition, the role of 18F-FDG PET/CT in identifying RS in the era of novel agent CLL therapy has evolved in recent years. Wang et al.58 have found that CLL patients receiving B-cell receptor pathway inhibitor (BCRi) therapy should undergo 18F-FDG PET/CT for the evaluation of potential disease progression. A biopsy should be considered in patients with suspected RS when an SUVmax ≥ 5.
The Utility of 18F-FDG PET/CT in Graft Versus Host Disease (GVHD) After Allo-HSCT
HSCT has been proved to be an important method for a variety of life-threatening malignancies, and it may be the only cure for most malignant blood diseases. However, HSCT is limited by the immunological recognition and destruction of host tissues, which is termed as GVHD. As one of the most serious complications, GVHD is the primary cause of non-recurrence death in patients after allo-HSCT but rare in those after autologous HSCT.59,60 GVHD affects 50–70% of patients receiving allo-HSCT.61 One remarkable feature of GVHD is its predilection for certain organ systems, such as the epithelial surfaces of the skin and mucous membranes, the crypts of the gastrointestinal tract, and the biliary ducts of the liver. About 80% of patients with acute GVHD have skin involvement, and more than 50% have gastrointestinal GVHD.62,63 Severe acute gastro-intestinal tract (GIT)-GVHD is likely the most serious manifestation, and it is a major determinant of long-term survival.64
Although clinical investigation, laboratory tests, and histology usually lead to an early diagnosis of skin or liver GVHD,65 GIT-GVHD can be difficult to diagnose because of its non-specific clinical symptoms, such as anorexia, nausea, vomiting, watery diarrhea, intestinal bleeding, abdominal pain, and ileus.66 Endoscopic examination and histology are mainly used to exclude differential diagnosis, and this method remains unsatisfactory in most cases.67 Furthermore, only the evolution of acute GIT-GVHD during the initial weeks will ultimately define the severity of GVHD.59 Non-invasive tests for assessment of GVHD activity are desirable but lacking. Some studies have indicated that 18F-FDG PET/CT may be a sensitive and specific non-invasive technique to assess the inflammatory activity associated with acute GIT-GVHD.
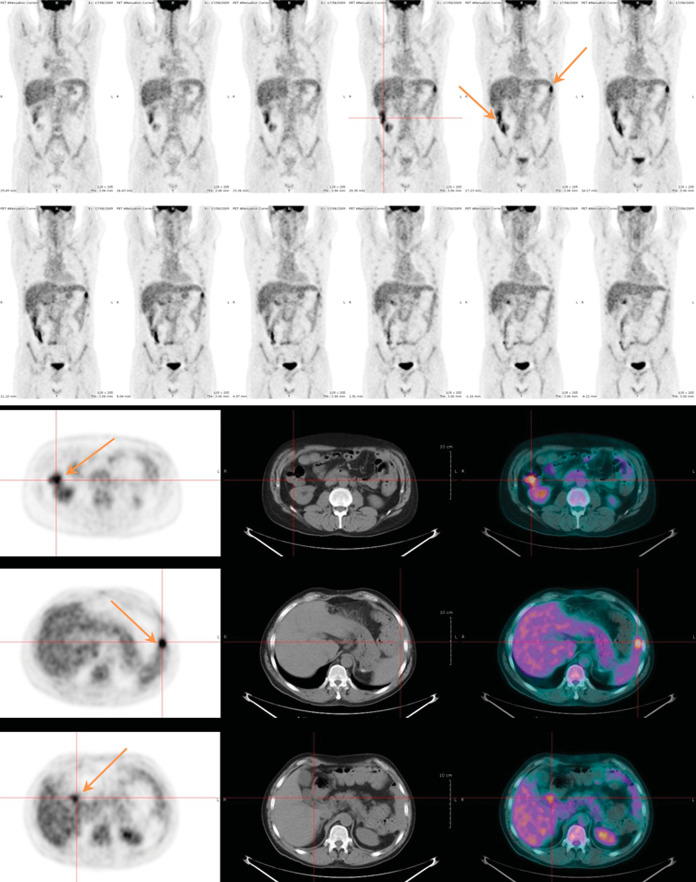
Stelljes et al.68 have observed 30 patients with suspected intestinal GVHD beyond 20 days after transplantation. Of the 17 histologically diagnosed patients, 14 patients show significant intestinal FDG uptake, mainly in the colon. Of the 13 patients without histological evidence, there is no increased FDG uptake. It shows for the first time that 18F-FDG PET/CT can be used as a sensitive and specific non-invasive method to diagnose and map the inflammatory component of intestinal GVHD, and monitor the treatment response of intestinal GVHD in patients after allo-HSCT (Figure 3). A prospective study by Bodet-Milin et al.69 has found that 9 of 10 (90%) patients with proven acute GIT-GVHD have a positive 18F-FDG PET/CT, including 5 patients before developing clinical symptoms, suggesting that this imaging technique would be potentially useful for early the diagnosis of acute GVHD in some cases. Dejanovic et al.70 have reported that a patient with extensive and multifocal involvement of the GIT in the whole body 18F-FDG PET/CT develops severe acute GVHD at 93 days post allo-SCT, demonstrating that 18F-FDG PET/CT can be valuable in mapping the activity and distribution of intestinal GVHD and directing targeted biopsies of involved regions.
Figure 3.
18FDG-PET/CT images demonstrating GVHD. A 46-year-old patient who presented with signs of acute GI-GVHD 30 days after allo-SCT. 18F-FDG PET/CT which performed at 25 days after allo-HSCT showed colon FDG uptake increased. (Reference: Bodet-Milin C, et al.69).
The Utility of PET With Other Radio Tracers in Leukemia
18F-Flurodeoxythymidine (18F-FLT)
18F-FLT is a thymidine analog that is resistant to in vivo degradation and accumulates predominantly in proliferating tissues. Some studies have reported that 18F- FLT, as a tracer of PET, is able to visualize extramedullary manifestation sites of AML and reflect the disease activity. Compared with 18F-FDG, 18F-FLT PET may reduce the false-positive manifestations due to infection or inflammation, and it may be appropriate for the detection of meningeal diseases because of the negligible background uptake in the brain and skull. This approach may be used in future studies of extramedullary AL.10.71
68Ga-Pentixafor
68Ga-Pentixafor is a novel PET radiotracer, which reflects the CXCR4 expression. Initially, a study by Herhaus et al. has demonstrated that in vivo application of 68Ga-Pentixafor PET is feasible in AML.72 Some researchers have found that 68Ga-Pentixafor uptake is elevated in BM of CLL patients compared with other oncological patients without BM infiltration. 68Ga-Pentixafor uptake may possibly play a role as an independent parameter for detection and treatment response assessment in CLL patients.1,73
11C-Choline
11C-Choline PET has been introduced for imaging various tumors, including prostate cancer, lung cancer and brain tumor.74 The negligible uptake of 11C-choline in the normal brain may be helpful for the diagnosis of extramedullary AL in brain. Qin et al.75 have reported a case of a 26-year-old man whose brain MRI suggests possible brain metastases, and AML is diagnosed after BM biopsy. The brain lesions are revealed by 11C-choline PET/CT obviously, while the FDG uptake level of brain lesions is similar to adjacent brain in 18F-FDG PET/CT.
In addition, novel targeted tracers for PET may have the potential to further improve the GVHD diagnosis and provide new insights into the mechanisms underlying the pathogenesis and management of intestinal GVHD.76-78 A summary table of PET radiotracers in leukemia as well as their main advantages and disadvantages can be seen in Table 1.
Table 1.
PET Radio Tracers in Leukemia as well as Their Main Advantages and Disadvantages.
| PET radiotracers | Advantages | Disadvantages |
|---|---|---|
| 18F -FDG |
18F-FDG can detectvarious hematologic neoplasms due to altered glucose consumption. Its half-live is appropriate for clinical use and it is inexpensive tomanufacture reliably |
Sometimes it is difficult to dis differentiate benign lesions such as inflammation from malignantdisease. And it may not be able to detect malignant involvement in areas of high HueD_Ref18glucose metabolism such as the meninges or the pericardium |
| 18F-FLT | 18F-FLT PET may reduce the false-positive manifestations due to infection or inflammation, and it may be appropriate for the detection of meningeal diseases because of the negligible background uptake in the brain and skull. | 18F-FLT uptake in bone marrow is unable to demonstrate leukemia infiltration due toit may be caused byboth neoplastic and normal hematopoietic cells |
| 68Ga-Pentixafor | 68Ga-Pentixafor may possibly be useful for detection, characterization, andtreatment response assessment in CLL patients | 68Ga-Pentixafor reflects the CXCR4 expression, and only used in CXCR4-based CLL |
| 11C-choline | The negligible uptake of 11C-Choline in the normal brain may be helpful for the diagnosis of extramedullary AL in brain | 11C-Choline was inappropriate for routine clinical use because of the rapid in vivo degradation and short half-life of 11C |
Conclusions
In summary, PET has many important applications in leukemia patients, especially 18F-FDG PET/CT. 18F-FDG PET/CT can be useful in diagnosing leukemia, mainly in patients with fever and anemia of unknown origin. Moreover, it is of great value for detecting extramedullary disease, monitoring extramedullary relapse and evaluating treatment response.18F-FDG PET/CT plays an important role in the early detection of RS and guiding biopsy, in which an SUVmax > 10 may be the optimal threshold for distinguishing RS in CLL. Furthermore, it can also be used to diagnose GVHD after allo-HSCT. Some novel radiopharmaceuticals for PET also have potential to be used in clinical practice to detect and assess the prognosis of leukemia.
PET may have great potential in the prediction of clinical outcomes in leukemia patients, and it deserves further research. In recent years, PET/MRI has also been increasingly used in clinical practice and shown important value in better visualization of BM without exposure to radiation. It may play more roles in the diagnosis and treatment of leukemia in the future. However, the studies about the use of PET in leukemia are still rare. We found that only few clinical trials are related to the application of PET in the diagnosis and treatment of leukemia, including18F-FDG PET/CT, PET/MRT and FLT PET in AML and in detecting GVHD, and most of them have no clinical data published (Table 2). Moreover, the sample size of the studies is not large enough so far, which can probably be related to the limitation of PET in the diagnosis of leukemia. Therefore, more trials should be designed, and prospective studies with larger sample size are needed in the future. For example, we can assess the use of 18F-FDG PET/CT to predict the clinical outcome in AL patients treated with allo-HSCT and the use of 18F-FDG PET/CT radiomic analysis in leukemia.
Table 2.
The Clinical Rials Performed in Different Types of Leukemias and GVHD.
| Number | Title of clinical rials | Outcome Measures | Status; Study Results |
|---|---|---|---|
| NCT03422731 | Multi-modality Imaging and Collection of Biospecimen Samples in Understanding Bone Marrow Changes in Patients With Acute Myeloid Leukemia Undergoing TBI and Chemotherapy |
|
• Recruiting • Start: February 6, 2018 • Completion: February 6, 2023 • No clinical data published |
| NCT02682732 | Molecular Imaging to CaptureDisease Heterogeneity in AcuteMyeloid Leukemia |
|
• Recruiting • Start: April 2016 • Completion: December 2020 • No clinical data published |
| NCT02390635 | PET/MRI, 18F-FDG PET/CT and Whole Body MRI in Finding Extramedullary Myeloid Leukemia in Patients With Newly Diagnosed Acute Myeloid Leukemia |
|
• Recruiting • Start: May 2, 2016 • Completion: May 31, 2021 • No clinical data published |
| NCT02392429 | FLT PET/CT in MeasuringResponse in Patients WithPreviously Untreated AcuteMyeloid Leukemia |
|
• Active, not recruiting • Study Start: December 8, 2015 • Primary Completion: July 31, 2020 • No clinical data published |
| NCT02103530 | Early Response Assessment of Induction Chemotherapy in Acute Myeloid Leukemia Patients Using F-18 FLT PET/CT |
|
• Completed • Start: April 2014 • Completion: December 2015 • No clinical data published |
| NCT01599429 | Study of the Predictive Marker FLT in Patients Suffering From AML |
|
• Unknown status • Start: October 2011 • Completion: October 2015 • No clinical data published |
| NCT01964625 | Positron Emission Tomography-Computed Tomography (PETCT) Scanning in Chronic Graft Versus Host Disease (cGvHD) |
|
• Terminated • Start: November 2010 • Completion: August 2013 • No clinical data published |
| NCT01596192 | PETCT for Diagnosing and Monitoring Acute GVHD |
|
• Unknow status • Start: May 2012 • Completion: June 2013 • No clinical data published |
| NCT02352064 | Evaluation of the Diagnostic Value of PET (18F-FDG) in Chronic Graft Versus Host Disease (cGVH) |
|
• Unknow status • Start: January 2015 • Completion: January 2018 • No clinical data published |
| NCT03546556 | 18-FLT PET/MR Imaging to Predict Graft Failure and GVHD in Bone Marrow Transplant Patients |
|
• Recruiting • Start: January 1, 2017 • Completion: June 2021 • No clinical data published |
| NCT03367962 | Detection of Graft Versus Host Disease With [18F]F-AraG |
|
• Recruiting • Start: May 15, 2018 • Completion: December 2020 • No clinical data published |
* Clinical trials which currently display an entry in www.clinicaltrials.gov.uk
Footnotes
Authors’ Note: Zixuan Zhao and Yanwen Hu contributed equally to this article as the co-first author. Our study did not require an ethical board approval because it did not contain human or animal trials.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Medical Youth Talent Project of Jiangsu Province (No. QNRC2016749) and Suzhou People’s Livelihood Science and Technology Project (SYS2019038).
ORCID iD: Zixuan Zhao  https://orcid.org/0000-0003-3990-7809
https://orcid.org/0000-0003-3990-7809
References
- 1. Mayerhoefer ME, Archibald SJ, Messiou C, Anton S, Dominik B, Heiko S. MRI and PET/MRI in hematologic malignancies. J Magn Reson Imaging. 2020;51(5):1325–1335. doi:10.1002/jmri.26848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez JV, Belka GK, Pan TC, et al. Oncogene pathway activation in mammary tumors dictates FDG-PET uptake. Cancer Res. 2014;74(24):7583–7598. doi:10.1158/0008-5472.Can-14-1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alam MS, Fu L, Ren YY, et al. 18F-FDG super bone marrow uptake: a highly potent indicator for the malignant infiltration. Medicine (Baltimore). 2016;95(52):e5579 doi:10.1097/MD.0000000000005579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vineeth Kumar PM, Verma GR, Mittal BR, et al. FLT PET/CT is better than FDG PET/CT in differentiating benign from malignant pancreat obiliary lesions. Clin Nucl Med. 2016;41(5):e244–e250. doi:10.1097/RLU.0000000000001163 [DOI] [PubMed] [Google Scholar]
- 5. Culverwell AD, Scarsbrook AF, Chowdhury FU. False-positive uptake on 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) positron-emission tomography/computed tomography (PET/CT) in oncological imaging. Clin Radiol. 2011;66(4):366–382. doi:10.1016/j.crad.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 6. Burton C, Ell P, Linch D. The role of PET imaging in lymphoma. Br J Haematol. 2004;126(6):772–784. doi:10.1111/j.1365-2141.2004.05069.x [DOI] [PubMed] [Google Scholar]
- 7. Seam P, Juweid ME, Cheson BD. The role of FDG-PET scans in patients with lymphoma. Blood. 2007; 110(10):3507–3516. doi:10.1182/blood-2007-06-097238 [DOI] [PubMed] [Google Scholar]
- 8. Karlin L, Itti E, Pautas C, et al. PET-imaging as a useful tool for early detection of the relapse site in the management of primary myeloid sarcoma. Haematologica. 2006;91(12 suppl):ECR54. [PubMed] [Google Scholar]
- 9. Zamagni E, Nanni C, Patriarca F, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92(1):50–55. doi:10.3324/haematol.10554 [DOI] [PubMed] [Google Scholar]
- 10. Buck AK, Bommer M, Juweid ME, et al. First demonstration of leukemia imaging with the proliferation marker 18F-fluorodeoxythymidine. J Nucl Med. 2008;49(11):1756–1762. doi:10.2967/jnumed.108.055335 [DOI] [PubMed] [Google Scholar]
- 11. Arimoto MK, Nakamoto Y, Nakatani K, et al. Increased bone marrow uptake of 18F-FDG in leukemia patients: preliminary findings. Springerplus. 2015;4:521 doi:10.1186/s40064-015-1339-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Endo T, Sato N, Koizumi K, et al. Localized relapse in bone marrow of extremities after allogeneic stem cell transplantation for acute lymphoblastic leukemia. Am J Hematol. 2004;76(3):279–282. doi:10.1002/ajh.20106 [DOI] [PubMed] [Google Scholar]
- 13. Arslan F, Yilmaz M, Cakir T, et al. Significant contribution of fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) in a case of acute lymphoblastic leukemia presenting with fever of unknown origin. Intern Med. 2014;53(7):789–791. doi:10.2169/ internalmedicine.53.1443 [DOI] [PubMed] [Google Scholar]
- 14. Ennishi D, Maeda Y, Niiya M, Katsuji SH, Mitsune T. Incidental detection of acute lymphoblastic leukemia on [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2009;27(36):e269–e270. doi:10.1200/JCO.2009.22.7769 [DOI] [PubMed] [Google Scholar]
- 15. Sugawara Y, Fisher SJ, Zasadny KR, et al. Preclinical and clinical studies of bone marrow uptake of fluorine-1-fluorodeoxyglucose with or without granulocyte colony-stimulating factor during chemotherapy. J Clin Oncol. 1998;16(1):173–180. doi:10.1200/JCO.1998.16.1.173 [DOI] [PubMed] [Google Scholar]
- 16. Blodgett TM, Ames JT, Torok FS, McCook BM, Carolyn CM. Diffuse bone marrow uptake on whole-body F-18 fluorodeoxyglucose positron emission tomography in a patient taking recombinant erythropoietin. Clin Nucl Med. 2004;29(3):161–163. doi:10.1097/01.rlu.0000115654.90324.02 [DOI] [PubMed] [Google Scholar]
- 17. Zhou M, Chen Y, Liu J, Gang H. A predicting model of bone marrow malignant infiltration in (18)F-FDG PET/CT images with increased diffuse bone marrow FDG uptake. J Cancer. 2018;9(10):1737–1744. doi:10.7150/jca.24836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li H, Xu C, Xin B, et al. (18)F-FDG PET/CT radiomic analysis with machine learning for identifying bone marrow involvement in the patients with suspected relapsed acute leukemia. Theranostics. 2019;9(16):4730–4739. doi:10.7150/thno.33841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118(14):3785–3793. doi:10.1182/blood-2011-04-347229 [DOI] [PubMed] [Google Scholar]
- 20. Yilmaz AF, Saydam G, Sahin F, et al. Granulocytic sarcoma: a systematic review. Am J Blood Res. 2013;3(4):265–270. [PMC free article] [PubMed] [Google Scholar]
- 21. Muss HB, Moloney WC. Chloroma and other myeloblastic tumors. Blood. 1973;42(5):721–728. [PubMed] [Google Scholar]
- 22. Cribe AS, Steenhof M, Marcher CW, Henrik P, Henrik F, Lone Smidstrup F. Extramedullary disease in patients with acute myeloid leukemia assessed by 18F-FDG PET. Eur J Haematol. 2013;90(4):273–278. doi:10.1111/ejh.12085 [DOI] [PubMed] [Google Scholar]
- 23. Ciarallo A, Makis W, Novales-Diaz JA, René PM. Extramedullary gastric relapse of acute lymphoblastic leukemia following allogeneic stem cell transplant: staging with F-18 FDG PET/CT. Clin Nucl Med. 2011;36(8):e90–e92. doi:10.1097/RLU.0b013e318217af1c [DOI] [PubMed] [Google Scholar]
- 24. Chong G, Byrnes G, Szer J, Grigg A. Extramedullary relapse after allogeneic bone marrow transplantation for haematological malignancy. Bone Marrow Transplant. 2000;26(9):1011–1015. doi:10.1038/sj.bmt.1702659 [DOI] [PubMed] [Google Scholar]
- 25. Tsimberidou AM, Kantarjian HM, Wen SJ, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer. 2008;113(6):1370–1378. doi:10.1002/cncr.23691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou WL, Wu HB, Wang LJ, et al. Usefulness and pitfalls of F-18-FDG PET/CT for diagnosing extramedullary acute leukemia. Eur J Radiol. 2016;85(1):205–210. doi:10.1016/j.ejrad.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 27. Arrigan M, Smyth L, Harmon M, Flynn C, Sheehy N. Imaging findings in recurrent extramedullary leukaemias. Cancer Imaging. 2013;13(1):26–35. doi:10.1102/1470-7330.2013.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aschoff P, Hantschel M, Oksuz M, et al. Integrated FDG-PET/CT for detection, therapy monitoring and follow-up of granulocytic sarcoma. Initial results. Nuklear Medizin. 2009;48(5):185–191. doi:10.3413/nukmed-0236 [DOI] [PubMed] [Google Scholar]
- 29. de Lima M, Porter DL, Battiwalla M, et al. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: part iii. Prevention and treatment of relapse after allogeneic transplantation. Biol Blood Marrow Transplant. 2014;20(1):4–13. doi:10.1016/j.bbmt.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mortimer J, Blinder MA, Schulman S, et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. J Clin Oncol. 1989;7(1):50–57. doi:10.1200/JCO.1989.7.1.50 [DOI] [PubMed] [Google Scholar]
- 31. Lee KH, Lee JH, Choi SJ, et al. Bone marrow vs extramedullary relapse of acute leukemia after allogeneic hematopoietic cell transplantation: risk factors and clinical course. Bone Marrow Transplant. 2003;32(8):835–842. doi:10.1038/sj.bmt.1704223 [DOI] [PubMed] [Google Scholar]
- 32. Lee KH, Lee JH, Kim S, Lee JS, Kim SH, Kim WK. High frequency of extramedullary relapse of acute leukemia after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2000;26(2):147–152. doi:10.1038/sj.bmt.1702488 [DOI] [PubMed] [Google Scholar]
- 33. Yoo SW, Chung EJ, Kim SY, et al. Multiple extramedullary relapses without bone marrow involvement after second allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia. Pediatric Transplant. 2012;16(4):E125–E129. doi:10.1111/j.1399-3046.2011.01546.x [DOI] [PubMed] [Google Scholar]
- 34. Elojeimy S, Stanescu AL, Parisi MT. Use of 18F-FDG PET-CT for detection of active disease in acute myeloid leukemia. Clin Nucl Med. 2016;41(3):E137–E140. doi:10.1097/Rlu.0000000000000999 [DOI] [PubMed] [Google Scholar]
- 35. Cistaro A, Saglio F, Asaftei S, Piercarlo F, Massimo B, Franca F. The role of 18F-FDG PET/CT in pediatric lymph-node acute lymphoblastic leukemia involvement. Radiol Case Rep. 2011;6(4):503 doi:10.2484/rcr.v6i4.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan G, Aslan A, Tazeler Z. FDG-PET/CT for detecting relapse in patients with acute lymphoblastic leukemia. JPN J Clin Oncol. 2016;46(1):96–97. doi:10.1093/jjco/hyv196 [DOI] [PubMed] [Google Scholar]
- 37. Stolzel F, Rollig C, Radke J, et al. 18F-FDG-PET/CT for detection of extramedullary acute myeloid leukemia. Haematologica. 2011;96(10):1552–1556. doi:10.3324/haematol.2011.045047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cunningham I, Kohno B. 18 FDG-PET/CT: 21st century approach to leukemic tumors in 124 cases. Am J Hematol. 2016;91(4):379–384. doi:10.1002/ajh.24287 [DOI] [PubMed] [Google Scholar]
- 39. Rao S, Langston A, Galt JR, Raghuveer KH. Extramedullary acute myeloid leukemia and the use of FDG-PET/CT. Clin Nucl Med. 2009;34(6):365–366. doi:10.1097/RLU.0b013e3181a3466d [DOI] [PubMed] [Google Scholar]
- 40. Doma A, Skerget M, Zagar I. . 18F-FDG PET/CT for staging and evaluation of therapy in a patient with unusual hairy cell leukemia presentation. Clin Nucl Med. 2019;44(7):e458–e460. doi:10.1097/RLU.0000000000002557 [DOI] [PubMed] [Google Scholar]
- 41. Matsui M, Yamanaka J, Shichino H, et al. FDG-PET/CT for detection of extramedullary disease in 2 pediatric patients with AML. J Pediatr Hematol Oncol. 2016;38(5):398–401. doi:10.1097/MPH.0000000000000447 [DOI] [PubMed] [Google Scholar]
- 42. Kaya Z, Akdemir OU, Atay OL, et al. Utility of 18-fluorodeoxyglucose positron emission tomography in children with relapsed/refractory leukemia. Pediatr Hematol Oncol. 2018;35(7-8):393–406. doi:10.1080/08880018.2018.1557306 [DOI] [PubMed] [Google Scholar]
- 43. Jain P, O’Brien S. Richter’s transformation in chronic lymphocytic leukemia. Oncology (Williston Park). 2012;26(12):1146–1152. [PubMed] [Google Scholar]
- 44. Shaikh F, Janjua A, Van Gestel F, Ahmad A. Richter transformation of chronic lymphocytic leukemia: a review of fluorodeoxyglucose positron emission tomography-computed tomography and molecular diagnostics. Cureus. 2017;9(1):e968 doi:10.7759/cureus.968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robertson LE, Pugh W, O’Brien S, et al. Richter’s syndrome: a report on 39 patients. J Clin Oncol. 1993;11(10):1985–1989. doi:10.1200/JCO.1993.11.10.1985 [DOI] [PubMed] [Google Scholar]
- 46. Brecher M, Banks PM. Hodgkin’s disease variant of Richter’s syndrome. Report of eight cases. Am J Clin Pathol. 1990;93(3):333–339. doi:10.1093/ajcp/93.3.333 [DOI] [PubMed] [Google Scholar]
- 47. Litz CE, Arthur DC, Gajl-Peczalska KJ, et al. Transformation of chronic lymphocytic leukemia to small non-cleaved cell lymphoma: a cytogenetic, immunological, and molecular study. Leukemia. 1991;5(11):972–978. [PubMed] [Google Scholar]
- 48. Richter MN. Generalized reticular cell sarcoma of lymph nodes associated with lymphatic leukemia. Am J Pathol. 1928;4(4):285–292. [PMC free article] [PubMed] [Google Scholar]
- 49. Tsimberidou AM, Keating MJ. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer. 2005;103(2):216–228. doi:10.1002/cncr.20773 [DOI] [PubMed] [Google Scholar]
- 50. Bekerman C, Hoffer PB, Bitran JD. The role of gallium-67 in the clinical evaluation of cancer. Semin Nucl Med. 1984;14(4):296–323. doi:10.1016/s0001-2998(84)80005-7 [DOI] [PubMed] [Google Scholar]
- 51. Partyka S, O’Brien S, Podoloff D, Kantarjian H, Keating MJ. The usefulness of high dose (7-10mci) gallium (67 Ga) scanning to diagnose Richter’s transformation. Leuk Lymphoma. 1999;36(1-2):151–155. doi:10.3109/10428199909145959 [DOI] [PubMed] [Google Scholar]
- 52. Mota PM, Dedes W, Yeoh SL, Soilleux E, Hague S. Ocular lymphoma with extrascleral extension as primary manifestation of Richter syndrome. Eye (Lond). 2012;26(6):891–893. doi:10.1038/eye.2012.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Papajik T, Myslivecek M, Urbanova R, et al. 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography examination in patients with chronic lymphocytic leukemia may reveal Richter transformation. Leuk Lymphoma. 2014;55(2):314–319. doi:10.3109/10428194.2013.802313 [DOI] [PubMed] [Google Scholar]
- 54. Jerusalem G, Beguin Y, Najjar F, et al. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) for the staging of low-grade non-Hodgkin’s lymphoma (NHL). Ann Oncol. 2001;12(6):825–830. doi:10.1023/a:1011169332265 [DOI] [PubMed] [Google Scholar]
- 55. Bruzzi JF, Macapinlac H, Tsimberidou AM, et al. Detection of Richter’s transformation of chronic lymphocytic leukemia by PET/CT. J Nucl Med. 2006;47(8):1267–1273. [PubMed] [Google Scholar]
- 56. Michallet AS, Sesques P, Rabe KG, et al. An 18F-FDG-PET maximum standardized uptake value > 10 represents a novel valid marker for discerning Richter’s syndrome. Leuk Lymphoma. 2016;57(6):1474–1477. doi:10.3109/10428194.2015.1099643 [DOI] [PubMed] [Google Scholar]
- 57. Sood A, Parihar AS, Lad D, Rajender K, Harmandeep S, Bhagwant RM. An unusual presentation of Richter’s transformation of chronic lymphocytic leukemia in liver and lung on 18f-labeled fluoro-2-deoxyglucose positron emission tomography/computed tomography. Indian J Nucl Med. 2020;35(1):70–71. doi:10.4103/ijnm.IJNM175_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Y, Rabe KG, Bold MS, et al. The role of 18F-FDG-PET in detecting Richter’s transformation of chronic lymphocytic leukemia in patients receiving therapy with a B-cell receptor inhibitor. Haematologica. 2020. doi:10.3324/haematol.2019.240564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75(4):1024–1030. [PubMed] [Google Scholar]
- 60. Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–414. doi:10.1182/blood.v100.2.406 [DOI] [PubMed] [Google Scholar]
- 61. Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–4126. doi:10.1182/blood-2006-12-041889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29–52. doi:10.1146/annurev.med.54.101601.152339 [DOI] [PubMed] [Google Scholar]
- 63. Martin PJ, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):320–327. doi:10.1016/j.bbmt.2003.12.304 [DOI] [PubMed] [Google Scholar]
- 64. Harris AC, Ferrara JLM, Braun TM, et al. Plasma biomarkers of lower gastrointestinal and liver acute GVHD. Blood. 2012;119(12):2960–2963. doi:10.1182/blood-2011-10-387357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29(3):259–277. doi:10.1016/s0301-472x (00)00677-9 [DOI] [PubMed] [Google Scholar]
- 66. Iqbal N, Salzman D, Lazenby AJ, Wilcox CM. Diagnosis of gastrointestinal graft-versus-host disease. Am J Gastroenterol. 2000;95(11):3034–3038. doi:10.1111/j.1572-0241.2000.03250.x [DOI] [PubMed] [Google Scholar]
- 67. Przepiorka D, Weisdorf D, Martin P, et al. Consensus conference on acute GVHD grading. Bone Marrow Transp. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 68. Stelljes M, Hermann S, Albring J, et al. Clinical molecular imaging in intestinal graft-versus-host disease: mapping of disease activity, prediction, and monitoring of treatment efficiency by positron emission tomography. Blood. 2008;111(5):2909–2918. doi:10.1182/blood-2007-10-119164 [DOI] [PubMed] [Google Scholar]
- 69. Bodet-Milin C, Lacombe M, Malard F, et al. 18F-FDG PET/CT for the assessment of gastrointestinal GVHD: results of a pilot study. Bone Marrow Transplant. 2014;49(1):131–137. doi:10.1038/bmt.2013.144 [DOI] [PubMed] [Google Scholar]
- 70. Dejanovic D, Amtoft A, Loft A. 18F-FDG PET/CT in extensive graft-versus-host disease of the gastrointestinal tract following autologous stem cell transplantation. Diagnostics (Basel). 2018;8(4):72 doi:10.3390/ diagnostics8040072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Holter JL, Thorp K, Smith ML, et al. [18F]-Fluorothymidine PET imaging in the diagnosis of leptomeningeal involvement with diffuse large B-cell lymphoma. Cancer Imaging. 2011;11(1):140–143. doi:10.1102/1470-7330.2011.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Herhaus P, Habringer S, Philipp-Abbrederis K, et al. Targeted positron emission tomography imaging of CXCR4 expression in patients with acute myeloid leukemia. Haematologica. 2016;101(8):932–940. doi:10.3324/haematol. 2016.142976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mayerhoefer ME, Jaeger U, Staber P, et al. [68 Ga]-Pentixafor PET/MRI for CXCR4 imaging of chronic lymphocytic leukemia: preliminary results. Invest Radiol. 2018;53(7):403–408. doi:10.1097/RLI.0000000000000469 [DOI] [PubMed] [Google Scholar]
- 74. Giovacchini G, Fallanca F, Landoni C, et al. C-11 choline versus F-18 fluorodeoxyglucose for imaging meningiomas: an initial experience. Clin Nucl Med. 2009;34(1):7–10. doi:10.1097/RLU.0b013e31818f4369 [DOI] [PubMed] [Google Scholar]
- 75. Qin C, Wu Z, Li J, Xun S, Xiaoli L. Differences in uptake of 18F-FDG and 11C-choline in a case of acute myeloid leukemia. Clin Nucl Med. 2016;41(10):799–801. doi:10.1097/RLU.0000000000001324 [DOI] [PubMed] [Google Scholar]
- 76. Cauchon N, Langlois R, Rousseau JA, et al. PET imaging of apoptosis with (64)Cu-labeled streptavidin following pretargeting of phosphatidylserine with biotinylated annexin-V. Eur J Nucl Med Mol Imaging. 2007;34(2):247–258. doi:10.1007/s00259-006-0199-y [DOI] [PubMed] [Google Scholar]
- 77. Faust A, Wagner S, Law MP, et al. The non peptidylcaspase binding radioligand (S)-1-(4-(2-[18F]Fluoroethoxy)-benzyl)-5-[1-(2-methoxymethylpyrrolidinyl)sulfonyl]isatin ([18F]CbR) as potential positron emission tomography-compatible apoptosis imaging agent. Q J Nucl Med Mol Imaging. 2007;51(1):67–73. [PubMed] [Google Scholar]
- 78. Aloya R, Shirvan A, Grimberg H, et al. Molecular imaging of cell death in vivo by a novel small molecule probe. Apoptosis. 2006;11(12):2089–2101. doi:10.1007/s10495-006-0282-7 [DOI] [PMC free article] [PubMed] [Google Scholar]