Abstract
BACKGROUND
Vitamin D metabolites support innate immune responses to Mycobacterium tuberculosis. Data from phase 3, randomized, controlled trials of vitamin D supplementation to prevent tuberculosis infection are lacking.
METHODS
We randomly assigned children who had negative results for M. tuberculosis infection according to the QuantiFERON-TB Gold In-Tube assay (QFT) to receive a weekly oral dose of either 14,000 IU of vitamin D3 or placebo for 3 years. The primary outcome was a positive QFT result at the 3-year follow-up, expressed as a proportion of children. Secondary outcomes included the serum 25-hydroxyvitamin D (25[OH]D) level at the end of the trial and the incidence of tuberculosis disease, acute respiratory infection, and adverse events.
RESULTS
A total of 8851 children underwent randomization: 4418 were assigned to the vitamin D group, and 4433 to the placebo group; 95.6% of children had a baseline serum 25(OH)D level of less than 20 ng per milliliter. Among children with a valid QFT result at the end of the trial, the percentage with a positive result was 3.6% (147 of 4074 children) in the vitamin D group and 3.3% (134 of 4043) in the placebo group (adjusted risk ratio, 1.10; 95% confidence interval [CI], 0.87 to 1.38; P = 0.42). The mean 25(OH)D level at the end of the trial was 31.0 ng per milliliter in the vitamin D group and 10.7 ng per milliliter in the placebo group (mean between-group difference, 20.3 ng per milliliter; 95% CI, 19.9 to 20.6). Tuberculosis disease was diagnosed in 21 children in the vitamin D group and in 25 children in the placebo group (adjusted risk ratio, 0.87; 95% CI, 0.49 to 1.55). A total of 29 children in the vitamin D group and 34 in the placebo group were hospitalized for treatment of acute respiratory infection (adjusted risk ratio, 0.86; 95% CI, 0.52 to 1.40). The incidence of adverse events did not differ significantly between the two groups.
CONCLUSIONS
Vitamin D supplementation did not result in a lower risk of tuberculosis infection, tuberculosis disease, or acute respiratory infection than placebo among vitamin D–deficient schoolchildren in Mongolia. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT02276755.)
THE END TB STRATEGY OF THE WORLD Health Organization (WHO) calls for an 80% decrease in the incidence of tuberculosis by 2030.1 The mainstay of tuberculosis control is treatment of active tuberculosis disease to reduce transmission; however, mathematical models indicate that this strategy alone cannot achieve the target reduction.2 This is because most cases of tuberculosis disease arise as a consequence of reactivation of asymptomatic latent Mycobacterium tuberculosis infection. It has been estimated that approximately 1.7 billion persons worldwide have latent tuberculosis infection,3 and about 10% of these persons will have progression to tuberculosis disease in their lifetime.4 Although reactivation of tuberculosis disease usually occurs in adulthood, primary infection is most commonly acquired in childhood; therefore, measures to prevent acquisition of latent tuberculosis infection in children will need to be implemented if desired reductions in tuberculosis incidence are to be achieved.2
Vitamin D supplementation has been proposed as an intervention to reduce the risk of acquiring latent tuberculosis infection in populations in which deficiency is prevalent.5 We have previously reported that vitamin D deficiency is associated with susceptibility to latent tuberculosis infection in schoolchildren6; we have also found that vitamin D supplementation boosts immunity to mycobacterial infection in persons in contact with others who have tuberculosis disease7 and reduces the risk of conversion to a positive result on a tuberculin skin test in schoolchildren.8 A recent meta-analysis of longitudinal studies has shown that vitamin D deficiency predicts the risk of tuberculosis disease in a concentration-dependent manner.9
We therefore hypothesized that vitamin D supplementation would reduce the risk of tuberculosis infection and tuberculosis disease in populations in which both vitamin D deficiency and tuberculosis are prevalent. We tested this hypothesis in a phase 3, double-blind, randomized, placebo-controlled trial of vitamin D supplementation in schoolchildren living in Mongolia, with development of M. tuberculosis infection as the primary outcome and the incidence of active tuberculosis and acute respiratory infection as secondary outcomes.
METHODS
TRIAL DESIGN, SETTING, AND PARTICIPANTS
We conducted the current trial in 18 public schools in Ulaanbaatar, Mongolia. Eligibility assessments were performed at participating schools by trial field workers. The main inclusion criteria were an age of 6 to 13 years at screening and attendance at a participating school; principal exclusion criteria were the presence of latent tuberculosis infection (as confirmed by a positive result on the QuantiFERON-TB Gold In-Tube assay [QFT, Qiagen] at screening) and the presence of clinical signs of rickets.
RANDOMIZATION
Eligible children were randomly assigned in a 1:1 ratio, with stratification according to school of attendance, to receive one capsule per week containing either 14,000 IU (0.35 mg) of vitamin D3 or placebo for 3 years. Group assignments were concealed from participants, clinicians, and all trial staff (including senior investigators and persons who assessed outcomes).
FOLLOW-UP ASSESSMENTS
During school terms, trial participants had weekly face-to-face visits at which vitamin D3 or placebo was administered and adverse events, including incident active tuberculosis and acute respiratory infection, were recorded. The final visit at the 3-year follow-up included the same assessments that were performed at baseline: a 5-ml blood specimen was obtained for QFT testing and for measurement of the serum 25-hydroxyvitamin D (25[OH]D) level. Children who were found to have a positive QFT result at the 3-year follow-up were referred to the Mongolia National Center for Communicable Diseases for clinical and radiographic screening for tuberculosis disease. Clinical and radiologic data for all children for whom antituberculosis therapy was prescribed were reviewed by members of the trial end-point committee, who classified the likelihood of tuberculosis disease as confirmed, probable, possible, or unlikely, according to published criteria.10
OUTCOMES
The primary outcome was a positive QFT result at the end of the trial (expressed as a proportion of children), as defined by an interferon-γ level that was at or above the manufacturer-recommended threshold value of 0.35 IU per milliliter. Secondary efficacy outcomes were a positive QFT result according to an interferon-γ level at or above the threshold value of 4.0 IU per milliliter at the end of the trial (a threshold value previously reported as indicating sustained conversion11), expressed as a proportion of children; the mean antigen-stimulated interferon-γ level; tuberculosis disease, as diagnosed by clinicians in Mongolia and adjudicated by the trial end-point committee; at least one hospitalization for treatment of acute respiratory infection; at least one episode of symptoms of acute respiratory infection; receipt of at least one course of antibiotic agents for treatment of acute respiratory infection; the mean serum 25(OH)D level at the end of the trial; and a serum 25(OH)D level of more than 20 ng per milliliter (50 nmol per liter) at the end of the trial. Safety outcomes were death, serious adverse events, adverse events resulting in discontinuation of the trial regimen, and other monitored safety conditions, including hypercalcemia (serum corrected calcium level, >2.55 mmol per liter [10.2 mg per deciliter], confirmed on two samples), hypervitaminosis D (25[OH]D level, >80 ng per milliliter [200 nmol per liter]), and renal stones.
STATISTICAL ANALYSIS
Assuming a 2% annual risk of tuberculosis infection, an 18% loss to follow-up, and 5% indeterminate QFT tests at the end of the trial, we calculated that enrollment of 8850 participants would give the trial 80% power at a type I error rate of 5% to detect a 25% reduction with supplementation in the percentage of children with a positive QFT result at the 3-year follow-up. Statistical analyses were performed according to the intention-to-treat principle, with a 5% significance level. Treatment effects for dichotomous outcomes were estimated with the use of the Mantel–Haenszel risk ratio, with stratification according to school, and are reported as risk ratios with 95% confidence intervals, adjusted for school of attendance. Additional details regarding statistical methods are provided in the Supplementary Appendix and the protocol, both available with the full text of this article at NEJM.org.
Results
PARTICIPANTS
From September 2015 through March 2017, a total of 11,475 children were invited to participate in the trial. Of these children, 9814 underwent QFT testing. Among the 8851 children who had a negative QFT result, 4418 were randomly assigned to receive vitamin D3 and 4433 to receive placebo (Fig. 1). The mean age of the children was 9.4 years, 49.3% were female, and 80.1% had a bacille Calmette–Guérin vaccination scar. The mean serum 25(OH)D level was 11.9 ng per milliliter (30 nmol per liter); 95.6% of children had a 25(OH)D level below 20 ng per milliliter, and 31.8% had a 25(OH)D level below 10 ng per milliliter (25 nmol per liter). The characteristics of the children were balanced between the two groups (Table 1).
Figure 1. Screening, Randomization, and Follow-up.
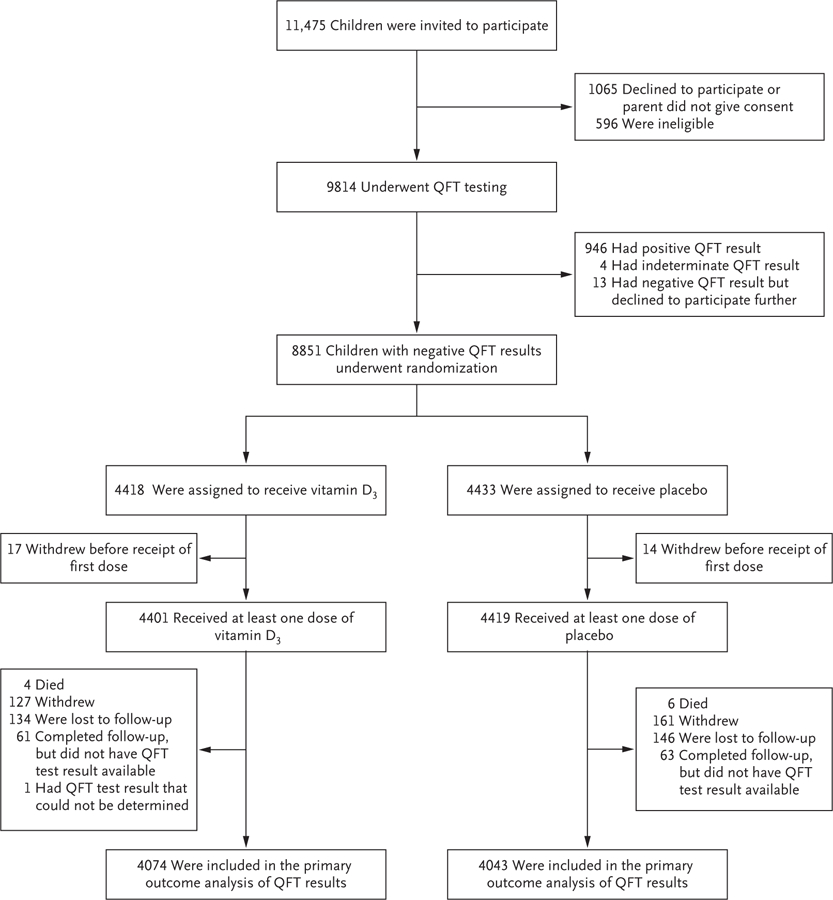
QFT denotes QuantiFERON-TB Gold In-Tube assay.
Table 1.
Baseline Characteristics of the Participants.*
| Characteristic | Overall (N = 8851) | Vitamin D (N = 4418) | Placebo (N = 4433) |
|---|---|---|---|
| Age — yr | 9.4±1.6 | 9.4±1.6 | 9.4±1.6 |
| Female sex — no. (%) | 4366 (49.3) | 2142 (48.5) | 2224 (50.2) |
| Ethnic group — no. (%)† | |||
| Khalkh | 8165 (92.2) | 4062 (91.9) | 4103 (92.6) |
| Other | 686 (7.8) | 356 (8.1) | 330 (7.4) |
| Highest educational level attained by either parent — no. (%) | |||
| Secondary school or lower | 4858 (54.9) | 2401 (54.3) | 2457 (55.4) |
| University or polytechnic institution | 3993 (45.1) | 2017 (45.7) | 1976 (44.6) |
| Type of residence — no. (%) | |||
| Yurt | 3271 (37.0) | 1643 (37.2) | 1628 (36.7) |
| House without central heating | 3387 (38.3) | 1665 (37.7) | 1722 (38.8) |
| House with central heating | 2193 (24.8) | 1110 (25.1) | 1083 (24.4) |
| Home owned by family — no. (%) | 6963 (78.7) | 3493 (79.1) | 3470 (78.3) |
| Monthly household income — U.S. dollars‡ | 848±579 | 851±554 | 846±604 |
| Tobacco smoking in the household — no. (%)§ | 3143 (35.5) | 1570 (35.5) | 1573 (35.5) |
| Child actively smoking — no. (%) | 47 (0.5) | 26 (0.6) | 21 (0.5) |
| History of pulmonary tuberculosis in a household member — no. (%) | 215 (2.4) | 108 (2.4) | 107 (2.4) |
| Bacille Calmette–Guérin scar — no. (%) | 7091 (80.1) | 3527 (79.8) | 3564 (80.4) |
| Body-mass index z score¶ | 0.2±1.1 | 0.2±1.0 | 0.2±1.1 |
| Height z score for age‖ | −0.3±1.0 | −0.3±1.0 | −0.3±1.0 |
| Serum 25(OH)D level — ng/ml | |||
| Mean value** | 11.9±4.2 | 11.9±4.2 | 11.9±4.2 |
| Distribution — no./total no. (%) | |||
| <10.0 | 2813/8846 (31.8) | 1393/4417 (31.5) | 1420/4429 (32.1) |
| 10.0–19.9 | 5640/8846 (63.8) | 2828/4417 (64.0) | 2812/4429 (63.5) |
| 20.0–29.9 | 381/8846 (4.3) | 188/4417 (4.3) | 193/4429 (4.4) |
| ≥30.0 | 12/8846 (0.1) | 8/4417 (0.2) | 4/4429 (0.1) |
Plus–minus values are means ±SD. To convert the 25-hydroxyvitamin D (25[OH]D) values to nanomoles per liter, multiply by 2.496. Percentages may not total 100 because of rounding.
Ethnic group was reported by the caregivers.
Data were missing for 1 household in each group.
Tobacco smoking in the household was defined as 1 or more persons in the household smoking indoors.
Data for body-mass index (the weight in kilograms divided by the square of the height in meters) z score were miss- ing for 1 child in the placebo group.
Data were missing for 1 child in the placebo group.
The 25(OH)D values have been adjusted for seasonal variation. Data were missing for 1 child in the vitamin D group and 4 children in the placebo group.
The median follow-up was 3.0 years (interquartile range, 2.9 to 3.1) in both groups, and the median number of doses of vitamin D or placebo that were administered was 152 (interquartile range, 138 to 158). Valid QFT results at the end of the trial were obtained for 8117 participants (91.7% of the children who underwent randomization) who were included in the analysis of the primary outcome; a total of 8819 children had at least one follow-up visit and had data included in the analyses of the incidence of active tuberculosis and the incidence of acute respiratory infection. Follow-up 25(OH)D levels were available for all children who had available QFT results at the end of the trial; the mean 25(OH)D level at 3 years was higher in the vitamin D group than in the placebo group (31.0 vs. 10.7 ng per milliliter [75 nmol per liter vs. 25 nmol per liter]; difference, 20.3 ng per milliliter [50 nmol per liter]; 95% confidence interval [CI] for difference, 19.9 to 20.6) (Fig. 2). At the end of the trial, 89.8% of children in the vitamin D group and 5.6% in the placebo group had a 25(OH)D level of 20 ng or higher per milliliter.
Figure 2. Mean Serum 25-Hydroxyvitamin D (25[OH]D) Level According to Time and Trial Group.
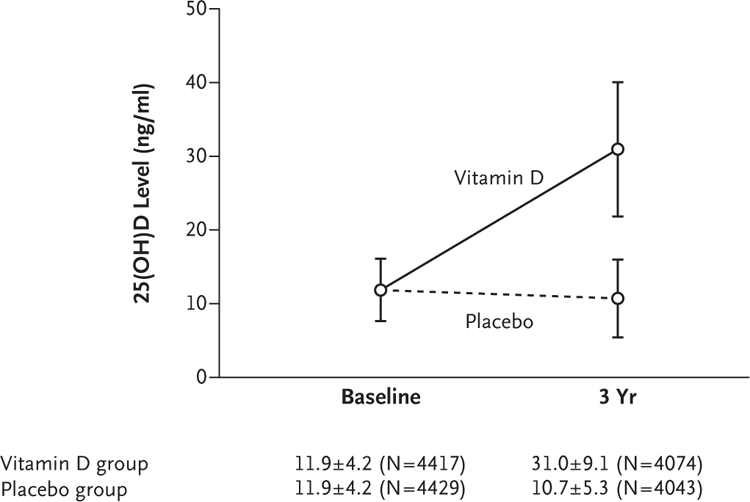
Levels of 25(OH)D were higher at the end of the trial in the group that received vitamin D supplementation than in the placebo group (mean between- group difference, 20.3 ng per milliliter; 95% CI, 19.9 to 20.6). To convert the 25(OH)D values to nanomoles per liter, multiply by 2.496. I bars indi- cate the standard deviation. The plus–minus values are means ±SD. The values in parentheses are the numbers of children.
PRIMARY AND SECONDARY OUTCOMES
At the 3-year follow-up, conversion to a positive QFT result, as defined by an interferon-γ level that was at or above the threshold value of 0.35 IU per milliliter (the primary outcome), occurred in 281 children. The percentage of children with a positive QFT result was similar in the two groups: 3.6% (147 of 4074 children) in the vitamin D group and 3.3% (134 of 4043) in the placebo group (adjusted risk ratio, 1.10; 95% CI, 0.87 to 1.38; P = 0.42) (Table 2). In the overall trial population, no significant between-group difference was observed in the proportion of children who had QFT conversion according to an interferon-γ level that was at or above the threshold value of 4.0 IU per milliliter (adjusted risk ratio, 0.67; 95% CI, 0.39 to 1.12). We found no evidence to suggest that assignment to the vitamin D group rather than to the placebo group had an effect on the mean antigen-stimulated interferon-γ level in QFT supernatants, either in the overall population (adjusted mean difference between groups, −0.01 IU; 95% CI, −0.04 to 0.02) or in the subgroup of children with a baseline 25(OH)D level below 10 ng per milliliter (adjusted mean difference between groups, −0.04 IU; 95% CI, −0.09 to 0.02) (Table S1 in the Supplementary Appendix).
Table 2.
Respiratory Infection.
| Variable | Vitamin D | Placebo | Adjusted Risk Ratio (95% CI)* |
|---|---|---|---|
| Tuberculosis infection according to positive QFT result† | |||
| Interferon-γ level ≥0.35 IU/ml — no./total no. (%)‡ | |||
| Overall | 147/4074 (3.6) | 134/4043 (3.3) | 1.10 (0.87–1.38)§ |
| Baseline 25(OH)D level <10 ng/ml | 64/1288 (5.0) | 64/1304 (4.9) | 1.01 (0.72–1.42) |
| Baseline 25(OH)D level ≥10 ng/ml | 83/2785 (3.0) | 70/2736 (2.6) | 1.17 (0.86–1.61) |
| Interferon-γ level ≥4.0 IU/ml — no./total no. (%) | |||
| Overall | 23/4074 (0.6) | 35/4043 (0.9) | 0.67 (0.39–1.12) |
| Baseline 25(OH)D level <10 ng/ml | 7/1288 (0.5) | 17/1304 (1.3) | 0.41 (0.17–0.99) |
| Baseline 25(OH)D level ≥10 ng/ml | 16/2785 (0.6) | 18/2736 (0.7) | 0.90 (0.46–1.77) |
| Tuberculosis disease status¶ | |||
| Starting treatment for tuberculosis disease — no./ total no. (%) | |||
| Overall | 21/4401 (0.5) | 25/4418 (0.6) | 0.87 (0.49–1.55) |
| Baseline 25(OH)D level <10 ng/ml | 10/1387 (0.7) | 15/1414 (1.1) | 0.67 (0.30–1.47) |
| Baseline 25(OH)D level ≥10 ng/ml | 11/3013 (0.4) | 10/3000 (0.3) | 1.17 (0.50–2.75) |
| Confirmed or probable tuberculosis disease, as adjudicated by the trial end-point committee — no./total no. (%) | |||
| Overall | 13/4401 (0.3) | 13/4418 (0.3) | 1.05 (0.49–2.27) |
| Baseline 25(OH)D level <10 ng/ml | 6/1387 (0.4) | 8/1414 (0.6) | 0.75 (0.26–2.14) |
| Baseline 25(OH)D level ≥10 ng/ml | 7/3013 (0.2) | 5/3000 (0.2) | 1.47 (0.47–4.61) |
| Acute respiratory infection¶ | |||
| Participant hospitalized for ≥1 episode — no./total no. (%) | |||
| Overall | 29/4401 (0.7) | 34/4418 (0.8) | 0.86 (0.52–1.40) |
| Baseline 25(OH)D level <10 ng/ml | 8/1387 (0.6)‖ | 10/1414 (0.7)** | 0.81 (0.32–2.09) |
| Baseline 25(OH)D level ≥10 ng/ml | 21/3013 (0.7)†† | 24/3000 (0.8)‡‡ | 0.86 (0.48–1.55) |
| Participant reported ≥1 episode — no./total no. (%) | |||
| Overall | 3783/4401 (86.0) | 3793/4418 (85.9) | 1.00 (0.98–1.02) |
| Baseline 25(OH)D level <10 ng/ml | 1195/1387 (86.2) | 1205/1414 (85.2) | 1.01 (0.98–1.04) |
| Baseline 25(OH)D level ≥10 ng/ml | 2587/3013 (85.9) | 2585/3000 (86.2) | 1.00 (0.98–1.02) |
| Participant received ≥1 course of antibiotics for episode — no./total no. (%) | |||
| Overall | 1272/4401 (28.9) | 1292/4418 (29.3) | 0.99 (0.93–1.05) |
| Baseline 25(OH)D level <10 ng/ml | 392/1387 (28.3) | 399/1414 (28.2) | 0.99 (0.88–1.12) |
| Baseline 25(OH)D level ≥10 ng/ml | 880/3013 (29.2) | 892/3000 (29.7) | 0.98 (0.91–1.06) |
The risk ratios were adjusted for school of attendance.
Data for the baseline 25(OH)D level were unavailable for 4 children included in the overall analysis of QFT results (1 of 4074 children in the vitamin D group and 3 of 4043 children in the placebo group).
The primary outcome was a positive QFT result, as defined by an interferon-γ level that was at or above the threshold value of 0.35 IU per milliliter, expressed as a proportion of children.
P = 0.42.
Data for the baseline 25(OH)D level were unavailable for 5 children included in the overall analysis of tuberculosis disease and acute respiratory infection (1 of 4401 children in the vitamin D group and 4 of 4418 children in the placebo group).
A total of 5 children had lower respiratory infections, and 3 had upper respiratory infections.
A total of 9 children had lower respiratory infections, and 1 had an upper respiratory infection.
A total of 18 children had lower respiratory infections, and 3 had upper respiratory infections.
A total of 21 children had lower respiratory infections, and 3 had upper respiratory infections.
POST HOC ANALYSIS
Subgroup analysis revealed no evidence to suggest heterogeneity of treatment effect for the outcome of QFT conversion according to an interferon-γ level at or above the threshold value of 0.35 IU per milliliter in children with a baseline 25(OH)D level of less than 10 ng per milliliter as compared with children with a level of 10 ng or higher per milliliter. However, subgroup analysis of QFT conversion at the threshold value of 4.0 IU per milliliter raised the possibility that in children with a baseline 25(OH)D level below 10 ng per milliliter, the risk of QFT conversion at this higher cutoff was lower among children assigned to receive vitamin D than among those assigned to placebo (adjusted risk ratio, 0.41; 95% CI, 0.17 to 0.99); this result was not seen in children with a baseline 25(OH)D level of 10 ng or higher per milliliter (adjusted risk ratio, 0.90; 95% CI, 0.46 to 1.77) (Table 2). The results of this subgroup analysis should be interpreted with caution, given that there was no adjustment for multiple comparisons and the analysis was performed post hoc.
TUBERCULOSIS DISEASE
Tuberculosis disease was diagnosed by local doctors in 46 children during the trial. These events were evenly distributed between the two groups: among children who had at least one follow-up visit at which they were assessed for tuberculosis disease, 21 of 4401 (0.5%) children in the vitamin D group and 25 of 4418 (0.6%) children in the placebo group received a diagnosis of tuberculosis (adjusted risk ratio, 0.87; 95% CI, 0.49 to 1.55). A total of 26 of these children were classified as having confirmed or probable tuberculosis disease by the trial end-point committee and were also evenly distributed between groups (13 of 4401 children in the vitamin D group and 13 of 4418 children in the placebo group; adjusted risk ratio, 1.05; 95% CI, 0.49 to 2.27). Subgroup analysis revealed no heterogeneity of treatment effect for either outcome in children with a baseline 25(OH)D level of less than 10 ng per milliliter or in children with a level of 10 ng or higher per milliliter (Table 2).
ACUTE RESPIRATORY INFECTION
Among children who had at least one follow-up visit at which they were assessed for acute respiratory infection, 63 children had at least one hospitalization for treatment of acute respiratory infection (29 of 4401 children in the vitamin D group and 34 of 4418 in the placebo group; adjusted risk ratio, 0.86; 95% CI, 0.52 to 1.40). No between-group difference was seen in the proportion of children who had at least one episode of symptoms of acute respiratory infection (adjusted risk ratio, 1.00; 95% CI, 0.98 to 1.02) or who were receiving at least one course of antibiotics for treatment of acute respiratory infection (adjusted risk ratio, 0.99; 95% CI, 0.93 to 1.05). No effect of the intervention was seen when the proportions of children who reported upper or lower acute respiratory infection were analyzed separately (Table S2). Subgroup analyses revealed no heterogeneity of treatment effect for acute respiratory infection outcomes in children with a baseline 25(OH)D level below 10 ng per milliliter or in those with a level of 10 ng or higher per milliliter (Table 2).
SENSITIVITY ANALYSES
With respect to all the outcomes described above, the results were materially unchanged when inverse probability weighting was used to correct for any potential bias due to missing data. Additional information is provided in Table S3.
ADVERSE EVENTS
A total of 10 children (4 in the vitamin D group and 6 in the placebo group) died during the trial, and 324 (142 in the vitamin D group and 182 in the placebo group) had one or more nonfatal serious adverse events (Table 3 and Table S4). None of these events were judged to be related to vitamin D or placebo. Symptomatic hypercalcemia developed in 1 child in the vitamin D group who had a corrected serum calcium level of 2.70 mmol per liter (10.8 mg per deciliter). The hypercalcemia manifested with nausea and epigastric discomfort; vitamin D was discontinued, and the hypercalcemia and symptoms resolved. Three children had a serum 25(OH)D level that was higher than 80 ng per milliliter at the 3-year follow-up (87.6 ng per milliliter [219 nmol per liter], 93.6 ng per milliliter [234 nmol per liter], and 95.7 ng per milliliter [239 nmol per liter]); none of the children had symptoms. A total of 15 nonfatal adverse events led to discontinuation of the trial regimen (in 10 children in the vitamin D group and 5 in the placebo group).
Table 3.
Adverse Events.*
| Variable |
Vitamin D (N = 4401) |
Placebo (N = 4419) |
Incidence Rate Ratio, Vitamin D vs. Placebo (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| No. of Events | Events/100 Person-Yr | Children with ≥1 Event | No. of Events | Events/100 Person-Yr | Children with ≥1 Event | ||
| Death | 4 | 0.03 | 4 | 6 | 0.05 | 6 | 0.67 (0.19–2.37) |
| Serious adverse event | 159 | 1.25 | 146 | 191 | 1.50 | 188 | 0.83 (0.68–1.03) |
| Nonfatal adverse event leading to discontinuation of trial regimen | 10 | 0.08 | 10 | 5 | 0.04 | 5 | 2.00 (0.68–5.86) |
| Other monitored safety conditions | |||||||
| Hypercalcemia† | 1 | 0.01 | 1 | 0 | 0 | 0 | NC |
| Hypervitaminosis D‡ | 3 | 0.02 | 3 | 0 | 0 | 0 | NC |
| Renal stones | 0 | 0 | 0 | 0 | 0 | 0 | NC |
Included are children who received at least one dose of vitamin D or placebo. Details about deaths and serious adverse events are provided in Table S4 in the Supplementary Appendix. NC indicates that the incidence rate ratio could not be calculated because of an absence of events in one or both groups.
Hypercalcemia was defined as a corrected serum calcium level higher than 2.55 mmol per liter (10.2 mg per deciliter), as confirmed on re- peat testing.
Hypervitaminosis D was defined as a serum 25(OH)D level higher than 80 ng per milliliter.
DISCUSSION
We report the results of a phase 3, randomized, controlled trial that investigated whether vitamin D supplementation reduces the risk of incident tuberculosis infection and disease; this large trial also assessed the effects of this intervention on the risk of acute respiratory infection. Vitamin D deficiency was highly prevalent in the trial population at baseline; at the end of the trial, 25(OH)D levels were elevated to the physiologic range among children assigned to the vitamin D group. However, we observed no between-group difference in the proportion of children with QFT conversion according to an interferon-γ level at or above the threshold value of 0.35 IU per milliliter (the primary outcome), either in the trial population as a whole or among children in the vitamin D group who had a baseline 25(OH) D level below 10 ng per milliliter (31.8%). The intervention also had no effect on the incidence of tuberculosis disease or acute respiratory infection. Oral vitamin D supplementation at a weekly dose of 14,000 IU was safe and did not cause unacceptable side effects. The incidence of adverse events was balanced between the two groups, and no serious adverse event was attributed to vitamin D or placebo.
The null finding for the primary outcome contrasts with positive findings from a cross-sectional analysis and a pilot intervention study that was performed in the same population.6,8 The results of cross-sectional studies are potentially vulnerable to the effects of unmeasured or residual confounding or reverse causality. The positive result of our pilot study may have been due to the type I error (the P value for the primary outcome of a conversion to a positive tuberculin skin test was 0.06) or the different methods used to assess M. tuberculosis infection (tuberculin skin test in the previous study and QFT in the current trial).
The current trial also showed no effect of vitamin D supplementation on the incidence of acute respiratory infection. This finding contrasts with results of our previous clinical trial involving Mongolian schoolchildren, in which the rate of participant-reported acute respiratory infection among children who were assigned to daily ingestion of milk fortified with 300 IU of vitamin D3 during the winter was 50% lower than that among children in the control group.12 The contrasting results of these two trials may reflect differences in the amount, frequency, and duration of vitamin D3 administration or the coadministration with milk in the previous trial.
Our trial has several strengths. In contrast to recent large clinical trials of vitamin D supplementation,13–15 the prevalence of vitamin D deficiency at baseline was very high among participants (95.6% of children had a 25[OH]D level of <20 ng per milliliter). We used a weekly regimen of vitamin D supplementation, which allowed for direct observation of administration to encourage adherence and avoided the large fluctuations in 25(OH)D levels that are seen when vitamin D is administered in longer and more widely spaced bolus doses.13,16 Therefore, our null results for the primary outcome of this trial cannot be readily attributed to a lack of participants with low vitamin D levels at baseline or to administration of a regimen that was potentially ineffective from a pharmacokinetic perspective. Other strengths include high retention (91.7%); use of QFT testing to assess tuberculosis infection (as opposed to tuberculin skin testing, which may yield false positive results because of previous bacille Calmette–Guérin vaccination or exposure to environmental mycobacteria); use of an externally accredited laboratory and the very low number of indeterminate results (the result of 1 of the 8118 follow-up QFT tests that were performed could not be determined); and standardization of serum 25(OH)D measurements17 with the use of standards provided by the Vitamin D External Quality Assessment Scheme.
Our trial also has some limitations. The 3.5% incidence of QFT conversion as defined by an interferon-γ level at or above the threshold value of 0.35 IU per milliliter was lower than the anticipated incidence of 5.9%, which rendered our trial as potentially underpowered. However, the 95% confidence interval for the adjusted risk ratio (0.87 to 1.38) effectively rules out a relative risk reduction of more than 13%. An effect of this size or less is unlikely to be considered of sufficient magnitude to warrant the population-level intervention that would be needed to elevate 25(OH)D levels into the physiologic range. An additional consideration is our use of the manufacturer-recommended cutoff of an interferon-γ level of 0.35 IU per milliliter to indicate QFT conversion. In the time since we designed the trial, other researchers have reported that reversion to a negative result is common at this threshold.18 By contrast, conversion at the higher threshold of 4.0 IU per milliliter has recently been reported to be more sustained than at the threshold of 0.35 IU per milliliter.11 We therefore prespecified QFT conversion at the threshold of 4.0 IU per milliliter as a secondary outcome in our analysis plan. No significant effect of the intervention on this outcome was seen in the trial population as a whole, but a post hoc subgroup analysis raised the possibility of an effect in children with baseline 25(OH)D levels below 10 ng per milliliter; however, the results of this subgroup analysis should be interpreted with caution, given that the analysis was performed post hoc and there was no adjustment for multiple comparisons. Additional follow-up is therefore planned to determine whether the intervention had an effect on sustained QFT conversion in the trial population.
In conclusion, we found that weekly oral supplementation with 14,000 IU of vitamin D3 for 3 years was effective in safely elevating 25(OH)D levels in a very large population of vitamin D–deficient schoolchildren in Mongolia. However, this intervention did not result in a lower risk of primary tuberculosis infection, as indicated by QFT conversion at the threshold value for the interferon-γ level of 0.35 IU per milliliter, than placebo.
Supplementary Material
Acknowledgments
Supported by an award (1R01HL122624-01) from the National Institutes of Health.
We thank all the children who participated in the trial and their parents and guardians; the independent members of the data and safety monitoring board (Prof. S.M. Fortune and Dr. P.L. Williams, Harvard T.H. Chan School of Public Health; Profs. M.F. Holick and C.R. Horsburgh, Boston University; Prof. P. Enkhbaatar, University of Texas; and Dr. E. Chadraa, Minnesota State University); independent members of the trial steering committee (Profs. W.C. Willett, E.L. Giovannucci, and B.R. Bloom, Harvard T.H. Chan School of Public Health; Dr. N. Naranbat, Gyals Medical Laboratory, Ulaanbaatar; and Dr. D. Malchinkhuu, National Center for Maternal and Child Health of Mongolia); board members at the Mongolian Health Initiative (Dr. J. Tuyatsetseg, Mongolian University of Science and Technology; Dr. J. Amarsanaa, Happy Veritas Laboratory, Ulaanbaatar; Drs. P. Erkhembulgan and G. Batbaatar, Mongolian National University of Medical Sciences; Prof. M.C. Elliott, Harvard University; Dr. K. Kraemer, Sight and Life Foundation; and T. Munh-Orgil, member of the Mongolian parliament); and S. Boldbaatar (Harvard University), Prof. F. Hu (Harvard T.H. Chan School of Public Health), M. Undrah (UBN Corporation), B. Tuguldur (First General Hospital, Ulaanbaatar), Dr. W. Burr (Anadyne Psychotherapy), and S. Tsend-Ayush (Mongolian State Inspection Agency) for advice and helpful discussions.
Appendix
The authors’ full names and academic degrees are as follows: Davaasambuu Ganmaa, Ph.D., Buyanjargal Uyanga, M.D., Xin Zhou, Ph.D., Garmaa Gantsetseg, M.D., Baigali Delgerekh, M.D., Davaasambuu Enkhmaa, Ph.D., Dorjnamjil Khulan, M.D., Saranjav Ariunzaya, M.D., Erdenebaatar Sumiya, B.Sc., Batbileg Bolortuya, M.D., Jutmaan Yanjmaa, Ph.D., Tserenkhuu Enkhtsetseg, M.D., Ankhbat Munkhzaya, M.D., Murneren Tunsag, M.D., Polyna Khudyakov, Ph.D., James A. Seddon, Ph.D., Ben J. Marais, Ph.D., Ochirbat Batbayar, M.D., Ganbaatar Erdenetuya, M.D., Bazarsaikhan Amarsaikhan, Ph.D., Donna Spiegelman, Sc.D., Jadambaa Tsolmon, M.D., and Adrian R. Martineau, Ph.D.
Footnotes
Contributor Information
D. Ganmaa, Harvard T.H. Chan School of Public Health, Boston; Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston; Mongolian Health Initiative, Ulaanbaatar, Mongolia
B. Uyanga, Mongolian Health Initiative, Ulaanbaatar, Mongolia
X. Zhou, Yale School of Public Health, New Haven, CT
G. Gantsetseg, Mongolian Health Initiative, Ulaanbaatar, Mongolia
B. Delgerekh, Global Laboratory, Ulaanbaatar, Mongolia
D. Enkhmaa, Mongolian Health Initiative, Ulaanbaatar, Mongolia
D. Khulan, Mongolian Health Initiative, Ulaanbaatar, Mongolia
S. Ariunzaya, Mongolian Health Initiative, Ulaanbaatar, Mongolia
E. Sumiya, Mongolian Health Initiative, Ulaanbaatar, Mongolia
B. Bolortuya, Mongolian Health Initiative, Ulaanbaatar, Mongolia
J. Yanjmaa, Mongolian Health Initiative, Ulaanbaatar, Mongolia
T. Enkhtsetseg, Mongolian Health Initiative, Ulaanbaatar, Mongolia
A. Munkhzaya, Global Laboratory, Ulaanbaatar, Mongolia
M. Tunsag, Royal Plaza, Bayanzurkh District, the National Center for Communicable Diseases, Ulaanbaatar, Mongolia
P. Khudyakov, Harvard T.H. Chan School of Public Health, Boston
J.A. Seddon, Faculty of Medicine, Imperial College, London; Desmond Tutu Tuberculosis Centre, Department of Pediatrics and Child Health, Stellenbosch University, Cape Town, South Africa
B.J. Marais, Faculty of Medicine and Health, University of Sydney, Sydney
O. Batbayar, Mongolian Health Initiative, Ulaanbaatar, Mongolia
G. Erdenetuya, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
B. Amarsaikhan, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
D. Spiegelman, Harvard T.H. Chan School of Public Health, Boston; Yale School of Public Health, New Haven, CT
J. Tsolmon, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
A.R. Martineau, Institute of Population Health Sciences, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London
References
- 1.Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet 2015; 385:1799–801. [DOI] [PubMed] [Google Scholar]
- 2.Dye C, Williams BG. Eliminating human tuberculosis in the twenty-first century. J R Soc Interface 2008;5:653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 2016;13(10):e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 2004; 350:2060–7. [DOI] [PubMed] [Google Scholar]
- 5.Martineau AR. Old wine in new bottles: vitamin D in the treatment and prevention of tuberculosis. Proc Nutr Soc 2012;71:84–9. [DOI] [PubMed] [Google Scholar]
- 6.Ganmaa D, Khudyakov P, Buyanjargal U, et al. Prevalence and determinants of QuantiFERON-diagnosed tuberculosis infection in 9810 Mongolian schoolchildren. Clin Infect Dis 2019;69:813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 2007;176:208–13. [DOI] [PubMed] [Google Scholar]
- 8.Ganmaa D, Giovannucci E, Bloom BR, et al. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. Am J Clin Nutr 2012;96:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aibana O, Huang CC, Aboud S, et al. Vitamin D status and risk of incident tuberculosis disease: a nested case-control study, systematic review, and individual-participant data meta-analysis. PLoS Med 2019;16(9):e1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease: consensus from an expert panel. J Infect Dis 2012;205: Suppl 2:S199–S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemes E, Geldenhuys H, Rozot V, et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med 2018;379:138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo CA Jr, Ganmaa D, Frazier AL, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012;130(3):e561–e567. [DOI] [PubMed] [Google Scholar]
- 13.Scragg R, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the Vitamin D Assessment Study: a randomized clinical trial. JAMA Cardiol 2017;2:608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med 2019;381:520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manaseki-Holland S, Maroof Z, Bruce J, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 2012;379:1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sempos CT, Betz JM, Camara JE, et al. General steps to standardize the laboratory measurement of serum total 25-hydroxyvitamin D. J AOAC Int 2017;100: 1230–3. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Xin H, Li X, et al. Reversion of QuantiFERON-TB Gold In-Tube test in individuals with and without prophylactic treatment for latent tuberculosis infection: a systematic review and meta-analysis. J Infect 2018;77:276–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


