Abstract
It is more difficult to develop the low-cost spinal cord injury repair materials with high stability and biocompatibility for the biomedical applications. Herein, for the first time, we demonstrated the functional restoration of an injured spinal cord by the nano CeO2 particles assembled onto poly (∊-caprolactone) (PCL)/resveratrol (RVL) were synthesized using the biocompatible ionic liquid. The as-prepared biocompatible nanomaterials were characterized and confirmed by using different instruments such as Fourier transform infra-red spectroscopy for functional groups identification, X-ray diffraction for crystalline nature, Scanning electron microscopy, transmission electron microscopy for morphological structure, Dynamic light scattering for size distribution of the nanoparticles and thermogravimetric analysis for thermal properties. The synergetic effect between the uniform distributions of nano-sized CeO2 particles onto the PCL polymer with RVL can remarkably enhance the catalytic performance. Biofabricated nano-cerium oxide loaded PCL with RVL revealed that treatment significantly preserved hydrogen peroxide and also good catalytic performance. This study presents a nano-sized cerium oxide particles loaded PCL with RVL biocompatible materials have been providing highly efficient regenerative activity and biocompatibility in spinal card regeneration.
Keywords: CeO2, poly (∊-caprolactone), resveratrol, biocompatible, spinal cord injury repair
Introduction
Inorganic metal or metal oxide nanoparticles embedded with synthetic and natural polymers are well acknowledged for the antimicrobial activity, antioxidant, autocatalytic, anti-inflammatory, and biocompatibility, these properties are necessary for the biomedical and environmental applications. Nanoparticles are used as the wound healing, anticancer activity, spinal cord injury repair, autoimmune, and cardiac disease treatment. Cerium oxide (CeO2) has one of the most significant catalytic materials in the field of nanotechnology and biomedical tissue engineering applications.1 Advantages of the nano CeO2 materials are used such as the biocompatibility, autocatalytic, and antioxidant properties and it is helpful for the spinal cord injury,2-4 and it is acting for an excellent protector against cellular breakdown system due to unnecessary free radicals, neurological disorders, pathological, and against radiations. In addition, less solubility of CeO2 conquered by the biocompatible cerium oxide embedded with biopolymers such as dextrin, folic acid, cyclodextrin, glucose unit, chitosan, and so on. Nowadays, researchers have been focused on increasing the solubility and biocompatibility behavior.5,6
Poly (∊-caprolactone) (PCL) polymer has chosen as the basal scaffold because of its good mechanical strength, nontoxic, odorless, and biocompatibility.7 Poly (∊-caprolactone) has demonstrated that easily produced to the (negative) charge vessels of tubular scaffolds and attached to the other polymeric compounds. Furthermore, it has been broadly applied to the drug delivery system in the many aspects. Recently, many researchers have been listening carefully on the many dietary and botanical usual materials, which contain antioxidant and antibacterial behavior that can be easily reduced the spinal cord repair from ischemic reperfusion injury.8
Resveratrol (RVL) (3, 4, 5-trihydroxystilbene), a polyphone compounds are founded in peanuts, plums, grapes, and various plants. The RVL is as well the attendance in red wines and it is too much lower content in white wines. It is used biological events such as cardioprotective, antioxidant, anti-inflammatory, and anticancer properties.9 It is showed to inhibit membrane lipid peroxidation, hydrogen peroxide scavenger free radicals through increased the antioxidant levels and inhibit platelet aggregation form and numerous organs are protected from ischemia reperfusion injury.10 However, many studies focused onto the RVL as an antioxidant inducer in different diseases, the important role of RVL is the defense of spinal cord reperfusion injury remnants unexplored.
Currently, many researchers’ have been growing various nanoparticles and polymer loaded bionanomaterials with highly efficient biological applications. In general, the mixture combines’ materials are acting as the physicochemical properties for synthetic polymers with metal oxide nanoparticles and biological properties for the natural polymers.11,12 The spinal cord injury applications used the biocompatible polymeric materials such as chitosan, dextran, cyclodextrin, folic acid, and so on. Specifically, the mixture of natural, synthetic and metal oxide bionanomaterials such as the PCL-collagen, poly (l-lactic acid)-b-poly (∊-caprolactone)-gelatin, polyurethane-gelatin, poly (lactic-co-glycolic acid)-collagen, PCL-gelatin, chitosan-CeO2, and PCL-CeO2 were broadly explored for the tissue engineering and spinal cord injury repair applications.13-21 According to our best knowledge, no one has reported on the “Novel approach for efficient recovery for spinal cord injury repair via Biofabricated nano-cerium oxide loaded PCL with RVL to improve in vitro biocompatibility and auto-recovery abilities.”
Herein, we demonstrate that a new biofabricate nano-cerium oxide loaded PCL polymer with RVL. The as-synthesized biocompatible nanomaterials have been characterized and confirmed by various analytical techniques including by Fourier transform infra-red spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), High Resolution-transmission electron microscopy (HR-TEM), and thermogravimetric (TG) analysis. Consequently, we have focused and established onto the fabrication of biocompatible nano-sized cerium oxide particles onto PCL matrix with RVL to increase their solubility, avoid the toxicity and biocompatibility for biomedical applications, it is like an antioxidant and autocatalytic performance for spinal cord injury repair.
Experimental Details
Materials
Poly (caprolactone) (PCL) polymer (Mn average 80 kDa) and cerium nitrate (Ce (NO3)3.6H2O) hexa hydrate (99.5%) were purchased from Sigma Aldrich. Resveratrol (99.8%) from Brazil and 1-Butyl-3-methylimidazolium tetrafluoroborate were purchased from Shanghai Chemical Reagent Corporation, China. Sodium hydroxide (98%), hydrogen peroxide (99.8%), and potassium dihydrogen phosphate (98%) were purchased from Sigma Aldrich. The chemicals and reagents were used as the analytical reagents grade.
Preparation of Nano Cerium Oxide (nano CeO2) Particles
Typical procedure, about 0.5 M of Ce (NO3)3.6H2O was dispersed in 100 mL of Millipore water with constant stirring for half an hour and then 2 mL of 1-Butyl-3-methylimidazolium tetrafluoroborate solution was added into the above reaction bath. And also, 100 mL of freshly prepared; (1 M) sodium hydroxide solution was added in dropwise, till the precipitation was completely formed. The obtained precipitate was continuously stirred for 4 hours and after completion; the precipitate was allowed to settle for one day. The remaining product was washed with water and ethanol, then filtered and dried in a hot air oven at 80 °C for 3 hours and annealed at 500 °C for 5 hours. The obtained final product was grounded well with a mortar and designated as nano CeO2.
Biofabrication of Cerium Oxide-POLY (Caprolactone) (CeO2-PCL/RVL) Nanomaterials
Take 1.2 g of nano cerium oxide and 1 g of PCL polymer powder were uniformly mixed cautiously 100 mL of Millipore water and using magnetic stir for 30 minutes. Subsequently, 0.01 g of RVL added into the above mixture. This hybrid blend was continuously stirred for 60 minutes and centrifuge at 8000 rpm for 5 minutes. Centrifuged nanoparticles were rinsing with Millipore water and dried in an oven at 80 °C for 3 hours. Finally, the obtained bionanomaterials CeO2-PCL/RVL for further study the spinal cord injury repair. Similar way, prepared to the nano CeO2-PCL bionanomaterials without RVL.
Characterizations
Functional groups identifications of as-synthesized nano CeO2, PCL polymer, and RVL bionanomaterials were confirmed by FT-IR spectra (Nicolet 380 FT-IR microscope). X-ray diffraction patterns were obtained from the crystalline behavior of biocompatible materials recorded using (Rigaku D/MAX-rA diffractometer). The morphological structure, shape, and distribution of nano CeO2 loaded onto the PCL polymer matrix and RVL drug molecules were evidenced by scanning electron microscope (JSM-7000F) and transmission electron microscope (Tecnai F20 at 200 kV). Thermal properties of bionanomaterials were recorded TG analysis (room temperature to 800 °C) using TG instrument SDT Q600 (10 °C per minutes and N2 gas). Dynamic light scattering (DLS) of nanomaterials was performed on a Zetasizer Nano instruments.
In Vitro Drug Release
In vitro release was carried out for the nano CeO2 embedded with PCL bionanomaterials and pure drug molecules. The dissolution assessment was measured in a Nova Ética tester equipped with paddles in 1 L of concerned buffer solution (pH range = 6.8, 22.5 mol·L−1 of sodium hydroxide and 50 mmol·L−1 of potassium dihydrogen phosphate) for 10 hours in triplicate.22,23 The whole schematic program was kept at a thermostatically controlled at 37 °C and magnetically stir for 75 rer·min−1. The whole process was detained under dark environment. At appropriate time intervals, processed materials were collected, filtered, and it was measured using ultraviolet (UV)-Vis spectrometer. The dissolution values were obtained from the amount of drug-released molecules.
In Vitro Cell Survival and Autocatalytic Study
In vitro cell and autocatalytic study of the nanoceria oxide particles were further established in a hydrogen peroxide–induced oxidative injury model utilizes the spinal cord model system. Firstly, 100 mM of hydrogen peroxide (H2O2) solution was added for 60 minutes to both a nano-Ceria oxide nanoparticles treated culture and a control culture at 15 days. Subsequent to 60 minutes of H2O2 treatment, the cell viability was assay using a live-dead kit.
Result and Discussions
Fourier Transform Infra-Red Spectroscopy
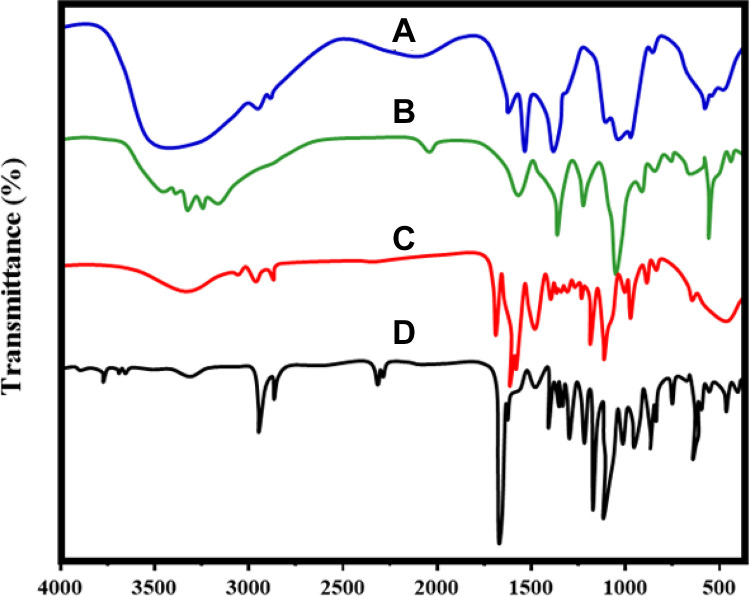
Fourier transform infra-red spectroscopy spectrum of nano CeO2, PCL, RVL, nano CeO2-PCL, and CeO2-PCL/RVL biocompatible nanomaterials are shown in Figure 1. The FT-IR spectrum shows the cerium oxide main functional groups of CeO2 strong peak obtained at 652 cm−1 which is ascribed txo the Ce-O-Ce stretching vibration confirms the CeO2 nanoparticles and introduction of ionic liquid, some characteristic functional groups peaks are observed.1 The PCL polymer showed the main characteristic peak at 1722 cm−1 for carbonyl (C-O) groups of stretching vibration and small intense characteristic peaks at 2865 cm−1 for symmetric and 2944 cm−1 for asymmetric CH2 stretching vibrations, respectively.20 The FT-IR spectra of RVL show a typical OH stretching peak at 3259 cm−1 and 3 small strong peaks at 1383 cm−1 for C–O stretching vibrations, 1587 cm−1 for C–C olefinic stretching and 1612 cm−1 for C–C aromatic double-bond stretching vibrations. And also, the typical peak was obtained at 962 cm−1 for Tran’s olefinic form. The nano CeO2-PCL composite shows the PCL polymer characteristic peaks are observed at the same region, but CeO2 peaks are the shift to higher wavenumbers at 658 cm−1, these confirm the CeO2 connection of PCL polymer matrix.7 It is interesting to note that FT-IR spectra of CeO2-PCL/RVL biocompatible nanomaterials, the characteristic peaks of RVL and PCL polymer matrix are observed at the same region and nano-sized CeO2 are slight shifts to the wavenumbers at 659 cm−1. This result confirms the presence of RVL, PCL polymer with nano-sized particles of cerium oxide. However, the occurrence of carbonyl and hydroxyl functional groups on nanomaterials surface is beneficial to the injury repair.
Figure 1.
FT-IR spectrum of (A) nano CeO2, (B) PCL, (C) RVL, (D) nano CeO2-PCL, and (E) CeO2-PCL/RVL. FT-IR indicates Fourier transform infra-red spectroscopy; PCL, poly (∊-caprolactone); RVL, resveratrol.
X-ray Diffraction Analysis
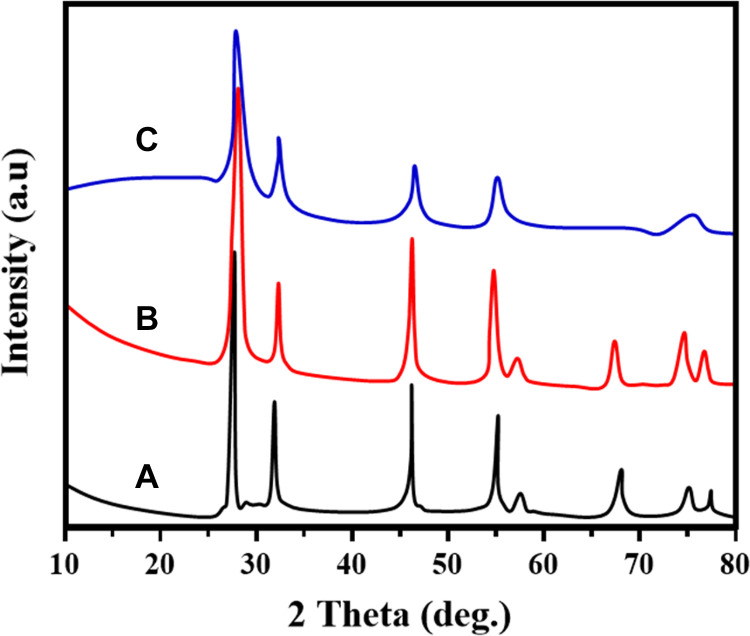
X-ray diffraction patterns of nano CeO2, PCL, RVL, nano CeO2-PCL, and CeO2-PCL/RVL biocompatible nanomaterials are shown in Figure 2. X-ray diffraction spectra of nano cerium oxide are well matched with the JCPDS card no. 75-0076.24 And the all information reveals that the as-fabricated bionanomaterials have the cubic phase arrangement confirmed. The nano CeO2 particles exhibited that the crystalline diffraction (111), (200, (220), (311), (222), (400), (331), (240), and (422), lattice planes, respectively. The PCL polymer matrix, 2 major sharp peaks are observed in the diffraction peaks at 21.2° and 23.6° that represent the planes of (110) and (200), which is a polyethylene such as semicrystalline structure, respectively.20
Figure 2.
XRD patterns of prepared samples of (A) nano CeO2, (B) nano CeO2-PCL, and (C) CeO2-PCL/RVL. PCL indicates poly (∊-caprolactone); RVL, resveratrol; XRD, X-ray diffraction.
X-ray diffraction patterns of RVL represent the different characteristic peaks connected to a crystalline structure, and major diffraction angle was observed at 2θ = 6.63°, 16.32°, 19.27°, 22.33°, 23.05°, and 28.57°, respectively.25 The XRD patterns of nano CeO2 loaded PCL matrix reveals that the similar to CeO2 crystalline lattice and small intense peaks are observed, these samples are well matched with (JCPDS card no. 75-0076) the CeO2 nanoparticles. The crystalline diffraction peaks of CeO2-PCL/RVL biocompatible nanomaterials are well matched to CeO2, PCL polymer, and RVL peaks. Thus, the results suggest that the biocompatible nanomaterials provide a significantly reduce the crystalline peaks of RVL leading to nano CeO2 loaded PCL polymer matrix.
Scanning Electron Microscope Analysis
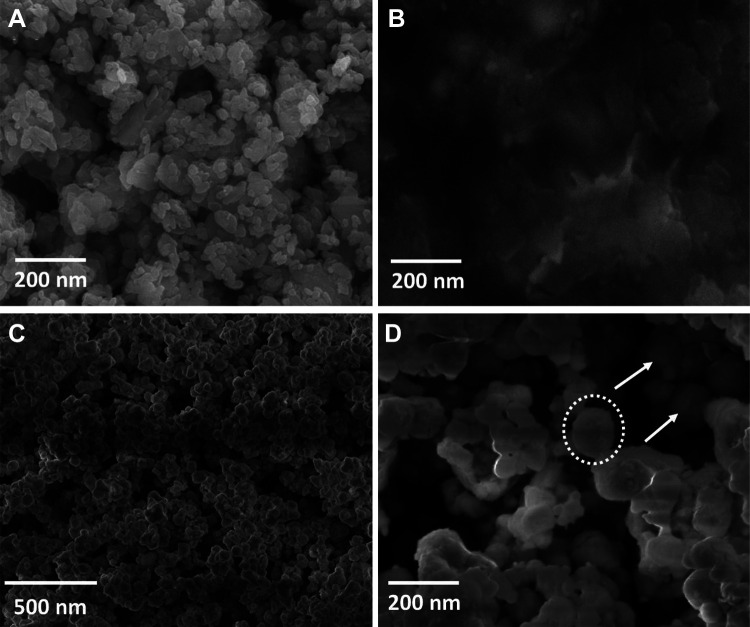
The morphological facial appearance of nano CeO2, PCL, nano CeO2-PCL, and CeO2-PCL/RVL bionanomaterials is shown in Figure 3. It can be clearly seen from the scanning electron microscope of nano CeO2 is the spherical shape,26 and PCL has porous structure revealed that the amorphous nature which is attribute the polymeric chain of PCL.20,27 The SEM images of nano CeO2-PCL polymer and CeO2-PCL/RVL bionanomaterials display the homogeneously distribution of spherical particles of nano CeO2 onto the PCL polymer and RVL surface, without any aggregation and the formed nanoparticles due to the capping or reducing behavior of PCL polymer and RVL, which is main role in the formation of bionanomaterials. These results are more in accordance with the XRD results.
Figure 3.
Scanning electron microscope images of (A) nano CeO2, (B) PCL, (C) nano CeO2-PCL, and (D) CeO2-PCL/RVL. PCL indicates poly (∊-caprolactone); RVL, resveratrol.
Transmission Electron Microscope Analysis
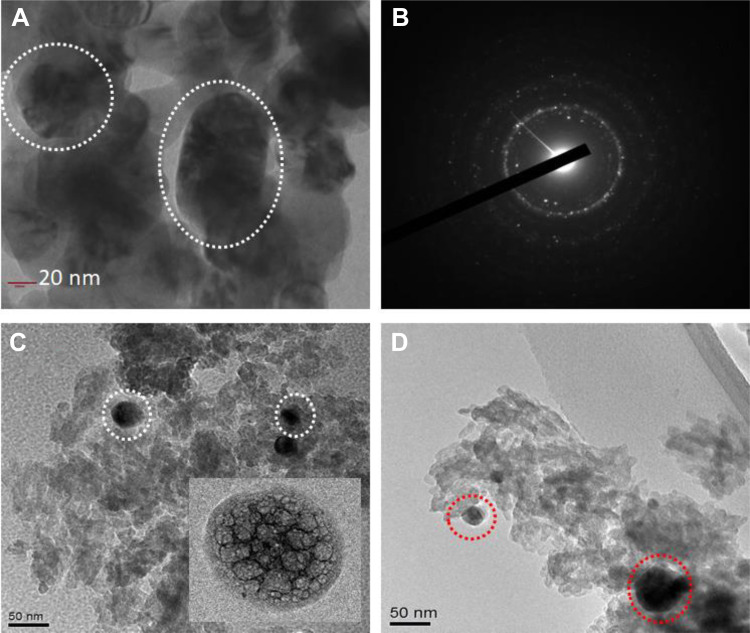
To demonstrate the effect on distribution of particle size morphological shape of the nano CeO2, PCL, nano CeO2-PCL, and CeO2-PCL/RVL bionanomaterials were analyzed by TEM images as shown in Figure 4. The observation TEM image of nano cerium oxide exhibits the particle size at 10 to 60 nm and the good contribution of sphere nanoparticles.28,29 The PCL polymer matrix reveals the porous nature of polymeric units. As clearly seen, TEM image of CeO2-PCL nanomaterials reveal that the porous nature of PCL polymer and their doping of nano cerium oxide particles visualized. It is interesting to note that the TEM image of CeO2-PCL/RVL bionanomaterials is similar morphological structure observed from the CeO2-PCL polymer matrix and surface slightly moderated. Hence, the result confirmed the construction of CeO2 nanoparticles loading PCL polymer and RVL. These nanostructure materials are beneficial to the injury repair biomedical applications.
Figure 4.
Transmission electron microscope images of (A and B) nano CeO2 and SAED pattern of nano CeO2, (C) nano CeO2-PCL, and (D) CeO2-PCL/RVL. PCL indicates poly (∊-caprolactone); RVL, resveratrol.
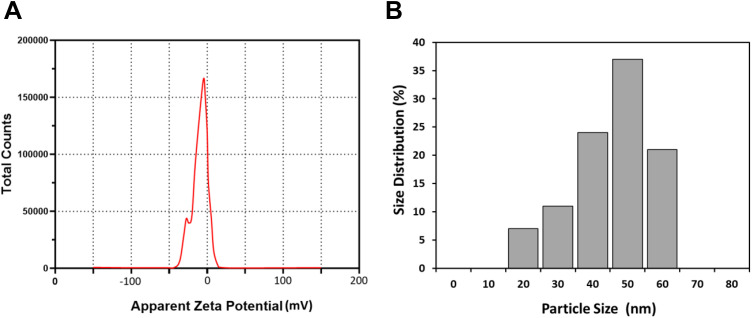
Size Distribution and Zeta Potential
As shown in Figure 5A, the CeO2-PCL/RVL had average diameters of 50 ± 3 nm observed by DLS and particle size ranges from 10 to 60 nm which is in agreement with the TEM analysis data. The zeta potential result of the CeO2-PCL/RVL nanocomposite is shown in Figure 5B, which indicates the stability of the colloidal dispersions. The magnitude of zeta potential values (−10.3 mV) indicates that the CeO2-PCL/RVL nanomaterials show incipient instability with a low rate of flocculation or coagulation. The negative value obtained for the zeta potential indicates that the CeO2-PCL/RVL nanomaterials surface is negatively charged.
Figure 5.
(A) Zeta potential value of CeO2-PCL/RVL and (B) size distribution. PCL indicates poly (∊-caprolactone); RVL, resveratrol.
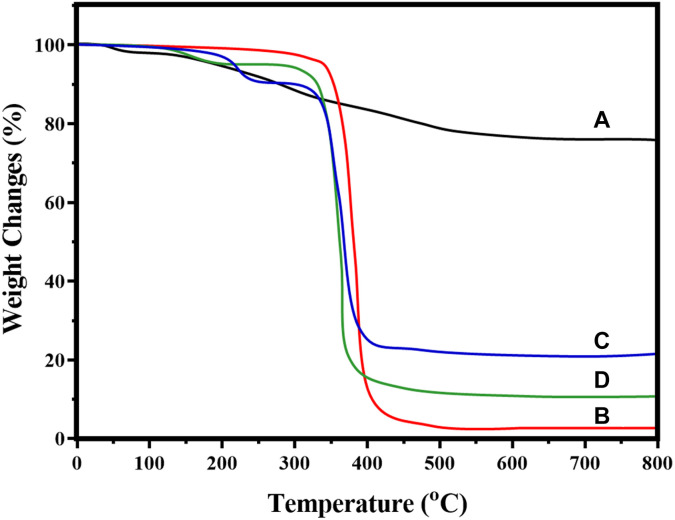
Thermogravimetric Analysis
The thermal properties of nano CeO2, PCL, nano CeO2-PCL, and CeO2-PCL/RVL were analyzed by the thermal gravimetric analysis (TGA). Figure 6 shows the weight loss and weight loss rate curves recorded with heating rate at 10 °C per minutes in nitrogen atmosphere between 27 °C and 800 °C. The first-stage weight loss obtained at 27 °C to 130 °C, which could be ascribed to the water dehydration from the entire polymeric structure unit.30 The second-stage weight decrease was observed the PCL polymer chain surrounded by the temperature range from 120 °C to 650 °C is the elimination of dopant decomposition from the biopolymeric structure, but the introduction of CeO2 nanoparticles does not decrease the thermal stability of PCL polymer chain at 250 °C.20 However, the CeO2-PCL/RVL was observed the 3 stages of weight loss, assigned to the 27 °C to 120 °C for dehydration polymeric chains, 120 °C to 650 °C for depolymerization and 650 °C to 800 °C for the polymer units decomposition. Thus, the thermal performance of CeO2-PCL/RVL composite is improved, when compared with CeO2-PCL and PCL polymer.
Figure 6.
Thermal Gravimetric Analysis curves of (A) nano CeO2, (B) PCL, (C) nano CeO2-PCL, and (D) CeO2-PCL/RVL. PCL indicates poly (∊-caprolactone); RVL, resveratrol.
It is well recognized that the weight derivative of bionanomaterials weight loss (%; 5.5%, 9.4%, 10.8%, and 80.8%) of the relevant materials is arranged in the order of CeO2 > CeO2-PCL/RVL > CeO2-PCL > PCL, respectively. Thus, resulting that the presence of nano CeO2 particles incorporated in the PCL and RVL has been improved the crystalline phase as well as thermal stability of the PCL-RVL polymer chain, which had been confirmed by XRD analysis.
Biological Evaluation
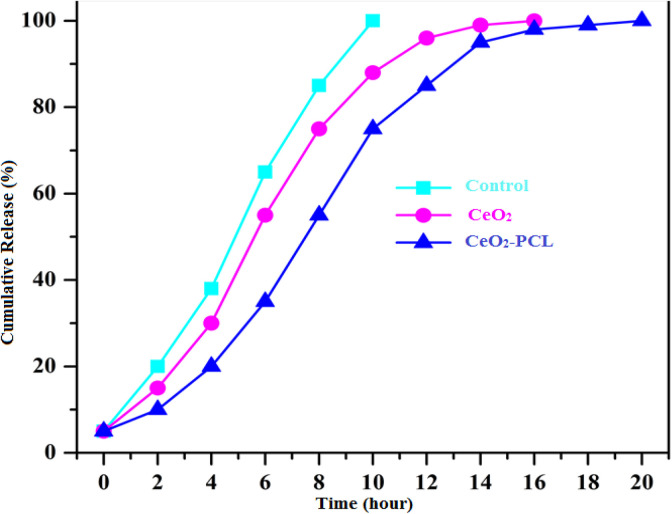
To study the in vitro drug-releasing profile, dissolution rates of nano CeO2 loaded onto the PCL bionanomaterials and pure drug molecules are shown in Figure 7. Shows the dissolution test, drug molecules free (RVL) contain simply released from the drug molecules within 10 hours. The molecule size is extremely low comparable to the drug carriers, so it may be certain amount of drug molecules unrestricted earlier in the drug delivery organization.
Figure 7.
In vitro drug release of nano CeO2 particles loaded PCL at 37 °C (pH 6.8 in Phosphate Buffer Solution (PBS) buffer). PCL indicates poly (∊-caprolactone).
However, in such a case, our container drug molecules were exhibit that continually released from the nano CeO2 particles loaded PCL matrix at 10 hours.1,31 The drug molecules releasing system rate of nano cerium oxide loaded PCL polymer materials was about (29%) comparable to CeO2 (48%). An arithmetical rate was designed among the CeO2 nanoparticles and CeO2 loaded PCL nanomaterials exhibit an arithmetical distinction value (*P < .05) by using the Student t test for first 10 hours. This drug release examination has been confirmed the main role of PCL polymer as efficient carriers in the current study.
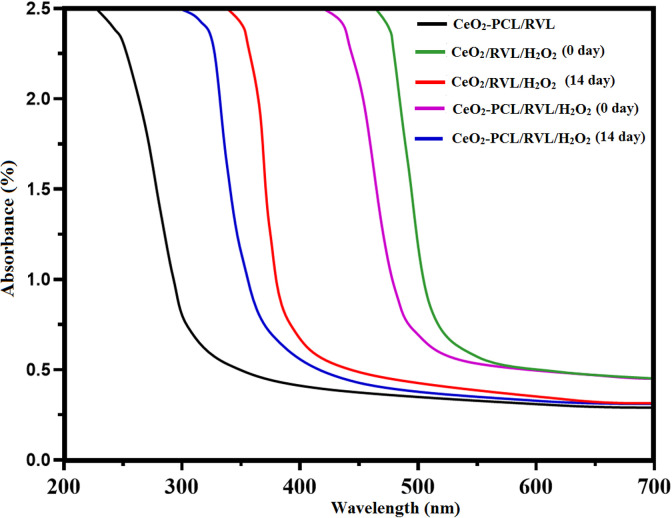
In addition, catalytic performance of nano cerium oxide loaded RVL and nano cerium oxide loaded PCL polymer with RVL was verified using UV-visible spectroscopy and conducted the H2O2 medium. In the activity measurement, the nano CeO2 particles loaded PCL polymer with RVL was used as a control solution, shown in Figure 8. After addition of the H2O2 as the standard solution, the intensity peak was shifts to lesser energy level. These shifts the spectrum hypothesizes value can be changed into the CeO2 oxidation state from Ce3+ to Ce4+ ions.32,33 Subsequently, the spectra of hydrogen peroxide solution were influence and shifted to the higher energy, following 14 days of duration kept in light off environmental conditions. This type of shifting demonstrates on a higher energy level that the reproduction of nano CeO2 particles from Ce4+ to Ce3+ ions is dependent on the size of nanomaterials. Consequently, UV-Vis spectra was recognized that adding together of CeO2 onto the PCL with RVL has enhance the catalytic oxidative revival from the UV-Vis spectra shifted from the lower energy level to higher energy level and higher energy level to lower energy level using the H2O2 solution.1 These specify that CeO2 nanoparticles are providing enhanced the catalytic activity in spinal card regeneration.
Figure 8.
Catalytic performance of nano CeO2 particles with hydrogen peroxide under UV-visible spectrometer. UV indicates ultraviolet.
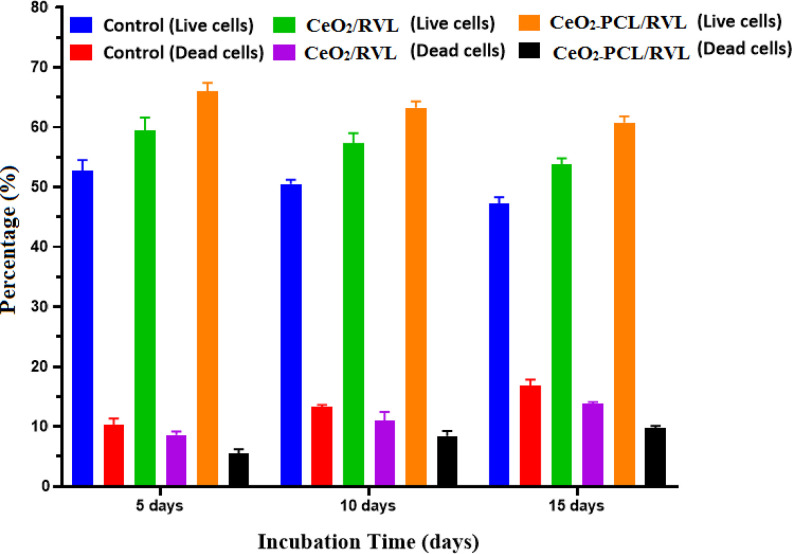
In cell viability, assessments were tested by using spinal card cells, being isolated from euthanize adult rats shown in Figure 9. The live-dead assays of RVL molecules loading with CeO2-PCL polymer have got large cell viability at (62 ± 2) for 5 days, (59 ± 2) for 10 days, and (57 ± 2) for 15 days as compared to drug molecules loaded nano CeO2. And the death cell also suggestively lower intensity level of RVL molecules connected nano cerium oxide and PCL polymer compared to the RVL molecules loaded nano CeO2.34 The valance states of cerium oxide (Ce3+ and Ce4+) ions on the surface of as-prepared nanomaterials are successfully involved in the antioxidant function, which had been the scavenger of the free radicals on the culture medium operation (hydrogen peroxide role act as the scavenger and autocatalytic agent). Subsequently, another chemical reaction seems involved that the nano cerium oxide and ions of culture medium possible to the reverse oxidation state from Ce4+ to Ce3+ ions. Thus, the results support to be a regenerative reaction and catalytic activity of drug molecules loaded nano CeO2-PCL polymer matrix.
Figure 9.
Cell viability on the Live-dead assay by adult rat spinal cord in different day’s intervals.
Conclusion
In summary, we have developed to a biofabricated nano-cerium oxide loaded PCL polymer with RVL. The as-prepared biocompatible nanomaterials were characterized and confirmed by FT-IR spectroscopy, XRD, SEM, TEM, DLS, Zeta potential, and Thermogravimetric analysis. The CeO2-PCL/RVL bionanomaterials were evaluated and established with its comparative studies on in vitro and in vivo viability analysis. The results of CeO2-PCL/RVL bionanomaterials were found to be excellent biocompatible without any harmful effect of neighboring implantation. Therefore, the CeO2-PCL/RVL bionanomaterials can be a feasible medical application material, functional recovery for spinal cord injury repair.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research work is financially supported by Natural Science Foundation of Shanxi-Young Program (2017JQ8036), China.
ORCID iD: Xiujin Chen  https://orcid.org/0000-0003-2522-7992
https://orcid.org/0000-0003-2522-7992
References
- 1. Fang X, Song H. Synthesis of cerium oxide nanoparticles loaded on chitosan for enhanced auto-catalytic regenerative ability and biocompatibility for the spinal cord injury repair. J Photoch Photobio B. 2019;191:83–87. [DOI] [PubMed] [Google Scholar]
- 2. Kang CE, Baumann MD, Tator CH, Shoichet MS. Localized and sustained delivery of fibroblast growth factor-2 from a nanoparticle-hydrogel composite for treatment of spinal cord injury. Cells Tissues Organs. 2012;197(1):55–63. [DOI] [PubMed] [Google Scholar]
- 3. Cerqueira SR, Lee Y, Cornelison RC, et al. Decellularized nerve matrix supports schwann cell transplants and axon growth following spinal cord injury. Biomaterials. 2018;19:2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cornelison RC, Rothi EG, Porvasnik SL, et al. Injectable hydrogels of optimized acellular nerve for injection in the injured spinal cord. Biomed Mater. 2018;13(3):034110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hassanneja H, Nouri A. Synthesis and evaluation of self-healing cerium-doped chitosan nanocomposite coatings on AA5083-H321. Int J Electrochem Sci.2016;11:2106–2118. [Google Scholar]
- 6. Wei D, Sun W, Qian W, Ye Y, Ma X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr Res. 2009;344(17):2375–2382. [DOI] [PubMed] [Google Scholar]
- 7. Magdalane CM, Kaviyarasu K, Vijaya JJ, Siddhardha B, Jeyaraj B. Photocatalytic activity of binary metal oxide nanocomposites of CeO2/CdO nanospheres: investigation of optical and antimicrobial activity. J Photochem Photobiol B. 2016;163:77–86. [DOI] [PubMed] [Google Scholar]
- 8. Alsarra IA. Chitosan tropical gel formulation in the management of burn wounds. Int J Biol. Macromol. 2009;45(1):16–21. [DOI] [PubMed] [Google Scholar]
- 9. Cheung DL. Aggregation of nanoparticles on one and two-component bilayer membranes. J Chem Phys. 2014;141(19):194908–194909. [DOI] [PubMed] [Google Scholar]
- 10. Azuma K, Izumi R, Osaki T, et al. Chitin, chitosan and its derivatives for wound healing: old and new materials. J Funct Biomater. 2015;6(1):104–142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Anjum S, Arora A, Alam MS, Gupta B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int J Pharm. 2016;508(1-2):92–101. [DOI] [PubMed] [Google Scholar]
- 12. Sudarshan N, Hoover D, Knorr D. Antibacterial action of chitosan. Food Biotechnol. 1992;6(3):257–272. [Google Scholar]
- 13. Duan X, Ma J, Lian J, Zheng W. The art of using ionic liquids in the synthesis of inorganic nanomaterials. Cryst Eng Comm. 2014;16:2550. [Google Scholar]
- 14. Liu H, Wang M, Wang Y, Liang Y, Cao W, Su Y. Ionic liquid-templated synthesis of mesoporous CeO2–TiO2 nanoparticles and their enhanced Photocatalytic activities under UV or visible light. J Photochem Photobiol A Chem. 2011;223(2-3):157–164. [Google Scholar]
- 15. Senthilkumar RP, Bhuvaneshwari V, kumar RR, Sathiyavimala S, Malayaman V, Chandarshekarda B. Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: as a bionanomaterials, department of biotechnology. Int J Biol Macromol. 2017;104(pt B):1746–1752. [DOI] [PubMed] [Google Scholar]
- 16. Fu S, Lv R, Wang L, Hou H, Liu H, Shao S. Resveratrol, an antioxidant, protects spinal cord injury in rats by suppressing MAPK pathway. J Biol Sci. 2018;25(2):259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajeshkumar S, Naik P. Synthesis and biomedical applications of Cerium oxide nanoparticles—a review. Biotechnol Rep. 2018;17:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ketzial J, Jasmine A, Nesaraj S. Synthesis of CeO2 nanoparticles by chemical precipitation and the effect of a sur-factant on the distribution of particle sizes. J Ceram Process Res. 2011;12(1):74–79. [Google Scholar]
- 19. Sisubalan N, Ramkumar VS, Pugazhendhi A. et al . ROS-mediated cytotoxic activity of ZnO and CeO2 nanoparticles synthesized using the rubia cordifolia L. lea extract on MG-63 human osteosarcoma cell lines. Environ Sci Pollut Res. 2017;25:1–11. [DOI] [PubMed] [Google Scholar]
- 20. Ahmad Rather H, Thakore R, Singh R, Jhala D, Singh S, Vasita R. Antioxidative Study of Cerium Oxide Nanoparticle Functionalized PCL-Gelatin Electrospun Fibers for Wound Healing Application. Bioactive Materials xxx; 2017:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Han J, Zhao Y, et al. Functionalized collagen scaffold implantation and cAMP administration collectively facilitate spinal cord regeneration. Acta Biomater. 2016;30:233–245. [DOI] [PubMed] [Google Scholar]
- 22. Sundararajan V, Dan P, Kumar A, et al. Drosophila melanogaster as an in vivo model to study the potential toxicity of cerium oxide nanoparticles. Appl Surf Sci. 2019;490:70–80. [Google Scholar]
- 23. Babin K, Antoine F, Goncalves DM, Girard D. TiO2, CeO2 and ZnO nanoparticles and modulation of the degranulation process in human neutrophils. Toxicol Lett. 2013;221(1):57–63. [DOI] [PubMed] [Google Scholar]
- 24. Sabelstrom H, Stenud M, Reu P, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342(6158):637–640. [DOI] [PubMed] [Google Scholar]
- 25. Novikova LN, Kolar MK, Kingham PJ, et al. Trimethylene carbonate-caprolactone conduit with poly-p-dioxanone microfilaments to promote regeneration after spinal cord injury. Acta Biomater. 2018;66:177–191. [DOI] [PubMed] [Google Scholar]
- 26. Soderblom C, Luo X, Blumenthal E, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci. 2013;33(34):13882–13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun J, Wang X, Wu J, et al. Biomimetic moth-eye nanofabrication: enhanced antireflection with superior self-cleaning characteristic. Sci Rep. 2018;8:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dumont CM, Carlson MA, Munsell MK, et al. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater. 2019;86:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang Z, Shi J, Xie J, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4(1):69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dumont CM, Margul DJ, Shea LD. Tissue engineering approaches to modulate the inflammatory milieu following spinal cord injury. Cells Tissues Organs. 2016;202(1-2):52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song YH, Agrawal NK, Griffin JM, Schmidt CE. Recent advances in nano therapeutic strategies for spinal cord injury repair. Adv Drug Deliv Rev. 2019;148:38–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Papa S, Ferrari R, Paola MD, et al. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release. 2014;174:15–26. [DOI] [PubMed] [Google Scholar]
- 33. Wan JM, Liu LL, Zhang JF, Lu JW, Li Q. Promotion of neuronal regeneration by using self-polymerized dendritic polypeptide scaffold for spinal cord tissue engineering. J Mater Sci Mater Med. 2018;29(1):2–8. [DOI] [PubMed] [Google Scholar]
- 34. Guan J, Zhu Z, Zhao RC, et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials. 2012;34(24):5937–5946. [DOI] [PubMed] [Google Scholar]