Abstract
Background
Despite the recent increase in the number of publications on diagnostic cerebral angiograms using transradial access (TRA), there have been relatively few regarding TRA for neurointerventional cases. Questions of feasibility and safety may still exist among physicians considering TRA for neurointerventional procedures.
Methods
A systematic literature review was performed following PRISMA guidelines. Three online databases (MedLine via PubMed, Scopus and Embase) were searched for articles published between January 2000 and December 2019. Search terms included “Transradial access”, “Radial Access”, “Radial artery” AND “Neurointerventions". The reference lists of selected articles and pertinent available non-systematic analysis were reviewed for other potential citations. Primary outcomes measured were access site complications and crossover rates.
Results
Twenty-one studies (n=1342 patients) were included in this review. Two of the studies were prospective while the remaining 19 were retrospective. Six studies (n=616 patients) included TRA carotid stenting only. The rest of the studies included treatment for cerebral aneurysms (n=423), mechanical thrombectomy (n=127), tumor embolization (n=22), and other indications (n=154) such as angioplasty and stenting for vertebrobasilar stenosis, balloon test occlusion, embolization of dural arteriovenous fistula and arteriovenous malformation, chemotherapeutic drug delivery, intra-arterial thrombolysis, and arterial access during a venous stenting procedure. Two (0.15%) major complications and 37 (2.75%) minor complications were reported. Sixty-four (4.77%) patients crossed over to transfemoral access for completion of the procedure. Seven (0.52%) patients crossed over due to access failure and 57 (4.24%) patients crossed over to TFA due to inability to cannulate the target vessel.
Conclusion
This systematic review demonstrates that TRA has a relatively low rate of access site complications and crossovers. With increasing familiarity, development of TRA-specific neuroendovascular devices, and the continued reports of its success in the literature, TRA is expected to become more widely used by neurointerventionalists.
Keywords: complication, device, intervention, technique, angiography
Introduction
Transradial access (TRA) has several distinct advantages over traditional transfemoral access (TFA). More than 10 years of experience reported by interventional cardiologists has demonstrated a reduction in the incidence of hemorrhagic access site complications with TRA compared with TFA.1–3 When hemorrhagic events arise, they are typically more easily managed given the radial artery’s superficial location and ease of compressibility. Additionally, post-procedure bedrest is not required, facilitating early ambulation and discharge following procedures not requiring hospital admission.4–6
Despite the widespread adoption and preference of TRA among cardiac interventionalists, neurointerventionalists have been slow to adopt this approach. There are 24 randomized controlled trials comparing TRA with TFA in the cardiac intervention literature compared with none in neurointervention. The overall superiority of TRA over TFA, especially in terms of access site complications, suggests that TRA for neurointervention should be explored in greater detail. We therefore sought to perform a systematic review to summarize the feasibility, complications, and crossover rates of TRA in various neurointerventional procedures.
Materials and methods
Data sources and searches
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed and the protocol was submitted to PROSPERO. Three online databases (MedLine via PubMed, Scopus and Embase) were searched using filters for English language articles published between 1 January 2000 and 31 December 2019. Medical subject headings and keyword searches included the terms “Transradial access”, “Radial Access”, “Radial Artery” AND “Neurointerventions”. In addition, the reference lists of selected articles and pertinent available non-systematic analysis were reviewed for other potential citations. Data from unpublished sources were not searched or included.
Study selection and data extraction
Two investigators (KCJ and ABF) conducted independent literature searches and data extractions using a standardized approach. Selected publications on TRA for neurointervention were reviewed by the same investigators to assess if the studies met the inclusion criteria: (1) studies with ≥5 patients; (2) studies involving any kind of neurointerventions including (but not limited to) aneurysm coiling, thrombectomies for acute ischemic stroke, and carotid artery stent (CAS). Studies which were (1) technical reports and case reports, (2) series with only diagnostic angiograms, and (3) cadaver and animal series were excluded. Studies with only distal radial access (dTRA), left radial access, or those describing repeat radial access were excluded. Two reviewers (KCJ and ABF) extracted information from articles including year of publication, indications for procedure, sample size, complications related to access, and crossover rates.
Data analysis and synthesis
Demographic and procedural data were analyzed from these papers and a systematic analysis was performed. Primary outcomes analyzed were complication rates, crossover to transfemoral rate, and procedural success rates. Secondary outcomes were to measure the procedural success rate with relation to the indications. Technical nuances with regard to indications, techniques used to improve puncture and access, size and characteristics of devices used, techniques used for hemostasis, and device selection for particular indications were noted. Heterogeneity testing was performed using Cochrane Q statistics to calculate I2 percentages; ≥50% would indicate statistically significant heterogeneity. Bias risk assessment was assessed using funnel plots.
Results
Search results
The initial search collectively resulted in 90 papers, of which 45 came from the MeSH PubMed search, 38 from Scopus and 7 from Embase, and an additional 15 articles came from a bibliographic search of the other articles. After removing duplicates, 74 studies remained. Through title and abstract review we narrowed the search to 37 articles and excluded 37 articles, which were assessed using the previously mentioned selection criteria. An additional 16 articles were excluded after full text review resulting in 21 articles that fit our inclusion standards. Of note, one patient who underwent arterial access using TRA for a case of venous stenting was included due to its inclusion in the source studies. The results of our literature screening are summarized in figure 1.
Figure 1.
PRISMA diagram summarizing the systematic process used to identify, screen, and include articles analyzed for this review.
Included studies
Twenty-one studies (n=1342) were included in this review. Baseline demographics were described in 14 studies (n=742), the median age was 66.21 years (range 48.5–86 years), and 315 (42.40%) were women. Six studies (616 patients) included TRA for CAS only. The remaining studies (n=726) included a variety of other neurointerventional procedures conducted through TRA. There were 423 aneurysms treated with TRA. Two studies (n=57) included patients treated with flow diverters (FD) for unruptured aneurysms. One hundred and twenty-seven patients were treated for acute ischemic stroke with mechanical thrombectomy (MT). The remaining cases included tumor embolization (n=22), treatment of vasospasm (n=26), angioplasty and stenting for vertebrobasilar stenosis (n=69), intracranial carotid angioplasty (n=4), balloon test occlusion (n=8), embolization of dural arteriovenous fistula (n=4), embolization of arteriovenous malformation (n=24), chemotherapeutic drug delivery (n=10), thrombolysis (n=5), vertebral artery sacrifice (n=1), middle meningeal artery embolization (n=2), and arterial access during a venous stenting procedure (n=1). Figure 2 shows the distribution of various indications of neurointerventional procedures performed by TRA. The side of lesion was mentioned in 692 cases: 391 (56.50%) interventions were performed on the right side, 300 (43.35%) were performed on the left side, and one was bilateral (0.14%). The location of the lesion was described as an anterior or posterior circulation lesion in 955 cases, of which 816 (85.44%) were located in the anterior circulation and 121 (14.55%) were located in the posterior circulation. The studies included are described in table 1.7–27
Figure 2.
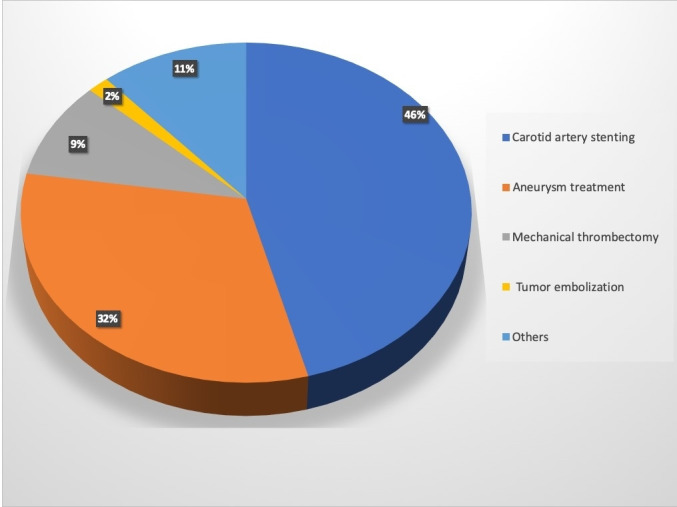
Pie chart demonstrating the various indications for neurointervention using the transradial access.
Table 1.
Brief description of the design and results of 21 eligible studies included in the systematic analysis
| Sample size | Indications | Laterality | Anterior/posterior | Crossover to TFA | Complications | |
| Almallouhi et al 7 | 19 | Aneurysm embolization (ruptured (n=3) and unruptured (n=8)), tumor embolization (n=2), CAS (n=2), balloon occlusion test (n=1), vertebral artery sacrifice (n=1), and AVM embolization (n=2) | Right 14 Left 4 Bilateral 1 |
17/2 | None | Minor complications 7, major complications 0 |
| Chen et al 8 | 49 | Flow diversion for aneurysms | Right 17 Left 32 |
37/12 | 2 patients due to radial artery spasm, 8 patients due to tortuosity of aorta | No complications reported |
| Chen et al 9 | 18 | Challenging vascular anatomy for mechanical thrombectomy of anterior circulation | N/A | 18/0 | None | No complications reported |
| Eskioglu et al 10 | 8 | Aneurysms (n=5), basilar stenosis (n=1), dural AV fistula (n=1), high flow AVM (n=1) | N/A | 1/7 | None | No complications reported |
| Gao et al 11 | 58 | Severe intracranial atherosclerotic vertebrobasilar stenosis. Of the 58 patients, 19 (32.8%) used the transradial approach due to poor iliofemoral artery access, 28 (48.3%) due to unfavorable brachiocephalic or subclavian artery anatomy, 11 (19%) due to unfavorable vertebral artery anatomy | N/A | 0/58 | None | 4 periprocedural minor complications of which one was asymptomatic |
| Goland et al 12 | 40 | Flow diverters (n=5) and coil embolization (n=35). Seven of these aneurysms were asymptomatic, whereas 33 had already ruptured | Right 24 Left 16 |
39/1 | None | No complications reported |
| Hanaoka et al 13 | 20 | CAS (n=11) and coil embolization of cerebral aneurysms (n=9) | N/A | 20/0 | None | One patient had asymptomatic RAO |
| Lee et al 14 | 30 | Balloon angioplasty and/or stent placement (n=18), aneurysm treatment (n=6), tumor embolization (n=3), mechanical thrombectomy (n=2), embolization of DAVF (n=1) | Right 26 Left 4 |
13/17 | None | 2 cases had minor puncture site hematoma |
| Lee et al 15 | 38 | 38 patients with documented internal carotid artery stenosis were selected for CAS via a sheathless TRA and compared with 61 patients who received CAS via the brachial artery: overall 99 patients | N/A | 38/0 | None | 1 patient in TRA group had TIA, no access site complications |
| Maud et al 16 | 10 | Mechanical thrombectomy for posterior circulation strokes | Right 9 Left 1 |
0/10 | None | No complications reported |
| Mendiz 17 | 79 | All patients underwent CAS, 46 patients were symptomatic and 34 were asymptomatic | Right 47 Left 41 Bilateral 1 |
79/0 | In 1 patient whounderwent ipsilateral TRA-CAS, right carotid artery had a steep angulation, with sheath kinking and stent delivery system fracture during withdrawal afterstent deployment. Sheath and stent delivery systems were completely removed andexchanged for a regular 6F hydrophilic radial sheath over the 0.014 wire, keeping distal protection filter in position. Balloon postdilatation wasthen performed and filter successfully removed with no guiding catheter or longsheath support and exchanged for a diagnostic catheter for final angiographicimaging. | There were no deaths, myocardial infarction, or radial access site complications. In all, 2 patients sustained a stroke, 1 hemorrhage, and 1 ischemia |
| Folmar et al 18 | 42 | CAS for stenosis greater than 80% and comorbid conditions increasing the risk of CEA | Right 29 Left 13 |
42/0 | 7 patients crossed over to TFA | 1 patient had a minor site-related complication |
| Ruzsa et al 19 | 130 | The clinical and angiographic outcomes of 265 consecutive patients with high risk for CEA treated by CAS with cerebral protection were evaluated in a prospective randomized multicenter study between 2010 and 2012. 130 of these patients underwent CAS through a TRA | N/A | 130/0 | 2 patients due to failure to access radial artery and 11 due to inability to engage the target artery | 1 patient with a known history of Buerger’s disease had a major access site-related complication. The patient had a symptomatic RAO. Minor access site complications occurred in 9 patients (7%) in the TRA group. The cause of minor vascular complications was small forearm hematoma in 1 patient (0.8%), and asymptomatic RAO in 8 patients (6.8%) |
| Montorosi et al 20 | 214 | 214 patients had CAS procedure with either Mo.MA proximal protection (n=61) or distal filter protection (n=153) | Right 112 Left 102 |
214/0 | 12 patients crossed over to TFA due to failure to engage the target vessel | Chronic RAO was detected by Doppler ultrasound in 2/30 (6.6%) Mo.MA patients and in 4/124 (3.2%) filter patients by clinical assessment (p=0.25) at 8.1±7.5 month follow-up |
| Pinter et al 21 | 20 | All patients underwent CAS, 7 patients were symptomatic and 13 were asymptomatic | Right 12 Left 8 |
20/0 | Procedural success was achieved in 18 patients (90%). Intense radial artery vasospasm resulted in one failure, and the second failure occurred in a patient with a left-sided carotid lesion and type I arch | The 30-day incidence of stroke, TIA, myocardial infarction, and death was 0%. RAO only occurred in the one patient because of the development of intense vasospasm during the procedure. One patient had persistent local pain requiring intravenous medication for relief |
| Snelling et al 22 | 105 | Mechanical thrombectomy (n=29), intracranial aneurysm treatments (n=33), and interventions such as angioplasty, balloon test occlusion, chemotherapy delivery, and thrombolysis (n=33) | Right 63 Left 42 |
81/24 | 2 patients developed radial artery spasm following sheath placement recalcitrant to antispasmodic medications, resulting in crossover to TFA. No occlusion, hand ischemia, or other sequelae were seen in these patients. 1 patient crossed over due to aortic arch tortuosity | Minor access site complications were seen in 2.85% (3/105) of patients. One patient had RAO on post-procedure testing following use of a 0.088 inch sheathless guide catheter (NeuronMax, Penumbra), despite anti-spasmolytics and patent hemostasis. However, no hand ischemia was seen. The patient eventually failed TFA due to significant aortic arch tortuosity |
| Sur et al 23 | 11 | 11 patients were identified who underwent a TRA for mechanical thrombectomy for anterior circulation occlusions | Right 7 Left 4 |
11/0 | None | No complications reported |
| Crockett et al 24 | 403 | 163 intracranial aneurysm treatments, 125 stroke interventions, 55 internal carotid artery stents, 26 vasospasm, 11 intracranial stenting/ angioplasty, 13 DAVF and AVM, 4 VA stent, 4 head and neck tumors, 2 MMA embolizations | N/A | N/A | None | 2 cases with RAO were reported, 1 following 6Fr sheath insertion and 1 following 8Fr sheath insertion. Both occlusions were asymptomatic, were identified on clinical examination and confirmed on ultrasound. 1 spontaneously recanalized after 36 hours |
| Chivot et al 26 | 64 | 62 patients with 64 aneurysms treated with TRA, 33 were treated on an emergency basis for a ruptured aneurysm and 29 underwent scheduled embolization for an unruptured aneurysm. Two patients had a second embolization after recanalization: One procedure was performed with coils and the other with flow diverters | Right 31 Left 33 |
56/8 | 2 patients had crossover to TFA, 1 due to the angle of origin ofthe left common carotid artery and the other due to subclavian occlusion | No complications reported |
| Catapano et al 25 | 58 | Retrospective chart review comparing standard TFA approach with TRA, with the primary outcome of complications analyzed via a propensity-adjusted analysis. 35 aneurysms treated, 9 thrombectomy, thrombolysis, CAS, or stent for stenosis/stroke, 12 embolizations other than aneurysms, 2 other treatments | N/A | N/A | 1 patient crossed over to TFA | 1 major access site complication (thromboembolic event) and 3 minor (forearm hematomas) were noted |
| Sweid Ahmad et al 27 | 18 | Retrospective analysis of aneurysms treated with flow diverters from 2010 to 2019. Also performed a logistic regression analysis to compare outcomes of aneurysms treated by TRA compared with TFA | N/A | N/A | 1 patient crossed over to TFA due to need for more support | No complications reported |
AV, arteriovenous; AVM, arteriovenous malformation; CAS, carotid artery stenting; RAO, radial artery occlusion; TFA, transfemoral approach; TIA, transient ischemic attack; TRA, transradial approach.
The Cochrane I2 test revealed no significant heterogeneity in studies reporting major complications (I2=20.7%); however, there was significant heterogeneity in studies reporting minor complications (I2=79.8%) and crossovers (I2=62.5%). Publication bias could not be calculated as there was significant heterogeneity in reported outcomes and no statistical effect size in most of the studies.
Complications
The studies were reviewed for access site complications, which were classified into minor (asymptomatic and found on routine follow-up or minimally symptomatic, which did not require readmission and intervention) and major (which were symptomatic and required further intervention such as blood transfusion or surgical intervention).
Two (0.15%) major complications were reported: one patient with Buerger’s disease had an acute symptomatic radial artery occlusion (RAO) and one patient had a large hematoma requiring blood transfusion.
There were 37 (2.75%) minor complications reported. Among the minor complication group, asymptomatic RAO was detected either clinically (through the absence of a palpable pulse) or on doppler in follow-up in 27 (72.97%) patients, severe radial artery spasm and pain in 4 (10.81 %) patients, and small forearm hematoma in 6 (16.21%) patients.
Crossovers
Sixty-four (4.77%) patients crossed over to TFA for completion of the procedure. Among the crossover group, seven (10.93%) patients crossed over due to failure to obtain radial artery access. In all of these cases intractable severe radial artery spasm was noted. Fifty-seven (89.06%) patients crossed over to TFA due to inability to catheterize the target vessel. The anatomical constraints impeding catherization of the target vessel were mentioned in 13 cases in which crossover to TFA was necessary; in eight cases aortic arch configuration precluded catheterization and in five cases acute angulation of the origin of the internal carotid artery (ICA) from the arch was noted. The results of the review are shown in table 2.
Table 2.
Results from systematic analysis of 21 studies on transradial approach for neurointervention
| Study variables | |
| Number of studies | 21 |
| Total number of patients | 1342 |
| Number of female patients* | 315/742 (42.4%) |
| Median age in years (range) | 66.2 (48.5–86) |
| Indication | |
| Aneurysm treatment | 423 |
| Mechanical thrombectomy | 127 |
| Carotid artery stenting | 616 |
| Tumor embolization | 22 |
| Others (vertebrobasilar angioplasty and stenting, embolization of arteriovenous malformation, dural arteriovenous fistula and tumor, balloon test occlusion) | 154 |
| Left-sided lesions † | 391/692 (56.5%) |
| Anterior lesions‡ | 816/955 (85.4 %) |
| Complications | |
| Major | 2 (0.15%) |
| Minor | 37 (2.75%) |
| Crossovers to transfemoral approach | |
| Secondary to access site issues | 7 (0.52%) |
| Difficult to engage in target vessel | 57 (4.24%) |
*Gender was reported only in 660 patients of the total 842 patients.
†Side of the lesion was reported only in 692 patients of the total 1342 patients.
‡Location of lesion was described only in 955 patients of the 1424 patients.
Discussion
Our results
There is extensive evidence in the interventional cardiology literature supporting the safety and efficacy of TRA over TFA.1–3 Only recently has TRA begun to be increasingly considered as a reasonable alternative to the more familiar TFA among neuroendovascular surgeons. However, its utility often has been mostly restricted to diagnostic cerebral angiography.28–30 Potential concerns with TRA for neurointerventional procedures include lack of familiarity, concern for placing larger access catheters in the radial artery, and a lack of devices with dimensions and specifications designed specifically for TRA.
We performed a systematic review of the literature on the use of TRA for various neurointerventional procedures. We found a total of 21 articles that qualified for our inclusion criteria and a total of 1342 patients treated through TRA. We found that TRA has been used for a wide variety of neurointerventional procedures, including the treatment of aneurysms, arteriovenous malformations, dural arteriovenous fistulas, carotid artery stenosis, intracranial stenosis, MT, and tumor embolization.
In the 1342 cases included in this review there were two (0.15%) major complications and 37 (2.75%) minor complications reported. In 64 cases (4.77%) crossover to TFA was required for completion of the procedure. Seven (0.52%) patients crossed over due to inability to access the radial artery and 57 (4.24%) patients crossed over due to the inability to catheterize the target vessel. Of the 423 aneurysms treated, 20 (4.73%) required crossover to TFA for completion of the procedure and, of the 616 CAS performed through TRA, 35 (5.68%) required crossover to TFA for completion of the procedure.
TRA for carotid artery stenting (CAS)
Recent studies have shown a high procedural success rate for CAS using TRA.31 32 A recent meta-analysis of seven CAS studies performed via TRA showed a pooled procedural successful outcome rate of 90.8% (657/723; 95% CI 86.7% to 94.2%).33 The mean procedural time pooled across five studies33 was 40.5±7.0 min compared with 54±18 min reported in a previous TFA study.34 The procedural success rates were higher for patients with type III or bovine arch morphology. As most neuroendovascular surgeons have experienced, these arch configurations pose particular technical challenges for the procedure using TFA. The technical success rate of TRA CAS is certainly lower than that reported in large TFA studies. A retrospective analysis of a large Japanese registry with 8458 eligible patients revealed a procedural success rate of 99.5%.35 Conversely, Gao et al included only patients with type III arch morphology and showed a 100% success rate in these patients with TRA compared with 90% using TFA.11 These findings suggest that TRA may have anatomical advantages in patients with type III and bovine arch morphology.
All studies included in our review used embolic protection devices for the CAS. Montorsi et al compared the feasibility and safety of using a proximal protection device (Mo.Ma) compared with a distal filter device in CAS.20 Crossover to TFA was required in 1/61 (1.6%) Mo.MA patients compared with 11/153 (7.1%) filter patients mainly due to technical difficulty in engaging the target vessel. They did not report any major vascular complications in the TRA group. Overall, CAS using TRA has comparable results to those done using TFA and can be considered especially in patients with type III or bovine arch morphology.
TRA for aneurysm treatment
In this review there were a total of 423 aneurysms treated. Reporting of rupture status at the time of presentation was done in only 260 cases, of which 95 were ruptured and 165 were unruptured. Chen et al 8 reported a series of 49 patients who underwent FD of unruptured intracranial aneurysms. Of the 49 patients, 39 underwent successful FD stent placement through TRA. In the 39 patients undergoing FD placement through TRA, 20 did so with the use of a triaxial system while 9 did so using a biaxial system. Ten patients were converted to TFA after failed attempts using TRA. There were no procedural complications. The reasons for failure included tortuosity of the left ICA or acute angulation of the left ICA origin at the aortic arch in eight patients and severe radial artery spasm resulting in inability to obtain radial access in two patients.
FD may pose unique challenges for TRA, particularly when it is deemed necessary to use a guide catheter larger than 6Fr. Increasing sheath diameters and smaller radial artery diameters (<2.5 mm) are shown to have higher rates of RAO.36 The routine confirmation of radial artery diameter >2.5 mm prior to sheath placement has been advocated by some authors.37 When larger guide catheters are required (eg, 0.88 inch outer diameter), it can be used alone, without placement of a sheath. Peterson et al 9 22 have described various combinations of catheters to form triaxial and quadriaxial systems in order to obtain adequate support to perform FD. These results suggest that the desire to use a triaxial system or a large guide catheter (ie, one that is too large for a 6Fr sheath) should not preclude TRA for the treatment of cerebral aneurysms.
TRA for mechanical thrombectomy (MT)
The decreased risk of access site hemorrhagic complications with TRA over TFA may be particularly relevant for MT, as intravenous thrombolysis is often administered concurrently. A recent report by Chen et al found no difference in the single-pass recanalization rate (54.5% vs 55.6%, p=0.949) and the average number of passes (1.9 vs 1.7, p=0.453) in patients undergoing MT via TFA or TRA, respectively.9 Additionally, there were no significant differences in mean access to reperfusion time (61.9 vs 61.1 min, p=0.920), successful revascularization rates (Thrombolysis in Cerebral Infarction score ≥2b 87.9% vs 88.9%, p=1.0), and functional outcomes (modified Rankin Scale score ≤2, 39.4% vs 33.3%, p=0.669) between TFA and TRA cohorts, respectively. TRA may be particularly well suited for stroke patients whose aortic arch anatomy would present a challenge using TFA (eg, type III or bovine aortic arch configurations).
Distal TRA (snuff box approach)
Distal TRA (dTRA) or the snuff box approach is a modification of TRA that has been adopted recently by neurointerventionalists. Cited advantages of dTRA include a reported lower rate of RAO; therefore, it may be especially useful when repeated endovascular procedures are anticipated. Additionally, it permits ergonomic access to the left radial artery—the physician can remain on the right side of the patient with the patient’s left arm resting across their abdomen without uncomfortable supination. The site of the arteriotomy in dTRA is distal to the origin of the superficial palmar arch; this is felt to reduce the risk of ischemic symptoms in the case of arterial occlusion at the access site.38 A recent paper comparing TRA (n=117) with dTRA (n=89) performed for both diagnostic and interventional procedures found no significant differences in access site crossovers or complication rates between the two approaches.25
Procedural failure and crossovers
The overall procedural success rate of TRA was 95.23%; there were 64 (4.77%) patients who crossed over to TFA. Of these, seven (10.93%) patients crossed over due to severe radial artery spasm resulting in the inability to obtain access. Fifty-seven patients (89.06%) crossed over due to the inability to catheterize the target vessel using TRA. However, in only 13 of these cases were the specific challenges discussed: in eight cases aortic arch configuration precluded catheterization and in five cases acute angulation of the ICA origin from the aortic arch was noted.
In a large meta-analysis of 23 randomized trials with 7020 patients comparing TRA versus TFA in patients undergoing coronary angiography or intervention, the crossover rate and procedural failure rate in the TRA group was 5.9% and 4.7%, respectively.1 Compared with cardiac interventions, TRA for neurointerventions has the additional challenge of navigating through tortuous arch into the carotid arteries, which often needs the use of reverse angle catheters and a theoretically higher risk of procedural failures.
Complications
Multiple prospective studies have shown that TRA is associated with a significant reduction in access site complications compared with TFA.1 3 5 Asymptomatic RAO is the most common complication reported after TRA. Previous studies in the interventional cardiology literature report rates of asymptomatic RAO between 1% and 10%.5 39 40 Proposed mechanisms for RAO include endothelial injury of the radial artery combined with decreased blood flow after sheath and catheter insertion.41 42 Post-procedural radial artery stenosis, which may further increase the risk of RAO, has been shown to occur within 2 days after TRA in 31% of patients while late development of RAO is reported to occur in up to 28%.43 In this review we found 27 (1.88%) patients who developed RAO. There was only one patient, with a history of Buerger’s disease, who developed severe symptomatic RAO.19 The relatively low rate of symptomatic RAO may, at least in part, be due to the robust collateral supply of the ulnar artery to the forearm and hand.
One of the primary benefits of TRA over TFA is the significant reduction in hemorrhagic access site complications. In a meta-analysis of 76 studies (15 randomized, 61 observational) involving a total of 761, 919 patients comparing TRA with TFA, TRA was associated with a 78% reduction in bleeding and 80% reduction in the need for blood transfusions.44 In our review, hemorrhagic access site complications were found to occur in six patients (0.47%), all of them being minor forearm hematomas and none requiring any transfusions or additional procedures. This demonstrates a significant reduction in hemorrhagic access site complications compared with studies in which TFA was used for neuroendovascular interventions where rates of hemorrhagic complications were reported to be as high as 7.9%.45
Limitations of study
There are several important limitations to the present study. Most importantly, there was significant heterogeneity in reporting primary outcomes (eg, procedural success) and secondary outcomes (eg, complication and crossover rates) among the various studies. The total number of studies included was small (n=21), with wide variation in sample size (n=8–214). Additionally, the results of this review are subject to significant selection and methodology bias, as some studies included only patients undergoing a specific procedure (eg, only CAS or FD). No study included in this review was a randomized controlled study.
Most of the studies included in this review are from institutions with experience in performing TRA for neuroendovascular interventions. Therefore, the results of this review to institutions beginning a transition to TRA from TFA may not capture the learning curve that inevitably occurs when implementing new techniques. Prospective randomized studies with larger sample size are needed objectively to assess the benefits of TRA over the traditional TFA.
Conclusion
In this review we show that TRA has a high rate of procedural success (95.23%) with a low complication rates (2.90%) when used for various neuroendovascular interventional procedures. These results provide some support that TRA may be a viable option beyond just diagnostic cerebral angiography. TRA may actually have advantages for neuroendovascular interventions, as many of these patients require platelet inhibition for concomitant cardiac disease or for their neurointerventional procedure (ie, intracranial stents), are on anticoagulants for coexisting conditions (ie, atrial fibrillation, deep venous thrombosis), or have received intravenous thrombolysis (ie, in the setting of acute ischemic stroke). While TRA is still undergoing growing adoption in the neuroendovascular field, increasing familiarity with the technique, its safety advantages, and the development of TRA-specific neuroendovascular devices are likely to underscore a trend that finds increasing use among neurointerventionalists.
Acknowledgments
We would like to thank Bichun Ouyang PhD for her help in statistical analysis.
Footnotes
Twitter: @dr_mchen
Contributors: All authors have made substantial contributions to the conception or design of the work or the acquisition, analysis or interpretation of data. They were involved in drafting the work or revising it critically for important intellectual content. All authors gave final approval of the version published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Jolly SS, Amlani S, Hamon M, et al. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J 2009;157:132–40. 10.1016/j.ahj.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 2. Ferrante G, Rao SV, Jüni P, et al. Radial versus femoral access for coronary interventions across the entire spectrum of patients with coronary artery disease: a meta-analysis of randomized trials. JACC Cardiovasc Interv 2016;9:1419–34. 10.1016/j.jcin.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 3. Feldman DN, Swaminathan RV, Kaltenbach LA, et al. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the National Cardiovascular Data Registry (2007-2012). Circulation 2013;127:2295–306. 10.1161/CIRCULATIONAHA.112.000536 [DOI] [PubMed] [Google Scholar]
- 4. Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J 1999;138:430–6. 10.1016/S0002-8703(99)70143-2 [DOI] [PubMed] [Google Scholar]
- 5. Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. The Lancet 2011;377:1409–20. 10.1016/S0140-6736(11)60404-2 [DOI] [PubMed] [Google Scholar]
- 6. Agostoni P, Biondi-Zoccai GGL, De Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: systematic overview and meta-analysis of randomized trials. ACC Current J Rev 2004;13:33–56. 10.1016/j.accreview.2004.08.091 [DOI] [PubMed] [Google Scholar]
- 7. Almallouhi E, Leary J, Wessell J, et al. Fast-Track incorporation of the transradial approach in endovascular neurointervention. J Neurointerv Surg 2020;12:176-180 10.1136/neurintsurg-2019-015127 [DOI] [PubMed] [Google Scholar]
- 8. Chen SH, Snelling BM, Shah SS, et al. Transradial approach for flow diversion treatment of cerebral aneurysms: a multicenter study. J Neurointerv Surg 2019;11:796–800. 10.1136/neurintsurg-2018-014620 [DOI] [PubMed] [Google Scholar]
- 9. Chen SH, Snelling BM, Sur S, et al. Transradial versus transfemoral access for anterior circulation mechanical thrombectomy: comparison of technical and clinical outcomes. J Neurointerv Surg 2019;11:874–8. 10.1136/neurintsurg-2018-014485 [DOI] [PubMed] [Google Scholar]
- 10. Eskioglu E, Burry MV, Mericle RA. Transradial approach for neuroendovascular surgery of intracranial vascular lesions. J Neurosurg 2004;101:767–9. 10.3171/jns.2004.101.5.0767 [DOI] [PubMed] [Google Scholar]
- 11. Gao F, Lo WJ, Sun X, et al. Selective use of transradial access for endovascular treatment of severe intracranial vertebrobasilar artery stenosis. Clin Neurol Neurosurg 2015;134:116–21. 10.1016/j.clineuro.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 12. Goland J, Doroszuk GF, Garbugino SL, et al. Transradial approach to treating endovascular cerebral aneurysms: case series and technical note. Surg Neurol Int 2017;8:73 10.4103/sni.sni_393_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanaoka Y, Koyama J-ichi, Nagm A, et al. Transradial neuroendovascular treatment for anterior circulation lesions: an initial experience with a 6 Fr guiding sheath. J Endovasc Ther 2018;12:532–41. 10.5797/jnet.oa.2018-0055 [DOI] [Google Scholar]
- 14. Lee DG, Lee DH, Shim JH, et al. Feasibility of the transradial or the transbrachial approach in various neurointerventional procedures. Neurointervention 2015;10:74–81. 10.5469/neuroint.2015.10.2.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee W-C, Fang H-Y, Chen H-C, et al. Comparison of a sheathless transradial access with looping technique and transbrachial access for carotid artery stenting. J Endovasc Ther 2016;23:516–20. 10.1177/1526602816640291 [DOI] [PubMed] [Google Scholar]
- 16. Maud A, Khatri R, Chaudhry MRA, et al. Transradial access results in faster skin puncture to reperfusion time than transfemoral access in posterior circulation mechanical thrombectomy. J Vasc Interv Neurol 2019;10:53–7. [PMC free article] [PubMed] [Google Scholar]
- 17. Mendiz OA, Sampaolesi AH, Londero HF, et al. Initial experience with transradial access for carotid artery stenting. Vasc Endovascular Surg 2011;45:499–503. 10.1177/1538574411405547 [DOI] [PubMed] [Google Scholar]
- 18. Folmar J, Sachar R, Mann T. Transradial approach for carotid artery stenting: a feasibility study. Catheter Cardiovasc Interv 2007;69:355–61. 10.1002/ccd.21049 [DOI] [PubMed] [Google Scholar]
- 19. Ruzsa Z, Nemes B, Pintér L, et al. A randomised comparison of transradial and transfemoral approach for carotid artery stenting: RADCAR (radial access for carotid artery stenting) study. EuroIntervention 2014;10:381–91. 10.4244/EIJV10I3A64 [DOI] [PubMed] [Google Scholar]
- 20. Montorsi P, Galli S, Ravagnani PM, et al. Carotid artery stenting with proximal embolic protection via a transradial or transbrachial approach: pushing the boundaries of the technique while maintaining safety and efficacy. J Endovasc Ther 2016;23:549–60. 10.1177/1526602816651424 [DOI] [PubMed] [Google Scholar]
- 21. Pinter L, Cagiannos C, Ruzsa Z, et al. Report on initial experience with transradial access for carotid artery stenting. J Vasc Surg 2007;45:1136–41. 10.1016/j.jvs.2007.02.035 [DOI] [PubMed] [Google Scholar]
- 22. Snelling BM, Sur S, Shah SS, et al. Transradial approach for complex anterior and posterior circulation interventions: technical nuances and feasibility of using current devices. Operative Neurosurgery 2019;17:293–302. 10.1093/ons/opy352 [DOI] [PubMed] [Google Scholar]
- 23. Sur S, Snelling B, Khandelwal P, et al. Transradial approach for mechanical thrombectomy in anterior circulation large-vessel occlusion. Neurosurg Focus 2017;42:E13 10.3171/2017.1.FOCUS16525 [DOI] [PubMed] [Google Scholar]
- 24. Crockett MT, Selkirk GD, Chiu AH, et al. Arterial access site complications in transradial neurointerventions: single center review of 750 consecutive cases. Clin Neuroradiol 2019. 10.1007/s00062-019-00866-1. [Epub ahead of print: 12 Dec 2019]. [DOI] [PubMed] [Google Scholar]
- 25. Catapano JS, Fredrickson VL, Fujii T, et al. Complications of femoral versus radial access in neuroendovascular procedures with propensity adjustment. J Neurointerv Surg 2019:neurintsurg-2019-015569 10.1136/neurintsurg-2019-015569 [DOI] [PubMed] [Google Scholar]
- 26. Chivot C, Bouzerar R, Yzet T. Transitioning to transradial access for cerebral aneurysm embolization. AJNR Am J Neuroradiol 2019;40:1947–53. 10.3174/ajnr.A6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sweid A, Starke RM, Herial N, et al. Transradial approach for the treatment of brain aneurysms using flow diversion: feasibility, safety, and outcomes. J Neurosurg Sci 2019;63:509–17. 10.23736/S0390-5616.19.04761-1 [DOI] [PubMed] [Google Scholar]
- 28. Levy EI, Boulos AS, Fessler RD, et al. Transradial cerebral angiography: an alternative route. Neurosurgery 2002;51:335–42. 10.1097/00006123-200208000-00007 [DOI] [PubMed] [Google Scholar]
- 29. Matsumoto Y, Hokama M, Nagashima H, et al. Transradial approach for selective cerebral angiography: technical note. Neurol Res 2000;22:605–8. 10.1080/01616412.2000.11740727 [DOI] [PubMed] [Google Scholar]
- 30. Matsumoto Y, Hongo K, Toriyama T, et al. Transradial approach for diagnostic selective cerebral angiography: results of a consecutive series of 166 cases. AJNR Am J Neuroradiol 2001;22:704–8. [PMC free article] [PubMed] [Google Scholar]
- 31. Snelling BM, Sur S, Shah SS, et al. Transradial cerebral angiography: techniques and outcomes. J Neurointerv Surg 2018;10:874–81. 10.1136/neurintsurg-2017-013584 [DOI] [PubMed] [Google Scholar]
- 32. Zussman BM, Tonetti DA, Stone J, et al. A prospective study of the transradial approach for diagnostic cerebral arteriography. J Neurointerv Surg 2019;11:1045–9. 10.1136/neurintsurg-2018-014686 [DOI] [PubMed] [Google Scholar]
- 33. Jaroenngarmsamer T, Bhatia KD, Kortman H, et al. Procedural success with radial access for carotid artery stenting: systematic review and meta-analysis. J Neurointerv Surg 2020;12:1378 10.1016/j.jvs.2019.07.013 [DOI] [PubMed] [Google Scholar]
- 34. Swerdlow NJ, Jones DW, Pothof AB, et al. Three-dimensional image fusion is associated with lower radiation exposure and shorter time to carotid cannulation during carotid artery stenting. J Vasc Surg 2019;69:1111–20. 10.1016/j.jvs.2018.07.038 [DOI] [PubMed] [Google Scholar]
- 35. Tokuda R, Yoshimura S, Uchida K, et al. Real-world experience of carotid artery stenting in Japan: analysis of 8458 cases from the JR-NET3 nationwide retrospective multi-center registries. Neurol Med Chir 2019;59:117–25. 10.2176/nmc.st.2018-0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rashid M, Kwok CS, Pancholy S, et al. Radial artery occlusion after transradial interventions: a systematic review and meta-analysis. J Am Heart Assoc 2016;5:e002686 10.1161/JAHA.115.002686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kherad B, Köhncke C, Spillmann F, et al. Postprocedural radial artery occlusion rate using a sheathless guiding catheter for left ventricular endomyocardial biopsy performed by transradial approach. BMC Cardiovasc Disord 2016;16:253–53. 10.1186/s12872-016-0432-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brunet M-C, Chen SH, Sur S, et al. Distal transradial access in the anatomical snuffbox for diagnostic cerebral angiography. J Neurointerv Surg 2019;11:710–3. 10.1136/neurintsurg-2019-014718 [DOI] [PubMed] [Google Scholar]
- 39. Agostoni P, Biondi-Zoccai GGL, de Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol 2004;44:349–56. 10.1016/j.jacc.2004.04.034 [DOI] [PubMed] [Google Scholar]
- 40. Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (radial versus femoral randomized investigation in ST-elevation acute coronary syndrome) study. J Am Coll Cardiol 2012;60:2481–9. 10.1016/j.jacc.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 41. Zwaan EM, IJsselmuiden AJJ, van Rosmalen J, et al. Rationale and design of the arcus: effects of trAnsRadial perCUtaneouS coronary intervention on upper extremity function. Catheter Cardiovasc Interv 2016;88:1036–43. 10.1002/ccd.26525 [DOI] [PubMed] [Google Scholar]
- 42. Kotowycz MA, Dzavík V. Radial artery patency after transradial catheterization. Circ Cardiovasc Interv 2012;5:127–33. 10.1161/CIRCINTERVENTIONS.111.965871 [DOI] [PubMed] [Google Scholar]
- 43. Wagener JF, Rao SV. Radial artery occlusion after transradial approach to cardiac catheterization. Curr Atheroscler Rep 2015;17:489 10.1007/s11883-015-0489-6 [DOI] [PubMed] [Google Scholar]
- 44. Bertrand OF, Bélisle P, Joyal D, et al. Comparison of transradial and femoral approaches for percutaneous coronary interventions: a systematic review and hierarchical Bayesian meta-analysis. Am Heart J 2012;163:632–48. 10.1016/j.ahj.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 45. Akins PT, Amar AP, Pakbaz RS, et al. Complications of endovascular treatment for acute stroke in the SWIFT trial with Solitaire and Merci devices. AJNR Am J Neuroradiol 2014;35:524–8. 10.3174/ajnr.A3707 [DOI] [PMC free article] [PubMed] [Google Scholar]


