Abstract
Introduction:
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the prevalent and disabling side effects of cancer treatment. However, management strategies for CIPN currently remain elusive, with treatment restricted to neuropathic pain medications, supportive care, and chemotherapy dosing adjustments. This overview explores evidence on the potential benefits and safety of nonpharmacological interventions in preventing and treating CIPN in cancer patients.
Methods:
Seven databases were searched for systematic reviews of randomized controlled trials (RCTs). The methodological quality of the selected reviews was assessed by AMSTAR 2, and the quality of evidence was judged by GRADE. Twenty-eight systematic reviews were considered eligible for this review.
Results:
It was found that nonpharmacological interventions (acupuncture, exercise, herbal medicine, nutritional supplements) provided potential benefits for patients with CIPN. Furthermore, Chinese herbal medicine, administered orally or externally, significantly prevented and/or relieved the incidence and severity of CIPN in comparison to control groups (no additional treatment, placebo, and conventional western medicine). However, the quality of evidence and strength of recommendations were compromised by the inconsistencies and imprecision of included studies. The main concerns regarding the quality of systematic reviews included the lack of sufficiently rigorous a priori protocols, and the lack of protocol registration adopted in the included studies.
Conclusions:
Though looking across reviews, Chinese herbal medicine appear generally effective in CIPN, uncertainty remains about the effects of many other nonpharmacological interventions. The evidence on what works was particularly compromised by reporting and methodological limitations, which requires further investigation to be more certain of their effects.
Keywords: overview, systematic review, meta-analysis, nonpharmacological intervention, chemotherapy-induced peripheral neuropathy
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the prevalent and disabling side effects of cancer treatment regimens including neurotoxic chemotherapeutic agents (eg, taxanes, platinum compounds, vinca alkaloids, proteasome inhibitors, immunomodulatory agents).1 The prevalence of CIPN varies from 30% to 80%, and many patients have chronic symptoms during treatment. CIPN manifestations include certain variation of numbness, tingling, shooting pain, stabbing pain, burning, and increased thermal sensitivity, which may lead to day-to-day functional comorbidity.2,3 Published reviews for the prevention and treatment of CIPN were dedicated to evaluating pharmacologic therapies.4-6 However, only duloxetine was recommended by the American Society of Clinical Oncology (ASCO) with limited effectiveness. Most pharmacologic medications, including tricyclic antidepressants and anticonvulsants, either present limited efficacy in CIPN or pose intolerable risk of adverse events to patients.7-10
Nonpharmacological therapies comprise a broad range of physical therapies, mind and body practices, natural products, and supplements. There is increasing interest in the effect of nonpharmacological therapies in integrative oncology.11,12 However, evidence remains incomplete for many of these therapies.13,14 To date, a number of systematic reviews (SRs) have been published broadly on the use of nutraceuticals, complementary and integrative remedies, whereas no reviews have comprehensively assessed the studies of these therapies to manage CIPN for cancer care. This was based on a preliminary search for existing overview of reviews on the topic conducted on the databases (ie, Cochrane Library, CINAHL, PubMed, and PROSPERO) searched on September 21, 2019. Furthermore, SRs may demonstrate varied scope, quality, population size, reporting of outcomes, and heterogeneous effects, making interpretation of the evidence on overall treatment efficacy difficult. Hence, this overview explores evidence on the potential benefits and safety of nonpharmacological interventions in preventing and treating CIPN in cancer patients.
Methods
Protocol and Registration
The protocol of this overview was registered on PROSPERO (CRD42019129145). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines15 and the Cochrane Collaboration Handbook16 were followed to undertake this overview of reviews.
Literature Search
A comprehensive literature search of SRs and meta-analysis of randomized controlled trials (RCTs) was performed in the MEDLINE, EMBASE, Cochrane Library, PROSPERO, CNKI, VIP, and Wanfang databases from inception to October 13, 2019. The literature search was composed of the Medical Subject Headings (MeSH) and free-text words for “CIPN,” “systematic review,” and “meta-analysis,” which were implemented for different databases. MEDLINE, EMBASE, and Cochrane Library search strategies are shown in the appendix (supplementary file, available online). The reference lists of all the appraised articles were screened for relevant citations that might have been missed from the electronic searches. There were language restrictions on SRs published with a title and abstract in English or Chinese.
Study Selection
Initially, all duplicates were removed from the references. Two independent researchers (JH and XZ) selected the relevant reviews by screening the titles and abstracts of the identified articles. The full texts of these were then retrieved for further assessment of their potential eligibility. Any disagreements about inclusion were resolved by discussion or consultation with a third assessor if a consensus was not reached (AB).
The inclusion criteria were as follows: (1) SRs and meta/analyses of clinical studies in which at least 1 RCT was included; (2) target population was any type of cancer participants with CIPN (any type of chemotherapy) where the nonpharmacological management was the primary focus of the review; (3) the interventions included were nonpharmacological approaches such as lifestyle interventions, physical therapy, nutritional supplements, and complementary medicine therapies (eg, acupuncture, herbal medicine); (4) comparator(s)/control were pharmacological control or any other forms of control (eg, placebo, no intervention); and (5) the main outcomes reported were neurotoxicity incidence and/or severity measured by standardized and validated clinical assessment tools, including, but not limited to, patient-reported outcomes, clinician-rated neuropathy assessments, and physical/functional measures. The additional outcomes were safety outcomes (eg, adverse events).
The exclusion criteria were as follows: (1) CIPN was assessed as a part of a broader topic; (2) interventions were administered intravenously only; and (3) control comparisons were related to nonpharmacological therapy. If there were duplicate publications, we selected the latest complete version.
Assessment of Quality of Included Reviews
Two reviewers (JH and XZ) independently assessed the methodological quality of the included reviews using the AMSTAR 2 (Assessment of Multiple Systematic Reviews) appraisal tool.17 Any discrepancies were resolved by consultation with a third reviewer (AB). This checklist can be used to appraise SRs that include RCTs of health care interventions and also those that include nonrandomized studies, or both. It includes 16 domain-specific questions, each referring to a relevant methodological aspect of the study. By assessing the potential impact of an inadequate rating for each item, the reviews were rated by the overall confidence (High/Moderate/Low/Critically low) detected on the number of critical and noncritical items of the review.17 The quality of evidence for each main and additional outcome across studies was individually determined by 2 assessors as per the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) recommendations.18 We downgraded the level of evidence if there were: risk of bias, unexplained heterogeneity, indirectness of evidence, imprecision of the pooled estimate, and publication bias. The overall quality of evidence was judged as either high, moderate, low, or very low.
Data Extraction and Analysis
Two reviewers independently extracted information from the reviews and cross-checked the other’s extracted data. Discrepancies were resolved via judgement from a third author. The following data were collected from the included SRs: authors, publication year, databases search, number of included clinical studies and patients, target population, type of chemotherapy, interventions, controls, outcomes and outcome measurements, risk of bias tool, statistical model for data pooling, estimates of effect size, heterogeneity, publication bias, and funding source. Descriptive summaries of the included studies and their methodological quality are displayed in Tables 1 and 2. In Table 3, we extracted the estimates of effect size from meta-analyses, and reported these as relative risk (RR), odds ratio (OR) for dichotomous outcomes, and mean difference (MD) or weighted/standardized MD (WMD/SMD) for continuous outcomes, with the 95% confidence intervals (CIs).
Table 1.
Characteristics of Included Systematic Reviews in the Overview.
| Author(s), year | Databases searched | No. of clinical CIPN studies/no. of patients | Target population | Included clinical study design | Type of chemotherapy | Intervention | Control | Outcomes (outcome measurements) | Risk of bias tool | Funding source |
|---|---|---|---|---|---|---|---|---|---|---|
| Eum et al19 | MEDLINE, EMBASE, CENTRAL the reference lists; NA | 5/319 | Cancer patients receiving chemotherapy | RCTs | Taxanes, cisplatin, carboplatin, oxaliplatin, combination | Oral vitamin E supplements | No treatment or placebo | Incidence of CIPN | The Jadad scale | NR |
| Franconi et al20 | MEDLINE, Google Scholar, Cochrane Database, CINAHL, CNKI, Wanfang Med Online, and ISI conference Proceedings; January 2012 reference lists | 7/265 | NR | RCTs, NRSIs, case series | Not specified | All types of acupuncture (electroacupuncture, auricular acupuncture; warm acupuncture, and moxibustion) | No control, placebo acupuncture and seeds, cobamamide, neurotrophin | VAS pain score, medication consumption, Questionnaire of CIPN/PN, WHO CIPN grade, QoL, neurotoxic symptoms, NCV | Not assessed | NR |
| He and Yang21 | CNKI, Wanfang, VIP; NA | 5/425 | Cancer patients receiving oxaliplatin chemotherapy | Prospective RCTs | Oxaliplatin | External use of Chinese herbal medicine | No treatment (nursing care) | Incidence of CIPN (WHO scale) | The modified Jadad scale | NR |
| Schloss et al22 | PubMed, the Cochrane Library, Science Direct, Scopus, EMBASE, MEDLINE, CINAHL; NA | 23/2075 | Cancer patients who had received or were undergoing chemotherapy | RCTs, NRSIs, case studies | Platinum derivates (oxaliplatin, carboplatin, cisplatin), taxanes (paclitaxel), combination Tx | Nutraceuticals (Magnesium and calcium, vitamin B6, vitamin E, glutathione, glutamine, N-acetyl cysteine, acetyl-l-carnitine, lipoic acid, omega-3 fatty acids, electroacupuncture included) | Placebo, current anti-CIPN treatment, no control | Incidence and/or severity of CIPN (TNS), electro-physiologic evaluation (NCV) | NHMRC clinical evidence assessment matrix | NICM/NHMRC funding; Bioconcepts Ltd Industry funding |
| Tian et al23 | MEDLINE, EMBASE, Cochrane Library, CBM, CNKI, VIP, Wanfang; December 2012 | 6/368 | Cancer patients receiving oxaliplatin chemotherapy | RCTs, quasi-RCTs | Oxaliplatin | Chinese herbal decoction (Huang Qi Gui Zhi Wu Wu decoction) | No treatment (nursing care), conventional therapeutic agents | Incidence and severity of CIPN (Levi scale), SNCV, AE | Cochrane Collaboration’s RoB tool | National Chinese Medicine Industry Research Project of China |
| Streckmann et al24 | PubMed, MEDPILOT (MEDLINE), Cochrane Database, reference lists; December 2013 | 18/837 | Lymphoma participants with CIPN | RCTs, NRSIs | Not specified | Exercise intervention (sensorimotor training, endurance and strength) | Not specified | QoL, peripheral deep sensitivity, incidence and severity of CIPN, balance control, aerobic performance level, level of activity | The Oxford levels of evidence by OCEBM | None |
| Brami et al25 | Web of Science, PubMed, CENTRAL; January 2005 to May 2015 | 13/1370 | Cancer adults diagnosed with CIPN | Prospective RCTs | Platinum derivates (oxaliplatin, carboplatin, cisplatin), taxanes (paclitaxel), vinca alkaloids, combination Tx | Natural products and complementary therapies (vitamin E, glutamate/glutamine, goshajinkigan, acetyl-l-carnitine, alpha-lipoic acid, omega-3 fatty acids, electro-acupuncture) | Not specified (non-supplemented, placebo, usual care alone, hydroelectric baths, vitamin B1/B6) | The incidence of PN (NSS, NDS), neurological exams (TNS), severity score questionnaires (NCI-CTCAEv2.0; EORTC QLQ-C30; NTX-FACT), NCV (SNCV, MNCV) | Not assessed | NR |
| Deng et al26 | MEDLINE (1982-2015), Cochrane Controlled Trials (2015, Issue 12), Springer (1997-2015), CNKI (1997-2015), CSPD (1998-2015), reference lists; January 2016 | 24/1552 | Cancer adults had received or were undergoing oxaliplatin chemotherapy | RCTs | Oxaliplatin | All types of Radix Astragali–based herbal interventions | Placebo, no intervention, conventional treatment | The severity and/or incidence rate of CIPN (WHO, Levi, NCI-CTCAE, DEB-NTC), remission rate (CR + PR), NCV (SNCV, MNCV), QoL | Improved Jadad scale | Beijing Municipal Science & Technology Commission; National Fund of Natural Science of China |
| Deng et al27 | MEDLINE (1982-2015), Cochrane Controlled Trials (2015, Issue 4), CNKI (1997-2015), CSPD (1989-2015), reference lists; May 2015 | 26/1682 | Cancer adults had received or were undergoing oxaliplatin chemotherapy | RCTs | Oxaliplatin | All types of Caulis Spatholobi–based herbal interventions | Placebo, no intervention, conventional treatment | The severity and/or incidence rate of CIPN (WHO, Levi, NCICTCAE, DEB-NTC), remission rate (CR + PR), NCV (SNCV, MNCV), QoL (KPS, ECOG) | Improved Jadad scale | Beijing Municipal Science & Technology Commission; National Fund of Natural Science of China |
| Huang et al28 | MEDLINE, EMBASE, CENTRAL, the reference lists; December 2013 | 6/353 | Cancer patients receiving chemotherapy | RCTs | Oxaliplatin, cisplatin, and other types of chemotherapy | Oral vitamin E supplements | Placebo or conventional treatment | The incidence of CIPN, safety of vitamin E administration | Cochrane Collaboration’s RoB tool | China Mianyang Central Hospital (Funding number: 2014YJ28) |
| Ji29 | MEDLINE, EMBASE, CENTRAL, CBM, CNKI, VIP, Wanfang, relevant journals; October 2015 | 75/2025 | Cancer patients receiving oxaliplatin chemotherapy | RCTs, quasi-RCTs | Oxaliplatin | Chinese herbal medicine | No treatment (nursing care), conventional therapeutic agents | Incidence and severity of CIPN (Levi, WHO, NCI-CTCAE, Sanofi-Synthelabo scale), SNCV, AE, incidence of severe digestive tract reaction/liver injury/kidney injury | Cochrane Collaboration’s RoB tool | National Natural Science Foundation of China (2017 publication) |
| Wei et al30 | PubMed, EMBASE, Cochrane Libraries, CNKI, VIP, Wanfang, reference lists; August 2015 | 3/193 | Cancer patients receiving oxaliplatin chemotherapy | RCTs | Oxaliplatin | Chinese herbal decoction (Dang Gui Si Ni decoction) | No treatment, conventional therapeutic agents | Incidence and severity of CIPN (Levi scale), SNCV, MNCV, AE | The modified Jadad scale | Tianjin (China) Municipal Health Bureau Research project |
| Wei et al31 | PubMed, EMBASE, Cochrane Libraries, CNKI, VIP, Wanfang, relevant journals; September 2015 | 8/489 | Cancer patients receiving oxaliplatin chemotherapy | RCTs | Oxaliplatin | Chinese herbal decoction (Bu Yang Huan Wu decoction) | Conventional therapeutic agents | Incidence and/or severity of CIPN (Levi, WHO scale), SNCV, MNCV | The modified Jadad scale | Tianjin (China) Municipal Health Bureau Research Project |
| Wei et al32 | PubMed, EMBASE, Cochrane Libraries, CNKI, VIP, Wanfang, relevant journals; August 2015 | 14/889 | Cancer patients receiving chemotherapy | RCTs | Not specified | Vitamin supplements | No treatment, placebo, conventional therapeutic agents | Incidence and severity of CIPN (Levi, WHO, NCI-CTCAE scale), TNS, NSS, SED | The modified Jadad scale | Tianjin (China) Municipal Health Bureau Research project |
| Derksen et al33 | PubMed, Embase, Google Scholar (1994-2015); December 2015 | 22/3093 | Colorectal cancer patients with chronic CIPN | RCTs, NRSIs, cohort studies, case series, cross-sectional studies, crossover studies | Oxaliplatin | Lifestyle related intervention (dietary supplements, physical activities, alternative and complementary therapies) | Not specified | Severity of CIPN (NCI-CTCAE, DEB-NTC, CIPNAT, EORTC QLQ-CIPN20, FACT/GOG-Ntx) | Not assessed | Alpe d’HuZes Foundation within the research program “Leven met kanker” of the Dutch Cancer Society; Kankeronderzoekfonds Limburg as part of Health Foundation Limburg |
| Brayall et al34 | CINAHL, PubMed, MEDLINE Complete, PEDro, Cochrane, Google Scholar, reference lists; January 2002 to January 2017 | 2/78 | Cancer patients with CIPN | RCTs | Not specified | Physical therapy (interactive sensor-based balance training; sensorimotor, endurance, strength training) | Not specified | Static/dynamic balance control; QoL peripheral deep sensitivity (gait speed and variability, sway of ankle, hip and COM with EO and EC in closed stance and semitandem stance; FES-I; EORTC QLQ-C30; IST, SGA) | STROBE scores | NR |
| Chen35 | PubMed, EMBASE, CBM, CNKI, Wanfang, VIP, dissertations, conference proceedings; October 2017 | 20/1452 | Cancer patients receiving oxaliplatin chemotherapy | RCTs | Oxaliplatin | Chinese herbal decoction (Huang Qi Gui Zhi Wu Wu decoction) | No intervention or western medicine | Incidence of OIPN; incidence of severe digestive tract reaction/liver injury/kidney injury; incidence of severe low white blood cell count/severe thrombocytopenia; AE | Cochrane Collaboration’s RoB tool | NR |
| Duregon et al36 | MEDLINE, Scopus, Bandolier, PEDro, Web of Science, reference lists; September 2017 | 5/147 | Cancer participants undergoing treatment diagnosed with CIPN | RCTs and pre- and postintervention comparison | Not specified | Physical exercise intervention (supervised-training intervention/home-based intervention) | Sensorimotor training, ankle point-to-point reaching, and virtual obstacle crossing tasks | CIPN symptoms (mTNS; FACT-Neurotoxicity); Static balance control (sway paths, mediolateral COM sway, hip sway, ankle sway, anteroposterior COM | Cochrane collaboration Back Review Criteria | Not funded by grants |
| sway, the Berg Balance Scale); dynamic balance control (sway paths); QoL (EORTC-QLQ-C-30, SF-36, FACT-O), fear of falling (FES-I), level of troublesome (McGill QOL Questionnaire) | ||||||||||
| Hoshino et al37 | Scopus, Ovid MEDLINE, CENTRAL, ICHUSHI, Google Scholar, reference lists; September 2016 | 5/386 | Cancer adults receiving hospital-based chemotherapy | RCTs | Oxaliplatin, docetaxel, paclitaxel | Goshajinkigan | Vitamin B12, placebo, no comparator | Incidence and/or severity of CIPN (CTCAE;DEB-NTC;VAS); incidence rate of AE with chemotherapy/SAE with Goshajinkigan/hematological toxicities (CTCAE), RECIST, rate of completion of chemotherapy | Cochrane Collaboration’s RoB tool | Japan Society for the Promotion of Science |
| Kuriyama and Endo38 | Medline, EMBASE, ICHUSHI, CENTRAL, Google scholar, reference lists; August, 2017 | 5/397 | Cancer adults receiving neurotoxic chemotherapy | RCTs | Oxaliplatin, docetaxel, paclitaxel | Goshajinkigan | Placebo, no intervention, and any agents that are currently known to not reduce or prevent CIPN (bathing in carbon dioxide-rich water; mecobalamin included) | Incidence rate of CIPN (CTCAE; DEB-NTC), response to chemotherapy, AEs to goshajinkigan, rate of completion of chemotherapy, disease control | Cochrane Collaboration’s RoB tool | NR |
| Liu et al39 | PubMed, Embase, CINAHL, AHMED, Cochrane Library, CBM, CQVIP, CNKI, Wanfang, reference lists; February 2018 | 63/4286 | Colorectal cancer adults had received or undergoing chemotherapy | Prospective RCTs | Oxaliplatin, cisplatin | Herbal medicines (orally and/or topically) used in traditional medicine in China, Korea, and/or Japan | Placebo, conventional chemotherapy, no additional intervention | The severity and/or incidence rate of CIPN (WHO, Levi, NCI-CTCAE, DEB-NTC), AE | Cochrane Collaboration’s RoB tool | The Australia International Research Centre for Chinese Medicine (CAIRCCM), the Foundation for Chinese Medicine and Technology Research of Guangdong Provincial Hospital of Chinese Medicine |
| Noh et al40 | MEDLINE, CENTRAL, EMBASE, AMED, CNKI, Wanfang, CQVIP, KSI, DBPIA, KISTI, the Research Information Centre for Health Database, KTKP, KoreaMed; May 17, 2017 | 28/2174 | Participants diagnosed with CIPN after chemotherapy | RCTs | Oxaliplatin, docetaxel, paclitaxel, NA | All types of herbal medicines | No treatment, placebo, conventional therapeutic agents | Remission rate (CR + PR), incidence rate (NCI-CTCAE, Levi), NCS, QoL | Cochrane Collaboration’s RoB tool | The Traditional Korean Medicine R&D Program funded by the Ministry of Health and Welfare through Korea Health Industry Development Institute (KHIDI) |
| Oh and Kim41 | PubMed, Cochrane Library CENTRAL, EMBASE, CINAHL, KoreaMed, KMbase, RISS, Nanet, KISS, Google Scholar; August 2017 | 22/954 | Cancer patients with CIPN | RCTs, NRSIs, case series, case reports, population-based survey, single-arm study, retrospective service evaluation | Not specified | Nondrug interventions | Not specified | Severity of CIPN (6MWT; ADL; CIPNAT; CTCAE; DGI; EC; EO; EORTC-QLQ CIPN20, EORTC-QLQ 30; EPIC; FACT/GOG-NTx; FACT-G; FES-I; HADS; LANSS; mCTSIB; MET; NPS; NRS; QOL; SF-12; TNSc; TNSr; TUG; VAS; VO2max; VPT) QoL, NCV, function tests, activity level | Cochrane Collaboration’s RoB tool | NR |
| Yan et al42 | CNKI, VIP, Wanfang, CBM, reference lists; July 2017 | 8/417 | Cancer patients with CIPN | RCTs | Not specified | Acupuncture | Placebo, conventional therapeutic agents | PNQ, NCV (SNCV, MNCV), VAS, FACT/GOG-NTX | The modified Jadad scale | NR |
| Yang et al43 | PubMed, CBM, Cochrane Library, CNKI, VIP, Wanfang; May 2017 | 8/805 | Cancer patients receiving oxaliplatin chemotherapy | RCTs | Oxaliplatin | External use of Chinese herbal medicine (herbal hand and foot baths; acupoint application) | Warm water bath, no treatment (nursing care) | Incidence and severity of CIPN (Levi scale) | Cochrane Collaboration’s RoB tool | National Natural Science Foundation of China |
| Li et al44 | PubMed, Cochrane Library, CNKI, CSPD; December 2018 | 20/1481 | Cancer adults diagnosed with CIPN | RCTs | Oxaliplatin, cisplatin, paclitaxel, NA | All types of ABDC herbal medicines | No additional control, placebo, conventional western medicine, warm water | The severity and/or incidence rate (WHO, NCI-CTCAE, Levi, CR + PR), NCV (SNCV, MNCV), QoL (KPS, ECOG), AE | Cochrane Collaboration’s RoB tool | Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents; Pro Program for the Cultivation of Youth talents in China Association of Chinese Medicine; Zhejiang Provincial Program for the Cultivation of the Young and Middle-Aged Academic Leaders in Colleges and universities; Zhejiang Pro Provincial Project for the key discipline of Traditional Chinese medicine |
| Li et al45 | EMBASE, Web of Science, MEDLINE, CENTRAL, CINAHL, the ClinicalTrials.gov Websites, the AcuTrials database, google scholar; May 2017 | 3/203 | Participants diagnosed with CIPN | RCTs | Taxanes, platinum derivative, vinca alkaloids | All type of acupuncture (acupuncture, electro-acupuncture, acupressure as an adjunctive or main intervention) | Placebo, sham acupuncture, conventional western medicine, hydroelectric bath | Pain, numbness, tingling, cold sensitivity, or any other signs of PN, subjective patient reports, surrogate markers, ADL, QoL, AE, changes in chemotherapy dosing | Cochrane Collaboration’s tool. The included studies generally had a low or unclear risk of bias | NR |
| Zhang et al46 | MEDLINE, EMBASE, CENTRAL, the US National Library of Health’s Clinical Trials registry, the WHO International Clinical Trials Registry Platform, February 2019 | 2/140 | Cancer patients undergoing chemotherapy | RCTs | Oxaliplatin, paclitaxel | Omega-3 polyunsaturated fatty acid oral supplements | Placebo or no intervention | Incidence of CIPN, NCS (SNCV, MNCV, SNAP, CMAP), AE | Cochrane Collaboration’s RoB tool | 2018 Melbourne Neuroscience Institute (MNI) Interdisciplinary Seed Fund grant |
Abbreviations: 6MWT, 6-minute walk test; ADL, activities of daily living; CBM, Chinese BioMedical Literature Database; CINAHL, Cumulative Index to Nursing and Allied Health Literature; CIPNAT, chemotherapy-induced peripheral neuropathy assessment tool; CMAP, compound motor action potential; CNKI, China National Knowledge Infrastructure; COM, center of mass; CQVIP, VIP Database for Chinese Technical Periodicals; CR, complete remission and the grade of CIPN reduced to 0 grade and all symptoms disappeared; CSPD, Wanfang Database of China Science Periodical Database; DEB-NTC, Neurotoxicity Criteria of Debiopharm; DGI, dynamic gait index; EC, eyes closed; EO, eyes opened; EORTC-QLQ (CIPN20/C30), European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy scale; EPIC, European Prospective Investigation into Cancer; FACT/GOG-NTx, Functional Assessment of Cancer Therapy/Gynecological Oncology Group–Neurotoxicity; FACT-G/O, Functional Assessment of Cancer Therapy–General/Ovarian Cancer-Specific Scale; FES-I, Falls Efficacy Scale International; HADS, Hospital Anxiety and Depression Scale; ICHUSHI, Japanese Database of Scientific Literature and Abstracts of Scientific Meetings; IST, Incremental Step Test; KISS, Korean Information Service System; KISTI, The Korea Institute of Science and Technology Information; KMbase, Korean studies Medical Database; KoreaMed, Korean Association of Medical Journal Editors; KSI, Korean Studies Information; LANSS, Leeds Assessment of Neuropathic Symptoms and Sign; mCTSIB, modified Clinical Test for Sensory Interaction in Balance; MET, metabolic equivalent; mTNS, Modified Total Neuropathy Score; Nanet, National Assembly Library of Korea; NCI-CTCAE, the National Central Cancer Institute Common Terminology Criteria for Adverse Events; NCS, nerve conduction studies; NCV, nerve conduction velocity; NHMRC, the Australian National Health and Medical Research; NPS, Neuropathy Pain Scale; NRS, Neuropathic Symptoms on Numerical Rating Scale; NSS, Neurological Severity Score; OCEBM, the Oxford Center for Evidence Based Medicine; PNQ, Patient Neurotoxicity Questionnaire; PR, partial remission; QOL, quality of life; RECIST, rate of response to chemotherapy; RISS, Research Information Service System; SED, symptom examination daily; SGA, Subjective Global Assessment; SF-12, Short-Form Health Survey–12; SF-36, 36-Item Short-Form Survey; SNAP, sensory nerve action potential; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; TNS, Total Neurological Score; TNSc, Clinical Total Neuropathy Score; TNSr, Total Neuropathy Score Reduced; TUG, timed up and go; VAS, Visual Analogue Scale; VO2max, maximal oxygen consumption; VPT, vibration perception threshold.
Table 2.
AMSTAR 2 Tool of Quality Assessment of the Included Systematic Reviews.
| Author | Q1 | Q2a | Q3 | Q4a | Q5 | Q6 | Q7a | Q8 | Q9a | Q10 | Q11a | Q12 | Q13a | Q14 | Q15a | Q16 | Overall rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eum et al19 | Yes | No | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | No | No | No | No | No | No | Critically low |
| Franconi et al20 | Yes | No | Yes | Partial yes | Yes | No | No | Partial yes | No | No | No meta-analysis conducted | No meta-analysis conducted | No | Yes | No meta-analysis conducted | Yes | Critically low |
| He and Yang21 | Yes | No | Yes | Partial yes | Yes | Yes | No | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Critically low |
| Schloss et al22 | No | Yes | Yes | Partial yes | Yes | No | No | Partial yes | Partial yes | No | No meta-analysis conducted | No meta-analysis conducted | Yes | Yes | No meta-analysis conducted | Yes | Low |
| Tian et al23 | Yes | No | Yes | Partial yes | No | Yes | No | No | Partial yes | No | Yes | No | Yes | Yes | Yes | Yes | Critically low |
| Streckmann et al24 | Yes (no C,O) | No | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | No meta-analysis conducted | No meta-analysis conducted | Yes | Yes | No meta-analysis conducted | Yes | Critically low |
| Brami et al25 | No | No | Yes | Partial yes | No | No | No | Partial yes | No | No | No meta-analysis conducted | No meta-analysis conducted | No | Yes | No meta-analysis conducted | Yes | Critically low |
| Deng et al26 | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Partial yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Low |
| Deng et al27 | Yes | No | Yes | Partial yes | Yes | Yes | No | Partial yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Critically low |
| Huang et al28 | Yes | No | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | Yes | Yes | Yes | Yes | No meta-analysis conducted | Yes | Critically low |
| Ji29 | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | Yes | No | Yes | Yes | Yes | Yes | Low |
| Wei et al30 | Yes | No | Yes | Partial yes | Yes | Yes | No | No | Partial yes | No | No | No | No | No | No | Yes | Critically low |
| Wei et al31 | Yes | No | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | Yes | No | Yes | Yes | No | Yes | Critically low |
| Wei et al32 | Yes | No | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | Yes | No | Yes | No | No | Yes | Critically low |
| Derksen et al33 | Yes | No | Yes | Partial yes | No | No | No | Partial yes | No | No | No meta-analysis conducted | No meta-analysis conducted | No | Yes | No meta-analysis conducted | Yes | Critically low |
| Brayall et al34 | Yes | No | No | Partial yes | No | Yes | No | Partial yes | Partial Yes | No | No meta-analysis conducted | No meta-analysis conducted | Yes | Yes | No meta-analysis conducted | No | Critically low |
| Chen35 | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Duregon et al36 | Yes | No | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | No meta-analysis conducted | No meta-analysis conducted | Yes | Yes | No meta-analysis conducted | Yes | Critically low |
| Hoshino et al37 | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | Yes | No | Yes | Yes | No | Yes | Low |
| Kuriyama and Endo38 | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Partial yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Low |
| Liu et al39 | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | Yes | Yes | Yes | Yes | No meta-analysis conducted | Yes | Low |
| Noh et al40 | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | No meta-analysis conducted | No meta-analysis conducted | Yes | Yes | No meta-analysis conducted | Yes | Low |
| Oh and Kim41 | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Yes | Partial yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Yan et al42 | No | No | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | Yes | No | No | Yes | No | Yes | Critically low |
| Yang et al43 | Yes | No | Yes | Partial yes | Yes | No | No | Partial yes | Partial yes | No | Yes | No | Yes | Yes | Yes | Yes | Critically low |
| Li et al44 | Yes | Yes | Yes | Partial yes | Yes | Yes | No | Partial yes | Partial yes | No | Yes | No | Yes | Yes | Yes | Yes | Low |
| Li et al45 | Yes | Partial yes | Yes | Partial yes | No | No | No | Partial yes | Partial yes | No | No meta-analysis conducted | No meta-analysis conducted | Yes | Yes | No meta-analysis conducted | Yes | Low |
| Zhang et al46 | Yes | Yes | Yes | Partial yes | Yes | Yes | Yes | Yes | Partial yes | Yes | Yes | Yes | Yes | Yes | No meta-analysis conducted | Yes | High |
Abbreviation: AMSTAR, Assessment of Multiple Systematic Reviews.
AMSTAR 2 critical domains.
Table 3.
GRADE Quality of Evidence Score for Significant Outcomes Reported in the Systematic Reviews Included in the Overview.
| Outcome | Author | Comparison | N/n | Statistical model | Pooled effects [95% CI] | Heterogeneity I2 | Quality of evidence |
|---|---|---|---|---|---|---|---|
| Incidence of grade ≥ 1 OIPN | |||||||
| Deng et al26 | RA herbal medicine (ad us ext; iv; po) vs control | 18/1155 | FE | OR: 0.20 [0.14 to 0.25] | 0% | Low 1.2.8 | |
| Deng et al26 | RA herbal medicine (ad us ext; iv; po) vs no intervention | 15/993 | FE | OR: 0.19 [0.14 to 0.25] | 0% | Low 1.2.8 | |
| Deng et al26 | RA herbal medicine (ad us ext; po) vs mecobalamin | 1/42 | FE | OR: 0.17 [0.03 to 0.94] | NA | Low 1.2.6.8 | |
| Deng et al26 | RA herbal medicine (ad us ext; po) plus western medications vs western medications | 2/120 | FE | OR: 0.42 [0.18 to 0.97] | 0% | Low 1.2.8 | |
| Deng et al27 | CS herbal medications (ad us ext; po) vs control | 17/1061 | FE | OR: 0.19 [0.14 to 0.25] | 0% | Low 1.2.8 | |
| Deng et al27 | High-dose CS herbal medications (ad us ext; po) vs control | 10/652 | FE | OR: 0.21 [0.14 to 0.30] | 0% | Low 1.2.8 | |
| Deng et al27 | Low-dose CS herbal medications (ad us ext; po) vs control | 7/409 | FE | OR: 0.19 [0.14 to 0.25] | 0% | Low 1.2.8 | |
| Ji29 | Herbal medicine (ad us ext; iv; po) vs no intervention/placebo | 60/3845 | FE | RR: 0.60 [0.56 to 0.64] | 19% | Low 1.2.8 | |
| Ji29 | Herbal medicine (ad us ext) vs no intervention/placebo | 20/1454 | FE | RR: 0.62 [0.57 to 0.67] | 19% | Low 1.2.8 | |
| Ji29 | Herbal medications (po) vs no intervention/placebo | 32/1860 | FE | RR: 0.58 [0.53 to 0.64] | 24% | Low 1.2.8 | |
| Ji29 | Herbal medications (ad us ext; po) vs western medications | 5/444 | FE | RR: 0.50 [0.41 to 0.62] | 14% | Low 1.2.8 | |
| Ji29 | herbs (ad us ext; po) in combined remedies vs the same western medications | 7/472 | FE | RR: 0.42 [0.32-0.54] | 0% | Low 1.2.8 | |
| WHO | Wei et al31 | BYHW herbal medicine (po) vs mecobalamine | 1/115 | NA | RR: 0.25 [0.12 to 0.53] | NA | Low 1.2.6.8 |
| He and Yang21 | Herbal medicine (ad us ext) vs no intervention | 5/425 | FE | OR: 0.26 [0.17 to 0.40] | 0% | Low 1.2.8 | |
| Liu et al39 | Herbal medicine (po) vs no intervention | 32/1853 | RE | RR: 0.78 [0.66 to 0.91] | 71% | Low 1.2.3.4.8. | |
| Levi | Liu et al39 | Herbal medicine (po) vs no intervention | 7/545 | RE | RR: 0.54 [0.38 to -0.76] | 82.30% | Low 1.2.8 |
| Liu et al39 | Herbal medicine (ad us ext) vs no intervention | 5/374 | RE | RR: 0.69 [0.50 to 0.95] | 68.80% | Low 1.2.3.4.8. | |
| Yang et al43 | Herbal medicine (ad us ext) vs control (no intervention; warm bath) | 8/805 | FE | OR: 0.23 [0.16 to 0.31] | 0% | Low 1.2.8 | |
| Tian et al23 | HQGZWW herbal medicine (ad us ext; po) vs no intervention | 5/297 | FE | OR: 0.10 [0.06 to 0.19] | 0% | Low 1.2.8 | |
| Tian et al23 | HQGZWW herbal medicine (po) or mecobalamine | 2/99 | FE | OR: 0.17 [0.05 to 0.61] | 0% | Low 1.2.8 | |
| Wei et al31 | BYHW herbal medicine (po) vs control | 7/374 | FE | RR: 0.50 [0.40 to 0.63] | 42% | Low 1.2.3.4.8 | |
| NCI-CTCAE | Liu et al39 | Herbal medicine (po) vs no intervention | 9/727 | RE | RR: 0.74 [0.58 to 0.94] | 13.50% | Low 1.2.8 |
| Incidence of grade ≥1 cisplatin-induced PN | |||||||
| Huang et al28 | Vitamin E (po) vs control (placebo/no intervention) | 3/98 | RE | RR: 0.31 [0.17 to 0.58] | 0% | Low 1.2.8 | |
| Incidence of grade ≥1 CIPN | |||||||
| Huang et al28 | Vitamin E (po) vs control (placebo/no intervention) | 6/353 | RE | RR: 0.55 [0.29 to 1.05] | 77% | Very low 1.2.4.7.8 | |
| Huang et al28 | Vitamin E (po) vs placebo | 3/264 | RE | RR: 1.03 [0.59 to 1.80] | 62% | Very low 1.2.7.8 | |
| TNSc | Zhang et al46 | Vitamin E (po) vs placebo | 2/128 | FE | RR: 0.58 [0.43 to 0.77] | 0% | Low 1.2.8 |
| NCI-CTCAE | Kuriyama and Endo38 | Goshajinkigan (po) vs control | 4/341 | RE | RR: 0.76 [0.50 to 1.17] | 84.9% | Very low 1.5.7.8 |
| DEB-NTC | Kuriyama and Endo38 | Goshajinkigan (po) vs control | 1/60 | RE | RR: 0.43 [0.27 to 0.66] | NA | Low 1.6.8 |
| Li et al44 | ABDC herbal medicine (ad us ext; iv; po) vs all types of control | 15/1093 | RE | OR: 0.26 [0.20 to 0.35] | 0% | Low 1.2.8 | |
| Li et al44 | ABDC herbal medicine (ad us ext; iv; po) vs no intervention/placebo | 8/617 | RE | OR: 0.22 [0.14 to 0.34] | 22% | Low 1.2.8 | |
| Li et al44 | ABDC herbal medicine (iv; po) vs western medications | 3/142 | RE | OR: 0.22 [0.09 to 0.54] | 0% | Low 1.2.8 | |
| Li et al44 | ABDC herbal medicine (ad us ext; iv; po) in combined remedies vs the same western medications | 4/334 | RE | OR: 0.36 [0.22 to 0.59] | 0% | Low 1.2.8 | |
| Incidence of grade ≥2 OIPN | |||||||
| Levi | Yang et al43 | Herbal medicine (ad us ext) vs control (no intervention; warm bath) | 8/805 | FE | OR: 0.41 [0.32 to 0.51] | 13% | Low 1.2.8 |
| Tian et al23 | HQGZWW herbal medicine (ad us ext; po) vs no intervention | 5/305 | FE | OR: 0.07 [0.04 to 0.14] | 44% | Low 1.2.3.8 | |
| Tian et al23 | HQGZWW herbal medicine (po) or mecobalamine | 2/99 | FE | OR: 0.14 [0.05 to 0.44] | 0% | Low 1.2.8 | |
| Wei et al31 | BYHW herbal medicine (po) vs control | 7/374 | FE | RR: 0.43 [0.28 to 0.65] | 0% | Low 1.2.8 | |
| 390 mg/m2 dose of OXA | Wei et al31 | BYHW herbal medicine (po) vs control | 2/129 | FE | RR: 0.32 [0.09 to 1.12] | 0% | Very low 1.2.7.8 |
| 680-780 mg/m2 dose of OXA | Wei et al31 | BYHW herbal medicine (po) vs control | 5/245 | FE | RR: 0.45 [0.29 to 0.70] | 0% | Low 1.2.8 |
| WHO | Wei et al31 | BYHW herbal medicine (po) vs mecobalamine | 1/115 | NA | RR: 0.19 [0.04 to 0.80] | NA | Low 1.2.6.8 |
| Incidence of grade ≥2 CIPN | |||||||
| NCI-CTCAE | Kuriyama and Endo38 | Goshajinkigan (po) vs control | 4/341 | RE | RR: 0.99 [0.53 to 1.85] | 79.6% | Very low 1.5.7.8 |
| Hoshino et al37 | Goshajinkigan (po) vs control | 4/341 | RE | RR: 0.94 [0.57 to 1.57] | 75% | Very low 1.2.5.7.8 | |
| DEB-NTC | Kuriyama and Endo38 | Goshajinkigan (po) vs control | 4/285 | RE | RR: 0.78 [0.36 to 1.72] | 94.7% | Very low 1.5.7.8 |
| Hoshino et al37 | Goshajinkigan (po) vs control | 3/287 | RE | RR: 0.74 [0.33 to 1.64] | 93% | Very low 1.2.5.7.8 | |
| NCI-CTCAE grade ≥3 CIPN | |||||||
| NCI-CTCAE | Kuriyama and Endo38 | Goshajinkigan (po) vs control | 4/341 | RE | RR: 0.95 [0.38 to 2.39] | 30.8% | Low 1.7.8 |
| Hoshino et al37 | Goshajinkigan (po) vs control | 4/341 | FE | RR: 1.08 [0.59 to 2.00] | 31% | Low 1.2.7.8 | |
| DEB-NTC | Kuriyama and Endo38 | Goshajinkigan (po) vs control | 2/105 | RE | RR: 0.42 [0.25 to 0.71] | 0% | Low 1.8 |
| Hoshino et al37 | Goshajinkigan (po) vs control | 3/287 | RE | RR: 0.65 [0.28 to 1.52] | 76% | Very low 1.2.5.7.8 | |
| NCI-CTCAE | Liu et al39 | Herbal medicine (po) vs no intervention | 7/561 | RE | RR: 0.65 [0.37 to 1.13] | 26.40% | Low 1.2.7.8. |
| WHO | Liu et al39 | Herbal medicine (po) vs no intervention | 14/955 | RE | RR: 0.42 [0.23 to 0.77] | 0% | Low 1.2.8 |
| Levi | Liu et al39 | Herbal medicine (po) vs no intervention | 6/485 | RE | RR: 0.28 [0.11 to 0.69] | 0% | Low 1.2.8 |
| Liu et al39 | Herbal medicine (ad us ext) vs no intervention | 6/374 | RE | RR: 0.35 [0.10 to 1.20] | 0% | Low 1.2.7.8. | |
| Incidence of grade ≥3 OIPN | |||||||
| Deng et al26 | RA herbal medicine (ad us ext; iv; po) vs all types of control | 18/1150 | FE | OR: 0.20 [0.12 to 0.34] | 0% | Low 1.2.8 | |
| Deng et al26 | RA herbal medicine (ad us ext; iv; po) vs no intervention | 14/931 | FE | OR: 0.17 [0.09 to 0.31] | 0% | Low 1.2.8 | |
| Deng et al26 | RA herbal medicine (ad us ext; po) vs mecobalamin | 2/99 | FE | OR: 0.60 [0.08 to 4.72] | 0% | Low 1.2.7.8 | |
| Deng et al26 | RA herbal medicine (ad us ext; po) plus western medications vs western medications | 2/120 | FE | OR: 0.34 [0.11 to 1.07] | 0% | Low 1.2.7.8 | |
| Li et al44 | ABDC herbal medicine (ad us ext; iv; po) vs all types of control | 16/1149 | RE | OR: 0.35 [0.22 to 0.57] | 0% | Low 1.2.8 | |
| Li et al44 | ABDC herbal medicine (ad us ext; iv; po) vs no intervention/placebo | 9/673 | RE | OR: 0.34 [0.20 to 0.61] | 0% | Low 1.2.8 | |
| Li et al44 | ABDC herbal medicine (iv; po) vs western medications | 3/142 | RE | OR: 0.26 [0.05 to 1.33] | 0% | Low 1.2.7.8 | |
| Li et al44 | ABDC herbal medicine (ad us ext; iv; po) in combined remedies vs the same western medications | 4/334 | RE | OR: 0.45 [0.14 to 1.44] | 0% | Low 1.2.7.8 | |
| Ji29 | Herbal medications (ad us ext; iv; po) vs no intervention/placebo | 59/3818 | FE | RR: 0.34 [0.28 to 0.43] | 0% | Low 1.2.8 | |
| Ji29 | Herbal medications (ad us ext; po) vs western medications | 6/504 | FE | RR: 0.51 [0.19 to 1.34] | 0% | Low 1.2.7.8 | |
| Ji29 | herbs (ad us ext; po) in combined remedies vs the same western medications | 8/532 | FE | RR: 0.32 [0.14 to 0.75] | 0% | Low 1.2.8 | |
| Deng et al27 | CS (ad us ext; po) herbal medications vs control | 12/773 | FE | OR: 0.22 [0.12 to 0.40] | 0% (0.98) | Low 1.2.8 | |
| Deng et al27 | High-dose CS herbal medications vs control | 9/591 | FE | OR: 0.26 [0.13 to 0.51] | 0% (0.97) | Low 1.2.8 | |
| Deng et al27 | Low-dose CS herbal medications vs control | 3/182 | FE | OR: 0.13 [0.04 to 0.47] | 0% (0.91) | Low 1.2.8 | |
| Curative effects | |||||||
| CR + PR | Deng et al26 | RA herbal medicine (ad us ext; po) vs control | 5/341 | FE | OR: 3.59 [2.16 to 5.95] | 0% | Low 1.2.8 |
| Deng et al26 | RA herbal medicine (ad us ext; po) plus western medications vs western medications | 3/213 | FE | OR: 4.84 [2.38 to 9.83] | 0% | Low 1.2.8 | |
| Deng et al26 | RA herbal medicine (ad us ext) vs mecobalamin | 1/60 | FE | OR: 2.51 [0.83 to 7.64] | NA | Very low 1.2.6.7.8 | |
| Deng et al26 | RA herbal medicine (ad us ext) vs no intervention | 1/68 | FE | OR: 2.61 [0.98 to 6.94] | NA | Very low 1.2.6.8 | |
| Li et al44 | ABDC herbal medicine vs all types of control | 6/418 | RE | OR: 4.30 [2.75 to 6.74] | 0% | Low 1.2.8 | |
| Li et al44 | ABDC herbal medicine (ad us ext) vs no intervention/placebo | 3/233 | RE | OR: 4.57 [2.48 to 8.40] | 0% | Low 1.2.8 | |
| Li et al44 | ABDC herbal medicine (po) vs western medications | 2/125 | RE | OR: 4.91 [1.10 to 21.81] | 61% | Low 1.2.3.8 | |
| Li et al44 | ABDC herbal medicine (ad us ext) in combined remedies vs the same western medications | 1/60 | RE | OR: 4.13 [1.39 to 12.27] | NA | Low 1.2.6.8 | |
| Deng et al27 | CS (ad us ext; po) herbal medications vs control | 9/577 | FE | OR: 4.27 [2.81 to 6.47] | 0% | Low 1.2.8 | |
| Deng et al27 | High-dose CS herbal medications vs control | 7/489 | FE | OR: 4.32 [2.72 to 6.87] | 0% | Low 1.2.8 | |
| Deng et al27 | Low-dose CS herbal medications vs control | 2/88 | FE | OR: 4.05 [1.56 to 10.50] | 0% (0.78) | Low 1.2.8 | |
| SN-PNQ | Yan et al42 | Acupuncture vs western medication (mecobalamin/cobamamide/B12 injection) | 5/313 | FE | OR: 2.51 [1.58 to 4.01] | 12% | Low 1.2.8 |
| MN-PNQ | Yan et al42 | Acupuncture vs western medication | 3/158 | FE | OR: 1.80 [0.70 to 4.67] | 0% | Very low 1.2.7.8. |
| Nerve Conduction Studies | |||||||
| SNCV | |||||||
| Deng et al26 | RA herbal medicine (ad us ext; po) vs all types of control | 6/374 | RE | MD: 4.42 [3.27 to 5.57] | 16% | Low 1.2.8 | |
| Deng et al26 | RA herbal medicine (ad us ext; po) vs no intervention | 3/168 | RE | MD: 4.44 [2.99 to 5.88] | 0% | Low 1.2.8 | |
| Deng et al26 | RA herbal medicine (ad us ext; po) vs mecobalamin | 2/116 | RE | MD: 3.77 [−0.47 to 8.00] | 77% | Very low 1.2.4.7.8 | |
| Deng et al26 | RA herbal medicine (ad us ext) plus western medications vs western medications | 1/90 | RE | MD: 4.81 [2.46 to 7.16] | NA | Low 1.2.6.8 | |
| Tian et al23 | HQGZWW herbal medicine (ad us ext; p.o.) vs no intervention | 2/102 | FE | MD: 5.49 [3.70 to 7.29] | 39% | Low 1.2.8 | |
| Fibular nerve | Li et al44 | ABDC herbal medicine (ad us ext; iv; po) vs all types of control | 8/498 | RE | MD: 4.59 [3.23 to 5.96] | 67% | Low 1.2.3.4.8 |
| Li et al44 | ABDC herbal medicine (ad us ext; po) vs no intervention/placebo | 2/137 | RE | MD: 4.59 [1.38 to 7.81] | 89% | Low 1.2.3.8 | |
| Li et al44 | ABDC herbal medicine (iv; po) vs western medications | 4/207 | RE | MD: 5.07[2.92 to 7.22] | 69% | Low 1.2.3.8 | |
| Li et al44 | ABDC herbal medicine (iv; po) in combined remedies vs the same western medications | 2/154 | RE | MD: 3.12 [0.81 to 5.43] | 0% | Low 1.2.8 | |
| Deng et al27 | CS (ad us ext; po) herbal medications vs control | 3/229 | FE | MD: 2.12 [1.04 to 3.20] | 43% | Low 1.2.3.8 | |
| Wei et al31 | BYHW herbal medicine (po) vs control | 1/38 | NA | MD: 3.32 [0.67 to 5.97] | NA | Low 1.2.6.8 | |
| Median nerve | Wei et al31 | BYHW herbal medicine (po) vs control | 1/38 | NA | MD: 3.18 [0.63 to 5.73] | NA | Low 1.2.6.8 |
| Wei et al30 | Herbal decoction (DGSN) vs control | 1/NR | NA | MD: 3.40 [0.58 to 6.22] | NA | Very low 1.2.6.7.9 | |
| Li et al44 | ABDC herbal medicine (ad us ext; po) vs all types of control | 6/392 | RE | MD: 4.00 [2.81 to 5.99] | 77% | Low 1.2.3.4.8 | |
| Li et al44 | ABDC herbal medicine (ad us ext) vs no intervention/placebo | 1/67 | RE | MD: 1.99[0.60 to 3.38] | NA | Low 1.2.6.8 | |
| Li et al44 | ABDC herbal medicine (po) vs western medications | 3/171 | RE | MD: 5.23[2.87 to 7.59] | 66% | Low 1.2.3.8 | |
| Li et al44 | ABDC herbal medicine (iv; po) in combined remedies vs the same western medications | 2/154 | RE | MD: 3.47[1.17 to 5.77] | 0% | Low 1.2.8 | |
| Ulnar nerve | Zhang et al46 | Omega-3 (po) vs placebo | 2/116 | FE | MD: 2.21[−0.64 to 5.06] | 0% | |
| Upper limbs | Yan et al42 | Acupuncture vs western medication | 3/216 | FE | MD: 3.17 [2.93 to 3.42] | 97% | Low 1.2.3.8. |
| Lower limbs | Yan et al42 | Acupuncture vs western medication | 3/216 | FE | MD: 2.40 [2.12 to 2.67] | 99% | Low 1.2.3.8. |
| MNCV | |||||||
| Deng et al26 | RA herbal medicine (ad us ext; po) vs all types of control | 4/267 | RE | MD: 1.79 [−1.45 to 5.03] | 92% | Very low 1.2.5.7.8 | |
| Deng et al26 | RA herbal medicine (ad us ext) vs no intervention | 1/60 | RE | MD: −1.22 [−2.80 to 0.36] | NA | Very low 1.2.6.7.8 | |
| Deng et al26 | RA herbal medicine (ad us ext) vs mecobalamin | 2/147 | RE | MD: 2.26 [−3.67 to 8.19] | 95% | Very low 1.2.5.7.8 | |
| Deng et al26 | RA herbal medicine (ad us ext) plus western medications vs western medications | 1/60 | RE | MD: 4.10 [1.70 to 6.50] | NA | Low 1.2.6.8 | |
| Fibular nerve | Li et al44 | ABDC herbal medicine vs all types of control | 7/428 | RE | MD: 4.53 [2.23 to 6.84] | 90% | Low 1.2.3.4.8 |
| Li et al44 | ABDC herbal medicine (ad us ext) vs no intervention/placebo | 1/67 | RE | MD: 3.36 [1.41 to 5.31] | NA | Low 1.2.6.8 | |
| Li et al44 | ABDC herbal medicine (iv; po) vs western medications | 4/207 | RE | MD: 5.15 [1.78 to 8.53] | 94% | Low 1.2.3.8 | |
| Li et al44 | ABDC herbal medicine (iv; po) in combined remedies vs the same western medications | 2/154 | RE | MD: 3.55 [1.29 to 5.81] | 0% | Low 1.2.8 | |
| Median nerve | Li et al44 | ABDC herbal medicine (ad us ext; iv; po) vs all types of control | 6/392 | RE | MD: 3.25 [1.07 to 5.42] | 84% | Low 1.2.3.4.8 |
| Li et al44 | ABDC herbal medicine (ad us ext) vs no intervention/placebo | 1/67 | RE | MD: 1.83 [0.09 to 3.57] | NA | Low 1.2.6.8 | |
| Li et al44 | ABDC herbal medicine (po) vs western medications | 3/171 | RE | MD: 3.36 [−0.52 to 7.24] | 92% | Very low 1.2.5.7.8 | |
| Li et al44 | ABDC herbal medicine (iv; po) in combined remedies vs the same western medications | 2/154 | RE | MD: 3.83 [1.50 to 6.16] | 0% | Low 1.8 | |
| Peroneal nerve | Zhang et al46 | Omega-3 (po) vs placebo | 2/116 | FE | MD: 1.99 [−0.51 to 4.49] | 0% | Low 1.8 |
| Ulnar nerve | Zhang et al46 | Omega-3 (po) vs placebo | 2/116 | FE | MD: 1.92 [−1.19 to 5.02] | 0% | Low 1.8 |
| Upper limbs | Yan et al42 | Acupuncture vs western medication | 3/216 | FE | MD: 1.04 [0.75 to 1.33] | 98% | Low 1.2.3.8. |
| Lower limbs | Yan et al42 | Acupuncture vs western medication | 3/216 | FE | MD: 2.02 [1.75 to 2.30] | 98% | Low 1.2.3.8. |
| SNAP amplitudes | |||||||
| Sural nerve | Zhang et al46 | Omega-3 supplements (po) vs placebo | 2/116 | FE | MD: 4.19 [2.19 to 6.19] | 0% | Low 8.10 |
| Ulnar nerve | Zhang et al46 | Omega-3 supplements (po) vs placebo | 2/116 | FE | MD: 5.57 [0.42 to 10.72] | 1% | Low 8.10 |
| Distal CMAP amplitudes | |||||||
| Peroneal nerve | Zhang et al46 | Omega-3 supplements (po) vs placebo | 2/116 | FE | MD: 1.08 [0.11 to 2.05] | 0% | Low 8.10 |
| Tibial nerve | Zhang et al46 | Omega-3 supplements (po) vs placebo | 2/116 | FE | MD: 2.36 [0.40 to 4.32] | 54% | Low 3.8.10 |
| Ulnar nerve | Zhang et al46 | Omega-3 supplements (po) vs placebo | 2/116 | FE | MD: 1.16 [−0.19 to 2.52] | 0% | Low 7.8.10 |
| Distal CMAP latencies | |||||||
| Peroneal nerve | Zhang et al46 | Omega-3 supplements (po) vs placebo | 2/116 | FE | MD: −1.02[−1.45 to −0.59] | 0% | Low 8.10 |
| Tibial nerve | Zhang et al46 | Omega-3 supplements (po) vs placebo | 2/116 | FE | MD: −0.27[−0.53 to −0.01] | 54% | Low 3.8.10 |
| Ulnar nerve | Zhang et al46 | Omega-3 supplements (po) vs placebo | 2/116 | FE | MD: −0.59 [−1.28 to 0.09] | 0% | Low 7.8.10 |
| Safety outcome | |||||||
| Incidence of adverse events | |||||||
| Severe leukopenia | Ji29 | Oral herbal medications vs control | 24/1604 | FE | RR: 0.46 [0.32 to 0.65] | 0% | Low 1.2.8 |
| Severe thrombocytopenia | Ji29 | Oral herbal medications vs control | 21/1445 | FE | RR: 0.66 [0.38 to 1.17] | 0% | Low 1.2.7.8 |
| Severe digestive tract reaction | Ji29 | Oral herbal medications vs control | 24/1485 | FE | RR: 0.63 [0.46 to 0.87] | 0% | Low 1.2.8 |
| Severe liver injury | Ji29 | Oral herbal medications vs control | 22/1493 | FE | RR: 0.50 [0.26 to 0.97] | 0% | Low 1.2.8 |
| Severe kidney injury | Ji29 | Oral herbal medications vs control | 17/1267 | FE | RR: 0.46 [0.11 to 2.00] | 0% | Low 1.2.7.8 |
| Skin allergies | Ji29 | Herbal hand and foot bath vs control | 6/548 | FE | RR: 3.61 [1.02 to 12.80] | 0% | Low 1.2.8 |
| All grades nausea | Hoshino et al37 | Goshajinkigan (po) vs control | 4/341 | NA | RR: 0.91 [0.77 to1.07] | 0% | Low 1.2.7.8 |
| Grade ≥3 nausea | Hoshino et al37 | Goshajinkigan (po) vs control | 4/297 | NA | RR: 1.18 [0.40 to 3.49] | 0% | Low 1.2.7.8 |
| All grades fatigue | Hoshino et al37 | Goshajinkigan (po) vs control | 4/341 | NA | RR: 0.97 [0.82 to 1.16] | 0% | Low 1.2.7.8 |
| Grade ≥3 fatigue | Hoshino et al37 | Goshajinkigan (po) vs control | 3/252 | NA | RR: 0.41 [0.08 to 2.07] | NA | Low 1.2.7.8 |
| All grades anorexia | Hoshino et al37 | Goshajinkigan (po) vs control | 4/341 | NA | RR: 0.98 [0.83 to 1.15] | 0% | Low 1.2.7.8 |
| Grade ≥3 anorexia | Hoshino et al37 | Goshajinkigan (po) vs control | 4/297 | NA | RR: 0.70 [0.24 to 2.03] | 0% | Low 1.2.7.8 |
| All grades leukocytopenia | Hoshino et al37 | Goshajinkigan (po) vs control | 3/331 | NA | RR: 0.93 [0.78 to 1.11] | 0% | Low 1.2.7.8 |
| Grade ≥3 leukocytopenia | Hoshino et al37 | Goshajinkigan (po) vs control | 3/331 | NA | RR: 0.95 [0.54 to 1.65] | 0% | Low 1.2.7.8 |
| All grades neutropenia | Hoshino et al37 | Goshajinkigan (po) vs control | 3/331 | NA | RR: 0.90 [0.76 to 1.06] | 0% | Low 1.2.7.8 |
| Grade ≥3 neutropenia | Hoshino et al37 | Goshajinkigan (po) vs control | 4/376 | NA | RR: 0.89 [0.67 to 1.18] | 0% | Low 1.2.7.8 |
| All grades anemia | Hoshino et al37 | Goshajinkigan (po) vs control | 3/331 | NA | RR: 1.05 [0.87 to 1.26] | 0% | Low 1.2.7.8 |
| Grade ≥3 anemia | Hoshino et al37 | Goshajinkigan (po) vs control | 3/331 | NA | RR: 0.62 [0.08 to 4.63] | 0% | Low 1.2.7.8 |
| All grades thrombocytopenia | Hoshino et al37 | Goshajinkigan (po) vs control | 3/331 | NA | RR: 1.11 [0.79 to 1.56] | 27% | Low 1.2.7.8 |
| Grade ≥3 thrombocytopenia | Hoshino et al37 | Goshajinkigan (po) vs control | 3/331 | NA | RR: 1.04 [0.15 to 7.26] | NA | Low 1.2.7.8 |
| Rate of response to chemotherapy | |||||||
| Hoshino et al37 | Goshajinkigan (po) vs control | 2/95 | NA | RR: 1.18 [0.83 to 1.69] | 0% | Low 1.2.7.8 | |
| CIPN symptoms, signs, and pain | |||||||
| Symptom and sign | Oh and Kim41 | Acupuncture vs control | 3/123 | RE | SMD: −0.71 [−1.09 to −0.33] | 6% | Low 1.2.8 |
| Oh and Kim41 | Exercise vs control | 2/35 | RE | SMD: −0.05 [−0.73 to 0.63] | 0% | Very low 1.2.7.8 | |
| Oh and Kim41 | Massage and foot bath vs control | 3/118 | FE | SMD: −0.68 [−1.05 to −0.30] | 19% | Low 1.2.8 | |
| Pain | Oh and Kim41 | Acupuncture vs control | 3/102 | RE | SMD: −0.73 [−1.13 to −0.32] | 0% | Low 1.2.8 |
| Muscle strength and endurance, balance | |||||||
| Balance | Oh and Kim41 | Exercise vs control | 3/63 | RE | SMD: 0.25 [−0.25 to 0.75] | 0% | Very low 1.2.7.8 |
| Muscle strength and endurance | Oh and Kim41 | Exercise vs control | 3/111 | RE | SMD: −0.55 [−0.93 to −0.17] | 0% | Low 1.2.8 |
Abbreviations: ad us ext, external use (hand and foot baths or fumigation or compress or gel); ABDC, activate blood and dredge collaterals; BYHW, Bu Yang Huan Wu; CI, confidence interval; CR, complete remission; CS, Caulis Spatholobi–based; DEB-NTC, Neurotoxicity Criteria of Debiopharm; FE, fixed-effects model; HQGZWW, Huang Qi Gui Zhi Wu Wu; iv, intravenous infusion; ND, mean difference; MN, motor nerve; MNCV, motor nerve conduction velocity; NCI-CTCAE, the National Central Cancer Institute Common Terminology Criteria for Adverse Events; NCV, nerve conduction velocity; OIPN, oxaliplatin-induced peripheral neuropathy; OR, odds ratio; PN, peripheral neuropathy; PNQ, Patient Neurotoxicity Questionnaire; po, oral dosage form; PR, partial remission; RA, Radix Astragali–based; RE, random-effects model; RR, risk ratio; SMD, standardized mean difference; SN, sensory nerve; SNCV, sensory nerve conduction velocity; TNSC, Clinical Total Neuropathy Score; WHO, World Health Organization.
Assessments of ‘Quality of Evidence’:
All/most trials with lack of blinding of participants and personnel.
Most trials with unclear random-sequence generation and/or allocation concealment.
High heterogeneity but with clear direction of effect.
High heterogeneity but might be explained by subgroup/sensitivity analyses.
High unexplained heterogeneity.
Impossible to calculate statistical heterogeneity.
Imprecision, 95% CI includes both benefit and harm.
Imprecision did not met optimal information size.
Impossible to calculate the optimal information size and presents small sample size (less than 2000 patients).
Selective outcome reporting.
Results
Literature Search
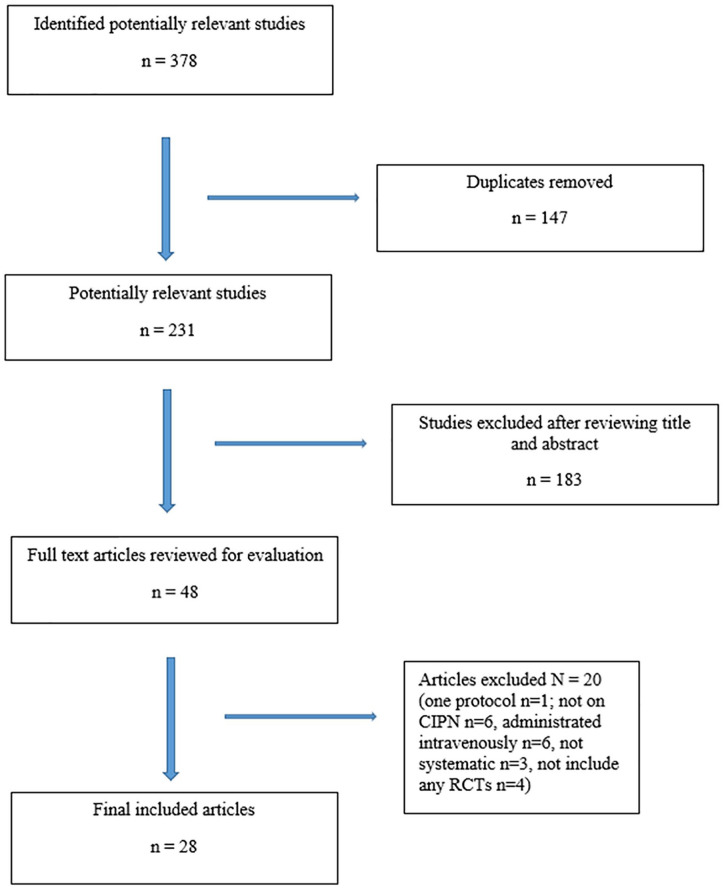
The literature search and cross-reference search retrieved 378 potentially relevant articles, of which 147 were duplicates. After screening the titles and abstracts, 183 records were excluded. Of the remaining 48 articles that were assessed as full text, 20 were excluded for the following reasons: 1 of them was only a protocol, 6 did not review on CIPN, 6 were administrated intravenously only, 4 were not systematic, and 4 did not include any RCTs as stated in the inclusion criteria. Finally, 28 SRs of RCT on nonpharmacological interventions for CIPN met the inclusion criteria (Figure 1).19-46
Figure 1.
Flow chart of the selection of systematic reviews included in the overview.
Characteristics of Included Systematic Reviews
Detailed characteristics of the included 28 SRs are presented in Table 1. The 28 SRs involved the following therapies: herbal medicine (n = 13), natural products and complementary therapies in general (n = 4), acupuncture (n = 3), physical exercise (n = 3), vitamins (n = 3), and omega-3 oral supplements (n = 1). All studies were published between 2013 and 2019, with 16 published in English and 11 published in Chinese and 1 published in Korean. RCTs and quasi-RCTs only were included by 21 reviews; the other 7 reviews also included controlled clinical trials, case studies, and other types of clinical studies. These SRs included a median of 8 trials (range = 2-75), involving a total of 25 719 participants, and each SR contained a median of 647 participants (range = 78-4286). Three authors (10.7%) did not assess the risk of bias of included studies. In contrast, 13 authors (46.4%) used Cochrane collaboration’s RoB assessment tool; 7 (25%) used improved Jadad scale; and 5 (17.9%) evaluated the quality with one each by Jadad scale, Cochrane Collaboration Back Review Criteria, National Health and Medical Research Council clinical evidence assessment matrix, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) scale, and OCEBM the Oxford Levels of Evidence. Ten studies (35.7%) did not report a source of funding, and the authors in 1 study (3.6%) received research support from industry. The MEDLINE database was the most used electronic source searched by the authors from 27 SRs (96.4%), followed by the Cochrane Register of Controlled Trials (CENTRAL)/Cochrane Library (23; 82.1%), EMBASE (16; 57.1%), CNKI (15; 53.6%), and Wanfang (12; 42.9%). The majority of authors (25; 89.3%) specified the date of literature searching; only 3 authors did not. Thirteen authors (46.4%) searched reference lists for additional studies as supplementary strategies, and another 2 (7.1%) also searched conference proceedings. Ten SRs (35.7%) were focused on oxaliplatin-induced peripheral neuropathy, 11 (39.3%) were reported on mixed chemotherapy regimens, and 7 (25%) were not detailed with the type of chemotherapy involved. Thirteen authors (46.4%) reported on preventive effects of nonpharmacological interventions, 11 (39.3%) reported on treatment efficacy, and the other 4 (14.3%) reported on the both. A variety of outcomes were reported, but mainly with neurotoxicity incidence and/or severity measured by patient-reported outcomes, clinician-rated assessments, and physical/functional measures. Clinician reported scales, such as Levi grade, World Health Organization (WHO) grade, National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), Neurotoxicity Criteria of Debiopharm (DEB-NTC), and Clinical version of the Total Neuropathy Score (TNSc) were used for the clinical grading of CIPN. Patient-reported CIPN Scales, such as Patient Neurotoxicity Questionnaire (PNQ), were also used. Clinical effectiveness was assessed by symptom remission completely or partially, in accordance with the grading of CIPN reduced at least over 1 grade or down to 0 grade.47,48 Changes in the parameters of the nerve conduction studies were reported. Adverse events were also extracted. Among all the measure tools, nerve conduction studies (13; 46.4%), Levi scale (11; 39.3%), and WHO Neurotoxicity scale (9; 32.1%) ranked the top 3 outcome instruments being applied to included studies.
Methodological Quality of the Included Systematic Reviews
The methodological quality of the reviews is displayed in Table 2, as determined using AMSTAR2 tool. As overall rating of quality was evaluated, only one study scored “high,” over half (16) of the studies scored “critically low,” and another 11 studies scored “low.” Most studies (27/28) showed shortcomings in reporting on the list of excluded studies (Q7), and the funding information of the included studies (Q10). In terms of individual domains of risk of bias, most authors (27/28) explained their selection of the study design in inclusion criteria (Q3). Twenty-three authors described the components of PICO (population, intervention, control, and outcome; 82.1%) in their research questions and inclusion criteria (Q1), but only 2 studies detailed all aspects of the included studies adequately (Q8). Twelve authors (42.9%) established an “a priori” design of review methods from a written protocol/guide (Q2). All studies executed a comprehensive literature search including at least 2 electronic databases, keywords of the search strategy, and publication restrictions. However, they were all rated “partial yes” because the searching did not include the references lists/bibliographies of included studies, trial registries, gray literature, and content from experts in the field (Q4). Twenty-three review authors (82.1%) stated that 2 independent authors determined the eligibility of studies for inclusion in SRs (Q5), of which 22 authors (78.6%) indicated 2 assessors independently performed data extraction (Q6). Twenty-four reviewers (85.7%) assessed the risk of bias for included individual studies, but largely from unconcealed allocation and lack of blinding of patients and assessors (Q9). Half of the authors properly justified combining the data in the meta-analysis (Q11), but only 8 of them (28.6%) assessed the potential impact of risk of bias in individual studies on the results of meta-analysis (Q12). By comparison, 2 studies did not use appropriate methods to conduct the meta-analysis (Q11). Nine SRs (32.1%) did not conduct a meta-analysis because of the heterogeneity in study design and treatments (Q11 and Q12). Twenty-one (75%) studies discussed the likely impact of risk of bias in individual studies on the results (Q13). Twenty-five (89.3%) authors provided a satisfactory explanation for heterogeneity in the results (Q14). Only 6 (21.4%) carried out an adequate investigation on publication bias (Q15). Most studies (26/28) reported on potential sources of conflict of interest (Q16).
Quality of Evidence in the Included Systematic Reviews Assessed by GRADE
On the basis of pooled data from 28 trials, evidence was graded as “low” or “very low” quality using the GRADE approach. The detailed information regarding the reason for downgrading of each outcome is presented in Table 3.
Incidence of Grade ≥1 CIPN/OIPN
A total of 12 studies summarized evidence on this outcome.21,23,26-29,31,38,39,43,44,46 Four studies reported on peripheral neuropathy (PN) caused by various types of chemotherapy, including one on cisplatin-induced neurotoxicity. Eight other studies reported evidence on overall incidence of oxaliplatin-induced peripheral neuropathy (OIPN). Regarding the incidence of grade ≥1 CIPN, Kuriyama and Endo38 found that it was significantly lower in oral goshajinkigan group when measured by DEB-NTC (RR = 0.43 [0.27-0.66]), but there was no statistical difference in NCI-CTCAE grade. Li et al44 concluded that this outcome was significantly decreased in all forms (externally:ad us ext; orally: po) of herbal medicine groups (OR = 0.26 [0.20-0.35]). These results were consistent with those of the subgroup analyses when the herbs were compared with no intervention/placebo (OR = 0.22 [0.14-0.34]) or western medications alone (OR = 0.22 [0.09-0.54]) or herbs in combined remedies compared with the same western medications (OR = 0.36 [0.22-0.59]). Huang et al28 found oral vitamin E supplements resulted in superior effects to placebo/no intervention group (RR = 0.31 [0.17-0.58]) in subgroup grade ≥1 cisplatin-induced PN, but not in CIPN. In contrast, Zhang et al46 reported that oral vitamin E supplements significantly reduced grade ≥1 TNSc, compared with placebo (RR = 0.58 [0.43-0.77]). In terms of incidence of grade ≥1 OIPN, 3 studies examined the pool effects between all forms (ad us ext; po) of herbal medicine and control. Deng et al26 investigated evidence on both Radix Astragali–based herbs and Caulis Spatholobi–based herbs for this outcome. Compared with all types of control, they reported a reduced incidence of grade ≥1 neurotoxicity in the Radix Astragali–based herbs group (OR = 0.20 [0.14-0.25]). In the relevant subgroup analyses, these results were consistent when compared with no intervention or mecobalamin alone. Another SR found the neurotoxicity incidence in the Caulis Spatholobi–based herbal group was lower than that of all the control groups (OR = 0.19 [0.14-0.25]), regardless of a low dose (15 g) or high dose (20 g-45 g) of Caulis Spatholobi included in the herbal medicine.27 Ji29 reported this outcome in favor of all forms of herbal medicine (ad us ext; po) compared with no intervention or placebo (RR = 0.60 [0.56-0.64]) or western medications (RR = 0.50 [0.41-0.62]); and herbs plus western medications to the same western medications (RR = 0.42 [0.32-0.54]). Two SRs pooled effects in oral and external herbs versus control.23,43 Both studies reported a reduction in the neurotoxicity incidence by the herbal medicine group (Yang et al43: OR = 0.23 [0.16-0.31]; Tian et al23: OR = 0.10 [0.06-0.19]). Orally applied herbal medicine was described in 3 studies (Ji29: RR = 0.58 [0.53-0.64]; Wei et al31: RR = 0.25 [0.12-0.53]; Liu et al39: RR = 0.78 [0.66-0.91], I2 = 71%; RR = 0.54 [0.38-0.76], I2 = 82.3%; RR = 0.74 [0.58-0.94]) with superior effects with this outcome compared with control groups. Three additional SRs summarized evidence of external use of herbal medicine versus no intervention/placebo control.29,31,39 External herbs had significant benefits (He and Yang21: OR = 0.26 [0.17-0.40]; Ji29: RR = 0.62 [0.57-0.67]; Liu et al39: RR = 0.69 [0.50-0.95], I2 = 68.8%). It should be noted that substantial heterogeneities were found in the meta-analyses from Liu et al’s study,39 but in the sensitivity analyses, the pooled result of studies remained significant without heterogeneity (I2 = 0%) after omitting 4 studies.49-52
Incidence of Grade ≥2 CIPN/OIPN
Five studies reported evidence on incidence of grade ≥2 neurotoxicity.23,31,37,38,43 Two studies reported on CIPN (evidence of very low quality). Another 3 studies focused on oxaliplatin (evidence of low quality). In terms of the incidence of grade ≥2 OIPN, Tian et al23 found that this outcome was in favor of Huang Qi Gui Zhi Wu Wu (HQGZWW) herbal medicine when compared with no intervention (OR = 0.07 [0.04-0.14]) or mecobalamine (OR = 0.14 [0.05-0.44]). Yet, there was moderate heterogeneity between HQGZWW (ad us ext; po) and no intervention control (I2 = 44%). Wei et al31 reported orally used Bu Yang Huan Wu (BYHW) herbal medicine was superior to control by different measures (Levi grade: RR = 0.43 [0.28-0.65]); WHO grade: RR = 0.19 [0.04-0.80]). But these results were only consistent with those of the subgroup analyses for oxaliplatin dose at 680 to 780 mg/m2 group, not for dose at 390 mg/m2 group. Yang et al43 also reported that the grade ≥2 neurotoxicity was significantly decreased in external herbal medicine group (OR = 0.41 [0.32-0.51]). Compared with control, goshajinkigan had no statistically significant difference in decreasing grade ≥2 CIPN irrespective of using NCI-CTCAE or DEB-NTC grade.37,38
Incidence of Grade ≥3 CIPN/OIPN
A total of 7 studies summarized evidence on this outcome (evidence of low quality).26,27,29,37,38,44 Three studies included 8 comparisons on CIPN. But only 3 comparsions reported a reduction in the neurotoxicity ≥3 grade of herbal medicine group.38 Kuriyama and Endo38 found that oral goshajinkigan decreased the incidence of DEB-NTC grade ≥3 CIPN (RR = 0.42 [0.25-0.71]). Liu et al39 reported that oral herbal medicine was superior to no intervention in grade ≥3 CIPN with WHO (RR = 0.42 [0.23-0.77]) and Levi scale (RR = 0.28 [0.11-0.69]). Four other studies were dedicated to oxaliplatin regimens. Three of the 4 studies reported that this outcome in favor of different types of herbal medicine compared with no intervention (Deng26: OR = 0.17 [0.09-0.31]; Ji29: RR = 0.34 [0.28-0.43]; Li et al44: OR = 0.34 [0.20-0.61]), but not to western medications. Only Ji29 reported add-on benefit to western medications (RR = 0.32 [0.14-0.75]). Additionally, the grade ≥3 neurotoxicity was significantly decreased in the oral and external use of Caulis Spatholobi–based herbal group (Deng et al: OR = 0.22 [0.12-0.40]), irrespective of high or low dose of Caulis Spatholobi.39
Curative Effects (Ratio of Complete and Partial Remission)
Four studies reported on curative effects, referring to the integral of complete remission plus partial remission.26,27,42,44 Three herbal studies assessed this outcome with the grading of CIPN and another one acupuncture study with PNQ. Herbal medicines were described in these 3 studies with superior effects on this outcome to the control group (Deng et al: OR = 3.59 [2.16-5.95]; Li et al: OR = 4.30 [2.75-6.74]; Deng et al: OR = 4.27 [2.81-6.47]).26,27,44 In subgroup analyses, compared with no intervention/placebo group, this benefit was only consistently reported in externally applied activating blood and dredging collaterals herbs group (Li et al44: OR = 4.57 [2.48-8.40]). Compared with western medication, curative effects were reported significantly improved in Radix Astragali–based herbs (ad us ext; po) plus western medication groups (Deng et al26: OR = 4.84 [2.38-9.83]), activating blood and dredging collaterals herbs group (po; OR = 4.91 [1.10-21.81], I2 = 61%) and activating blood and dredging collaterals herbs plus western medications groups (Li et al44: OR = 4.13 [1.39-12.27]). Also compared with western medicine, Yan et al42 reported that acupuncture significantly enhanced the curative effects by PNQ sensory scale (OR = 2.51 [1.58-4.01]).
Sensory Nerve Conduction Velocity (SNCV)
Six studies summarized evidence on this outcome.23,26,27,31,42,44 Three herbal studies reported on SNCV of both median nerve and fibular nerve and 1 acupuncture study of upper limbs and lower limbs. Other 2 herbal studies did not specify either the nerve or the body area for SNCV testing. With herbal medicine, compared with control, both forms of activate blood and dredge collaterals herbal medicine and oral BYHW herbal decoction showed a significant improvement in SNCV of both fibular nerve (Li et al: MD = 4.59 [3.23-5.96]; Wei et al: MD = 3.32 [0.67-5.97]) and median nerve (Li et al: MD = 4.00 [2.81-5.99]; Wei et al: MD = 3.18 [0.63-5.73]).31,44 Caulis Spatholobi–based herbal medicine administered orally or externally had beneficial influences on improving the SNCV of the fibula nerve (Deng et al: MD = 2.12 [1.04-3.20]).27 However, heterogeneity was significant or not reported in these meta-analysis. Besides that, 2 authors (Tian et al: MD = 5.49 [3.70-7.29]; Deng et al: MD = 4.42 [3.27-5.57]) reported an increase of SNCV in Radix Astragali–based herbal medicine and HQGZWW herbal decoction, administered orally or externally.23,26 One study found that acupuncture enhanced the SNCV of upper limbs (MD = 3.17 [2.9- 3.42]) and lower limbs (MD = 2.40 [2.12-2.67]), but these results were represented with extremely high heterogeneity.42
Motor Nerve Conduction Velocity (MNCV)
Three studies summarized evidence on this outcome.26,42,44 One herbal study reported on MNCV of both median nerve and fibular nerve and 1 acupuncture study of upper limbs and lower limbs. Another one herbal study did not specify either the nerve or the body area for MNCV testing. Compared with control, all forms of activate blood and dredge collaterals herbal medicine were found with a significant improvement in MNCV of both fibular nerve (MD = 4.53 [2.23-6.84]) and median nerve (MD = 3.25 [1.07-5.42]).44 With Radix Astragali–based herbal medicine comparisons, only with western medications, RA plus western medications was superior to improving the MNCV (Deng et al: MD = 4.10 [1.70-6.50]).26 Additionally, Yan et al42 reported that acupuncture increased MNCV of upper limbs (MD = 1.04 [0.75-1.33]) and lower limbs (MD = 2.02 [1.75-2.30]), compared with western medication. However, heterogeneity was significant or not reported in all these meta-analyses.
Sensory Nerve Action Potential (SNAP) Amplitudes
Zhang et al46 reported the superior effect of omega-3 polyunsaturated fatty acid (PUFA) oral supplements compared with placebo on SNAP amplitudes of the ural nerve (MD = 4.19 [2.19-6.19]) and ulnar nerve (MD = 5.57 [0.42-10.72]).
Distal Compound Motor Action Potential (CMAP) Amplitudes and Latencies
Zhang et al46 reported significant differences in distal CMAP amplitudes, favoring the omega-3 group over placebo, in both the peroneal nerve (MD = 1.08 [0.11-2.05]) and tibial nerve (MD = 2.36 [0.40-4.32]). Similarly, omega-3 PUFA oral supplements have shown to better preserve CMAP latencies of the peroneal nerve (MD = −1.02 [−1.45 to −0.59]) and tibial nerve (MD = −0.27 [−0.53 to −0.01]). However, heterogeneities were evident (I2 = 54%) in CMAP amplitudes and latencies of tibial nerve.46
Safety Outcome
Two studies reported on safety outcomes with regard to incidence of adverse events and hematological toxicities.29,37 One also reported on the rate of response to chemotherapy. Hoshino et al37 reported that goshajinkigan did not influence the risk of grade ≥1 and ≥3 nausea, fatigue, anorexia, leukocytopenia, neutropenia, anemia, thrombocytopenia, or rate of response to chemotherapy. Ji29 found that oral herbal medications lowered the incidence of severe leukopenia (RR = 0.46 [0.32-0.65]), severe digestive tract reaction (RR = 0.63 [0.46-0.87]), and severe liver injury (RR = 0.50 [0.26-0.97]), but there was no statistically significant difference in severe thrombocytopenia and severe kidney injury. As well, no significant differences were observed on incidence of skin allergies between herbal hand and foot bath and control. However, the evidence reported was of low-grade quality.
CIPN Symptoms and Signs
One study reported that this outcome measured by a mix of multiple scales.41 Compared with control group, CIPN symptoms and signs were relieved significantly with acupuncture (SMD = −0.71 [−1.09 to −0.33]) and massage and foot bath (SMD = −0.68 [−1.05 to −0.30]). Acupuncture was also statistically effective in reducing CIPN pain (SMD = −0.73 [−1.13 to −0.32]).
Muscle Strength, Endurance, and Balance
One study reported that exercises were effective in improving muscle strength and endurance (SMD = −0.55 [−0.93 to −0.17]).41
Discussion
Key Findings From the Overview
Twenty-eight SRs of varied methodological quality including nonpharmacological treatment modalities for the clinical management of CIPN were identified. The strengths and weaknesses of each study were evaluated, and the level of evidence was summarized. We did not set any restrictions on the CIPN diagnoses or cancer types of included SRs. This approach reflects real-world practice and improves the external validity of this overview.
There was some evidence to suggest the superior effects of Chinese herbal medicine on preventing the development of CIPN, but evidence was mainly limited to low-quality trials. Radix Astragali, Caulis Spatholobi–based herbal combination, and the additional use of herbal medicine with active components promoting blood circulation presented superiority in reducing the incidence of grade ≥1 OIPN/CIPN. BYHW and HQGZWW herbal decoction was effective in both grade ≥1 and ≥2 OIPN. In SRs synthesizing evidence on decreasing grade ≥3 OIPN, Caulis Spatholobi–based herbal combination played an active role. Regarding treatment effects, evidence indicated that Radix Astragali–based herbal combination plus western medication, Caulis Spatholobi–based herbal combination and herbs promoting blood circulation action presented curative effects in OIPN. Radix Astragali–based herbal combination, Radix Astragali–based herbal combination plus western medication, Caulis Spatholobi–based herbal combination, BYHW, and HQGZWW herbal decoction had the potential of being more effective in improving sensory nerve conduction velocity. With regard to MNCV, positive results were observed on Radix Astragali–based herbs plus western medications and herbs with promoting blood circulation. In term of safety outcomes, 2 meta-analyses that reported on the incidence of adverse events agreed that herbal medications did not increase this risk.
There was insufficient evidence to make any judgements on the efficacy of vitamin E, omega-3 supplementation, exercise, massage, and foot baths in the treatment of CIPN with low certainty from single meta-analysis. Although evidence from 2 meta-analyses reported that acupuncture has the potential to alleviate CIPN symptoms and enhance nerve conduction velocity, the confidence of it remains low due to poor reporting quality, little details on acupuncture procedures, and how outcomes were measured. In addition, available evidence cannot demonstrate clear/consistent add-on benefits of vitamin E administration for CIPN. Goshajinkigan also did not appear to reduce the risk of CIPN nor did it reduce the severity of CIPN.
In general, results from the identified SRs demonstrated add-on protection and benefit potentials from acupuncture, natural products (including vitamins, omega-3 PUFAs), herbal medicine, and physical exercise for CIPN symptoms control. Seventeen (60.7%) of the 28 reviews in this overview reported a meta-analysis with the topic. A significant proportion of meta-analyses indicated that herbal medicine, regardless of being administered orally or externally, reduced the overall OIPN incidence significantly compared with no intervention, placebo, or pharmaceuticals, thus increased adherence to chemotherapy. Acupuncture, omega-3 supplementation, vitamin E supplementation, massage and foot baths, and exercise showed some positive effects, but their effects were taken with caution as low certainty or less consistent overall and so need further study to be more certain of their effects.
Potentials of Nonpharmacological Interventions
The underlying mechanisms of these nonpharmacological interventions on CIPN have not yet been fully understood. Acupuncture may relieve neuropathic pain by improving central neurotransmission of GABA-ergic, serotoninergic, and adrenergic.53-57 It may also downregulate nerve growth factor signaling with a parallel decrease in sensory neurons hypersensitization.58 Balance exercises, such as sensorimotor training or whole-body vibration, have shown the highest effect on the crucial side effects of nonmetabolic peripheral neuropathic disorders.24 It may work through inducing neural adaptations and nerve fibers regeneration,59 activating deafferented neurons,60 lowering the excitability threshold,61 or activating supraspinal learning effects.59,62 In vivo, HQGZWW decoction, a classic Chinese herbal formula, may relieve pain and ameliorate sciatic nerve conduction velocity in rats with CIPN. Its extract AC591 reduced oxaliplatin-induced cold hyperalgesia, mechanical allodynia as well as morphological damage of dorsal root ganglion.63 Hydroalcoholic Astragali Radix extract (50%) was also found to reduce oxaliplatin-induced cold hypersensitivity, effectively block the onset of the pro-allodynia action, and protect pain induced by neuro damage in rat models.64,65 Potential nerve growth–promoting factors in peripheral nerves regeneration were also found in other vitro and vivo studies with Radix Paeoniae alba extract, puerarin from Pueraria lobata and Tanshinone IIA from Salvia miltiorrhiza.66-68 Ginkgo extract EGb761 was observed to promote faster nerve conduction velocity. This was probably due to its neuroprotective effect on pathological changes of decrease in somatic and nuclear size, nucleolar segregation, and multinucleolation.69 Explanations of the standardized herbal granule goshajinkigan (Pilula renales plantaginis et achyranthis), well known as Niu Che Shen Qi Wan in China, included its antioxidant and C fiber activation properties for inhibiting the development of CIPN.70-72 In animal models of diabetic neuropathy, it is possible that the action of omega-3 PUFAs lead to beneficial effects on enhancing nerve conduction velocity,72 and attenuating adverse changes in nerve structure and function.73,74 In other vitro and in vivo experiments, omega-3 PUFAs has been found to reduce neuronal cell death, recover peripheral nerve injury,75 and stimulate neurite growth in sensory neurons.76
Strength and Limitations
This is the first overview that summarizes evidence on nonpharmacological therapies for one of the most common side effects of conventional cancer care (CIPN). We comprehensively summarized and critically appraised all available evidence on this topic. Using the GRADE tool, we generally judged the efficacy and safety as being supported by low quality of evidence (and some very low). Thus, further studies are required to enhance confidence in the estimate of effect. The presence of high heterogeneity, small sample size, unclear random allocation concealment, inadequate blinding and follow-up, and selective outcome reporting were identified by authors as possible risk factors for downgrading the level of evidence. Additionally, none of the studies discussed the clinical appropriateness of combining the RCTs prior to choosing a fixed- or random-effects model. Although we attempted to include all key outcomes reporting on CIPN, most included studies from these SRs reported on subjective clinician-rated scales, which may become less optimal when blinding was not well executed. The definitions of PN grades differ between the scales, NCI-CTCAE, WHO, and TNSc scales place focus on the severity of a range of objective neurotoxicity, whereas Levi and DEB-NTC scales place emphasis on the duration of neurotoxicity. Only a small number of SRs included objective neurological assessments and patient-reported questionnaires. Furthermore, reporting safety outcomes (adverse events) of included SRs was unsatisfactory, which may be a result of minimal details provided in the primary studies.
This overview has been designed, conducted, and reported rigorously, but presents with its own limitations. First, the retrieval language limited to SRs with an abstract in English and Chinese can result in a potential risk of positive publication bias.77 Besides, our evaluation depended on what was reported in the SRs. The authors may have designed and conducted their article more rigorously but removed some key details that we were looking for in their reports. In this instance, the reporting quality of the included article may have had an impact on our results. Future SR should be in accordance with the PRISMA statement.15 Finally, as the majority of results reporting on herbal medicine therapies came from the Chinese population, the generalizability of current findings is limited. It is worth noting that the Chinese herbs included in SRs, even with the same name of category group, were heterogeneous in treatment duration, formula composition, and doses of individual herbs, likely because one of the features in traditional Chinese medicine is to use the different prescriptions accommodating for different individuals.
Research with the aim of proving the benefits of nonpharmacological interventions has not made much advancement in the CIPN area; however, if a review study fails to include such results, nonpharmacological interventions (eg herbal therapies) would not find acceptance and integration in conventional therapies. Reliable studies are essential to make future research valuable. To provide more rigorous evidence on the effectiveness of nonpharmacological interventions, prudent methods and high reporting standards must be complied within future study design.
Conclusions
This overview compiles the clinical evidence of one of the most common side effects from chemotherapy in a systematic and comprehensive way. It provides a coherent summary of the totality of evidence from SRs of nonpharmacological interventions for CIPN management. This may help busy clinicians, policy makers, and patients. However, the evidence is not sufficiently robust because of the unsatisfactory flaws in both reporting and methodology. Based on the identified evidence, published SRs for CIPN described the potential benefits of nonpharmacological interventions as follows: acupuncture, exercise, and herbal medicine, nutritional supplements. As an adjuvant option to prevent and relieve CIPN, herbal medicine showed promise but required further study to be more certain of their effects. The variability in the use of various intervention methods and outcome measures in different trials was a major challenge in assembling this overview and the individual SRs on which it is based. This made it difficult to pool results and derive conclusions. Readers with a particular interest in one specific intervention would probably look up the relevant SRs and refer to the primary articles.
Supplemental Material
Supplemental material, References_to_studies_excluded_in_this_review for Effects of Nonpharmacological Interventions in Chemotherapy-Induced Peripheral Neuropathy: An Overview of Systematic Reviews and Meta-Analyses by Jie Hao, Xiaoshu Zhu and Alan Bensoussan in Integrative Cancer Therapies
Supplemental material, Search_strategies for Effects of Nonpharmacological Interventions in Chemotherapy-Induced Peripheral Neuropathy: An Overview of Systematic Reviews and Meta-Analyses by Jie Hao, Xiaoshu Zhu and Alan Bensoussan in Integrative Cancer Therapies
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Western Sydney University Research Training Scheme (Grant Number UWS18405473); China Scholarship Council Higher Degree (Doctor of Philosophy) Program (Grant Number CSC201506550006).
ORCID iD: Jie Hao  https://orcid.org/0000-0003-3450-9254
https://orcid.org/0000-0003-3450-9254
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Argyriou AA, Bruna J, Genazzani AA, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity: management informed by pharmacogenetics. Nat Rev Neurol. 2017;13:492-504. doi: 10.1038/nrneurol.2017.88 [DOI] [PubMed] [Google Scholar]
- 2. Pachman DR, Watson JC, Loprinzi CL. Therapeutic strategies for cancer treatment related peripheral neuropathies. Curr Treat Options Oncol. 2014;15:567-580. doi: 10.1007/s11864-014-0303-7 [DOI] [PubMed] [Google Scholar]
- 3. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461-2470. doi: 10.1016/j.pain.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 4. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941-1967. doi: 10.1200/JCO.2013.54.0914 [DOI] [PubMed] [Google Scholar]
- 5. Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 2017;81:772-781. doi: 10.1002/ana.24951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gewandter JS, Freeman R, Kitt RA, et al. Chemotherapy-induced peripheral neuropathy clinical trials: review and recommendations. Neurology. 2017;89:859-869. doi: 10.1212/WNL.0000000000004272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kautio AL, Haanpaa M, Saarto T, Kalso E. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage. 2008;35:31-39. doi: 10.1016/j.jpainsymman.2007.02.043 [DOI] [PubMed] [Google Scholar]
- 8. Rao RD, Flynn PJ, Sloan JA, et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled trial (N01C3). Cancer. 2008;112:2802-2808. doi: 10.1002/cncr.23482 [DOI] [PubMed] [Google Scholar]
- 9. Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer. 2007;110:2110-2118. doi: 10.1002/cncr.23008 [DOI] [PubMed] [Google Scholar]
- 10. Tsavaris N, Kopterides P, Kosmas C, et al. Gabapentin monotherapy for the treatment of chemotherapy-induced neuropathic pain: a pilot study. Pain Med. 2008;9:1209-1216. doi: 10.1111/j.1526-4637.2007.00325.x [DOI] [PubMed] [Google Scholar]
- 11. Hunter J, Ussher J, Parton C, et al. Australian integrative oncology services: a mixed method study exploring the views of cancer survivors. BMC Complement Altern Med. 2018;18:153. doi: 10.1186/s12906-018-2209-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shalom-Sharabi I, Lavie O, Samuels N, et al. Can complementary medicine increase adherence to chemotherapy dosing protocol? A controlled study in an integrative oncology setting. J Cancer Res Clin Oncol. 2017;143:2535-2543. [DOI] [PubMed] [Google Scholar]
- 13. Rossi E, Noberasco C, Picchi M, et al. Complementary and integrative medicine to reduce adverse effects of anticancer therapy. J Altern Complement Med. 2018;24:993-994. doi: 10.1089/acm.2018.0143 [DOI] [PubMed] [Google Scholar]
- 14. Ben-Arye E, Ali-Shtayeh MS, Nejmi M, et al. Integrative oncology research in the Middle East: weaving traditional and complementary medicine in supportive care. Support Care Cancer. 2012;20:557-564. doi: 10.1007/s00520-011-1121-0 [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009;339:b2535. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Updated March 2011. Accessed March 21, 2019 https://handbook-5-1.cochrane.org
- 17. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or nonrandomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook. Handbook for grading the Quality of Evidence and the Strength of Recommendations using the GRADE approach. Updated October 2013. Accessed September 12, 2018 http://www.guidelinedevelopment.org/handbook
- 19. Eum S, Choi HD, Chang MJ, et al. Protective effects of vitamin E on chemotherapy induced peripheral neuropathy: a meta-analysis of randomized controlled trials. Int J Vitam Nutr Res. 2013;83:101-111. doi: 10.1024/0300-9831/a000149 [DOI] [PubMed] [Google Scholar]
- 20. Franconi G, Manni L, Schröder S, Marchetti P, Robinson N. A systematic review of experimental and clinical acupuncture in chemotherapy-induced peripheral neuropathy. Evid Based Complement Alternat Med. 2013;2013:516916. doi: 10.1155/2013/516916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He B, Yang YF. Meta-analysis of effects of external treatments of TCM on oxaliplatin-induced neurotoxicity. J Liaoning Univ Tradit Chin Med. 2013;15:146-148. doi: 10.13194/j.jlunivtcm.2013.06.148.heb.055 [DOI] [Google Scholar]
- 22. Schloss J, Colosimo M, Airey C, et al. Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review. Clin Nutr. 2013;32:888-893. doi: 10.1016/j.clnu.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 23. Tian J, Yao XQ, Wu XY, et al. Systematic review and meta-analysis on efficacy of Huangqi Guizhi Wuwu decoction for oxaliplatin-induced peripheral neurotoxicity. Chin J Exp Tradit Chin Formulae. 2013;19:325-330. [Google Scholar]
- 24. Streckmann F, Zopf EM, Lehmann HC, et al. Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med. 2014;44:1289-1304. doi: 10.1007/s40279-014-0207-5 [DOI] [PubMed] [Google Scholar]
- 25. Brami C, Bao T, Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Crit Rev Oncol Hematol. 2016;98:325-334. doi: 10.1016/j.critrevonc.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng B, Jia L, Cheng Z. Radix astragali-based Chinese herbal medicine for oxaliplatin-induced peripheral neuropathy: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:2421876. doi: 10.1155/2016/2421876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deng B, Jia L, Cheng Z, et al. Effects of Jixueteng on oxaliplatin induced peripheral neuropathy. Chin Arch Tradit Chin Med. 2016;34:20-26. doi: 10.13193/j.issn.1673-7717.2016.01.005 [DOI] [Google Scholar]
- 28. Huang H, He M, Liu LH, Huang L. Vitamin E does not decrease the incidence of chemotherapy-induced peripheral neuropathy: a meta-analysis. Contemp Oncol (Pozn). 2016;20:237-241. doi: 10.5114/wo.2016.61567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ji Y. Prevention of Oxaliplatin-Induced Peripheral Neuro-pathy by Traditional Chinese Medicine: A Systematic Review and Meta-Analysis [dissertation]. Nanjing University of Chinese Medicine; 2016. [Google Scholar]
- 30. Wei XC, Wang H, Zhu LQ, et al. Dang Gui Si Ni decoction for preventing oxaliplatin-induced peripheral neuropathy: a systematic review and meta-analysis. Shandong Med J. 2016;56:77-78. [Google Scholar]
- 31. Wei XC, Wang H, Zhu LQ, et al. Systematic evaluation of efficacy and safety of Buyang Huanwu Tang in preventing oxaliplatin-induced peripheral neurotoxicity in cancer patients. Chin J Exp Tradit Med Formulae. 2016;22:186-190. doi: 10.13422/j.cnki.syfjx.2016220186 [DOI] [Google Scholar]
- 32. Wei XC, Zhu LQ, Wang CG, et al. Meta-analysis of the efficacy and safety of vitamins in preventing chemotherapy-induced peripheral neurotoxicity in cancer patients. Chin J Mod Appl Pharm. 2016;33:476-484. doi: 10.13748/j.cnki.issn100-7693.2016.04.022 [DOI] [Google Scholar]
- 33. Derksen TM, Bours MJ, Mols F. Lifestyle-related factors in the self-management of chemotherapy-induced peripheral neuropathy in colorectal cancer: a systematic review. Evid Based Complement Alternat Med. 2017;2017:7916031. doi: 10.1155/2017/7916031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brayall P, Donlon E, Doyle L, Leiby R, Violette K. Physical therapy-based interventions improve balance, function, symptoms, and quality of life in patients with chemotherapy induced peripheral neuropathy: a systematic review. Rehab Oncol. 2018;36:161-166. doi: 10.1097/01.REO.0000000000000111 [DOI] [Google Scholar]
- 35. Chen SS. Prevention of Oxaliplatin-Induced Peripheral Neuropathy by Huangqi Guizhi Wuwu Decoction: A Systematic Review and Meta-Analysis [dissertation]. Nanjing University of Chinese Medicine; 2018. [Google Scholar]
- 36. Duregon F, Vendramin B, Bulloa V, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90-100. doi: 10.1016/j.critrevonc.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 37. Hoshino N, Ganeko R, Hida K, Sakai Y. Goshajinkigan for reducing chemotherapy induced peripheral neuropathy: a systematic review and meta-analysis. Int J Clin Oncol. 2018;23:434-442. doi: 10.1007/s10147-017-1229-4 [DOI] [PubMed] [Google Scholar]
- 38. Kuriyama A, Endo K. Goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Support Care Cancer. 2018;26:1051-1059. doi: 10.1007/s00520-017-4028-6 [DOI] [PubMed] [Google Scholar]
- 39. Liu Y, May BH, Zhang AL, et al. Integrative herbal medicine for chemotherapy-induced peripheral neuropathy and hand-foot syndrome in colorectal cancer: a systematic review and meta-analysis. Integr Cancer Ther. Published online December 10, 2018. doi: 10.1177/1534735418817833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noh H, Yoon SW, Park B. A systematic review of herbal medicine for chemotherapy induced peripheral neuropathy. Evid Based Complement Alternat Med. 2018;2018:6194184. doi: 10.1155/2018/6194184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oh PJ, Kim YL. Effectiveness of non-pharmacologic interventions in chemotherapy induced peripheral neuropathy: a systematic review and meta-analysis. J Korean Acad Nurs. 2018;48:123-142. doi: 10.4040/jkan.2018.48.2.123 [DOI] [PubMed] [Google Scholar]
- 42. Yan Y, Liu HF, Wu J. Acupuncture for treatment of chemotherapy-induced peripheral neurotoxicity: a meta-analysis of randomised controlled trials. Jiangxi J Tradit Chin Med. 2018;49:61-65. [Google Scholar]
- 43. Yang LN, Wu WY, Li Q. Meta-analysis on efficacy of external treatment of TCM for oxaliplatin-induced peripheral neurotoxicity. J Basic Chin Med. 2018;24:1101-1105. [Google Scholar]
- 44. Li Z, Jin H, Yan Q, et al. The method of activating blood and dredging collaterals for reducing chemotherapy-induced peripheral neuropathy: a systematic review and meta analysis. Evid Based Complement Alternat Med. 2019;2019:1029626. doi: 10.1155/2019/1029626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li K, Giustini D, Seely D. A systematic review of acupuncture for chemotherapy induced peripheral neuropathy. Curr Oncol. 2019;26:e147-e154. doi: 10.3747/co.26.4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang CA, De Silva MEH, MacIsaac RJ, et al. Omega-3 polyunsaturated fatty acid oral supplements for improving peripheral nerve health: a systematic review and meta-analysis. Nutr Rev. 2020;78:323-341. doi: 10.1093/nutrit/nuz054 [DOI] [PubMed] [Google Scholar]
- 47. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. doi:10.1002/1097-0142(19810101)47:1< 207::aid-cncr2820470134>3.0.co;2-6 [DOI] [PubMed] [Google Scholar]
- 48. Shi Y, Sun Y. Manual of Clinical Oncology. 6th ed. People’s Medical Publishing House; 2015. [Google Scholar]
- 49. Mao ZJ, Zhu LM, Lu YL, Shen KP. The effect of Jianpi therapy plus FOLFOX on cancer-related fatigue, Th1/Th2 immune response balance and peripheral neuropathy in postoperative colon cancer patients. Mod J Integr Trad Chin West Med. 2017;26:4027-4030. [Google Scholar]
- 50. Zeng JQ, Li ZP, Wang X. Clinical observation of Chinese medicine plus chemotherapy for advanced colorectal cancer in 30 cases. J Jiangxi Uni Tradit Chin Med. 2008;20:39-41. [Google Scholar]
- 51. Wang Q. Clinical study of the prevention and treatment effects of hand and foot baths of Huangqiguizhiwuwu decoction plus with Ca-Mg Infusion on oxaliplatin induced peripheral neuropathy. Mod J Integr Trad Chin West Med. 2015;24:3. [Google Scholar]
- 52. Wang QY, He W, Lin Q, et al. Clinical observation of Chinese medicine plus chemotherapy for advanced colon cancer. Mod Digest Interv. 2015;20:387-389. [Google Scholar]
- 53. Sung HJ, Kim YS, Kim IS, et al. Proteomic analysis of differential protein expression in neuropathic pain and electroacupuncture treatment models. Proteomics. 2004;4:2805-2813. doi: 10.1002/pmic.200300821 [DOI] [PubMed] [Google Scholar]
- 54. Xing GC, Liu FY, Qu XX, Han JS, Wan Y. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol. 2007;208:323-332. doi: 10.1016/j.expneurol.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 55. Fusumada K, Yokoyama T, Miki T, et al. C-Fos expression in the periaqueductal gray is induced by electroacupuncture in the rat, with possible reference to GABAergic neurons. Okajimas Folia Anat Jpn. 2007;84:1-10. doi: 10.2535/ofaj.84.1 [DOI] [PubMed] [Google Scholar]
- 56. Silva JRT, Silva ML, Prado WA. Analgesia induced by 2- or 100-Hz electroacupuncture in the rat tail-flick test depends on the activation of different descending pain inhibitory mechanisms. J Pain. 2011;12:51-60. doi: 10.1016/j.jpain.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 57. Park JH, Han JB, Kim SK, et al. Spinal GABA receptors mediate the suppressive effect of electroacupuncture on cold allodynia in rats. Brain Res. 2010;1322:24-29. doi: 10.1016/j.brainres.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 58. Manni L, Florenzano F, Aloe L. Electroacupuncture counteracts the development of thermal hyperalgesia and the alteration of nerve growth factor and sensory neuromodulators induced by streptozotocin in adult rats. Diabetologia. 2011;54:1900-1908. doi: 10.1007/s00125-011-2117-5 [DOI] [PubMed] [Google Scholar]
- 59. Taube W, Gruber M, Beck S, et al. Cortical and spinal adaptations induced by balance training: correlation between stance stability and corticospinal activation. Acta Physiol (Oxf). 2007;189:347-358. doi: 10.1111/j.1365-201X. 2007.01665.x [DOI] [PubMed] [Google Scholar]
- 60. Taube W, Gruber M, Gollhofer A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol (Oxf). 2008;193:101-116. doi: 10.1111/j.1748-1716.2007.01665.x [DOI] [PubMed] [Google Scholar]
- 61. Gollhofer A. Proprioceptive training: considerations for strength and power production. In: Komi PV, ed. Strength and Power in Sport. 2nd ed. Blackwell; 2003:331-342. [Google Scholar]
- 62. Schröder S, Beckmann K, Franconi G, et al. Can medical herbs stimulate regeneration or neuroprotection and treat neuropathic pain in chemotherapy-induced peripheral neuropathy? Evid Based Complement Alternat Med. 2013;2013:423713. doi: 10.1155/2013/423713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cheng X, Huo J, Wang D, et al. Herbal medicine AC591 prevents oxaliplatin-induced peripheral neuropathy in animal model and cancer patients. Front Pharmacol. 2017;8:344. doi: 10.3389/fphar.2017.00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Di Cesare Mannelli L, Zanardelli M, Bartolucci G, et al. In vitro evidence for the use of astragali radix extracts as adjuvant against oxaliplatin-induced neurotoxicity. Planta Med. 2015;81:1045-1055. [DOI] [PubMed] [Google Scholar]
- 65. Di Cesare Mannelli L, Pacini A, Micheli L, et al. Astragali radix: could it be an adjuvant for oxaliplatin-induced neuropathy? Sci Rep. 2017;7:42021. doi: 10.1038/srep42021 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66. Hsiang SW, Lee HC, Tsai FJ, et al. Puerarin accelerates peripheral nerve regeneration. Am J Chin Med. 2011;39:1207-1217. doi: 10.1142/S0192415X11009500 [DOI] [PubMed] [Google Scholar]
- 67. Huang KS, Lin JG, Lee HC, et al. Paeoniae alba radix promotes peripheral nerve regeneration. Evid Based Complement Alternat Med. 2011;2011:109809. doi: 10.1093/ecam/nep115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu Y, Wang L, Li X, et al. Tanshinone IIA improves impaired nerve functions in experimental diabetic rats. Biochem Biophys Res Commun. 2010;399:49-54. doi: 10.1016/j.bbrc.2010.07.037 [DOI] [PubMed] [Google Scholar]
- 69. Oztürk G, Anlar O, Ender E, et al. The effect of Ginkgo extract EGb761 in cisplatin induced peripheral neuropathy in mice. Toxicol Appl Pharmacol. 2014;196:169-175. doi: 10.1016/j.taap.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 70. Niwa Y, Miyachi Y. Antioxidant action of natural health products and Chinese herbs. Inflammation. 1986;10:79-91. [DOI] [PubMed] [Google Scholar]
- 71. Kim BJ, Kim JH, Kim HP, Heo MY. Biological screening of 100 plant extracts for cosmetic use (II): antioxidative activity and free radical scavenging activity. Int J Cosmet Sci. 1997;19:299-307. doi: 10.1046/j.1467-2494.1997.171726.x [DOI] [PubMed] [Google Scholar]
- 72. Coste TC, Gerbi A, Vague P, Pieroni G, Raccah D. Neuroprotective effect of docosahexaenoic acidenriched phospholipids in experimental diabetic neuropathy. Diabetes. 2003;52:2578-2585. doi: 10.2337/diabetes.52.10.2578 [DOI] [PubMed] [Google Scholar]
- 73. Gerbi A, Maixent JM, Ansaldi JL, et al. Fish oil supplementation prevents diabetes induced nerve conduction velocity and neuroanatomical changes in rats. J Nutr. 1999;129:207-213. doi: 10.1093/jn/129.1.207 [DOI] [PubMed] [Google Scholar]
- 74. Yee P, Weymouth AE, Fletcher EL, Vingrys AJ. A role for omega-3 polyunsaturated fatty acid supplements in diabetic neuropathy. Invest Ophthalmol Vis Sci. 2010;51:1755-1764. doi: 10.1167/iovs.09-3792 [DOI] [PubMed] [Google Scholar]
- 75. Gladman SJ, Huang W, Lim SN, et al. Improved outcome after peripheral nerve injury in mice with increased levels of endogenous ω-3 polyunsaturated fatty acids. J Neurosci. 2012;32:563-571. doi: 10.1523/JNEUROSCI.3371-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Robson LG, Dyall S, Sidloff D, Michael-Titus AT. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurones throughout development and in aged animals. Neurobiol Aging. 2010;31:678-687. doi: 10.1016/j.neurobiolaging.2008.05.027 [DOI] [PubMed] [Google Scholar]
- 77. Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials. 1998;19:159-166. doi: 10.1016/s0197-2456(97)00150-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, References_to_studies_excluded_in_this_review for Effects of Nonpharmacological Interventions in Chemotherapy-Induced Peripheral Neuropathy: An Overview of Systematic Reviews and Meta-Analyses by Jie Hao, Xiaoshu Zhu and Alan Bensoussan in Integrative Cancer Therapies
Supplemental material, Search_strategies for Effects of Nonpharmacological Interventions in Chemotherapy-Induced Peripheral Neuropathy: An Overview of Systematic Reviews and Meta-Analyses by Jie Hao, Xiaoshu Zhu and Alan Bensoussan in Integrative Cancer Therapies