Abstract
Background: Rapid repeat pregnancy (RRP) is common among adolescents and is associated with adverse maternal and infant outcomes. Despite evidence that use of long-acting forms of contraception before hospital discharge can help minimize RRP rates, barriers to placement existed within the state of Indiana. We sought to determine state-specific RRP and induced abortion rates for adolescents based on chosen postpartum contraception to inform policy change.
Methods: We examined a retrospective cohort of 227 adolescents (ages 12–18 years) who gave birth in Indiana between 2010 and 2012. Demographics, postpartum contraception, and subsequent pregnancies or abortions after the sentinel delivery were obtained. Rates of RRP based on type of immediate postpartum contraception, etonogestrel (ENG) contraceptive implant, depo-medroxyprogesterone acetate (DMPA) injection, and short-acting methods were compared. Bivariate and logistic regression analyses were conducted.
Results: RRP rates were 3.7% for those with ENG contraceptive implant, 22.6% for those with DMPA, and 39.1% for those who choose short-acting methods (p = 0.01). Adolescents who did not choose an ENG contraceptive implant were significantly more likely to have an RRP (adjusted odds ratio [aOR] = 11.8, 95% confidence interval: 2.74–110.3), compared with other contraceptive methods, even after adjusting for covariates such as age, prior pregnancies, and postpartum visit attendance.
Conclusions: Immediate postpartum receipt of ENG implant was significantly associated with a lower likelihood of RRP in adolescents in Indiana. These data facilitated state policy change regarding insurance reimbursement to improve statewide access for all women, regardless of age, showing how local data can inform policy change.
Keywords: postpartum contraception, repeat pregnancy, adolescents, advocacy, contraception
Introduction
Rapid repeat pregnancy (RRP), defined as an interpregnancy interval of 18 months or fewer between a previous delivery and conception of the subsequent pregnancy, is associated with adverse maternal and neonatal outcomes, including obstetrical complications, preterm delivery, and infant mortality.1 In Indiana, 17.1% of adolescents aged 15–19 years have RRP within 12 months and 22.5% have RRP within 18 months after delivery.2 Effective interventions to decrease RRP rate is essential for this high-risk group.
Although postpartum contraception traditionally was provided at the postpartum visit (e.g., 4–6 weeks after the delivery), low attendance rates for adolescents has led to studies focusing on provision before hospital discharge.3,4 These studies have found that providing contraception, specifically access to long-acting reversible contraception (LARC) methods, such as the etonogestrel (ENG) contraceptive implant, before hospital discharge can increase utilization and decrease RRP rates.4–8
Despite this evidence, the implementation of immediate postpartum LARC presents its own challenges. Given the global reimbursement for obstetrical care, bundling of other inpatient procedures and the cost of stocking devices can be cost- prohibitive for hospitals.9,10 This logistical barrier was preventing patients in our state from accessing LARC methods before hospital discharge.
Our primary objective was to determine state-specific RRP rates for adolescents based on chosen postpartum contraception to inform policy change. We hypothesized that adolescents with access to immediate postpartum contraception, in particular the ENG contraceptive implant, would have lower RRP rates compared with other groups. Access to ENG contraceptive implant was prioritized given the preference of this LARC method in the adolescent population.4–8
Methods
Study population
We used the Indiana Network for Patient Care (INPC) database, which includes information from more than 90% of medical encounters within the greater Indianapolis area, to create a retrospective cohort of all adolescents aged 12–18 years who gave birth between January 1, 2010, and July 1, 2012.11 Only patients who delivered and received prenatal and postpartum care within a three-hospital network were included in this study. At the time of the study, all women, regardless of insurance, were able to receive a contraceptive implant if it were chosen within this hospital network. This study was approved by the Institutional Review Board (IRB) at Indiana University School of Medicine (IUSM) with a waiver of consent. In addition, Planned Parenthood of Indiana and Kentucky provided access to state-specific data after the study had IRB approval.
Data collection
We performed chart reviews to confirm eligibility and obtain demographic characteristics (age, race, and insurance status), pregnancy-related information (gravity, parity, and mode of delivery), pregnancy complications (pregnancy-related hypertensive disorders, pre-eclampsia, abnormal labor, preterm delivery, fetal anomalies, cephalopelvic disproportion, maternal illness of infection, or gestational diabetes), what if any contraceptive method was provided or prescribed before discharge from the hospital, and postpartum visit attendance. As described above, postplacental intrauterine devices were not offered at the time of this study.
We identified subsequent pregnancies within 18 months of the sentinel delivery that resulted in birth or induced abortion and defined those as RRPs. Since over 50% of induced abortions performed in the state of Indiana are provided at Planned Parenthood, their database was cross-referenced to identify those who received subsequent reproductive health care, including induced abortion.
Statistical analysis
We performed bivariate analyses to examine factors associated with RRP. t-Tests and Fisher's exact tests are reported. Logistic regression was used to estimate the odds of RRP by immediate postpartum contraception method type while adjusting for significant covariates. To address issues with small-sample bias and sparsity of the data, Firth's penalized likelihood approach was used to estimate parameters in the logistic regression model.12,13 All analyses were completed in SAS V9.4 (Cary, NC).
Results
Of the 330 charts that were identified initially with inclusion criteria, 277 adolescents had complete prenatal and postpartum information and were included in the final analysis. More than half of the patients left the hospital without any form of contraception (54.5%). However, 9.7% of patients received an immediate postpartum ENG contraceptive implant, and 19.1% received Depo injection before hospital discharge. The remainder received a prescription for a short-acting method such as oral contraceptive pills, vaginal ring, patch, or reported they would use barrier methods such as condoms (16.7%).
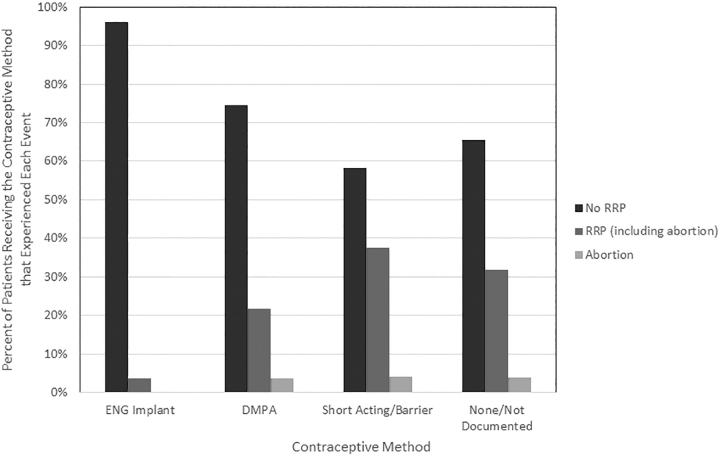
The overall RRP in our study population was 28.5% (79/277). RRP rates were 3.7% (1/27) for those who received an immediate postpartum ENG contraceptive implant, 22.6% (12/53) for those who received immediate postpartum depo-medroxyprogesterone acetate (DMPA) injection, 39.1% (18/46) for those who received a short-acting or barrier method, and 31.8% (48/151) for those who received no contraception or did not have a documented method (p = 0.01) (Fig. 1).
FIG. 1.
Outcomes by contraceptive method. DMPA, depo-medroxyprogesterone acetate; ENG, etonogestrel; RRP, rapid repeat pregnancy.
In the multivariable model, the association of RRP with contraception method remained statistically significant (p = 0.01) even after controlling for age, gravidity, and postpartum visit attendance. Variables significantly associated with a higher RRP rate included having a previous abortion (p = 0.03) or pregnancy (p < 0.01), living children (p = 0.05), older age (p ≤ 0.01), and attendance at postpartum visit (p ≤ 0.01) (Table 1). The odds of RRP for patients who do not receive any contraception were 11 times higher than those who received ENG contraceptive implant before hospital discharge (adjusted odds ratio [aOR] = 11.8, 95% confidence interval [CI]: 2.74–110.3). In comparison, the odds ratio for RRP for no method compared with DMPA was aOR = 2.41 (95% CI: 1.13–5.44) and compared with short-acting or barrier methods was aOR = 1.29 (95% CI: 0.60–2.80).
Table 1.
Description of Study Subjects and Rates of Rapid Repeat Pregnancy
| Variable | RRP |
|
|
|---|---|---|---|
| Yes |
No |
|
|
| n = 79 |
n = 198 |
p | |
| n (%) | n (%) | ||
| Race | |||
| Hispanic | 17 (21.5) | 43 (21.7) | 0.56 |
| Black | 28 (35.4) | 86 (43.4) | |
| White | 27 (34.2) | 53 (26.8) | |
| Other | 7 (8.9) | 16 (8.1) | |
| Insurance status | |||
| Public | 61 (77.2) | 156 (78.8) | 0.72 |
| Private | 5 (6.3) | 16 (8.1) | |
| Not documented | 13 (16.5) | 26 (13.1) | |
| Gravida | |||
| 1 | 52 (65.8) | 167 (84.3) | <0.01 |
| >1 | 27 (34.2) | 31 (15.7) | |
| Previous induced abortion | |||
| Yes | 16 (20.3) | 19 (9.6) | 0.03 |
| No | 63 (79.7) | 179 (90.4) | |
| Other living children | |||
| Yes | 13 (16.4) | 15 (7.6) | 0.05 |
| No | 66 (83.5) | 183 (92.4) | |
| Mode of sentinel delivery | |||
| Vaginal | 59 (74.7) | 166 (83.8) | 0.09 |
| C-section | 20 (25.3) | 32 (16.2) | |
| Pregnancy complicationa | |||
| Yes | 29 (38.7) | 78 (41.8) | 0.70 |
| No | 46 (61.3) | 111 (62.0) | |
| Attendance at postpartum visit | |||
| Yes | 41 (51.9) | 145 (73.2) | <0.01 |
| No/not documented | 38 (48.1) | 53 (26.8) | |
| Continuous variables | |||
| Mean (SD) | Mean (SD) | ||
| Age at delivery | |||
| n = 277 | 17.4 (0.8) | 16.8 (1.3) | <0.01 |
| Gestational age | |||
| n = 273 | 38.4 (3.5) | 38.7 (2.4) | 0.42 |
Pregnancy complications included pregnancy-induced hypertensive disorders, pre-eclampsia, labor abnormalities, preterm delivery, fetal anomalies, cephalopelvic disproportion, maternal illness or infection, gestational diabetes, and multifetal gestation.
RRP, rapid repeat pregnancy.
Only one adolescent had the ENG contraceptive implant removed (for breakthrough bleeding) during the 18-month study period, which resulted in an RRP. There was no difference in subsequent induced abortion rates between the groups (p = 0.73), and no abortions documented in the ENG contraceptive implant group.
Discussion
This study demonstrates that patients who received an immediate postpartum ENG contraceptive implant had lower RRP rates than patients who did not. This result is consistent with growing evidence that the provision of immediate postpartum ENG contraceptive implant is essential in decreasing RRP, especially in adolescents.7,14 Although previous studies have published evidence to support this intervention, local policy makers were interested in from within our state and among their constituents to show the benefits. The importance of research informing policy has been discussed and is more relevant now that larger pools of data within specific health care systems are available.15 Our study highlights the importance of providing specific local data to augment published literature and impact local policy.
There are limitations in our study. Adolescents could have obtained care outside of the INPC or Planned Parenthood network, and we may have missed subsequent pregnancies, including spontaneous abortions, and intended pregnancies. Furthermore, there was no way to confirm sustained use of any method during the study period and the rate of removal for ENG contraceptive implant was low compared with other published data, which could suggest removal happened outside of our clinical data network. The low rates of RRP in the ENG contraceptive implant group led to wide CIs, although we did use a small-sample bias correction. Finally, immediate postpartum contraception was determined by chart review, and we did not verify prescriptions for short-acting methods or patient adherence.
Data from this study were presented to the Indiana Medicaid Medical Advisory Cabinet in 2014. This interdisciplinary team of clinicians and researchers was created in 2006 to provide research-based policy advice to the Indiana Office of Medicaid Policy and Planning. Given the strong evidence that LARC, specifically the ENG contraceptive implant, was effective in preventing adolescent RRP in our state, the recommendation was to uncouple these payments to increase LARC access for all women after childbirth. This insurance reimbursement policy change took effect in 2015 and was further expanded to some private insurers within the state. At the time of our study conception in 2012, only 1 state Medicaid plan had published policies regarding immediate postpartum LARC placement and now there are 40 states with such policies.16 The importance of providing specific local data to augment published literature and impact local policy was critical.
Our study reaffirms that immediate postpartum placement of the ENG contraceptive implant is effective in preventing RRP in adolescents. Furthermore, we demonstrate how research can lead to state-wide insurance policy change that provided access to all LARC methods for women in our state before hospital discharge. The importance of partnerships between policy makers, public health officials, clinicians, and researchers is integral to assuring that policy is reflective and responsive to recent and local evidence. These partnerships are vital as efforts continue to assure all women are able to access the contraception they desire. Following this policy change, next steps include assuring that implementation and improvements to access are realized.
Acknowledgments
We thank Dr. Brownsyne Tucker-Edmonds, Dr. Elizabeth Ferries-Rowe, Dr. Debra Kirkpatrick, Indiana Perinatal Quality Improvement Collaborative and Planned Parenthood of Indiana-Kentucky, for their support of this research and advocacy initiative.
Author Disclosure Statement
Drs. N.T.Q. and J.W.S. are Merck NEXPLANON trainers, but are not currently paid for their work. The other authors report that no competing financial interests exist.
Funding Information
No funding was received for this article.
References
- 1. World Health Organization. Report of a Technical Consultation on Birth Spacing. Geneva, Switzerland: World Health Organization, 2005 [Google Scholar]
- 2. Short Interpregnancy Intervals and Rick of Adverse Birth Outcomes in Indiana: Statistics from the Live Birth Data 1990–2005. IN: Indiana State Department of Health, Maternal and Child Special Health Care Services, 2008
- 3. Ogburn JA, Espey E, Stonehocker J. Barriers to intrauterine device insertion in postpartum women. Contraception 2005;72:426–429 [DOI] [PubMed] [Google Scholar]
- 4. Wilson EK, Fowler CI, Koo HP. Postpartum contraceptive use among adolescent mothers in seven states. J Adolesc Health 2013;52:278–283 [DOI] [PubMed] [Google Scholar]
- 5. Cizek S, Postlethwaite D, Alabaster A, Greenfield I, Gupta P. Immediate postpartum contraception in adolescents: Intention, retention, and repeat pregnancies [35A]. Obstet Gynecol 2017;129:18S [Google Scholar]
- 6. Cohen R, Sheeder J, Arango N, Teal SB, Tocce K. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception 2016;93:178–183 [DOI] [PubMed] [Google Scholar]
- 7. Baldwin MK, Edelman AB. The effect of long-acting reversible contraception on rapid repeat pregnancy in adolescents: A review. J Adolesc Health 2013;52(4 Suppl):S47–S53 [DOI] [PubMed] [Google Scholar]
- 8. Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: Do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol 2012;206:481..e481–e487. [DOI] [PubMed] [Google Scholar]
- 9. Clinical Practice: Expanding Coverage and Reimbursement for Immediate Postpartum LARC. The American college of Obstetricians and Gynecologists, 2017
- 10. Okoroh EM, Kane DJ, Gee RE, et al. Policy change is not enough: Engaging provider champions on immediate postpartum contraception. Am J Obstet Gynecol 2018;218:590..e591–590.e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Indiana Health Information Exchange. Requested Data for IRB Study #1302010575-Impact of Inpatient LARC on Rate of Rapid Repeat Pregnancy. Available at: https://www.ihie.org/ Accessed December20, 2018
- 12. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993;80:27–38 [Google Scholar]
- 13. Heinze G, Puhr R. Bias-reduced and separation-proof conditional logistic regression with small or sparse data sets. Stat Med 2010;29:770–777 [DOI] [PubMed] [Google Scholar]
- 14. Rodriguez MI, Evans M, Espey E. Advocating for immediate postpartum LARC: Increasing access, improving outcomes, and decreasing cost. Contraception 2014;90:468–471 [DOI] [PubMed] [Google Scholar]
- 15. Clancy CM, Glied SA, Lurie N. From research to health policy impact. Health Serv Res 2012;47(1 Pt 2):337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Medicaid Reimbursement for Postpartum LARC by State. The American College of Obstetricians and Gynecologists. Available at: https://www.acog.org/About-ACOG/ACOG-Departments/Long-Acting-Reversible-Contraception/Immediate-Postpartum-LARC-Medicaid-Reimbursement?IsMobileSet=false Accessed October23, 2017