Abstract
Background: Hürthle cell/oncocytic change is commonly reported on thyroid fine-needle aspiration (FNA) and may be considered an “atypical cell” by clinicians. This study aims to delineate the association between Hürthle cells in preoperative cytology and subsequent pathology of the indexed thyroid nodule and to report rates of malignancy.
Methods: Retrospective review of records of 300 patients with Hürthle cell/oncocytic change on FNA and final surgical pathology at a tertiary referral center between 2000 and 2013 was performed and compared with a multi-institutional FNA cohort. The degree of Hürthle cell presence was correlated with histopathologic diagnoses.
Results: In the Hürthle cell FNA group, Bethesda System for Reporting Thyroid Cytopathology (BSRTC) categories were as follows: I (nondiagnostic) 14 (4.7%); II (benign) 113 (37.7%); III (atypia of undetermined significance/follicular lesion of undetermined significance) 33 (11%); IV (follicular neoplasm/suspicious for a follicular neoplasm) 125 (41.6%); V (suspicious for malignancy) 12 (4%); and VI (malignant) 3 (1%). When categorized based on the degree of Hürthle cell change, 59 (29%) were classified as mild, 13 (6%) moderate, and 131 (65%) as predominant. When comparing the results with a multi-institutional FNA cohort (all with surgical confirmation), the presence of Hürthle cells was found to be associated with a lower risk of malignancy in all BSRTC categories, with a statistically significant difference in the BSRTC IV and V groups. The sole exception was when Hürthle cell presence was classified as predominant (defined as >75% of the cellular population); the rate of malignancy was significantly elevated in FNAs interpreted as benign/Bethesda II.
Conclusions: Although Hürthle cells have been considered by clinicians as an “atypical cell,” their presence does not increase the risk of malignancy within BSRTC categories overall. However, when predominant Hürthle cell change is present, the risk of malignancy is increased in the benign cytology/BSRTC category II.
Keywords: Hürthle cell, fine-needle aspiration, thyroid malignancy, Bethesda System for Reporting Thyroid Cytopathology, rate of malignancy, oncocytes
Introduction
Fine-needle aspiration (FNA) is a widely accepted technique and is commonly utilized for the preoperative evaluation of thyroid nodules (1,2). FNA results are reported in a standardized manner using the Bethesda System for Reporting Thyroid Cytopathology (BSRTC) criteria, which have recently been updated, and provide rate of malignancy information that is crucial to guide subsequent treatment (3).
The presence of Hürthle cell/oncocytic change in BSRTC categories further poses a diagnostic challenge for cytopathologists, endocrinologists, and surgeons. Hürthle cells, also known as oncocytes, are large polygonal cells with abundant granular eosinophilic cytoplasm and prominent nucleoli (4). Hürthle cell/oncocytic change has been shown to be associated with both benign and malignant thyroid lesions, including benign conditions such as Hashimoto thyroiditis (HT), multinodular goiter, as well as neoplasms including Hürthle cell adenoma, and Hürthle cell carcinoma (5). One cannot reliably distinguish between Hürthle cell adenoma and Hürthle cell carcinoma using cytomorphologic features alone (6,7). The 2017, the BSRTC classification denotes Hürthle cells as a subcategory in the follicular neoplasm or suspicious for a follicular neoplasm (FN/SFN) category but does not provide specific information to the clinician as to its implication with respect to the rate of malignancy. Moreover, the degree of Hürthle cell presence in FNA cytology can vary greatly, but little is known about the correlation of the degree of Hürthle cell features with the rate of malignancy on final pathology (8).
Given that Hürthle cells may be interpreted by clinicians as “atypical cells” and are frequently reported on FNA reports, clinicians may be uncertain as to how to interpret the presence of Hürthle cells as it relates to the BSRTC classification and the implied rates of malignancy. Many clinicians assume that the presence of Hürthle cells increases the risk of malignancy beyond the risk predicted by a given BSRTC category. This study aimed to (i) determine the histopathologic outcome and risk of malignancy in a series of thyroid FNAs containing Hürthle cell/oncocytic change classified by the standard BSRTC criteria; (ii) determine the histological outcomes and risks of malignancy in the same FNA dataset subcategorized based on the degree of Hürthle cell changes; and (iii) ultimately, help inform clinicians how to optimally interpret Hürthle cell information in the context of the BSRTC classification.
Materials and Methods
This retrospective study was approved by our Institutional Review Board (Protocol No. 14-050H). A list of all patients with thyroid FNA biopsy containing Hürthle cell/oncocytes was obtained by searching the institutional pathology database from 2000 to 2013. Data acquisition from this time frame provided the largest dataset that was available with optimally detailed data fields needed for our study and thus afforded the most robust dataset. A search of all FNA reports with mention of Hürthle cells or oncocytes yielded 1421 reports. During pre-2009 years, the reporting system that was used at our institution was analogous to the BSRTC. Since 2009, our institution uses the BSRTC for cytology reporting. Medical records of these 1421 cases were reviewed, and 377 subjects were identified to have a subsequent surgical resection of the biopsied nodule. Further, 77 patients with FNA reports that did not include the BSRTC category were excluded. The remaining 300 subjects were ultimately selected as the study cohort. Electronic medical records of the cohort were also retrospectively reviewed to collect demographic information.
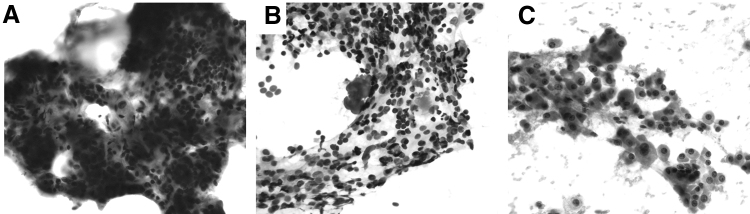
FNA reports were characterized using two different systems. First, based on the FNA reports, these 300 subjects were categorized by their BSRTC class: I. nondiagnostic, II. benign, III. atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS), IV. FN/SFN, V. suspicious for malignancy (SM), and VI. malignant. Of 300 FNAs, 203 slides were available for review. These 203 FNA slides were reviewed by the senior thyroid cytopathologist (W.C.F.) to further classify them based on the degree of Hürthle cells present. The groups included (i) mild Hürthle cell presence: Hürthle cells comprised of <25% of the cellular content; (ii) moderate Hürthle cell presence: Hürthle cells accounted for 25% to 75% of the cellular content; and (iii) predominant Hürthle cell presence: Hürthle cells accounted for >75% of the cellular content. Representative cytopathology images for these groups are shown in Figure 1. A published multi-institutional study with a cohort of 1827 continuous thyroid FNA cases between January 1, 2013 and June 30, stratified with BSRTC from five institutions: Geneva University Hospitals, Johns Hopkins Hospital, University Hospital of Lausanne, Massachusetts General Hospital (Boston, MA), and the Hospital of the University of Pennsylvania, was used as a control.
FIG. 1.
Representative cytology images of (A) mild, (B) moderate, and (C) predominant Hürthle presence. Mild is defined as Hürthle cells making up <25% of the cellular content of the smear, moderate is defined as a smear comprised of 25–75% Hürthle cells, and predominant is defined as a smear comprised of >75% Hürthle cell change.
Statistical analysis was performed using a t-test. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed using SPSS (IBM, Inc., Armonk, NY).
Results
A total of 300 subjects with “Hürthle cell” or “oncocytes” mentioned in the FNA reports and meeting the inclusion criteria were studied. The male-to-female ratio was 1:5, and the mean age at the time of surgery was 54 years (range: 16–92 years). In total, 300 FNA reports between 2000 and 2013 were reviewed and analyzed by the BSRTC, and 203 of these reports were further subcategorized by the degree of Hürthle cell presence (Tables 1 and 2). When segregating Hürthle cell FNA samples according to BSRTC, the FNA was I. nondiagnostic in 4.7% (n = 14), II. benign in 37.7% (n = 113), III. AUS/FLUS in 11% (n = 33), IV. FN/SFN in 41.6% (n = 125), V. SM in 4% (n = 12), and VI. malignant in 1% (n = 3). Of the 203 Hürthle cell FNA cases classified based on Hürthle cell presence, 29% (n = 59) of specimens demonstrated a mild degree of Hürthle cells, 6% (n = 13) showed a moderate degree of Hürthle cells, and 65% (n = 131) showed a predominant Hürthle cell population.
Table 1.
Distribution of Hürthle Cell Fine-Needle Aspirations by the Bethesda System (n = 300)
| Diagnostic category | No. of cases (%) |
|---|---|
| I. Nondiagnostic | 14 (4.7) |
| II. Benign | 113 (37.7) |
| III. AUS/FLUS | 33 (11) |
| IV. FN/SFN | 125 (41.6) |
| V. SM | 12 (4) |
| VI. Malignant | 3 (1) |
AUS, atypia of undetermined significance; FLUS, follicular lesion of undetermined significance; FN, follicular neoplasm; FNA, fine-needle aspiration; PTC, papillary thyroid carcinoma; SFN, suspicious for a follicular neoplasm; SM, suspicious for malignancy.
Table 2.
Distribution of Hürthle Cell Fine-Needle Aspirations by the Degree of Hürthle Cell Presence (n = 203)
| Degree of Hürthle cell presence | No. of cases (%) |
|---|---|
| Mild (<25%) | 59 (29) |
| Moderate (25–75%) | 13 (6) |
| Predominant (>75%) | 131 (65) |
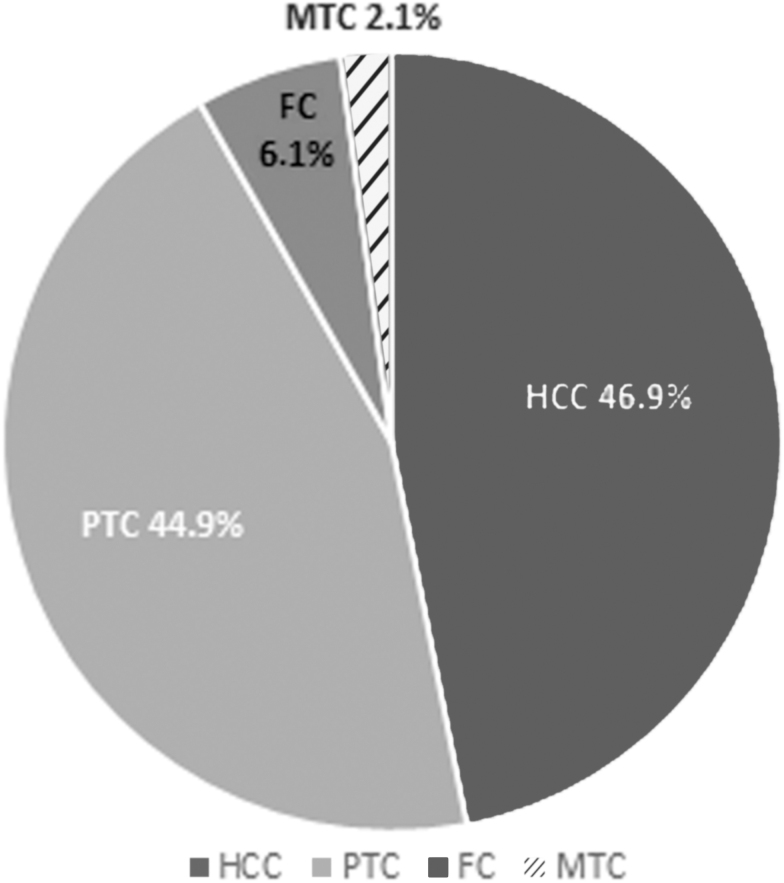
Distribution of the cytological diagnoses and histopathologic outcomes of all 300 study subjects are described in Table 3. Of these 300 lesions, 49 were diagnosed as malignant on final histopathology. Figure 2 shows the distribution of various malignancies in these 49 cases. The overall risk of malignancy of each cytologic subcategory was assessed. The malignancy risk for the nondiagnostic category was 7% (n = 1 of 14); the histopathologic diagnosis for this case was papillary thyroid carcinoma (PTC). The overall malignancy risk for the benign category was 7% (n = 8 of 113). Correlation of these benign FNA specimens with final histopathology demonstrated 5 cases of PTC, 2 cases of Hürthle cell carcinoma (HCC), and 1 case of follicular carcinoma (FC). In the AUS/FLUS category, the malignancy risk was 15% (n = 5 of 33). Histopathologic analysis of the AUS/FLUS group showed 4 HCC cases and 1 case of PTC. The malignancy risk for the FN/SFN category was 21% (n = 26 of 125); 15 of these cases were diagnosed as HCC, 9 were PTC, 1 was FC, and 1 was medullary thyroid carcinoma (MTC). The malignancy risk for the SM category was 50% (n = 6 of 12), 5 of which were diagnosed as PTC, and 1 was FC. Finally, all 3 patients with malignant FNA cytology had malignancy on pathology, thus with a malignancy risk of 100%, with 2 cases of HCC and 1 case of PTC.
Table 3.
Correlation of Fine-Needle Aspiration, Histopathologic Diagnoses, and Risk of Malignancy in Surgically Resected Specimens Classified by Bethesda (n = 300)
| FNA | Histopathologic diagnoses, n |
ROM, % | ||||
|---|---|---|---|---|---|---|
| HCC | FC | PTC | MTC | Benigna | ||
| I. ND (n = 14) | 0 | 0 | 1 | 0 | 13 | 7 |
| II. Benign (n = 113) | 2 | 1 | 5 | 0 | 105 | 7 |
| III. AUS/FLUS (n = 33) | 4 | 0 | 1 | 0 | 28 | 15 |
| IV. FN/SFN (n = 125) | 15 | 1 | 9 | 1 | 99 | 21 |
| V. SM (n = 12) | 0 | 1 | 5 | 0 | 6 | 50 |
| VI. Malignant (n = 3) | 2 | 0 | 1 | 0 | 0 | 100 |
| Total (n = 300) | 23 (7.7) | 3 (1) | 22 (7.3) | 1 (0.3) | 251 (83.7) | 16 |
Includes benign nodules, thyroiditis, follicular adenoma, and Hürthle cell adenoma.
FC, follicular carcinoma; FN, follicular neoplasm; HCC, Hürthle cell carcinoma; MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma; ROM, risk of malignancy.
FIG. 2.
Distribution of various malignancies (N = 49) in lesions with Hürthle cell presence in cytology (N = 300). FC, follicular carcinoma; HCC, Hürthle cell carcinoma; MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma.
Of the 300 study subjects, 203 patients were classified by the degree of Hürthle cell presence on FNA (Table 4). Among the 59 cases with mild degree of Hürthle cells, two cases had malignancy on final histopathology, one FC and one PTC, corresponding to a malignancy risk of 3%. Of 13 patients with a moderate degree of Hürthle cells, 2 patients were diagnosed as malignant, 1 patient had HCC, and the other had PTC, with a malignancy risk of 15%. Twenty-eight cases out of 131 Hürthle cell predominant cases were diagnosed as malignant, 13 patients had HCC, 12 had PTC, 1 had MTC, and 2 had FC, with an overall malignancy risk of 21.4%.
Table 4.
Malignancy Risks Correlated with Hürthle Cell Fine-Needle Aspirations According to the Bethesda System and Degree of Hürthle Cell Presence (n = 203)
| Diagnostic category | Degree of Hürthle cell presence, n (ROM, %) |
||
|---|---|---|---|
| Mild | Moderate | Predominant | |
| I. Nondiagnostic (n = 9) | 3 (0/3, 0) | 0 (0/0, 0) | 6 (1/6, 16.7) |
| II. Benign (n = 67) | 50 (2/50, 4) | 6 (0/6, 0) | 11 (3/11, 27.3) |
| III. AUS/FLUS (n = 26) | 4 (0/4, 0) | 3 (1/3, 33.3) | 19 (4/19, 21.1) |
| IV. FN/SFN (n = 94) | 2 (0/2, 0) | 1 (0/1, 0) | 91 (17/91, 18.7) |
| V. SM (n = 6) | 0 (0/0, 0) | 3 (1/3, 33.3) | 3 (2/3, 66.7) |
| VI. Malignant (n = 1) | 0 (0/0, 0) | 0 (0/0, 0) | 1 (1/1, 100) |
| Total (n = 203) | 59 (2/59, 3.4) | 13 (2/13, 15.4) | 131 (28/131, 21.4) |
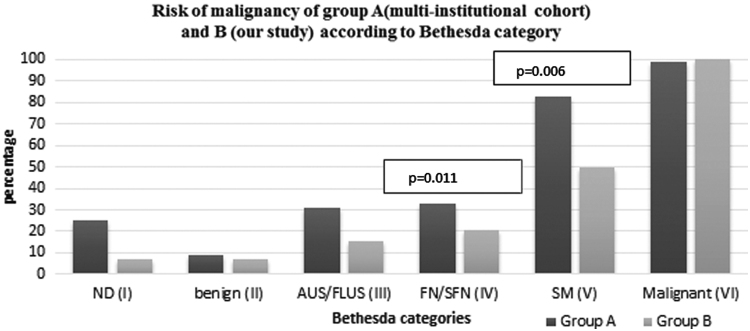
Risk of malignancy based on FNA cytopathology using the BSRTC, as well as the degree of Hürthle cells classification systems, was calculated (Fig. 3). We compared the risk of malignancy in the study population with the risk of malignancy in a large multi-institutional FNA cohort with 1827 cases (9). All patients in this cohort underwent surgery (i.e., they had FNA with corresponding pathology correlation, similar to our study cohort) (9). This comparison is shown in Table 5 and Figure 4. The overall risk of malignancy in our study when compared with this multi-institutional FNA cohort with all FNAs (i.e., with and without Hürthle cell presence) is lower in each BSRTC diagnostic category except in the malignant category, where it is equivalent. The differences are statistically significant only in the FN/SFN and SM categories (with p-values of 0.011 and 0.006, respectively). In each BSRTC diagnostic category, Hürthle cell presence does not increase the risk of malignancy.
FIG. 3.
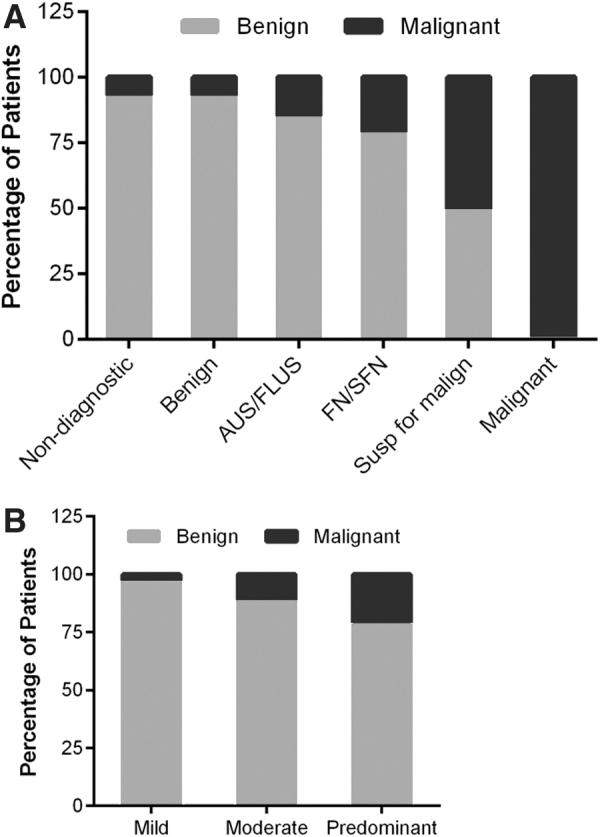
Risk of malignancy based on FNA cytopathology using (A) BSRTC or (B) degree of Hürthle cell presence classification systems. AUS, atypia of undetermined significance; BSRTC, Bethesda System for Reporting Thyroid Cytopathology; FLUS, follicular lesion of undetermined significance; FN, follicular neoplasm; SFN, suspicious for a follicular neoplasm.
Table 5.
Comparison of the Risk of Malignancy in BSRTC Categories and by Degree of Hürthle Cell Presence Between (A) Multi-Institutional FNA Cohort (All FNAs with All Patients Going to Surgery)* Results and (B) the Current Study (All FNAs with Hürthle Cell Change with All Patients Going to Surgery)
| Diagnostic category | A. ROM in all FNAs (n = 1827), % | B. ROM in Hürthle cell FNAs (n = 300), % | p† | Degree of Hürthle cell presence (n = 203) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mild, n (ROM %) | p | Moderate, n (ROM %) | p | Predominant, n (ROM %) | p | ||||
| I. Nondiagnostic | 25.3 | 7.1 | 0.138 | 3 (0) | 0.32 | 0 (0) | N/A | 6 (16.7) | 0.64 |
| II. Benign | 9.3 | 7.1 | 0.464 | 50 (4) | 0.21 | 6 (0) | 0.434 | 11 (27.3) | 0.048 |
| III. AUS/FLUS | 31.2 | 15.2 | 0.054 | 4 (0) | 0.179 | 3 (33.3) | 0.938 | 19 (21.1) | 0.352 |
| IV. FN/SFN | 33.2 | 20.8 | 0.011 | 2 (0) | 0.32 | 1 (0) | 0.48 | 91 (18.7) | 0.01 |
| V. SM | 82.6 | 50 | 0.006 | 0 (0) | N/A | 3 (33.3) | 0.029 | 3 (66.7) | 0.474 |
| VI. Malignant | 99.1 | 100 | 0.869 | 0 (0) | N/A | 0 (0) | N/A | 1 (100) | 0.924 |
Faquin et al. (9).
The p-values were calculated with the two-tailed t-test; significance was set at <0.05.
N/A, not applicable.
FIG. 4.
A bar graph showing risk of malignancy in the multi-institutional cohort (group A) (9) and our study (group B). The p-values were calculated with the two-tailed t-test; significance was set at <0.05. ND, non diagnostic; SM, suspicious for malignancy.
We noted an exception to the finding of lower risk of malignancy in the Hürthle cell FNAs when risk of malignancy in the mild, moderate, and predominant Hürthle cell groups within a BSRTC category was individually compared with the risk of malignancy in the corresponding BSRTC category in the multicenter control cohort (N = 1827). This comparison revealed that the predominant Hürthle cell group within the benign category in our study cases was the only exception, and had a higher risk of malignancy as compared with the benign category in the multicenter control cohort with 27.3% versus 9.3% (p = 0.048) (Table 5).
When the final histopathologic diagnosis in the Hürthle cell cohort was categorized as benign versus malignant, 83.7% had benign disease, and 16.3% had malignant disease among the 300 biopsied lesions (Table 3). In the FNA specimens containing predominant Hürthle cells, 78.6% (103/131) were benign, and 21.4% (28/131) were malignant (Table 4). Hürthle cell adenoma was the most common benign diagnosis overall (44%), and in the predominant Hürthle cell category it was 54%. Hürthle cell carcinoma was the most common malignant diagnosis overall (47%), and in patients with predominant Hürthle cells it was 46.4%.
Discussion
The study addresses the knowledge gap in the interpretation of Hürthle cell presence in thyroid cytology, as it relates to the existing BSRTC diagnostic category. Hürthle cells are frequently mentioned in FNA reports and may ultimately represent a range of lesions, including HT, adenomatous/multinodular goiter with Hürthle cell metaplasia, or Hürthle cell neoplasia including adenoma or Hürthle cell carcinoma. There is little information in the literature that discusses how the presence of Hürthle cells affects rates of malignancy within the BSRTC diagnostic categories. Recent work has shown that the presence of nonmacrofollicular architecture, absence of background colloid, absence of chronic inflammation, and presence of transgressing blood vessels increase the risk of Hürthle cell neoplasia (10,11). In the 2017 BSRTC update, Hürthle cell atypia is described as an additional important criterion in raising concern for malignancy in the setting of Hürthle cell changes. Hürthle cell atypia may be large cell or small cell dysplasia, which involves specific cytoplasmic and nuclear features (8).
In the 2017 BSRTC, FNA specimens that are suspicious for Hürthle cell neoplasm are distinguished from those suspicious for non-Hürthle cell follicular neoplasm because of the striking morphologic differences between the two cytologic patterns, as well as the divergent genetic underpinnings of follicular and Hürthle cell carcinomas. According to the 2017 BSRTC, the terms used are “follicular neoplasm, Hürthle cell type” or the equally acceptable “suspicious for follicular neoplasm, Hürthle cell type.” The interpretation of follicular neoplasm Hürthle cell type or suspicious for a follicular neoplasm Hürthle cell type refers to cellular aspirates that consist exclusively or almost exclusively of Hürthle cells. The World Health Organization guidelines suggest only follicular neoplasms comprised of >75% Hürthle cell change be termed Hürthle cell neoplasm (11,12). It is noted that the specificity of FNA for detection of Hürthle cell carcinoma is low with most nodules diagnosed as suspicious for follicular neoplasm Hürthle cell type (also known as follicular neoplasm Hürthle cell type) with a risk of malignancy ranging between 10% and 40%. In the recent 2017 BSRTC, there is no other commentary as to the effect of Hürthle cell presence on the risk of malignancy. To the best of our knowledge, our study is the largest series that exclusively reports on FNAs with Hürthle cell presence and corresponding histopathology control.
Upon comparing the risks of malignancy between the BSRTC categories in a multi-institutional FNA cohort and our study, the overall risk of malignancy was lower in our study as compared with the control cohort across all BSRTC categories. Our findings suggest that clinicians should not consider Hürthle cell as an added atypical feature of the aspirate that increases the risk of malignancy beyond that of the underlying BSRTC category. The rate of malignancy is most accurately predicted based on the BSRTC diagnostic categories.
There was one exception to the Hürthle cell lower risk of malignancy effect. The malignancy risk in benign FNAs (BSRTC II) with a predominance of Hürthle cells was 27.3%, which was significantly higher than the overall risk of 9.3% for all benign FNAs derived from the multi-institutional control cohort (p = 0.048). One may be concerned with our reporting of cases with an aspirate consisting of predominantly Hürthle cell as Bethesda II (benign) category. We specifically reviewed all these cases, and despite having predominant Hürthle cell presence, the cellular and architectural features favored the cytological diagnosis of a benign lesion in all these cases.
Other reports have focused on attempting to identify preoperative clinical features that correlate with an increased likelihood of malignant disease on surgical pathology. These included patient demographics such as age and sex, sonographic nodule size, and the presence of cytologic features such as atypia or metaplasia. In patients with more advanced age and tumors >4 cm, there was a higher risk of malignancy, whereas a low rate of malignancy was found if the tumor was <2 cm (13). In a retrospective study of 70 FNA specimens containing Hürthle cells, factors including male sex, nodules >2 cm, presence of a solitary nodule, and presence of metaplasia were associated with an increased risk of malignancy. However, none reached statistical significance (14). Another study questioned whether Hürthle cell lesions have a higher risk of malignancy compared with non-Hürthle cell follicular lesions on FNA cytology, and concluded that it did not (8), findings that are in agreement with the series presented here.
In several series reported to date, the risk of malignancy varied widely from 10% to 45% in patients with cytology consisting of Hürthle cells and available corresponding final pathology (15,16). While the data presented in this study focused only on FNA specimens that contained Hürthle cell/oncocytic changes, the overall risk of malignancy of 16% is in agreement with the data reported thus far. In the study presented here, the Hürthle cell presence overall did not increase the risk of malignancy when compared with the multi-institutional FNA cohort inclusive of all FNAs regardless of Hürthle cell presence when analyzed by BSRTC categories. In a smaller series of 128 FNAs with Hürthle cells compared with 582 FNAs without Hürthle cells, the presence of Hürthle cells altered the expected distribution of Bethesda cytological diagnoses, with a higher number of cases in the AUS/FLUS and FN/SFN categories, and an increased risk of malignancy on final pathology in FNAs that were either benign (6.0–15.1%) or FN/SFN (21.9–63.6%) (17). In our series, Hürthle cell presence was not found to increase the risk of malignancy overall. While our control cohort included all FNAs regardless of Hürthle cell presence, the control cohort in the study noted above consisted of FNAs without Hürthle cells (17).
This study focused on the correlation of Hürthle cell presence in cytology and final pathology of malignancy—hence we did not review other factors that can influence the rate of malignancy. For example, Silva de Morais et al. have reported an association of HT with an increased risk of malignancy (18). However, some researchers believe that this is a result of selection bias resulting from inclusion of only surgical cases (19). Radetti et al. emphasize that the relation between HT and thyroid cancer remains unclear (20). In their study in children and adolescents, the authors report association of HT with an increased risk of developing thyroid nodules but not with thyroid malignancy (20). Prospective studies with longer follow-up may further elucidate this association.
There are several limitations of our study. First, our report is based on a single cohort from a single institution, thus introducing institutional bias such as referral bias, surgeon bias, and assessment bias. Second, our statistical analysis is limited by the fact that it is not adjusted for multiple comparisons. Third, only the subjects who underwent subsequent surgical resection of the indexed thyroid nodule were included in the study. There may have been additional clinical features leading to the decision to proceed with surgery. This selection bias may be associated with an increased risk of malignancy. However, the fact that Hürthle cell presence did not increase the risk of malignancy even in a cohort with lesions with an inherently increased risk of malignancy strengthens the findings. Next, we have utilized a control group that included cytologies with and without Hürthle cell presence rather than a Hürthle cell negative group. This is a limitation of our study, but this would understate the effect of Hürthle cell presence in lowering the rate of malignancy. Finally, the use of an independent cohort could perhaps be a limitation, but we feel that the large control group from a multi-institutional FNA cohort with surgical resection of all index nodules is a suitable comparable cohort.
Conclusion
This study focused on the consequences of Hürthle cell presence on the risk of malignancy with an understanding that many clinicians consider Hürthle cell presence as an atypical finding that may increase the risk of malignancy. This series indicates that the presence of Hürthle cells does not increase the malignancy risk in any Bethesda categories. Thus, the Hürthle cell descriptor is not additively helpful beyond the categorization into Bethesda categories in the prediction of malignancy.
Author Disclosure Statement
None of the authors have anything to disclose.
Funding Information
The study was supported by the Ruane Thyroid Research Fund.
References
- 1. Lowhagen T, Willems JS, Lundell G, Sundblad R, Granberg PO. 1981. Aspiration biopsy cytology in diagnosis of thyroid cancer. World J Surg 5:61–73 [DOI] [PubMed] [Google Scholar]
- 2. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB, Goellner JR, Hay ID, Hertzberg BS, Intenzo CM, Jeffrey RB, Langer JE, Larsen PR, Mandel SJ, Middleton WD, Reading CC, Sherman SI, Tessler FN. 2005. Management of thyroid nodules detected at US: society of Radiologists in Ultrasound consensus conference statement. Radiology 237:794–800 [DOI] [PubMed] [Google Scholar]
- 3. Cibas ES, Ali SZ. 2009. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 19:1159–1165 [DOI] [PubMed] [Google Scholar]
- 4. Cannon J. 2011. The significance of hurthle cells in thyroid disease. Oncologist 16:1380–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mete O, Asa SL. 2010. Oncocytes, oxyphils, Hurthle, and Askanazy cells: morphological and molecular features of oncocytic thyroid nodules. Endocr Pathol 21:16–24 [DOI] [PubMed] [Google Scholar]
- 6. Skoog L, Tani E. 2002. Hurthle cell carcinoma: time for a drastic change? Cancer 96:259–260 [DOI] [PubMed] [Google Scholar]
- 7. Alaedeen DI, Khiyami A, McHenry CR. 2005. Fine-needle aspiration biopsy specimen with a predominance of Hurthle cells: a dilemma in the management of nodular thyroid disease. Surgery 138:650–656; discussion 656–657. [DOI] [PubMed] [Google Scholar]
- 8. Pu RT, Yang J, Wasserman PG, Bhuiya T, Griffith KA, Michael CW. 2006. Does Hurthle cell lesion/neoplasm predict malignancy more than follicular lesion/neoplasm on thyroid fine-needle aspiration? Diagn Cytopathol 34:330–334 [DOI] [PubMed] [Google Scholar]
- 9. Faquin WC, Wong LQ, Afrogheh AH, Ali SZ, Bishop JA, Bongiovanni M, Pusztaszeri MP, VandenBussche CJ, Gourmaud J, Vaickus LJ, Baloch ZW. 2016. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in The Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol 124:181–187 [DOI] [PubMed] [Google Scholar]
- 10. Elliott DD, Pitman MB, Bloom L, Faquin WC. 2006. Fine-needle aspiration biopsy of Hurthle cell lesions of the thyroid gland: a cytomorphologic study of 139 cases with statistical analysis. Cancer 108:102–109 [DOI] [PubMed] [Google Scholar]
- 11. Faquin WC, Michael CW, Renshaw AA, Vielh P. 2017 Follicular neoplasm, Hürthle cell (oncocytic) type/suspicious for a follicular neoplasm, Hürthle cell (oncocytic) type. In: Ali SZ, Cibas ES (eds) The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria and Explanatory Notes. Second edition. New York: Springer, pp. 81–100 [Google Scholar]
- 12. DeLellis R, Lloyd RV HP, Eng C. 2017. Pathology and genetics of tumours of endocrine organs. WHO Classification of Tumours. Third Edition, Volume 8 Lyon: IARC Press [Google Scholar]
- 13. Zhang YW, Greenblatt DY, Repplinger D, Bargren A, Adler JT, Sippel RS, Chen H. 2008. Older age and larger tumor size predict malignancy in hurthle cell neoplasms of the thyroid. Ann Surg Oncol 15:2842–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turanli S, Pirhan Y, Ozcelik CK, Cetin A. 2011. Predictors of malignancy in patients with a thyroid nodule that contains Hurthle cells. Otolaryngol Head Neck Surg 144:514–517 [DOI] [PubMed] [Google Scholar]
- 15. Sorrenti S, Trimboli P, Catania A, Ulisse S, De Antoni E, D'Armiento M. 2009. Comparison of malignancy rate in thyroid nodules with cytology of indeterminate follicular or indeterminate Hurthle cell neoplasm. Thyroid 19:355–360 [DOI] [PubMed] [Google Scholar]
- 16. Auger M. 2014. Hurthle cells in fine-needle aspirates of the thyroid: a review of their diagnostic criteria and significance. Cancer Cytopathol 122:241–249 [DOI] [PubMed] [Google Scholar]
- 17. Yazgan A, Balci S, Dincer N, Kiyak G, Tuzun D, Ersoy R, Cakir B, Guler G. 2014. Hurthle cell presence alters the distribution and outcome of categories in the Bethesda system for reporting thyroid cytopathology. Cytopathology 25:185–189 [DOI] [PubMed] [Google Scholar]
- 18. Silva de Morais N, Stuart J, Guan H, Wang Z, Cibas ES, Frates MC, Benson CB, Cho NL, Nehs MA, Alexander CA, Marqusee E, Kim MI, Lorch JH, Barletta JA, Angell TE, Alexander EK. 2019. The impact of Hashimoto thyroiditis on thyroid nodule cytology and risk of thyroid cancer. J Endocr Soc 3:791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jankovic B, Le KT, Hershman JM. 2013. Clinical Review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab 98:474–482 [DOI] [PubMed] [Google Scholar]
- 20. Radetti G, Loche S, D'Antonio V, Salerno M, Guzzetti C, Aversa T, Cassio A, Cappa M, Gastaldi R, Deluca F, Vigone MC, Tronconi GM, Corrias A. 2019. Influence of Hashimoto thyroiditis on the development of thyroid nodules and cancer in children and adolescents. J Endocr Soc 3:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]