Abstract
Management of residual and persistent cardiovascular disease (CVD) risk among statin-treated individuals has emerged as an important preventive strategy. The purpose of this article is to review the unique landscape of CVD in women and relevant prior prevention trials, and to discuss how the recent results of the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) might apply to the contemporary management of CVD risk among statin-treated women. Women have unique risk factors that may impact CVD and its prevention. Historically, women have been underrepresented in CVD trials, posing a challenge to development of clinical recommendations for women. Low-density lipoprotein cholesterol-targeting treatments have demonstrated CVD risk reduction, with comparable effects in both sexes. In contrast, triglyceride-lowering treatments (niacin, fenofibrate, and omega-3 fatty acids) have reported mixed findings for CVD risk reduction. Recent clinical trials of combination omega-3 fatty acids (docosahexaenoic acid/eicosapentaenoic acid [EPA]) have not found significant CVD risk reduction. The recently published REDUCE-IT study found that icosapent ethyl, an EPA-only omega-3 fatty acid, in combination with statins, significantly reduced CVD events in high-risk patients. The icosapent ethyl group had a significantly lower occurrence of the primary composite CVD endpoint (17.2%) than the placebo group (22.0%; hazard ratio 0.75; 95% confidence interval 0.68–0.83; p < 0.001). CVD risk reduction with icosapent ethyl treatment was comparable between women and men (p for interaction, 0.33). Data from REDUCE-IT suggest women benefit similarly to men with respect to icosapent ethyl, a novel therapy for prevention of CVD.
Keywords: cardiovascular disease, eicosapentaenoic acid, icosapent ethyl, sex differences, women
Introduction
Despite considerable progress in reducing the risk of cardiovascular disease (CVD) with evidence-based therapies such as statins, CVD remains the leading cause of death among women, with substantial human and economic toll.1 The fact that sex-based differences in biology can impact health and disease has been recognized2; however, historically, women have been underrepresented in CVD trials, which has led to challenges in development of clinical guidelines to reduce CVD risk in women.3,4
Addressing residual CVD risk among statin-treated individuals who have controlled levels of low-density lipoprotein cholesterol (LDL-C) has broadly been recognized as an important clinical strategy. However, studies targeting lipids beyond LDL-C, such as triglycerides (TG), using fibrates, niacin, or combination omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]), have yielded disappointing or inconsistent results; some have failed to show clinical benefit in combination with a statin overall,5–8 and one even suggested potential harm among women.6
Recently, addition of an omega-3 fatty acid (EPA; icosapent ethyl) to statins in high-risk individuals demonstrated reductions in major CVD events in the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT).9 While these findings are promising, it is important to examine the efficacy and safety of icosapent ethyl in women, and to place results in the context of other therapies proven to prevent CVD events. The purpose of this article is to review the unique experiences of CVD in women and relevant prior CVD trials in women, and to discuss how the results of REDUCE-IT might apply to the contemporary management of residual CVD risk in women.
CVD Experience in Women
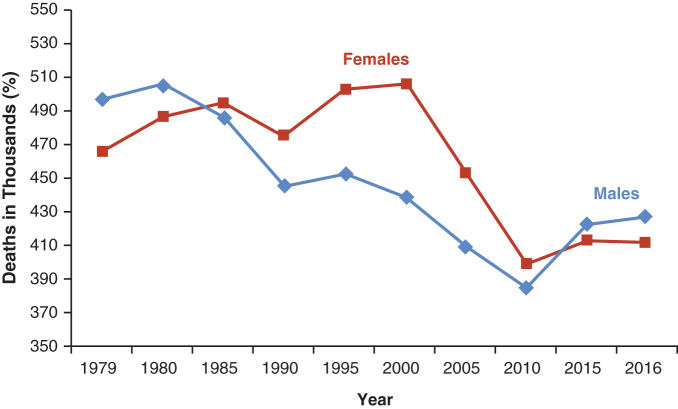
Despite substantial reductions in CVD death over the past 40 years, there has been a concerning upward trend in mortality over the most recent decade (Fig. 1).1 While rates of decline in CVD mortality have diminished in recent decades for both men and women <55 years of age, the rates of decline have been consistently lower for women than for men.1
FIG. 1.
Change in cardiovascular deaths in men and women since 1979. Reprinted with permission, Benjamin et al., ©2019 American Heart Association, Inc.1
Recent trends in CVD death may be related, in part, to the rise in diabetes, with the overall prevalence of diagnosed diabetes mellitus having increased from 5.0% in 1999–2000 to 7.8% in 2009–2010.1 In women, the prevalence estimates for diagnosed diabetes, undiagnosed diabetes, and prediabetes are 8.9%, 2.8%, and 31.3%, respectively, based on The National Health and Nutrition Examination Survey (NHANES) 2013–2016 data.1 The relative risk of CVD in patients with diabetes versus without diabetes is more pronounced in women (risk ratio [RR] 3.57) than in men (RR 1.93).10
The greater association between diabetes and CVD risk in women compared with men may be due to the more deleterious effects of diabetes and lipids on blood pressure in women11,12; the interplay between lipids and insulin resistance may contribute to endothelial dysfunction, which has the potential to impact hypertension and diabetes risk.13–17
Women develop CVD at an older age compared with men, and factors such as diabetes and hypertriglyceridemia appear to better predict the risk of CVD in women compared with men.18,19 Women's sex hormones may play a role in CVD risk; before menopause, women have greater subcutaneous adipose tissue and greater TG clearance, but this is reduced postmenopause, and associated with increased TG and metabolic syndrome,20 which may both contribute to elevated CVD risk.
Beyond lipids, a number of other uniquely important factors may have implications for CVD risk in women, including psychosocial risk factors such as depression, poor sleep quality, inadequate hours of sleep, complications of pregnancy, poor adherence, and fear of side effects.10,21–24 Risk factors that cause stress, such as depression and post-traumatic stress disorder, as well as social isolation and greater family responsibilities, are more prevalent in women than men, and have demonstrated robust associations with CVD.10,25 Thus, while CVD risk management should focus on areas where intervention has been proven to ameliorate risk, it is important to consider the potential impact of psychosocial risk factors on CVD risk and on adherence to proven therapies.
Suboptimal preventive care in women
CVD risk may be underestimated in women, posing a barrier to optimal preventive care. An American Heart Association (AHA) national survey of 500 physicians (primary care physicians, obstetricians/gynecologists, and cardiologists) found that the main driver of CVD prevention was proper assessment of baseline risk; however, women, despite similar calculated risk to men, were more likely to be assigned a lower risk category.26 The American College of Cardiology (ACC)/AHA CVD risk calculator has been shown to underperform in women (especially young women), potentially causing patients and physicians to misjudge risk.27
Differences in the presentation and symptoms of CVD in women compared with men18,19 may further contribute to a misconception that women have a lower CVD risk. Even though women have a recurrence rate of myocardial infarction (MI) that is three times greater than men,28 women with acute coronary syndrome (ACS) or MI are less likely to undergo revascularization, receive appropriate medication, or receive cardiac rehabilitation than men.29–36
Underrepresentation in clinical trials
Historically, a major challenge in the management of CVD risk in women has been the lack of data compared with men owing to the lower enrollment rates for women in clinical trials. Many reasons underlie the underrepresentation of women in CVD trials. Women tend to present with CVD on average 10 years later than men,34 which may contribute to exclusion from trials due to upper age limits3,37 as well as exclusions due to pregnancy-related concerns for younger women. Moreover, women may have logistical barriers to participation due to lack of transportation and/or caregiving responsibilities.37,38
In an effort to address the sex-based disparities in research and clinical studies, the National Institutes of Health and the Institute of Medicine published recommendations to promote inclusion of women in clinical research and sex-/gender-based subpopulation analyses, and to make sex-specific data more readily available.2,39 Progress has been made, with recent CVD trials showing a trend in enrolling larger numbers of women.40 Critical evaluation of new and ongoing CVD outcome studies, with attention to sex-based differences, and the application of these findings to clinical practice may help to guide CVD management in women.
Examining Sex-Based Differences in CVD Trials
Statin trials
Analyses of sex-based differences in statin CVD outcome trials have generally found comparable outcomes between women and men, although sample sizes are typically powered to detect differences in treatment effect for the overall study population and may be insufficient for identifying statistically significant differences between sexes.38
The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) (N = 17,802; 38.2% women) is of interest, given the large population of women.41 This trial studied rosuvastatin for primary prevention of CVD events in women ≥60 years of age and men ≥50 years of age with LDL-C <130 mg/dL.40 After 12 months, changes in LDL-C and TG were similar for women and men. A significant reduction versus placebo in the primary composite endpoint (MI, stroke, hospitalization for unstable angina, arterial revascularization, or CVD death) was reported for both men (hazard ratio [HR] 0.58; 95% confidence interval [CI] 0.45–0.73; p < 0.001) and women (HR 0.54; 95% CI 0.37–0.80; p = 0.002), with no significant treatment-by-sex interaction (p = 0.80).
A meta-analysis of 11 randomized, double-blind, placebo-controlled CVD outcome trials of statins for secondary prevention found that statins were effective for prevention of CVD endpoints in women and men overall; stratification by sex found that statins did not achieve significance versus placebo for the endpoints of all-cause mortality and stroke in women.42 A systematic review of primary prevention studies supported that statins have benefits for primary prevention even among low-risk groups such as women.43
The largest meta-analysis comparing statin outcomes by sex (27 randomized clinical trials; N = 174,149; 26.8% women) found that, although women had a lower CVD risk than men, the proportional reduction in major vascular events with statin treatment among women was comparable to the reduction among men (adjusted p = 0.33 for heterogeneity).44 Statin effects were comparable for women and men with a definite history of CVD (secondary prevention: adjusted p = 0.431 for heterogeneity); however, the risk reduction among individuals with no known history of CVD was smaller in women (primary prevention: adjusted p = 0.02 for heterogeneity). These data support that statins reduce major vascular events comparably in women and men with equivalent CVD risk.
Nonstatin treatments for lowering LDL-C
Add-on therapy to statins for further LDL-C reduction has emerged as an effective strategy for addressing residual CVD risk. The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) (N = 18,144; 24.3% women) demonstrated that ezetimibe, in combination with simvastatin, reduced the primary composite endpoint (CVD death, nonfatal MI, rehospitalization for unstable angina, or coronary revascularization) in patients >50 years of age, who had been hospitalized within 10 days of randomization for ACS.45,46 After 7 years of follow-up, the HR for the reduction in CVD events for ezetimibe versus placebo was 0.88 (95% CI 0.79–0.99) for women and 0.95 (95% CI 0.90–1.01) for men, with no statistically significant difference between sexes (p = 0.26).
The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) study (N = 27,564; 24.6% women) demonstrated that the PCSK9 inhibitor evolocumab, in combination with a statin, significantly reduced the primary composite endpoint (CVD death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) in patients 40 to 85 years of age with clinically evident atherosclerotic CVD. A sex-based subanalysis found that the HR for the relative risk reduction in the primary endpoint was 0.81 (95% CI 0.69–0.95) for women and 0.86 (95% CI 0.80–0.94) for men, with no difference between sexes (p for heterogeneity was not significant).47 These data suggest that the benefits of ezetimibe and evolocumab as add-on therapy in statin-treated patients are comparable between women and men.
Nonstatin treatments: beyond targeting LDL-C
Beyond LDL-C, TG has been a long-standing theoretical therapeutic target for addressing residual CVD risk. However, high-profile trials of TG-lowering treatments have reported inconsistent findings overall and/or with respect to women (Table 1).5–9,48–54 Studies of the TG-lowering drug fenofibrate yielded mixed results. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study (N = 9,795; 37.3% women) randomized patients with type 2 diabetes who were not using a statin to fenofibrate or placebo.5 Improvements with fenofibrate relative to placebo were greater in women versus men for both LDL-C (9.8% vs. 3.3% at study end, p < 0.001) and total cholesterol (9.5% vs. 5.2%, p < 0.001); changes in high-density lipoprotein cholesterol (HDL-C) and TG were similar for women and men. In the overall population, there was no significant improvement in the primary composite endpoint (HR 0.89; 95% CI 0.75–1.05; p = 0.16). Although the p-value for the interaction analysis was nonsignificant (p = 0.3), in subgroup analyses, total CVD events were reduced by 20% in women (HR 0.8; 95% CI 0.64–0.99; p = 0.04) and nonsignificantly by 8% in men (HR 0.92; 95% CI 0.81–1.05; p = 0.2). Among patients who did not have CVD at study entry, fenofibrate reduced the risk of total CVD events by 26% in women and 16% in men (both p = 0.04), with no significant interaction by sex (p = 0.45).
Table 1.
Summary of Results From Select Cardiovascular Outcome Trials
| Trial |
Intervention |
Patient population |
Primary endpoint |
Sex-based findings for primary endpoint |
|---|---|---|---|---|
| Fibrates | ||||
| FIELD5,48 | Fenofibrate vs. placebo for primary prevention | • N = 9,795 • n = 3,657 women; n = 6,138 men • 50–75 years of age with type 2 diabetes, and not using a statin |
• First occurrence of nonfatal MI or death from coronary heart disease • Primary endpoint met = No |
• Women: HR 0.80 (95% CI 0.64–0.99; p = 0.04) • Men: HR 0.92 (95% CI 0.81–1.05; p = 0.2) • p = 0.3 for difference by sex |
| ACCORD6 | Fenofibrate + statin vs. placebo + statin | • N = 5,518 • n = 1,694 women; n = 3,824 men • Type 2 diabetes • 40–79 years of age if clinical CVD, or 55–79 years of age if subclinical CVD or ≥2 additional risk factors |
• First occurrence of major cardiovascular event, including nonfatal MI, nonfatal stroke, or death from cardiovascular causes • Primary endpoint met = No |
• Women: event rate 9.05% fenofibrate vs. 6.64% placebo • Men: event rate 11.18% fenofibrate vs. 13.30% placebo • p = 0.01 for difference by sex |
| Niacin | ||||
| AIM-HIGH8 | Niacin + statin vs. placebo + statin | • N = 3,414 • n = 504 women; n = 2,910 men • ≥45 years of age with established ASCVD and atherogenic dyslipidemia |
• Composite of the first event of death from coronary heart disease, nonfatal MI, ischemic stroke, hospitalization (for >23 hours) for an ACS, or symptom-driven coronary or cerebral revascularization • Primary endpoint met = No |
NR |
| HPS2-THRIVE49 | Niacin + laropiprant added to statin vs. placebo added to statin for secondary prevention | • N = 25,673 • n = 4,444 women; n = 21,229 men • 50–80 years of age with a history of MI, cerebrovascular disease, PAD, or diabetes with evidence of symptomatic coronary disease |
• Major vascular events (nonfatal MI, death from coronary causes, stroke of any type, or coronary or noncoronary revascularization) • Primary endpoint met = No |
• Women: event rate 13.4% niacin+laropiprant vs. 12.3% placebo • Men: event rate 13.2% niacin+laropiprant vs. 14.0% placebo • p = 0.07 for difference by sex |
| Omega-3 fatty acids | ||||
| JELIS50 | EPA-only omega-3 fatty acid 1.8 g/day + statin vs. statin alone for primary and secondary prevention | • N = 18,645 • n = 12,786 postmenopausal women up to 75 years of age with hypercholesterolemia • n = 5,859 men 40–75 years of age |
• Major coronary events, including sudden cardiac death, fatal and nonfatal MI, unstable angina pectoris, angioplasty, stenting, or coronary artery bypass grafting • Primary endpoint met = Yes |
• Women: HR 0.87 (0.68–1.13) • Men: HR 0.76 (95% CI 0.62–0.94) • p = 0.43 for difference by sex |
| OMEGA51 | Combination omega-3 fatty acid (DHA+EPA) ∼1 g/day vs. placebo for secondary prevention | • N = 3,818 • n = 977 women; n = 2,841 men • >18 years of age and admitted to the hospital for acute MI • Patients all received “guideline-adjusted treatment for acute MI” • Majority were on statins |
• Sudden cardiac death and/or sudden cardiac arrest with initially successful cardiopulmonary resuscitation and subsequent death during the hospital stay within 3 weeks • Primary endpoint met = No |
Stated that “sex-based differences were not present” |
| ORIGIN52 | Combination omega-3 fatty acid (DHA+EPA) 900 mg vs. placebo for primary prevention | • N = 12,536 • n = 4,386 women; n = 8,150 men • ≥50 years of age with diabetes/dysglycemia and additional CVD risk factors • ∼50 on statins at baseline |
• Death from cardiovascular causes • Primary endpoint met = No |
NR |
| Risk and Prevention Study53 | Combination omega-3 fatty acid (DHA+EPA) 1 g/day vs. placebo for primary prevention | • N = 12,505 • n = 4,818 women; n = 7,687 men • CVD or high risk for CVD • ∼40% on statins at baseline |
• Composite of time to death from cardiovascular causes or hospital admission for cardiovascular causes • Primary endpoint met = No |
• Women: HR, 0.82; 95% CI, 0.67–0.99 • Men: HR 1.04; 95% CI 0.92–1.17 • p = 0.04 for interaction |
| ASCEND54 | Combination omega-3 fatty acid (DHA+EPA) <1 g/day for primary prevention | • N = 15,480 • n = 5,796 women; n = 9,684 men • ≥40 years of age with diabetes but no evidence of CVD • ∼75% on statins at baseline |
• Composite of nonfatal MI or stroke (excluding confirmed intracranial hemorrhage), TIA, or vascular death, excluding intracranial hemorrhage • Primary endpoint met = No |
• Women: HR 0.99 (0.89–1.11) • Men: HR 1.0 (0.84–1.18) • p = 0.99 for heterogeneity |
| VITAL7 | Combination omega-3 fatty acid (DHA+EPA) <1 g/day and vitamin D vs. placebo for primary prevention | • N = 25,871 • n = 13,085 women >55 years of age • n = 12,786 men >50 years of age • Approximately one-third on statins at baseline |
• Major cardiovascular events (composite of MI, stroke, and death from cardiovascular causes) and invasive cancer of any type • Primary endpoint met = No |
• Women: HR 0.93 (95% CI 0.76–1.15) • Men: HR 0.91 (95% CI 0.76–1.10) • p = 0.88 for difference by sex |
| REDUCE-IT9 | Icosapent ethyl (EPA) 4 g/day + statin vs. statin alone for primary or secondary prevention | • N = 8,179 • n = 2,357 women; n = 5,822 men • ≥45 years of age with established CVD or ≥50 years of age with diabetes and ≥1 additional risk factor(s) |
• Composite of cardiovascular death, nonfatal MI (including silent MI), nonfatal stroke, coronary revascularization, or unstable angina in a time-to-event analysis • Primary endpoint met = Yes |
• Women: HR 0.82 (95% CI 0.66–1.01) • Men: HR 0.73 (95% CI 0.65–0.82) • p = 0.33 for interaction |
ACCORD, Action to Control Cardiovascular Risk in Diabetes; ACS, acute coronary syndrome; AIM-HIGH, Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes; ASCEND, A Study of Cardiovascular Events in Diabetes; ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FIELD, Fenofibrate Intervention and Event Lowering in Diabetes; HDL, high-density lipoprotein; HPS2-THRIVE, Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events; HR, hazard ratio; JELIS, Japan EPA Lipid Intervention Study; MI, myocardial infarction; NR, not reported; ORIGIN, Outcome Reduction with an Initial Glargine Intervention; PAD, peripheral artery disease; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial; TIA, transient ischemic attack; VITAL, Vitamin D and Omega-3 Trial.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study (N = 5,518; 30.7% women) found that addition of fenofibrate to a statin did not reduce the rate of CVD endpoints compared with statin alone in the majority of high-risk patients with diabetes mellitus. A significant interaction by sex favoring men was found (p = 0.01): women had a higher CVD event rate with fenofibrate plus statin than with placebo plus statin (9.1% vs. 6.6%), whereas men had a lower CVD event rate with fenofibrate plus statin than with placebo plus statin (11.2% vs. 13.3%).6 These collective data show that fibrates did not reduce CVD risk overall, with potentially negative findings regarding effects in women.
Initial interest in niacin derived from the theory that drugs that increase HDL-C could potentially reduce CVD risk.55 Studies of niacin in the secondary prevention of CVD were highly anticipated, yet had disappointing findings.8,49 The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study (N = 3,414; <15% women) found that addition of niacin to statin treatment failed to reduce CVD events in patients with atherosclerotic CVD and LDL-C <70 mg/dL despite increases in HDL-C and decreases in TG.8
Subsequently, the Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) (N = 25,673; <18% women) found that adding a combination of niacin with laropiprant to simvastatin ± ezetimibe did not reduce major vascular events, and increased the risk of serious adverse events.49 A sex-based subanalysis of HPS2-THRIVE showed a trend (p = 0.07) toward worse CVD outcomes among niacin-treated women.
Investigations of omega-3 fatty acids for CVD risk reduction have reported mixed findings. The Japan EPA Lipid Intervention Study (JELIS), an open-label trial conducted in a Japanese population (N = 19,466; 69% women) with hypercholesterolemia, randomized patients to EPA 600 mg three times a day on a background of statin treatment or to statin treatment alone.50 The EPA group had a significant relative risk reduction of 19% in major CVD events compared with the control group (HR 0.81; 95% CI 0.69–0.95; p = 0.011) over 5 years. The CVD event rate was lower for women than men in both the control and EPA-treated groups. The HR for the reduction in CVD events with EPA versus placebo was 0.87 (95% CI 0.68–1.13) for women and 0.76 (95% CI 0.62–0.94) for men, with no interaction by sex (p = 0.43). EPA was associated with a significant improvement in secondary prevention of CVD events (HR 0.81; 95% CI 0.66–1.0; p = 0.048), but did not reach statistical significance for primary prevention (HR 0.82; 95% CI 0.63–1.06).
Subsequent omega-3 fatty acid CVD outcome trials investigating DHA+EPA combinations have failed to achieve their primary endpoints (Table 1).7,51–54 Among outcome trials of DHA+EPA that reported sex-based subanalyses, only the Risk and Prevention Study found a difference between sexes (p = 0.04 for interaction): a significantly lower rate of CVD events was found among women receiving DHA+EPA versus placebo (HR 0.82; 95% CI 0.67–0.99), with no significant finding in men (HR 1.04; 95% CI 0.92–1.17).53
The most recent combination omega-3 fatty acid outcome trial, the Vitamin D and Omega-3 Trial (VITAL) (N = 25,871; 51% women), randomized patients in a factorial design to receive a low dose (1 g/day) of DHA+EPA, vitamin D3 (200 IU) supplementation, or placebo.7,56 After 5.3 years of follow-up, neither combination omega-3 fatty acids nor vitamin D3 was associated with a significantly lower incidence of major CVD events or invasive cancer. An analysis of major CVD events by sex subgroup, comparing the combination omega-3 fatty acid group with the placebo group, found that the HRs for major CVD events for women and men were 0.93 (95% CI 0.76–1.15) and 0.91 (95% CI 0.76–1.10), respectively (p = 0.88 for interaction).
The contrast between positive findings in JELIS for EPA in CVD risk reduction and subsequent negative studies of DHA+EPA combination omega-3 fatty acids suggests that EPA may have differential effects on CVD risk reduction. Insights regarding the effects of EPA in women can be gained from analyses of the Multi-Center, PlAcebo-Controlled, Randomized, Double-BlINd, 12-week study with an open-label Extension (MARINE) and ANCHOR studies, which found that in women at elevated risk of CVD with TG ≥500 mg/dL (MARINE) and ≥200 to <500 mg/dL (ANCHOR), icosapent ethyl 4 g/day significantly reduced TG in women by 21.5% and 22.7% in MARINE and ANCHOR, respectively, without an increase in LDL-C; these findings were generally comparable to findings in the overall study populations.57 Similar results were seen in a post hoc analysis of women with diabetes from the ANCHOR study.58 Changes in other atherogenic lipid, lipoprotein, and inflammatory parameters were favorable and generally consistent with results of the overall study that included women and men.57–63
Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial
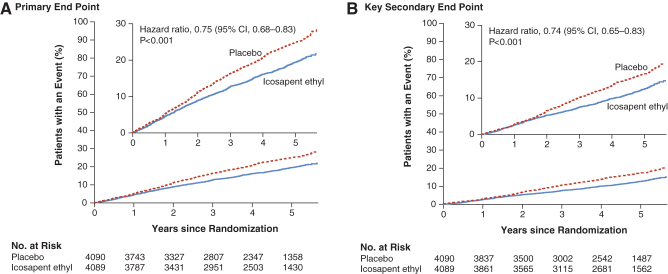
The recently published REDUCE-IT study (N = 8,179; 29% women) investigated CVD outcomes with icosapent ethyl 4 g/day, in addition to statin treatment in patients with elevated TG (135–499 mg/dL) who were ≥45 years of age with established CVD or ≥50 years of age with type 2 diabetes and at least one additional risk factor.9 Patients were randomized to icosapent ethyl or placebo. The primary composite endpoint (CVD death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina) occurred in significantly fewer patients in the icosapent ethyl group (17.2%) compared with the placebo group (22.0%) (HR 0.75; 95% CI 0.68–0.83; p < 0.001) after a median 4.9 years of follow-up (Fig. 2A). These data correspond to a number needed to treat to avoid one primary endpoint event of 21 (95% CI 15–33).
FIG. 2.
Cumulative incidence of cardiovascular events in REDUCE-IT.9 Kaplan-Meier event curves for the primary efficacy endpoint, defined as a composite of CVD death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina in a time-to-event analysis (A); and the key secondary endpoint, defined as a composite of CVD death, nonfatal MI, or nonfatal stroke in a time-to-event analysis (B). In each panel, the inset shows the same data on an expanded y axis. The curves were visually truncated at 5.7 years because a limited number of events occurred beyond that time point; all patient data were included in the analyses. CVD, cardiovascular disease; MI, myocardial infarction; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial. From Bhatt et al., ©2019 Massachusetts Medical Society.9 Reprinted with permission from Massachusetts Medical Society.
Similarly, the key secondary composite endpoint (CVD death, nonfatal MI, or nonfatal stroke) occurred in 11.2% of patients in the icosapent ethyl group and 14.8% in the placebo group (HR 0.74; 95% CI 0.65–0.83; p < 0.001; Fig. 2B). The number needed to treat to prevent one key secondary endpoint was 28 (95% CI 20–47).9
As reported in JELIS, the overall rate of coronary events was lower with women than with men. A subgroup analysis by sex in REDUCE-IT found that, in women, event rates for the primary endpoint were 13.3% in the icosapent ethyl arm and 15.6% in the placebo group (HR 0.82; 95% CI 0.66–1.01), whereas for men, the event rates were 18.8% and 24.7% in the icosapent ethyl and placebo groups, respectively (HR 0.73; 95% CI 0.65–0.82) (Table 2). The p-value for interaction by sex was not statistically significant (p = 0.33), suggesting that both women and men benefitted equally from treatment.
Table 2.
Gender Differences in Primary and Secondary Endpoints in Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial9
| HR (95% CI) | p-Value for interaction | |
|---|---|---|
| Primary endpoint: composite of cardiovascular death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina | ||
| Male | 0.73 (0.65–0.82) | 0.33a |
| Female | 0.82 (0.66–1.01) | |
| Key secondary endpoint: composite of cardiovascular death, nonfatal MI, or nonfatal stroke | ||
| Male | 0.72 (0.62–0.82) | 0.44a |
| Female | 0.80 (0.62–1.03) | |
Interaction p-value not statistically significant, denoting comparable benefit.
CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
Comparable findings were reported for the key secondary endpoint when analyzed by sex (Table 2). Overall, the adverse events and serious adverse events in REDUCE-IT were low and similar to placebo. An issue that has been raised is that the placebo (mineral oil) was associated with small increases in LDL-C and high-sensitivity C-reactive protein levels; however, an independent review suggested this could not explain the observed benefit associated with the intervention.64 Moreover, the CVD event rate in the placebo group was similar to that expected for comparable trials.
Based on the efficacy and safety profile demonstrated in REDUCE-IT, the United States Food and Drug Administration recently approved icosapent ethyl for an expanded indication as an adjunct to maximally tolerated statin therapy to reduce the risk of MI, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated TG levels (≥150 mg/dL) and established CVD, or diabetes mellitus and two or more additional risk factors for CVD.65 Following suit, Health Canada recently approved the use of icosapent ethyl to reduce the risk of cardiovascular events (cardiovascular death, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina) in statin-treated patients with elevated TG who are at high risk of cardiovascular events due to established CVD or diabetes and at least one other cardiovascular risk factor.66
In contrast to the positive findings in REDUCE-IT and JELIS for stable prescription EPA-only products, recent CVD outcome trials of combination omega-3 fatty acids have not demonstrated reduced CVD risk. A differentiating factor between these clinical studies could be that combination omega-3 fatty acids contain DHA, which has been associated with increases in LDL-C,67,68 which may mitigate the beneficial effects in CVD risk reduction.
A number of ongoing studies will provide additional insights into the role of omega-3 fatty acids in CVD risk reduction.69–71 The STRENGTH trial, which utilized 4 g/day of an EPA ± DHA mixture, was recently stopped due to futility.72 Trial details should be forthcoming from the investigators; however, the lack of benefit of the combined EPA ± DHA mixture is consistent with other combination omega-3 fatty acid trials, with published data showing lack of efficacy.
Conclusions
Women have unique barriers to the prevention of CVD. Underestimation of CVD risk in women may hinder appropriate preventive therapy. Women with CVD have been shown to have greater risk of adverse CVD outcomes than men, and women have a high risk of CVD associated with diabetes; therefore, application of evidence-based therapies in women is of paramount importance. Statins and other LDL-C–lowering treatments appear to have comparable benefits in high-risk women and men. In contrast, trials of treatments targeting other lipids have had mixed or negative results. The recently published REDUCE-IT study showed that addition of icosapent ethyl to statin treatment significantly reduced CVD events in patients at high risk of CVD, with comparable residual CVD risk reduction in women and men.
Acknowledgments
The genesis of this article was based on presentations from co-authors that took place during a Medical Advisory board supported by Amarin Pharma, Bedminster, NJ.
Author Disclosure Statement
L.M.: Consultant, Speaker's Bureau, Equity Investment: Amarin Pharma, Inc. Consultant: Livongo.
A.M.N.: Grant/research support: Amarin Pharma, Inc., Amgen, Regeneron, Sanofi, and Janssen; Consultant: Amarin Pharma, Inc., Amgen, Regeneron, Sanofi, Janssen, NovoNordisk, and AstraZeneca. Other financial or material support: NHLBI K01HL133416.
N.K.W.: No disclosures to report.
Funding Information
Medical writing assistance was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and supported by Amarin Pharma, Inc, Bedminster, NJ, USA.
References
- 1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019;139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 2. Institute of Medicine Committee on Understanding the Biology of Sex and Gender Differences. The National Academies Collection: Reports funded by National Institutes of Health. Wizemann TM, Pardue ML, eds. Exploring the biological contributions to human health: Does sex matter? Washington, DC: National Academies Press (US), 2001 [PubMed] [Google Scholar]
- 3. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. American Heart Association scientific statement. Arterioscler Thromb Vasc Biol 2004;24:e29–e50 [DOI] [PubMed] [Google Scholar]
- 4. Institute of Medicine. Women's health research: Progress, pitfalls, and promise. Washington, DC: The National Academies Press, 2010 [PubMed]
- 5. d'Emden MC, Jenkins AJ, Li L, et al. Favourable effects of fenofibrate on lipids and cardiovascular disease in women with type 2 diabetes: Results from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 2014;57:2296–2303 [DOI] [PubMed] [Google Scholar]
- 6. The ACCORD Study Group, Ginsberg HN, Elam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manson JE, Cook NR, Lee IM, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The AIM-HIGH Investigators, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267 [DOI] [PubMed] [Google Scholar]
- 9. Bhatt DL, Steg G, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22 [DOI] [PubMed] [Google Scholar]
- 10. Humphries KH, Izadnegahdar M, Sedlak T, et al. Sex differences in cardiovascular disease—Impact on care and outcomes. Front Neuroendocrinol 2017;46:46–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mosca L, Manson JE, Sutherland SE, Langer RD, Manolio T, Barrett-Connor E. Cardiovascular disease in women: A statement for healthcare professionals from the American Heart Association. Writing Group. Circulation 1997;96:2468–2482 [DOI] [PubMed] [Google Scholar]
- 12. Manson JE, Spelsberg A. Risk modification in the diabetic patient. In: Manson JE, Ridker PM, Gaziano JM, Hennekens CH, eds. Prevention of myocardial infarction. New York, NY: Oxford University Press, 1996:241–273 [Google Scholar]
- 13. Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manage 2007;3:853–876 [PMC free article] [PubMed] [Google Scholar]
- 14. Ghosh A, Gao L, Thakur A, Siu PM, Lai CWK. Role of free fatty acids in endothelial dysfunction. J Biomed Sci 2017;24:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L, Manson JE, Forman JP, Gaziano JM, Buring JE, Sesso HD. Dietary fatty acids and the risk of hypertension in middle-aged and older women. Hypertension 2010;56:598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gerber RT, Holemans K, O'Brien-Coker I, et al. Cholesterol-independent endothelial dysfunction in virgin and pregnant rats fed a diet high in saturated fat. J Physiol 1999;517 (Pt 2):607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vogel RA. Coronary risk factors, endothelial function, and atherosclerosis: A review. Clin Cardiol 1997;20:426–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berger JS, Elliott L, Gallup D, et al. Sex differences in mortality following acute coronary syndromes. JAMA 2009;302:874–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hochman JS, Tamis JE, Thompson TD, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global use of strategies to open occluded coronary arteries in acute coronary syndromes IIb Investigators. N Engl J Med 1999;341:226–232 [DOI] [PubMed] [Google Scholar]
- 20. Bessesen DH, Cox-York KA, Hernandez TL, et al. Postprandial triglycerides and adipose tissue storage of dietary fatty acids: Impact of menopause and estradiol. Obesity (Silver Spring) 2015;23:145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karalis DG, Wild RA, Maki KC, et al. Gender differences in side effects and attitudes regarding statin use in the Understanding Statin Use in America and Gaps in Patient Education (USAGE) study. J Clin Lipidol 2016;10:833–841 [DOI] [PubMed] [Google Scholar]
- 22. Walsh MN, Joynt KE. Delays in seeking care: A women's problem? Circ Cardiovasc Qual Outcomes 2016;9(2 Suppl 1):S97–S99 [DOI] [PubMed] [Google Scholar]
- 23. Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the American Heart Association. Circulation 2011;123:1243–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. St-Onge MP, Grandner MA, Brown D, et al. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American Heart Association. Circulation 2016;134:e367–e386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Low CA, Thurston RC, Matthews KA. Psychosocial factors in the development of heart disease in women: Current research and future directions. Psychosom Med 2010;72:842–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mosca L, Linfante AH, Benjamin EJ, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation 2005;111:499–510 [DOI] [PubMed] [Google Scholar]
- 27. Navar-Boggan AM, Peterson ED, D'Agostino RB, Sr., Pencina MJ, Sniderman AD.. Using age- and sex-specific risk thresholds to guide statin therapy: One size may not fit all. J Am Coll Cardiol 2015;65:1633–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kannel WB, Sorlie P, McNamara PM. Prognosis after initial myocardial infarction: The Framingham study. Am J Cardiol 1979;44:53–59 [DOI] [PubMed] [Google Scholar]
- 29. Bhatt DL, Roe MT, Peterson ED, et al. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: Results from the CRUSADE Quality Improvement Initiative. JAMA 2004;292:2096–2104 [DOI] [PubMed] [Google Scholar]
- 30. Yan AT, Yan RT, Tan M, et al. Management patterns in relation to risk stratification among patients with non-ST elevation acute coronary syndromes. Arch Intern Med 2007;167:1009–1016 [DOI] [PubMed] [Google Scholar]
- 31. Poon S, Goodman SG, Yan RT, et al. Bridging the gender gap: Insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am Heart J 2012;163:66–73 [DOI] [PubMed] [Google Scholar]
- 32. Donataccio MP, Puymirat E, Parapid B, et al. In-hospital outcomes and long-term mortality according to sex and management strategy in acute myocardial infarction. Insights from the French ST-elevation and non-ST-elevation Myocardial Infarction (FAST-MI) 2005 Registry. Int J Cardiol 2015;201:265–270 [DOI] [PubMed] [Google Scholar]
- 33. Vaccarino V, Rathore SS, Wenger NK, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med 2005;353:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Canto JG, Rogers WJ, Goldberg RJ, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012;307:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nanna MG, Wang TY, Xiang Q, et al. Sex differences in the use of statins in community practice. Circ Cardiovasc Qual Outcomes 2019;12:e005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peters SAE, Colantonio LD, Zhao H, et al. Sex differences in high-intensity statin use following myocardial infarction in the United States. J Am Coll Cardiol 2018;71:1729–1737 [DOI] [PubMed] [Google Scholar]
- 37. Kim ES, Menon V. Status of women in cardiovascular clinical trials. Arterioscler Thromb Vasc Biol 2009;29:279–283 [DOI] [PubMed] [Google Scholar]
- 38. Scott PE, Unger EF, Jenkins MR, et al. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J Am Coll Cardiol 2018;71:1960–1969 [DOI] [PubMed] [Google Scholar]
- 39. National Institutes of Health. NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. 2001. Available at: https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm Accessed January31, 2020
- 40. Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: Results from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010;121:1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207 [DOI] [PubMed] [Google Scholar]
- 42. Gutierrez J, Ramirez G, Rundek T, Sacco RL. Statin therapy in the prevention of recurrent cardiovascular events: A sex-based meta-analysis. Arch Intern Med 2012;172:909–919 [DOI] [PubMed] [Google Scholar]
- 43. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013:CD004816. [DOI] [PMC free article] [PubMed]
- 44. Fulcher J, O'Connell R, Voysey M, et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397–1405 [DOI] [PubMed] [Google Scholar]
- 45. Kato ET, Cannon CP, Blazing MA, et al. Efficacy and safety of adding ezetimibe to statin therapy among women and men: Insight from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). J Am Heart Assoc 2017;6:e006901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397 [DOI] [PubMed] [Google Scholar]
- 47. Sever PS, Gouni-Berthold I, Keech A, et al. Benefit of LDL-C lowering with evolocumab on cardiovascular outcomes by age & sex: An analysis of the FOURIER trial [abstract 5002]. Eur Heart J 2018;39(suppl 1):1041–1042 [Google Scholar]
- 48. The FIELD Study Investigators, Keech A, Simes RJ, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005;366:1849–1861 [DOI] [PubMed] [Google Scholar]
- 49. The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–212 [DOI] [PubMed] [Google Scholar]
- 50. Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–1098 [DOI] [PubMed] [Google Scholar]
- 51. Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010;122:2152–2159 [DOI] [PubMed] [Google Scholar]
- 52. ORIGIN Trial Investigators. n-3 Fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309–318 [DOI] [PubMed] [Google Scholar]
- 53. The Risk and Prevention Study Collaborative Group. n-3 Fatty acids in patients with multiple cardiovascular risk factors: The Risk and Prevention Study Collaborative Group. N Engl J Med 2013;368:1800–1808 [DOI] [PubMed] [Google Scholar]
- 54. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379:1540–1550 [DOI] [PubMed] [Google Scholar]
- 55. D'Andrea E, Hey SP, Ramirez CL, Kesselheim AS. Assessment of the role of niacin in managing cardiovascular disease outcomes: A systematic review and meta-analysis. JAMA Network Open 2019;2:e192224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mosca L, Ballantyne CM, Bays HE, et al. Usefulness of icosapent ethyl (eicosapentaenoic acid ethyl ester) in women to lower triglyceride levels (results from the MARINE and ANCHOR trials). Am J Cardiol 2017;119:397–403 [DOI] [PubMed] [Google Scholar]
- 58. Brinton EA, Ballantyne CM, Guyton JR, et al. Lipid effects of icosapent ethyl in women with diabetes mellitus and persistent high tiglycerides on statin treatment: ANCHOR trial subanalysis. J Womens Health (Larchmt) 2018;27:1170–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol 2011;108:682–690 [DOI] [PubMed] [Google Scholar]
- 60. Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol 2012;110:984–992 [DOI] [PubMed] [Google Scholar]
- 61. Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: Effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs 2013;13:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ballantyne CM, Bays HE, Braeckman RA, et al. Icosapent ethyl (eicosapentaenoic acid ethyl ester): Effects on plasma apolipoprotein C-III levels in patients from the MARINE and ANCHOR studies. J Clin Lipidol 2016;10:635–645 [DOI] [PubMed] [Google Scholar]
- 63. Ballantyne CM, Bays HE, Philip S, et al. Icosapent ethyl (eicosapentaenoic acid ethyl ester): Effects on remnant-like particle cholesterol from the MARINE and ANCHOR studies. Atherosclerosis 2016;253:81–87 [DOI] [PubMed] [Google Scholar]
- 64. FDA Briefing Document Endocrinologic and Metabolic Drugs Advisory Committee Meeting. 2019. Available at: https://www.fda.gov/media/132477/download Accessed January31, 2020
- 65. Vascepa [package insert]. Bridgewater, NJ: Amarin Pharma, Inc., 2019 [Google Scholar]
- 66. Vascepa [product monograph]. Etobicoke, Canada: HLS Therapeutics, 2019 [Google Scholar]
- 67. Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr Atheroscler Rep 2011;13:474–483 [DOI] [PubMed] [Google Scholar]
- 68. Jacobson TA, Glickstein SB, Rowe JD, Soni PN. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: A review. J Clin Lipidol 2012;6:5–18 [DOI] [PubMed] [Google Scholar]
- 69. Budoff M, Brent Muhlestein J, Le VT, May HT, Roy S, Nelson JR. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499 mg/dL) on statin therapy: Rationale and design of the EVAPORATE study. Clin Cardiol 2018;41:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Randomized trial for evaluation in secondary prevention efficacy of combination therapy—Statin and eicosapentaenoic acid UMIN000012069. UMIN Clinical Trials Registry 2016. Available at: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000014051&type=summary&language=E Accessed January31, 2020
- 71. Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin treated patients with high levels of triglycerides and low levels of high density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin Cardiol 2018;41:1281–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. AstraZeneca ends cardiovascular outcomes study of Epanova as unlikely to show benefit [press release]. 2020. Available at: https://m.firstwordpharma.com/astrazeneca-ends-cardiovascular-outcomes-study-epanova-unlikely-show-benefit Accessed January31, 2020