Abstract
There have been several episodes of viral infection evolving into epidemics in recent decades, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the latest example. Its high infectivity and moderate mortality have resulted in an urgent need to find an effective treatment modality. Although the category of immunosuppressive drugs usually poses a risk of infection due to interference of the immune system, some of them have been found to exert antiviral properties and are already used in daily practice. Recently, hydroxychloroquine and baricitinib have been proposed as potential drugs for SARS-CoV-2. In fact, there are other immunosuppressants known with antiviral activities, including cyclosporine A, hydroxyurea, minocycline, mycophenolic acid, mycophenolate mofetil, leflunomide, tofacitinib, and thalidomide. The inherent antiviral activity could be a treatment choice for patients with coexisting rheumatological disorders and infections. Clinical evidence, their possible mode of actions and spectrum of antiviral activities are included in this review article.
Lay summary
Immunosuppressants often raise the concern of infection risks, especially for patients with underlying immune disorders. However, some disease-modifying antirheumatic drugs (DMARDs) with inherent antiviral activity would be a reasonable choice in the situation of concomitant viral infections and flare up of autoimmune diseases. This review covers DMARDs of treatment potential for SARS-CoV-2 in part I, and antiviral mechanisms plus trial evidence for viruses other than SARS-CoV-2 in part II.
Keywords: baricitinib, COVID-19, hydroxychloroquine, immunosuppressant, thalidomide, virus
Introduction
Infections are a common concern of immunosuppressive drugs. However, some immunosuppressants or disease-modifying antirheumatic drugs (DMARDs) show antiviral activity and may be safely used or even beneficial in patients with selected concomitant viral infections. Certain DMARDs may even be considered as an alternative treatment for recalcitrant infections. Moreover, the concomitant use of immunosuppressants and antiviral agents was proved to be more effective than antiviral agent monotherapy in some reports.1 The antiviral property of immunosuppressants may act through (a) direct virucidal activity, (b) blockage of receptors, (c) inhibition of necessary molecules for viral replication in the hosts, or (d) amelioration of inflammatory symptoms. Also, control of inflammation may decrease the susceptibility or enhance host ability to defend against viral infection. The following review focuses on the immunosuppressants/DMARDs which have antiviral potential through the first three mode of actions. Antiviral agents with immunosuppressive activity such as ribavirin2 are beyond the scope of this review. In view of the imperative demand to control the recently discovered severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), DMARDs with treatment potential are covered in part I. For other viruses, including We also conducted a two-step research as follows: common ones in daily practice and those without specific target therapy, but life threatening, evidence is covered in part II.
Method
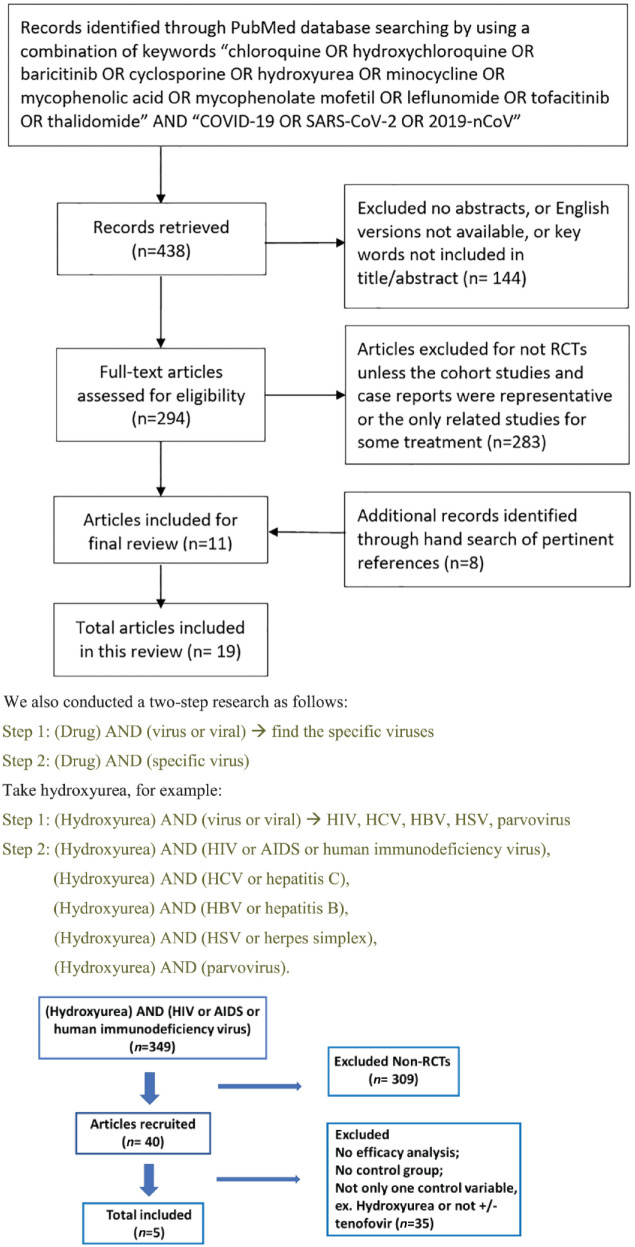
A literature search of the PubMed database using the keywords (chloroquine OR hydroxychloroquine OR baricitinib OR cyclosporine OR hydroxyurea OR minocycline OR mycophenolic acid OR mycophenolate mofetil OR leflunomide OR tofacitinib OR thalidomide) AND (virus OR viral) was performed from inception to 13 May 2020 (Figure 1). Reference lists of pertinent articles were hand searched for additional studies of interest.
Figure 1.
Article selection flowchart.
AIDS, acquired immunodeficiency syndrome; COVID-19, coronavirus disease 2019; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; RCT, randomized controlled trial; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Part I: DMARDs for severe acute respiratory syndrome coronavirus 2
Coronavirus disease 2019 (COVID-19) is a newly emerged lethal pandemic caused by SARS-CoV-2. It is transmitted efficiently by droplets and is contagious between humans. While most patients experience mild symptoms, some develop acute respiratory distress syndrome, multi-organ failure, or even death. As of 20 May 2020, more than 4.90 million cases have been reported, causing a total of 0.32 million deaths.
SARS-CoV-2 is a single-strand ribonucleic acid (RNA) virus belonging to betacoronaviruses. It has three structural proteins, S (spike), E (envelope), M (membrane), anchoring on the lipid bilayer membrane. The spike protein binds on host receptors and mediates membrane fusion.3
Given that there has been no effective and specific therapy to meet the urgent need, several DMARDs were repurposed on the basis of potential anti-SARS-CoV-2 activity and for modulating the cytokine storm.
Chloroquine and hydroxychloroquine
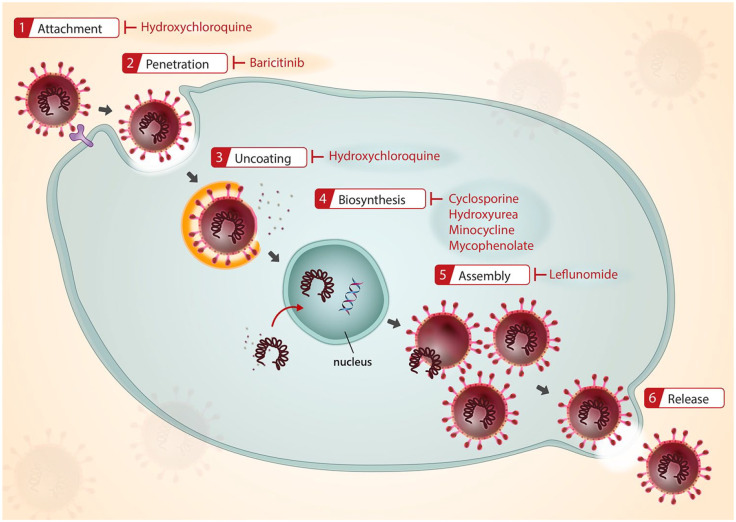
Chloroquine (CQ) and hydroxychloroquine (HCQ), both derivatives of 4-aminoquinoline, are indicated to treat and prevent malaria. They are also used as DMARDs for rheumatoid arthritis, lupus erythematosus, and porphyria cutanea tarda. In addition, the application for viral infections in off-label use has recently been investigated vigorously. The antiviral activity is through blocking the virus/cell fusion via increasing endosomal pH and hindering the glycosylation of cellular receptors (Figure 2).4
Figure 2.
Proposed target of antiviral activities by DMARDs and immunosuppressants.
DMARDs, disease-modifying anti-rheumatic drugs.
In vitro CQ revealed low half-maximal effective concentration (EC50) and high half-cytotoxic concentration (CC50) for COVID-19.5 A preliminary study conducted in China showed benefits in pneumonia image, shortening of disease course, and promoting a virus-negative conversion compared with control group.6 Then, four completed clinical studies demonstrated favorable outcomes in clinical and radiologic amelioration, while another two randomized controlled trials (RCTs) illustrated no statistically significant change compared with control arms.7–12 Based on the inhibitory effect of azithromycin against Ebola and Zika viruses in vitro, and the possibility of preventing from progressing to severe respiratory tract infections, two French trials which combined the use of azithromycin and HCQ revealed better efficacy.7,9
However, further studies are still needed to draw conclusions because most of these studies bear limitations including selection bias, allocation bias, or insufficient case numbers. Several multicenter, double-blind, and well-designed controlled trials are already underway to assess the efficacy and safety of CQ or HCQ in the treatment of COVID-19 pneumonia. In the absence of other confirmed effective therapy specific to SARS-CoV-2, both drugs are currently still listed in the treatment guidelines (Table 1).
Table 1.
Potential antiviral efficacy of DMARDs and immunosuppressants for SARS-CoV-2.
| Medications | Proposed antiviral mechanisms | In vitro | Clinical report |
|---|---|---|---|
|
Chloroquine
HCQ |
(1) Increase endosomal pH required for virus/cell fusion (2) Interfere with the glycosylation of cellular receptors |
✓ | ✓ Cohort (n = 42): negative RT-PCR rate on day 6 HCQ group: 70% (13/20) Control group: 12.5% (2/16) HCQ + azithromycin group: 100% (6/6)7 ✗ RCT (n = 30): no significant difference8 ✓ Open label, no control group (n = 80): with azithromycin, 65/80 improved clinical outcomes9 ✓ RCT (n = 62): significant improvement in time to clinical recovery and radiologic change (p < 0.05)10 ✓ RCT (n = 22): shorten hospital days and greater radiologic improvement, but not significant compared to control (Lopinavir/Ritonavir)11 ✗ RCT (n = 150): only significant in CRP reduction (p = 0.045)12 |
| Baricitinib | (1) Regulate endocytosis of virus by inhibiting AAK1, GAK. (2) Reduce cytokines including IL-2, IL-6, IL-10, G-CSF, and IFN-γ |
✓ n = 4, improved clinically and in laboratory data.13
✓ Placebo-controlled, open-label study (n = 24): significant improvement in baricitinib group14 |
|
| Cyclosporine A | (1) Target cyclophilin D to inhibit MPTP opening and rescues mitochondria from apoptosis. (2) MDA5, a putative cytoplasmic receptor of SARS-CoV-2, could be reversed by calcineurin inhibitors |
||
|
MMF
MPA |
Inhibit DHODH and IMPDH | ✓ | |
| Thalidomide | Suppress pro-inflammatory cytokines (TNF-α, IL-8) through inhibition of NF-κB | ✓ n = 1: 45 years, woman15 |
AAK1, AP2-associated protein kinase 1; CRP, C-reactive protein; DHODH, dihydroorotate dehydrogenase; GAK, cyclin G-associated kinase; G-CSF, granulocyte-colony-stimulating factor; IL, interleukin; HCQ, hydroxychloroquine; IFN, interferon; IMPDH, inosine monophosphate dehydrogenase; MDA5, melanoma-differentiation-activated protein 5; MMF/MPA, mycophenolate mofetil/mycophenolic acid; MPTP, mitochondrial permeability transition pore opening; RCT, randomized-controlled trial; RT-PCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor.
Baricitinib
Baricitinib, blocking Janus kinase (JAK)1 and JAK2, is approved for rheumatoid arthritis and has been investigated in atopic dermatitis.
SARS-CoV-2 binds on the angiotensin-converting enzyme 2 (ACE2) receptors and enters lung cells through receptor-mediated endocytosis. Some of the numb-associated kinase (NAK) family members, AP2-associated protein kinase 1 (AAK1) and cyclin G-associated kinase (GAK), are hypothesized to regulate the ACE2-mediated endocytosis. Baricitinib demonstrated high affinity to AAK1 and GAK and was identified as a potential treatment for COVID-19 by artificial intelligence. Intriguingly, other JAK inhibitors such as tofacitinib and upadacitinib did not illustrate affinities to NAKs.13,16
A case series reported four Italian patients with moderate-to-severe unstable COVID-19 infections. Except one female nurse, the other three male individuals were aged 51–76 with high body mass index, and two of them had chronic obstructive pulmonary disease plus hypertension histories. Under baricitinib 2 mg or 4 mg for 10–12 days, all patients improved in clinical symptoms (fever, cough, and dyspnea) and in laboratory data [interleukin-6 (IL-6), C-reactive protein, ferritin, liver enzymes, D-dimer, and viral loads].13
In one controlled open-label study (n = 24), patients were given either baricitinib 4 mg/day plus lopinavir–ritonavir or antiretroviral plus hydroxychloroquine (control group) for 2 weeks. Significant improvement of symptoms and laboratory results, no intensive care unit transfer (versus 33% transfer in control cases), and 58% discharge from wards (versus 8% in control) was shown among the baricitinib-treated individuals.14
In addition to antiviral property, baricitinib has been suggested as an approach for a cytokine storm syndrome, which features hypercytokinemia and multi-organ failure. Elevated ferritin and IL-6 in COVID-19 cases were predictive of a high mortality rate according to a China retrospective study.17 Baricitinib inhibits cytokines including IL-2, IL-6, IL-10, interferon gamma (IFN-γ), and granulocyte-colony-stimulating factor (G-CSF)13,18 and may bring the benefit of immune reconstruction which could be used in rapidly progressive diseases.
However, there are competing ideas about the interference of JAK inhibitors with IFN-mediated antiviral activities. IFNs prohibit viral spreading in the early phase of infections. In animal models of SARS and Middle East respiratory syndrome (MERS), IFN-α and IFN-β showed benefit at the early stage but were harmful at the late phase. Patients with severe SARS who died of hypoxemia revealed high IFN-α, -γ, while those discharged from hospital had low IFN-α, -γ. Therefore, some experts suggested baricitinib’s use in the situation of hyperinflammation and cytokine syndrome, rather than in those with mild diseases.
In fact, clinical trials have commenced to evaluate the optimal timing, duration, and safety of baricitinib in viral infections, including SARS-CoV-2.19–21
Cyclosporine A
Cyclosporine A (CsA) is indicated for rheumatoid arthritis, psoriasis, organ transplants to prevent injection, and keratoconjunctivitis sicca. It is also used in severe atopic dermatitis, chronic urticaria, pyoderma gangrenosum Kimura disease, acute systemic mastocytosis, and ulcerative colitis. CsA inhibits lymphocyte function, mainly T cells, by forming a complex with cyclophilin. Cyclophilin–CsA complex binds on the calcineurin, which blocks the dephosphorylation of nuclear factor of activated T cells (NF-AT). This interferes with entry of NF-AT into the T-cell nucleus and further suppresses cytokine production such as IL-2.
Proposed antiviral mechanisms: Betacoronaviruses, including SARS-CoV-2, replicate in cytosol, where RIG-1 like receptor (RLR) helicases bind on virus RNA and activate mitochondrial antiviral proteins (MAVs). MAVs then promote the production of IFNs and cytokines to defend against viral infections. Thus, mitochondria appear to play a vital role for protection; in other words, mitochondrial failure could lead to severe COVID-19. Experimentally, CsA targets cyclophilin D to inhibit mitochondrial permeability transition pore (MPTP) opening and rescues mitochondria from apoptosis.22–24
Moreover, melanoma-differentiation-activated protein 5 (MDA5), an RLR helicase and putative cytoplasmic receptor of SARS-CoV-2, is also the target antigen of clinically amyopathic dermatomyositis (CADM). Patients with MDA5 plus CADM have higher risks of developing rapidly progressive interstitial lung diseases and respiratory failure, while this could be reversed by calcineurin inhibitors. Based on these hypothetical functions, CsA was proposed as a modulator for cytokine storm syndrome in COVID-19 infections.25
Mycophenolate mofetil and mycophenolic acid
Mycophenolic acid (MPA), an active metabolite of mycophenolate mofetil (MMF), inhibits inosine monophosphate dehydrogenase (IMPDH), an essential enzyme in the de novo purine synthesis pathway. IMPDH inhibition especially influences T and B lymphocytes because they use almost a de novo pathway to synthesize (minimally use a salvage pathway). MMF and MPA are utilized in organ transplantation, Crohn’s disease, and as steroid-sparing agents for conditions such as pemphigus, Behçet’s disease, and lupus erythematosus. Although they were associated with higher risk of opportunistic infections including herpes zoster, cytomegalovirus (CMV), and BK virus (BKV) nephropathy, literature also revealed its possible benefit for HIV and influenza virus.26,27
In vitro: MMF showed low EC50 (0.47 μmol/l) in SARS-CoV-2-infected Vero E6 cells, while the EC50 of remdesivir, as a positive control, was 0.77 μmol/l. Besides, MMF probably inhibited SARS-CoV-2 through IMPDH and especially dihydroorotate dehydrogenase (DHODH). DHODH is another essential enzyme for pyrimidine synthesis, and MMF might control viral infection by depleting the intracellular pyrimidine pools.28
Thalidomide
Thalidomide, a derivative of glutamic acid, is approved for erythema nodosum leprosum and is also used in many conditions such as prurigo nodularis, pyoderma gangrenosum, Bechet’s disease, lupus erythematosus and erythema multiforme. It exerts anti-inflammatory effect through cereblon E3 ubiquitin ligase as the primary target and thus inhibits chemotaxis of leukocytes, monocytes as well as the production of tumor necrosis factor (TNF)-alpha, IL-8, and IL-12.
Case report: A 45-year-old woman with critical symptoms of COVID-19 was treated by thalidomide 100 mg every 24 h. After the first day use of thalidomide, clinical conditions including oxygen index improved. Cytokines such as IL-6, IL-10, IFN-γ all decreased to normal range.15 Proposed mechanisms are as follows: thalidomide inhibits NF-κB, which further suppresses the production of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and IL-8, and prevents the cytokine surge. It also regulates immune function by activating T cells and T-cell receptors. Moreover, the sedative and antiemetic property of thalidomide helps anxious patients calm down, which reduces oxygen consumption.15
Now, at least one clinical trial has been conducted to investigate the efficacy and safety of thalidomide as an adjuvant therapy for COVID-19 pneumonia.29
Part II: DMARDs with antiviral potential other than SARS-CoV-2
Many oral DMARDs have inherent antiviral activity and could be the treatment of choice for patients with coexisting immune-based diseases and infections. Especially when the infection is still in progression, choosing DMARDs with anti-microbial evidence would bring double benefits for better infection control without sacrificing underlying disease management. The antimicrobial mechanisms of DMARDs are often distinct from their immunomodulatory pathway, and the efficacy is different in viral species (Tables 2, 3 and 4).
Table 2.
Suggestions for selecting DMARDs/immunosuppressants when immune-based diseases concomitant with viral infection.
| Drugs Virus |
Chloroquine/HCQ | Baricitinib | CsA | Hydroxyurea | Minocycline | MMF/MPA | Leflunomide | Tofacitinib | Thalidomide |
|---|---|---|---|---|---|---|---|---|---|
| Retrovirus | |||||||||
| HIV | △ | △ | • | • | • | ||||
| HTLV-1 | • Animal | ||||||||
| RNA virus | |||||||||
| SARS-CoV-2 | △ | • | • In vitro | • Case report | |||||
| Influenza | △ | • In vitro | • Animal | ||||||
| Dengue virus | △ | • In vitro | • In vitro | ||||||
| JEV | • | ||||||||
| HCV | • Case report | • | • | ||||||
| RSV | • In vitro | • Animal | |||||||
| DNA virus | |||||||||
| HSV | • In vitro | • Case reports | |||||||
| CMV | • | ||||||||
| HHV-8 | • | ||||||||
| HBV | △ | ||||||||
| BKV | △ | ||||||||
| Parvovirus B19 | • | ||||||||
• Evidence showed positive results.
△ Literature revealed both positive and negative results.
BKV, BK virus; CMV, cytomegalovirus; CsA, cyclosporine A; DMARDs, disease-modifying anti-rheumatic drugs; DNA, deoxyribonucleic acid; GAK, cyclin G-associated kinase; HCQ, hydroxychloroquine; HBV, hepatitis B virus; HCV, hepatitis C virus; HHV-8, human herpesvirus 8; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HTLV-1, human T-cell–lymphotrophic virus-1; IMPDH, inosine monophosphate dehydrogenase; JEV, Japanese encephalitis virus; MDA5, melanoma differentiation activated protein 5; MMF/MPA, mycophenolate mofetil/mycophenolic acid; MPTP, mitochondrial permeability transition pore opening; RCT, randomized-controlled trial; RNA, ribonucleic acid; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 3.
Mechanisms regarding antiviral activities of DMARDs and immunosuppressants.
| Medications | Viral susceptibility | Proposed antiviral mechanisms |
|---|---|---|
|
Chloroquine
HCQ |
SARS-CoV, HIV, dengue virus, chikungunya virus, influenza A virus, HCV, Zika virus | (1) Increase endosomal pH required for virus/cell fusion (2) Interfere with the glycosylation of cellular receptors |
| Cyclosporine A | HIV | Inhibit cyclophilins to incorporate into new virion, which is essential for virus infectivity |
| HCV genotype 1 | Inhibit host cyclophilins to form replication complex with NS5A/B of HCV, and influence protein folding and trafficking | |
| Flavivirus (Zika virus, dengue virus, West Nile virus, yellow fever virus) | Block the interaction between host cyclophilins and flaviviral NS5 protein | |
| Betaretrovirus | Interrupt life cycle from: (1) viral protein synthesis (2) gag and envelope assembly (3) particle budding |
|
| Hydroxyurea | HIV | (1) Inhibit DNA synthesis, slowing production of viral DNA (2) Deplete dNTP pools, which increase competitive ability of NRTIs to incorporate into HIV-1 DNA chain (3) Enhance NRTI phosphorylation, reducing resistance to NRTIs (4) Reduce cellular division of CD4+ T lymphocytes |
| HCV | Inhibit HCV RNA replication | |
| HBV | Unknown, inhibit HBV replication | |
| HSV | Inhibit HSV DNA replication | |
| Parvovirus B19 | Unknown | |
| Minocycline | HIV | (1) High affinity to HIV integrase and interaction with HIV integrase suppress the virus (2) Decrease viral expression from CD4+ T cells |
| Japanese encephalitis virus | Inhibit microglial activation and neuronal apoptosis | |
| Dengue virus | Reduce viral RNA synthesis, intracellular envelope protein expression, and the production of infectious virions | |
| RSV | (1) Reduce RSV-mediated cytopathic effects (2) Prevent RSV infection by affecting RSV F protein production or maturation |
|
| Enterovirus 71 | Reduce cytopathic effects and viral protein expressions | |
| Influenza virus | Reverse H7N9 replication | |
| West Nile virus | Anti-apoptotic properties result in neuroprotection. | |
| Reovirus | Reduce apoptosis and antigen expression | |
| Rabies | Reduce CD3+ cells may impair the host to control disease | |
| Mycophenolate mofetil/mycophenolic acid | HIV | Inhibit the dividing CD4+ T cells, and hence cytostatic and antiviral effect by depletion of this substrate |
| Influenza virus | Inhibit viral mRNA and protein expression via inhibition of cellular IMPDH | |
| MERS-CoV | Unknown | |
| Leflunomide | HSV, HIV, molluscum and verruca, CMV, BKV, RSV | Inhibit nucleocapsid tegumentation and thus prevents virion assembling |
| Tofacitinib | HTLV-1 | HTLV-1-induced ATLL is associated with JAK3 mutations; tofacitinib inhibits JAK3 |
| Thalidomide | HHV-8 | Unknown, suspect anti-angiogenesis and make immune system able to trigger antiviral response |
AAK1, AP2-associated protein kinase 1; ATLL, adult T-cell lymphoma/leukemia; BKV, BK virus; CMV, cytomegalovirus; DENV, dengue virus; DMARDs, disease-modifying anti-rheumatic drugs; DNA, deoxyribonucleic acid; dTNP, deoxynucleoside triphosphate; GAK, cyclin G-associated kinase; HAART, highly active antiretroviral therapy; HBV, hepatitis B virus; HCQ, hydroxychloroquine; HHV-8, human herpesvirus 8; HIV, human immunodeficiency virus; HTLV-I, human T-cell lymphotrophic virus-1; IMPDH, inosine monophosphate dehydrogenase; JAK, Janus kinase; MERS-CoV, Middle East respiratory syndrome coronavirus; mRNA, messenger RNA; NRTI, nucleoside analog reverse-transcriptase inhibitor; RCT, randomized-controlled trial; RNA, ribonucleic acid; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 4.
Clinical studies regarding antiviral activities of DMARDs and immunosuppressants.
| Medications | Viral susceptibility | In vitro | Animal study | Clinical report |
|---|---|---|---|---|
|
Chloroquine
HCQ |
SARS-CoV | Freund et al.,30 Keyaerts et al.,31 Vincent et al.32 | ||
| HIV | ✓ Savarino et al.,33 Boelaert et al.34 | ✓ RCT (n = 40): moderate efficacy35
✓ Cohort (n = 287): decreased HIV vertical transmission36 ✓ RCT (n = 12): reduced T-cell immune activation; no effect on viral load37 ✓ Prospective (n = 20): significant reduction immune activation38 ✗ RCT (n = 83): no efficacy39 ✗ Single-arm, pilot (n = 20): not beneficial40 |
||
| Dengue virus | ✓ Farias et al.41 | ✓ Farias et al.42 | ✓ RCT (n = 37): improve dengue-related symptoms43
✗ RCT (n = 307): reduced fever period; no efficacy44 |
|
| Chikungunya virus | ✓ Kaur and Chu45 | ✓ Open pilot study (n = 10): 50% improved clinically46
✗ RCT (n = 54): no significant decrease in viremia47 |
||
| Influenza A virus | ✓ Fedson48 | ✓ Yan et al.49 | ✓ Prospective study (n = 555): beneficial50
✗ RCT (n = 1496): no preventive effect51 |
|
| HCV | ✓ Ashfaq et al.52 | ✓ n = 1: 42 years, male with liver transplantation; PCT improved53 | ||
| Zika virus | ✓ Li et al.54 | ✓ Li et al.54 | ||
| Cyclosporine A | HIV | ✓ Thali et al.55 | ✓ Retrospective study (n = 27): effective56
✗ RCT (n = 28): no benefits under low-dose CsA57 |
|
| HCV genotype 1 | ✓ Liu et al.,58 Watashi et al.59 | ✓ Placebo-controlled trial (n = 120): increased sustained virological response60 | ||
| Flavivirus (Zika virus, Dengue virus, West Nile virus, Yellow fever virus) | ✓ Qing et al.,61 Barrows et al.62 | |||
| Betaretrovirus | ✓ Montano-Loza et al.63 | |||
| Hydroxyurea | HIV | ✓ Lori and Lisziewicz64 | ✓ RCT (n = 57): greater decrease in viral load65
✓ RCT (n = 144): greater decrease of HIV RNA levels66 ✓ RCT (n = 69): higher rate reached HIV RNA level <200 and <2067 ✓ RCT (n = 134): greater decrease of HIV RNA levels68 ✓ RCT (n = 21): HIV RNA significant decrease, but no added benefit compared with placebo active comparator69 |
|
| HCV | ✓ Nozaki et al.70 | ✓ phase I (n = 9): 8 people achieved moderate decrease in serum HCV RNA levels71 | ||
| HBV | ✓ n = 4: significant decrease of viral loads in all patients72
✗ n = 1, HBV reactivation73 |
|||
| HSV | ✓ Rosenkranz and Becker,74 Sergerie and Boivin75 | |||
| Parvovirus B19 | ✓ Bonvicini et al.76 | ✓ Retrospective review (n = 120): require fewer transfusions; higher nadir Hb concentration77 | ||
| Minocycline | HIV | ✓ Si et al.78 | ✓ Less degeneration of axons and less CNS replication of virus 79 | ✗ RCT (n = 107): no difference in cognitive function80
✗ RCT (n = 73): no difference in cognitive function81 |
| Japanese encephalitis virus | ✓ Mishra and Basu82 | ✓ Reduce viral titers, neuronal apoptosis83 | ✓ RCT (n = 44), 1–13 years: duration of fever, unconsciousness, and hospital stay significantly reduced; mortality rate unchanged84
✓ RCT (n = 281): a trend towards better outcomes85 |
|
| Dengue virus | ✓ Leela et al.86 | |||
| RSV | ✓ Bawage et al.87 | |||
| Enterovirus 71 | ✓ Liao et al.88 | ✓ Decrease mortality rates, clinical scores, viral titers68 | ||
| Influenza virus | ✓ Josset et al.89 | |||
| West Nile virus | ✓ Michaelis et al.90 | |||
| Reovirus | ✓ Delay encephalitis onset; reduce mortality91 | |||
| Rabies | ✗ Worse, higher mortality rate92 | |||
| Mycophenolate mofetil/Mycophenolic acid | HIV | ✓ Chapuis et al.93 | ✓ Inhibition of virus isolation from purified CD4+ T-cell populations.93 | ✓ RCT (n = 17): combine MMF and HAART delayed viral load rebound and improved control of viral replication26 |
| Influenza virus | ✓ To et al.27 | ✓ All mice survived; viral titers in lungs markedly reduced94 | ||
| MERS-CoV | ✓ Chan et al.95 | ✗ Severe and fatal disease; higher viral loads than the untreated animals96 | ||
| Leflunomide | HSV | ✓ Knight et al.97 | ✓ n = 1: 42 years, male with HIV98
✓ n = 1: 52 years, male with HIV99 |
|
| HIV | ✓ Hossain and Margolis,100 Schlapfer et al.101 | ✓ RCT (n = 18): stable CD4+ T-cell counts102 | ||
| Molluscum and verruca | ✓ n = 3 with AD103
✓ n = 3 with renal transplantation104 |
|||
| CMV | ✓ Chacko and John105 | ✓ Chong et al.106 | ✓ Review (n = 45): viremia clearance in 73% of patients107 | |
| BK virus | ✓ Phase II RCT (n = 46): viremia decreased, no improvement of renal function108 | |||
| RSV | ✓ Dunn et al.109 | ✓ Viral loads reduced109 | ||
| Tofacitinib | HTLV-1 | ✓ Prolong survival duration110 | ||
| Thalidomide | HHV-8 | ✓ Phase II (n = 17): 35% partial response rate111
✓ Phase II (n = 20): 40% partial responses and 10% stable disease112 |
AD, atopic dermatitis; CMV, cytomegalovirus; CsA, cyclosporine A; DENV, dengue virus; DMARDs, disease-modifying anti-rheumatic drugs; HAART, highly active antiretroviral therapy; Hb, hemoglobin; HBV, hepatitis B virus; HCQ, hydroxychloroquine; HCV, hepatitis C virus; HHV-8, human herpesvirus 8; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HTLV-I, human T cell lymphotrophic virus-1; MERS-CoV, Middle East respiratory syndrome coronavirus; MMF, mycophenolate mofetil; PCT, porphyria cutanea tarda; RCT, randomized-controlled trial; RSV, respiratory syncytial virus; RNA, ribonucleic acid; SARS-CoV, severe acute respiratory syndrome coronavirus.
Leflunomide
Leflunomide is approved for rheumatoid arthritis and psoriatic arthritis (not in the United States). Leflunomide inhibits the synthesis of pyrimidine via acting on the mitochondrial enzyme DHODH; therefore, rapidly dividing cells, especially lymphocytes, are suppressed. On the other hand, leflunomide showed antiviral activity at least for CMV, BKV, and HIV. It works by teriflunomide, the active metabolite of leflunomide, which disrupts nucleocapsid tegumentation, and thus prevents virion assembling, rather than influences the de novo pyrimidine synthesis pathway.97,100,101,105
Herpes simplex virus
A case report with perianal HSV-2 lesions in an acyclovir-resistant HIV patient significantly improved with leflunomide 40 mg, twice a day.98 Another HIV patient with HSV-1/HSV-2 pseudo-tumors on the perineum and scrotum only slightly improved with valacyclovir, foscarnet, and imiquimod. After 9 months of leflunomide, complete regression of the lesions was noted. Leflunomide has both immunomodulation and antiviral activities in the HSV pseudotumors because pseudotumor formation is an immune reconstruction phenomenon in HIV patients.99
Human immunodeficiency virus
An RCT (n = 18) demonstrated leflunomide decreased the activation and cycling of CD4+ T cells. The expression of HIV co-receptors CCR5 and CXCR4 was also reduced compared with placebo.102
Molluscum and verruca
Three patients with atopic dermatitis treated with azathioprine developed multiple verrucae and molluscum contagiosum. Due to treatment resistance, azathioprine was switched to leflunomide (100 mg loading 3 days, then 20 mg/day). All the lesions subsided in three patients within 2 months of leflunomide treatment.103
Multiple recalcitrant verrucae in three and molluscum in one of renal allograft recipients cleared after switching from MMF to leflunomide.104
Leflunomide can serve as a potential option for patients with skin warts or molluscum concomitant with immune conditions that require immunosuppressants.
Cytomegalovirus
A review article collected 45 transplant recipients with CMV infection treated by leflunomide. Among them, the plasma CMV viral load became undetectable in 33 patients (73%). Most of the patients had ganciclovir-resistance mutation.107
A prospective study evaluated 17 renal transplant recipients. A loading dose of 100 mg for the first 3 days and then 20 mg per day was given. The result showed 15 patients (88%) responded to leflunomide with involved organ healing and viremia clearance.113
Leflunomide was regarded as an add-on treatment for multi-drug-resistance CMV infection. In vitro anti-CMV properties of leflunomide were not through blocking the replication of viral DNA, so it is effective even in patients with direct antiviral drug-resistance history.105,106
BK virus
BK polyomavirus is widespread (80% of the population has the latent form), and often causes mild diseases except in immunocompromised patients, especially kidney-transplant recipients. The virus disseminates to the urinary-tract system and lives there persistently. A sudden increase of BK-virus-associated nephropathy is related to the administration of potent immunosuppressants such as MMF and tacrolimus. Leflunomide is now generally accepted as a second choice after reduction of immunosuppressive agents. However, in a phase II RCT (n = 46), viremia was decreased in the group of leflunomide active metabolites, but no significant improvement of renal function was noted.108
Respiratory syncytial virus (RSV)
Treatment options for RSV are limited to supportive care or ribavirin with only marginal effectiveness. Leflunomide showed a potent, dose-dependent anti-RSV activity in cell cultures.92 Also, pulmonary viral loads were prominently reduced in cotton rats, even if there was a 3-day delay of leflunomide administration after viral inoculation.109
Leflunomide offers a dual benefit of both viral-load reduction and anti-inflammatory effects that attenuate the destruction of cytokine-related diseases.
Tofacitinib
Tofacitinib, a JAK1 and JAK3 inhibitor, is indicated in rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis. It is also used off label for vitiligo and alopecia areata. Tofacitinib treats inflammatory diseases by interfering with the activation of the JAK/signal transducers and activators of transcription (STAT) pathway, which inhibits gene transcription, and cytokine production is thereby reduced.
Human T-cell lymphotrophic virus-1 (HTLV-1)
HTLV-1, a retrovirus, has been linked to diseases such as adult T-cell lymphoma/leukemia (ATLL), HTLV-1-associated myelopathy (HAM), and uveitis. Ex vivo and animal studies revealed positive results of tofacitinib for ATLL and HAM.110 HTLV-1-encoded tax protein activates IL-2, -9, -15, which further trigger JAK3-STAT5 pathway. Accumulating data demonstrated a major role of JAK3 in the pathophysiology of ATLL.114 As a result, tofacitinib targeting JAK3 has been suggested as a therapeutic strategy in future studies.
Hydroxyurea
Hydroxyurea, a deoxyribonucleic acid (DNA)-synthesis inhibitor, belongs to the antineoplastic medications. However, it may be used as a second-line drug for psoriasis and palmoplantar pustulosis115 based on the ability to slow down the rapid division of keratinocytes. Bone marrow suppression is the major and common adverse effect of hydroxyurea.
Human immunodeficiency virus
Hydroxyurea demonstrated promising results in reducing HIV RNA viral loads in five placebo-controlled clinical trials. Among all the trials, hydroxyurea was combined with didanosine, a nucleoside analog reverse-transcriptase inhibitor (NRTI). However, one should be reminded that decreased CD4 counts were noted in some studies. Therefore, close follow up of hematologic change is required in daily practice.65–69
In vitro studies demonstrated the antiviral modes of hydroxyurea. First, hydroxyurea depletes deoxynucleoside triphosphate (dNTP) pools, which impedes DNA synthesis and in turn slows down the production of viral DNA. Second, hydroxyurea enhances NRTI phosphorylation and reduces resistance to NRTIs. This may partially explain the benefits of adding hydroxyurea to NRTI for viral control. Finally, cytotoxic effect of hydroxyurea makes cellular division of CD4+ T cells decline. This enables hydroxyurea to block HIV proliferation, because HIV could only replicate in dividing CD4+ T cells.64
Hepatitis C virus, hepatitis B virus, herpes simplex virus (HSV), parvovirus B19 (B19V)
Although the mode of action of hydroxyurea for HCV, HBV, HSV, and B19V is unknown, viral replications were inhibited by hydroxyurea in in vitro studies.70,74–76 Small-scaled clinical trials showed significant reduction of HCV RNA levels and HBV viral loads in chronic HCV and HBV carriers, respectively.71,72 However, there is a case report of an elderly patient with essential thrombocythemia experiencing reactivation of HBV during treatment with hydroxyurea.73 A retrospective review of children with sickle cell anemia demonstrated decreased requirement of blood transfusion and attenuation of clinical symptoms when using hydroxyurea in patients with B19V infection.77
Minocycline
Minocycline, a second-generation of tetracyclines, is frequently used for bacterial infections, acne, and rheumatoid arthritis. The small size and lipophilic nature facilitate its penetration into blood–brain barrier easily. The neuroprotection and anti-inflammation effects116,117 brought interest in the treatment of virus-induced encephalitis such as HIV, Japanese encephalitis virus (JEV), and reovirus. The antiviral property is not clearly known but seems to be diverse, including neuroprotective, antiapoptotic, interference of viral protein expression, and anti-inflammatory effects.
Human immunodeficiency virus
In microglial cell culture, minocycline reduced viral replication by 71–96%.78 In vivo, macaque monkeys treated with minocycline showed less destruction of axons and less replication of viruses in the central nervous system. The experiment suggested that the antiviral effect of minocycline was through reducing the activation of monocytes and hence, viral replication was blocked.79 Nevertheless, two double-blind, randomized, placebo-controlled human studies revealed that under minocycline 100 mg twice daily, there was no difference in cognitive function compared with placebo.80,81
Japanese encephalitis virus
Minocycline showed high efficacy in animal models and in vitro studies for the treatment of JEV.82,83 A double-blind, RCT allocated 44 pediatric patients into a minocycline group or placebo group. The results demonstrated minocycline significantly reduced the duration of unconsciousness, fever, and hospital-stay days, while neurologic deficits and mortality rate were unchanged.84 Another RCT (n = 281) revealed that minocycline led to a trend of better outcomes, especially those who survived the first hospital day.85
Dengue virus, respiratory syncytial virus, enterovirus 71, influenza virus, West Nile virus, reovirus, rabies
Minocycline showed antiviral activity in a broad spectrum of other viruses, but most of the evidence was limited to animal or in vitro studies.86–91 On the contrary, an animal experiment with rabies virus infection treated by minocycline caused the exacerbation of encephalomyelitis and higher mortality rate.92
Chloroquine and hydroxychloroquine
Severe acute respiratory syndrome coronavirus (SARS-CoV)
CQ and HCQ are reported to have strong antiviral property for SARS-CoV in vitro. Similar to COVID-19, CQ interferes with glycosylation of cellular receptor ACE2 of SARS-CoV.30 Interestingly, the inhibitory effect was noted before and after viral entry, which means CQ may offer benefit for both prophylaxis and treatment.31,32
Human immunodeficiency virus (HIV), dengue virus, chikungunya virus, influenza A virus, hepatitis C virus (HCV)
Some in vitro studies and clinical trials investigated the efficacy of CQ or HCQ for different viruses, especially HIV.33,34 Four clinical trials showed positive results of HIV control, either in the reduction of immune activation or lowering the vertical transmission.35–38 However, another two trials (one RCT, one single-arm) revealed no efficacy.39,40 As for dengue,41–44 chikungunya,45–47 influenza A viruses,48–51 and HCV,52,53 paradoxical outcomes were found in the literature. Therefore, the utilization of CQ in these viral diseases still needs further investigation.
Zika virus
The major concern of Zika virus infection is that it can transmit from placenta to fetus and cause microcephaly or congenital defects. CQ prevented Zika virus internalization in cell cultures and reduced morbidity or mortality in mice. In addition, it prevented fetal mice from microcephaly.54 Therefore, CQ might be a potential treatment waiting for clinical verification.
HCQ had been reported to downregulate the expression of IFN genes and reduce the production of type I IFNs. This phenomenon was noted in vitro118 and in human studies of autoimmune diseases.119 Since IFNs are crucial in innate immunity to defend viral infections, the usage of HCQ may raise concerns about the counter effects in viral control. Nevertheless, opposite results were also presented: HCQ activated IFN-β signaling pathways in cell studies of dengue virus.120 Furthermore, blocking type I IFN receptors attenuated the efficacy of HCQ in the treatment of dengue-virus-infected cells.120 On the other hand, a case-control study revealed patients with lupus erythematosus were 16 times less likely to develop serious infections if taking antimalarials.121 Another retrospective study showed significantly lower infection rate in patients with lupus nephritis and exposure to antimalarials.122 HCQ is generally thought of as having protective effect for viral infection,123 but their relationship with IFNs still needs further investigation.
Cyclosporine A
Human immunodeficiency virus
In vitro cyclophilin A interacts with the gag protein of HIV and its incorporation into a new virion plays an essential role for virus infectivity. CsA binds to cyclophilin A and inhibits virus replication by interfering with the incorporation.55
A retrospective study included 27 HIV patients with CD4 T-cell counts 300–600/µl. After a median exposure time of 11 months on CsA (7.5 mg/kg per day), stable CD4 numbers were demonstrated, and none of the patients progressed to AIDS. Conversely, CD4 counts declined at a rate of 5 cells/mm3 per year after cessation of CsA.56 Nevertheless, an RCT (n = 28) allocated HIV patients with CD4 numbers greater than 500/µl into a CsA 2 mg/kg per day group, or placebo group. The results demonstrated that CD4 numbers did not increase under low-dose CsA, but HIV RNA levels raised slightly (that was statistically significant) instead.57
Hepatitis C virus
A controlled trial enrolled 120 chronic HCV carriers into two groups: IFN-α alone or a combination of IFN and CsA. The combination group significantly increased the sustained virological response rate with similar safety profiles in the two groups. The benefit was especially marked in patients with HCV genotype 1 and high viral loads.60
NS5A and NS5B are two important non-structural proteins that several direct-acting antiviral agents target. Cyclophilin A and B bind to NS5A and NS5B of HCV to form a replication complex. This complex modulates folding and trafficking of HCV proteins. Thus, CsA influences HCV replication by inhibiting cyclophilins’ function.58,59
Flavivirus
Flavivirus is an arthropod virus transmitted by infected arthropods such as mosquito or tick. In vitro, CsA showed efficacy against several viruses belonging to this genus (dengue virus, West Nile virus, yellow fever virus and Zika virus). The efficacy of CsA is through the inhibition of cyclophilins that interact with the NS5 protein of flavivirus to facilitate protein folding.61,62
Betaretrovirus
Betaretrovirus is regarded as one of the environmental factors triggering the recurrence of primary biliary cirrhosis (PBC) after liver transplantation. Earlier and more severe recurrence of PBC occurred with tacrolimus compared with CsA as an immunosuppressant. This may be partially explained by the antiviral activity of CsA. According to an in vitro study, it was suggested that CsA interrupted viral replication through inhibiting viral protein synthesis, gag and envelope assembly, and particle budding.63
Mycophenolate mofetil and mycophenolic acid
Human immunodeficiency virus
The combination of MMF and highly active antiretroviral therapy improved the control of viral replication and delayed viral-load rebound in a randomized pilot study (n = 17).26 In vitro and in vivo studies showed cytostatic and antiviral effect by depletion of the dividing CD4+ T cells.93
Influenza virus
MMF had antiviral activity for influenza A(H1N1), A(H3N2), A(H7N9), and B viruses in many in vitro studies.27 The effect of MMF on influenza virus was through the inhibition of viral messenger RNA and protein expression by inhibiting the cellular IMPDH. Besides, MMF markedly reduced viral loads in lungs and all mice survived.94
Middle East respiratory syndrome coronavirus (MERS-CoV)
MERS-CoV causes respiratory infection called MERS or camel flu. Mortality rate is around 33%, and there is no specific treatment or vaccine for the disease till now. MMF is one of the retrieved medications investigated for its antiviral efficacy for MERS. Although good inhibitory effects were noted in in vitro studies,95 all marmosets treated with MMF experienced severe and fatal disease.96
Thalidomide
Human herpesvirus 8 (HHV-8)
HHV-8 is the cause of Kaposi’s sarcoma (KS). Case reports showed encouraging experience of thalidomide in all types of KS (classic, iatrogenic, and HIV related).124 Two phase II clinical trials investigating HIV-related KS revealed 35% and 40% of partial responders. Serum titers of HHV-8 were decreased in all patients.111,112
The effectiveness of thalidomide for KS might be related to anti-angiogenesis, and experts hypothesized the modulation of the immune system to trigger an antiviral action.
Conclusion
The treatment of immune-based diseases has been revolutionized by the introduction of target therapy, mainly biologics. Compared with biologics, conventional synthetic DMARDs exert broad-spectrum functionality. DMARDs work through immunosuppressive and anti-inflammatory effects with the possibility of higher infection risk. However, many none-biologic DMARDs demonstrate antiviral activities instead. Although in most instances, the antiviral activity of DMARDs is based on in vitro or small-scale controlled studies, this property would be useful in the choice of DMARDs for patients with concomitant viral infections. Also, the combinational use of antiviral drugs and DMARDs has been shown to be more effective and less resistant in the control of some viral infections. Furthermore, in the face of novel viral infection, such as SARS-CoV-2, screening of existing chemicals, including DMARDs, may prove to be fruitful.
Supplemental Material
Supplemental material, Contributors_form for Oral disease-modifying antirheumatic drugs and immunosuppressants with antiviral potential, including SARS-CoV-2 infection: a review by Y. C. Tsai and T. F. Tsai in Therapeutic Advances in Musculoskeletal Disease
Footnotes
Conflict of interest statement: Dr Tsen-Fang Tsai has conducted clinical trials or received honoraria for serving as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, EliLilly, Galderma, GSK-Stiefel, Janssen-Cilag, Leo-Pharma, Merck, Novartis, Pfizer Inc., and UCB Pharma. Dr Ya-Chu Tsai has delivered speeches held by AbbVie, EliLilly, Janssen-Cilag, Leo-Pharma, Novartis, Pfizer.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: YC Tsai  https://orcid.org/0000-0002-0853-8636
https://orcid.org/0000-0002-0853-8636
Contributor Information
Y. C. Tsai, Department of Dermatology, Far Eastern Memorial Hospital, New Taipei city, Taiwan
T. F. Tsai, Department of Dermatology, National Taiwan University Hospital and National Taiwan University College of Medicine, No. 7, Zhongshan S. Rd, Zhongzheng District, Taipei City 100, Taiwan.
References
- 1. Fujiwara K, Yasui S, Yonemitsu Y, et al. Efficacy of combination therapy of antiviral and immunosuppressive drugs for the treatment of severe acute exacerbation of chronic hepatitis B. J Gastroenterol 2008; 43: 711–719. [DOI] [PubMed] [Google Scholar]
- 2. Potter CW, Phair JP, Vodinelich L, et al. Antiviral, immunosuppressive and antitumour effects of ribavirin. Nature 1976; 259: 496–497. [DOI] [PubMed] [Google Scholar]
- 3. Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. Epub ahead of print 27 February 2020. DOI: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect 2017; 5: e00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30: 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020; 14: 72–73. [DOI] [PubMed] [Google Scholar]
- 7. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Epub ahead of print 20 March 2020. DOI: 1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen J, Liu D, Liu L, et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020; 49: 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gautret P, Lagier JC, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis 2020; 34: 101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. Epub ahead of print 2020. DOI: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 11. Huang M, Tang T, Pang P, et al. Treating COVID-19 with chloroquine. J Mol Cell Biol 2020; 12: 322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020; 369: m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Justin S, Venkatesh K, De Bono S, et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med. Epub ahead of print 15 April 2020. DOI: 10.21203/rs.3.rs-23195/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cantini F, Niccoli L, Matarrese D, et al. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. Epub ahead of print 23 April 2020. DOI: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen C, Qi F, Shi K, et al. Thalidomide combined with low-dose short-term glucocorticoid in the treatment of critical Coronvirus Disease 2019. Clin Transl Med. Epub ahead of print 4 June 2020. DOI: 10.1002/ctm2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020; 395: e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanchez GAM, Reinhardt A, Ramsey S, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest 2018; 128: 3041–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Favalli EG, Biggioggero M, Maioli G, et al. Baricitinib for COVID-19: a suitable treatment? Lancet Infect Dis. Epub ahead of print 3 April 2020. DOI: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Praveen D, Puvvada RC, Vijey Aanandhi M. Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19. Int J Antimicrob Agents 2020; 55: 105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richardson PJ, Corbellino M, Stebbing J. Baricitinib for COVID-19: a suitable treatment?—Authors’ reply. Lancet Infect Dis. Epub ahead of print 3 April 2020. DOI: 10.1016/S1473-3099(20)30270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanchez-Pernaute O, Romero-Bueno FI, Selva-O’Callaghan A. Why choose cyclosporin A as first-line therapy in COVID-19 pneumonia. Reumatol Clin. Epub ahead of print 16 April 2020. DOI: 10.1016/j.reuma.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lebedev I, Nemajerova A, Foda ZH, et al. A novel in vitro CypD-mediated p53 aggregation assay suggests a model for mitochondrial permeability transition by chaperone systems. J Mol Biol. 2016; 428: 4154–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baines CP, Kaiser RA, Purcell NH, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005; 434: 658–662. [DOI] [PubMed] [Google Scholar]
- 25. Go DJ, Park JK, Kang EH, et al. Survival benefit associated with early cyclosporine treatment for dermatomyositis-associated interstitial lung disease. Rheumatol Int 2016; 36: 125–131. [DOI] [PubMed] [Google Scholar]
- 26. García F, Plana M, Arnedo M, et al. Effect of mycophenolate mofetil on immune response and plasma and lymphatic tissue viral load during and after interruption of highly active antiretroviral therapy for patients with chronic HIV infection: a randomized pilot study. J Acquir Immune Defic Syndr 2004; 36: 823–830. [DOI] [PubMed] [Google Scholar]
- 27. To KK, Mok KY, Chan AS, et al. Mycophenolic acid, an immunomodulator, has potent and broad-spectrum in vitro antiviral activity against pandemic, seasonal and avian influenza viruses affecting humans. J Gen Virol 2016; 97: 1807–1817. [DOI] [PubMed] [Google Scholar]
- 28. He Y, Pei R, Xu Z, et al. Mycophenolate mofetil is active against SARS-CoV-2 in vero E6 cells. Preprints 2020, 2020040380. [Google Scholar]
- 29. US National Library of Medicine. The efficacy and safety of thalidomide in the adjuvant treatment of moderate new coronavirus (COVID-19) pneumonia. ClinicalTrials.gov identifier: NCT04273529, https://clinicaltrials.gov/ct2/show/NCT04273529.
- 30. Freund NT, Roitburd-Berman A, Sui J, et al. Reconstitution of the receptor-binding motif of the SARS coronavirus. Protein Eng Des Sel 2015; 28: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keyaerts E, Vijgen L, Maes P, et al. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun 2004; 323: 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005; 2: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Savarino A, Gennero L, Chen HC, et al. Anti-HIV effects of chloroquine: mechanisms of inhibition and spectrum of activity. AIDS 2001; 15: 2221–2229. [DOI] [PubMed] [Google Scholar]
- 34. Boelaert JR, Sperber K, Piette J. The additive in vitro anti-HIV-1 effect of chloroquine, when combined with zidovudine and hydroxyurea. Biochem Pharmacol 2001; 61: 1531–1535. [DOI] [PubMed] [Google Scholar]
- 35. Sperber K, Louie M, Kraus T, et al. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin Ther 1995; 17: 622–636. [DOI] [PubMed] [Google Scholar]
- 36. Neely M, Kalyesubula I, Bagenda D, et al. Effect of chloroquine on human immunodeficiency virus (HIV) vertical transmission. Afr Health Sci 2003; 3: 61–67. [PMC free article] [PubMed] [Google Scholar]
- 37. Murray SM, Down CM, Boulware DR, et al. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol 2010; 84: 12082–12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Piconi S, Parisotto S, Rizzardini G, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood 2011; 118: 3263–3272. [DOI] [PubMed] [Google Scholar]
- 39. Paton NI, Goodall RL, Dunn DT, et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA 2012; 308: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Routy JP, Angel JB, Patel M, et al. Assessment of chloroquine as a modulator of immune activation to improve CD4 recovery in immune nonresponding HIV-infected patients receiving antiretroviral therapy. HIV Med 2015; 16: 48–56. [DOI] [PubMed] [Google Scholar]
- 41. Farias KJ, Machado PR, Da Fonseca BA. Chloroquine inhibits dengue virus type 2 replication in Vero cells but not in C6/36 cells. ScientificWorldJournal 2013; 2013: 282734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farias KJ, Machado PR, Muniz JA, et al. Antiviral activity of chloroquine against dengue virus type 2 replication in Aotus monkeys. Viral Immunol 2015; 28: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borges MC, Castro LA, Fonseca BA. Chloroquine use improves dengue-related symptoms. Mem Inst Oswaldo Cruz 2013; 108: 596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tricou V, Minh NN, Van TP, et al. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis 2010; 4: e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaur P, Chu JJ. Chikungunya virus: an update on antiviral development and challenges. Drug Discov Today 2013; 18: 969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brighton SW. Chloroquine phosphate treatment of chronic chikungunya arthritis. An open pilot study. S Afr Med J 1984; 66: 217–218. [PubMed] [Google Scholar]
- 47. De Lamballerie X, Boisson V, Reynier JC, et al. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis 2008; 8: 837–839. [DOI] [PubMed] [Google Scholar]
- 48. Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis 2008; 8: 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yan Y, Zou Z, Sun Y, et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res 2013; 23: 300–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borba EF, Saad CG, Pasoto SG, et al. Influenza A/H1N1 vaccination of patients with SLE: can antimalarial drugs restore diminished response under immunosuppressive therapy? Rheumatology (Oxford) 2012; 5: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 51. Paton NI, Lee L, Xu Y, et al. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis 2011; 11: 677–683. [DOI] [PubMed] [Google Scholar]
- 52. Ashfaq UA, Javed T, Rehman S, et al. Lysosomotropic agents as HCV entry inhibitors. Virol J 2011; 8: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pellicelli AM, Morrone A, Barbieri L, et al. Porphyria cutanea tarda in an HCV-positive liver transplant patient: a case report. Ann Hepatol 2012; 11: 951–954. [PubMed] [Google Scholar]
- 54. Li C, Zhu X, Ji X, et al. Chloroquine, a FDA-approved drug, prevents zika virus infection and its associated congenital microcephaly in mice. EBioMedicine 2017; 24: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thali M, Bukovsky A, Kondo E, et al. Functional association of cyclophilin A with HIV-1 virions. Nature 1994; 372: 363–365. [DOI] [PubMed] [Google Scholar]
- 56. Levy R, Jais JP, Tourani JM, et al. Long-term follow-up of HIV positive asymptomatic patients having received cyclosporin A. Adv Exp Med Biol 1995; 374: 229–234. [DOI] [PubMed] [Google Scholar]
- 57. Calabrese LH, Lederman MM, Spritzler J, et al. Placebo-controlled trial of cyclosporin-A in HIV-1 disease: implications for solid organ transplantation. J Acquir Immune Defic Syndr 2002; 29: 356–362. [DOI] [PubMed] [Google Scholar]
- 58. Liu Z, Yang F, Robotham JM, et al. Critical role of cyclophilin A and its prolylpeptidyl isomerase activity in the structure and function of the hepatitis C virus replication complex. J Virol 2009; 83: 6554–6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Watashi K, Ishii N, Hijikata M, et al. Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol Cell 2005; 19: 111–122. [DOI] [PubMed] [Google Scholar]
- 60. Inoue K, Sekiyama K, Yamada M, et al. Combined interferon alpha2b and cyclosporin A in the treatment of chronic hepatitis C: controlled trial. J Gastroenterol 2003; 38: 567–572. [DOI] [PubMed] [Google Scholar]
- 61. Qing M, Yang F, Zhang B, et al. Cyclosporine inhibits flavivirus replication through blocking the interaction between host cyclophilins and viral NS5 protein. Antimicrob Agents Chemother 2009; 53: 3226–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barrows NJ, Campos RK, Powell ST, et al. A screen of FDA-approved drugs for inhibitors of zika virus infection. Cell Host Microbe 2016; 20: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Montano-Loza AJ, Wasilenko S, Bintner J, et al. Cyclosporine A inhibits in vitro replication of betaretrovirus associated with primary biliary cirrhosis. Liver Int 2010; 30: 871–877. [DOI] [PubMed] [Google Scholar]
- 64. Lori F, Lisziewicz J. Rationale for the use of hydroxyurea as an anti-human immunodeficiency virus drug. Clin Infect Dis 2000; 30(Suppl. 2): S193–S197. [DOI] [PubMed] [Google Scholar]
- 65. Lori F, Malykh AG, Foli A, et al. Combination of a drug targeting the cell with a drug targeting the virus controls human immunodeficiency virus type 1 resistance. AIDS Res Hum Retroviruses 1997; 13: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 66. Rutschmann OT, Opravil M, Iten A, et al. A placebo-controlled trial of didanosine plus stavudine, with and without hydroxyurea, for HIV infection. The Swiss HIV cohort study. AIDS 1998; 12: F71–F77. [DOI] [PubMed] [Google Scholar]
- 67. Lafeuillade A, Hittinger G, Chadapaud S, et al. The HYDILE trial: efficacy and tolerance of a quadruple combination of reverse transcriptase inhibitors versus the same regimen plus hydroxyurea or hydroxyurea and interleukin-2 in HIV-infected patients failing protease inhibitor-based combinations. HIV Clin Trials 2002; 3: 263–271. [DOI] [PubMed] [Google Scholar]
- 68. Frank I, Bosch RJ, Fiscus S, et al. Activity, safety, and immunological effects of hydroxyurea added to didanosine in antiretroviral-naive and experienced HIV type 1-infected subjects: a randomized, placebo-controlled trial, ACTG 307. AIDS Res Hum Retroviruses 2004; 20: 916–926. [DOI] [PubMed] [Google Scholar]
- 69. Stebbing J, Nelson M, Orkin C, et al. A randomized trial to investigate the recycling of stavudine and didanosine with and without hydroxyurea in salvage therapy (RESTART). J Antimicrob Chemother 2004; 53: 501–505. [DOI] [PubMed] [Google Scholar]
- 70. Nozaki A, Morimoto M, Kondo M, et al. Hydroxyurea as an inhibitor of hepatitis C virus RNA replication. Arch Virol 2010; 155: 601–605. [DOI] [PubMed] [Google Scholar]
- 71. Nozaki A, Numata K, Morimoto M, et al. Hydroxyurea suppresses HCV replication in humans: a phase I trial of oral hydroxyurea in chronic hepatitis C patients. Antivir Ther 2010; 15: 1179–1183. [DOI] [PubMed] [Google Scholar]
- 72. Kashani HH, Vossoughi A, Adibi P, et al. Amazing results with hydroxyurea therapy in chronic hepatitis B: a preliminary report. Hepat Mon 2010; 10: 215–217. [PMC free article] [PubMed] [Google Scholar]
- 73. Watanabe N, Yasuda H, Aota Y, et al. Reactivation of hepatitis B virus during treatment with hydroxyurea in an elderly patient with essential thrombocythemia. Geriatr Gerontol Int 2015; 15: 812–813. [DOI] [PubMed] [Google Scholar]
- 74. Rosenkranz HS, Becker Y. Reversible inhibition of herpes simplex virus replication by hydroxyurea. Antimicrob Agents Chemother 1973; 3: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sergerie Y, Boivin G. Hydroxyurea enhances the activity of acyclovir and cidofovir against herpes simplex virus type 1 resistant strains harboring mutations in the thymidine kinase and/or the DNA polymerase genes. Antiviral Res 2008; 77: 77–80. [DOI] [PubMed] [Google Scholar]
- 76. Bonvicini F, Bua G, Conti I, et al. Hydroxyurea inhibits parvovirus B19 replication in erythroid progenitor cells. Biochem Pharmacol 2017; 136: 32–39. [DOI] [PubMed] [Google Scholar]
- 77. Hankins JS, Penkert RR, Lavoie P, et al. Original research: parvovirus B19 infection in children with sickle cell disease in the hydroxyurea era. Exp Biol Med (Maywood) 2016; 241: 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Si Q, Cosenza M, Kim MO, et al. A novel action of minocycline inhibition of human immunodeficiency virus type 1 infection in microglia. J Neurovirol 2004; 10: 284–292. [DOI] [PubMed] [Google Scholar]
- 79. Zink MC, Uhrlaub J, DeWitt J, et al. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 2005; 293: 2003–2011. [DOI] [PubMed] [Google Scholar]
- 80. Sacktor N, Miyahara S, Deng L, et al. Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology 2011; 77: 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nakasujja N, Miyahara S, Evans S, et al. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology 2013; 80: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mishra MK, Basu A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem 2008; 105: 1582–1595. [DOI] [PubMed] [Google Scholar]
- 83. Dutta K, Kumawat KL, Nazmi A, et al. Minocycline differentially modulates viral infection and persistence in an experimental model of Japanese encephalitis. J Neuroimmune Pharmacol 2010; 5: 553–565. [DOI] [PubMed] [Google Scholar]
- 84. Singh A, Mehta A, Kushwaha KP, et al. Minocycline trial in Japanese encephalitis: a double blind, randomized placebo study. Int J Pediatr Res 2016; 3: 371–377. [Google Scholar]
- 85. Kumar R, Basu A, Sinha S, et al. Role of oral minocycline in acute encephalitis syndrome in India: a randomized controlled trial. BMC Infect Dis 2016; 16: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leela SL, Srisawat C, Sreekanth GP, et al. Drug repurposing of minocycline against dengue virus infection. Biochem Biophys Res Commun 2016; 478: 410–416. [DOI] [PubMed] [Google Scholar]
- 87. Bawage SS, Tiwari PM, Pillai S, et al. Antibiotic minocycline prevents respiratory syncytial virus infection. Viruses 2019; 11: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liao YT, Wang SM, Chen SH. Anti-inflammatory and antiviral effects of minocycline in enterovirus 71 infections. Biomed Pharmacother 2019; 118: 109271. [DOI] [PubMed] [Google Scholar]
- 89. Josset L, Zeng H, Kelly SM, et al. Transcriptomic characterization of the novel avian-origin influenza A (H7N9) virus: specific host response and responses intermediate between avian (H5N1 and H7N7) and human (H3N2) viruses and implications for treatment options. mBio 2014; 5: e01102–e01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Michaelis M, Kleinschmidt MC, Doerr HW, et al. Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J Antimicrob Chemother 2007; 60: 981–986. [DOI] [PubMed] [Google Scholar]
- 91. Richardson-Burns SM, Tyler KL. Minocycline delays disease onset and mortality in reovirus encephalitis. Exp Neurol 2005; 192: 331–339. [DOI] [PubMed] [Google Scholar]
- 92. Jackson AC, Scott CA, Owen J, et al. Therapy with minocycline aggravates experimental rabies in mice. J Virol 2007; 81: 6248–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chapuis AG, Paolo Rizzardi G, D’Agostino C, et al. Effects of mycophenolic acid on human immunodeficiency virus infection in vitro and in vivo. Nat Med 2000; 6: 762–768. [DOI] [PubMed] [Google Scholar]
- 94. Cho J, Yi H, Jang EY, et al. Mycophenolic mofetil, an alternative antiviral and immunomodulator for the highly pathogenic avian influenza H5N1 virus infection. Biochem Biophys Res Commun 2017; 494: 298–304. [DOI] [PubMed] [Google Scholar]
- 95. Chan JF, Chan KH, Kao RY, et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect 2013; 67: 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chan JF, Yao Y, Yeung ML, et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis 2015; 212: 1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Knight DA, Hejmanowski AQ, Dierksheide JE, et al. Inhibition of herpes simplex virus type 1 by the experimental immunosuppressive agent leflunomide. Transplantation 2001; 71: 170–174. [DOI] [PubMed] [Google Scholar]
- 98. Henao-Martínez AF, Weinberg A, Waldman WJ, et al. Successful treatment of acyclovir-resistant herpes simplex virus type 2 proctitis with leflunomide in an HIV-infected man. J Clin Virol 2012; 54: 276–278. [DOI] [PubMed] [Google Scholar]
- 99. Roger MR, Anstead GM. Leflunomide in the treatment of a pseudotumoral genital herpes simplex virus infection in an HIV patient. Case Rep Infect Dis 2017; 2017: 1589356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hossain MM, Margolis DM. Inhibition of HIV replication by A77 1726, the active metabolite of leflunomide, in combination with pyrimidine nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr 2001; 28: 199–201. [DOI] [PubMed] [Google Scholar]
- 101. Schlapfer E, Fischer M, Ott P, et al. Anti-HIV-1 activity of leflunomide: a comparison with mycophenolic acid and hydroxyurea. AIDS 2003; 17: 1613–1620. [DOI] [PubMed] [Google Scholar]
- 102. Read SW, DeGrezia M, Ciccone EJ, et al. The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants. PLoS One 2010; 5: e11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Smith KJ, Germain M. Leflunomide: an immune modulating drug that may have a role in controlling secondary infections with review of its mechanisms of action. J Drugs Dermatol 2015; 14: 230–236. [PubMed] [Google Scholar]
- 104. Nguyen L, McClellan RB, Chaudhuri A, et al. Conversion from tacrolimus/mycophenolic acid to tacrolimus/leflunomide to treat cutaneous warts in a series of four pediatric renal allograft recipients. Transplantation 2012; 94: 450–455. [DOI] [PubMed] [Google Scholar]
- 105. Chacko B, John GT. Leflunomide for cytomegalovirus: bench to bedside. Transpl Infect Dis 2012; 14: 111–120. [DOI] [PubMed] [Google Scholar]
- 106. Chong AS, Zeng H, Knight DA, et al. Concurrent antiviral and immunosuppressive activities of leflunomide in vivo. Am J Transplant 2006; 6: 69–75. [DOI] [PubMed] [Google Scholar]
- 107. Verkaik NJ, Hoek RA, Van Bergeijk H, et al. Leflunomide as part of the treatment for multidrug-resistant cytomegalovirus disease after lung transplantation: case report and review of the literature. Transpl Infect Dis 2013; 15: E243–E249. [DOI] [PubMed] [Google Scholar]
- 108. Guasch A1, Roy-Chaudhury P, Woodle ES, et al. Assessment of efficacy and safety of FK778 in comparison with standard care in renal transplant recipients with untreated BK nephropathy. Transplantation 2010; 90: 891–897. [DOI] [PubMed] [Google Scholar]
- 109. Dunn MC, Knight DA, Waldman WJ. Inhibition of respiratory syncytial virus in vitro and in vivo by the immunosuppressive agent leflunomide. Antivir Ther 2011; 16: 309–317. [DOI] [PubMed] [Google Scholar]
- 110. Ju W, Zhang M, Jiang JK, et al. CP-690,550, a therapeutic agent, inhibits cytokine-mediated Jak3 activation and proliferation of T cells from patients with ATL and HAM/TSP. Blood 2011; 117: 1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fife K, Howard MR, Gracie F, et al. Activity of thalidomide in AIDS-related Kaposi’s sarcoma and correlation with HHV8 titre. Int J STD AIDS 1998; 9: 751–755. [DOI] [PubMed] [Google Scholar]
- 112. Little RF, Wyvill KM, Pluda JM, et al. Activity of thalidomide in AIDS-related Kaposi’s sarcoma. J Clin Oncol 2000; 18: 2593–2602. [DOI] [PubMed] [Google Scholar]
- 113. John GT, Manivannan J, Chandy S, et al. A prospective evaluation of leflunomide therapy for cytomegalovirus disease in renal transplant recipients. Transplant Proc 2005; 37: 4303–4305. [DOI] [PubMed] [Google Scholar]
- 114. Elliott NE, Cleveland SM, Grann V, et al. FERM domain mutations induce gain of function in JAK3 in adult T-cell leukemia/lymphoma. Blood 2011; 118: 3911–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hattel T, Sondergaard J. Pustulosis palmaris et plantaris treated with hydroxyurea. Acta Derm Venereol 1974; 54: 152–154. [PubMed] [Google Scholar]
- 116. Popovic N, Schubart A, Goetz BD, et al. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol 2002; 51: 215–223. [DOI] [PubMed] [Google Scholar]
- 117. Song Y, Wei EQ, Zhang WP, et al. Minocycline protects PC12 cells from ischemic-like injury and inhibits 5-lipoxygenase activation. Neuroreport 2004; 15: 2181–2184. [DOI] [PubMed] [Google Scholar]
- 118. Sacre K, Criswell LA, McCune JM. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther 2012; 14: R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bodewes ILA, Gottenberg JE, van Helden-Meeuwsen CG, et al. Hydroxychloroquine treatment downregulates systemic interferon activation in primary Sjögren’s syndrome in the JOQUER randomized trial. Rheumatology (Oxford) 2020; 59: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang LF, Lin YS, Huang NC, et al. Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery. J Interferon Cytokine Res 2015; 35: 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ruiz-Irastorza G, Olivares N, Ruiz-Arruza I, et al. Predictors of major infections in systemic lupus erythematosus. Arthritis Res Ther 2009; 11: R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sisó A, Ramos-Casals M, Bové A, et al. Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus 2008; 17: 281–288. [DOI] [PubMed] [Google Scholar]
- 123. Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus 2013; 22: 1286–1294. [DOI] [PubMed] [Google Scholar]
- 124. Rubegni P, Sbano P, De Aloe G, et al. Thalidomide in the treatment of Kaposi’s sarcoma. Dermatology 2007; 215: 240–244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Contributors_form for Oral disease-modifying antirheumatic drugs and immunosuppressants with antiviral potential, including SARS-CoV-2 infection: a review by Y. C. Tsai and T. F. Tsai in Therapeutic Advances in Musculoskeletal Disease