Abstract
Background: Active surveillance is established as an alternative to surgery for papillary thyroid microcarcinomas, but inclusion criteria and mortality risk for pursuing a nonsurgical approach have not been clearly defined. To gauge the feasibility of expanding active surveillance thresholds, we investigated the effects of increasing size and age on disease-specific survival (DSS) in a large nonoperative thyroid cancer cohort, compared against a matched group of surgical patients.
Methods: Papillary thyroid carcinoma patients staged T1-4N0M0 were identified in the Surveillance, Epidemiology, and End Results (SEER) database between 1975 and 2015, stratified by nonsurgical and surgical management. Propensity score matching was performed to adjust for imbalances in covariates. Multivariable models were constructed using restricted cubic splines to model nonlinear relationships of age and tumor size with DSS.
Results: Overall, 1453 nonoperative patients and 54,718 surgical patients met the inclusion criteria. Collectively, increasing age and size after certain thresholds independently led to greater differences in DSS between nonsurgical and surgical patients. For younger ages (14–55 years), surgical approach compared with nonsurgical approach was not associated with any difference in the 10-year DSS among 0–4 cm cancers (99.8% vs. 100%, p = 0.470), 4.1–6 cm cancers (98.8% vs. 100%, p = 0.599), or >6 cm cancers (97.3% vs. 100%, p = 0.718). Older patients with larger tumors (>75 years, >6 cm) demonstrated the greatest difference in DSS (48.1% vs. 91.3%, p < 0.001). Similar results were found when applying propensity score matching. For age, restricted cubic spline plots showed minimal relative survival hazard in nonoperative cases beginning after age 60 years, with a change point illustrating acceleration in relative hazard beyond age 72 years. For size, relative survival hazard was observed after 2.0 cm and increased slowly with nodule growth up to an inflection point of 4.5 cm. Beyond this, mortality risk escalated with each additional year without plateau.
Conclusions: Increasing age and size lead to progressively greater mortality risk without surgery, but only beyond certain thresholds. We define escalating gradients at which a nonsurgical approach may be deemed appropriate, and beyond which survival benefits from surgery become apparent. Such findings reconcile controversial observations regarding age and size in active surveillance and further reshape evolving treatment paradigms in thyroid cancer.
Keywords: thyroid cancer, active surveillance, papillary thyroid carcinoma, low-risk cancer, nonsurgical treatment
Introduction
An outsized surge in thyroid cancer incidence has been reported across numerous countries, tied principally to the incidental detection of small papillary thyroid carcinomas (PTCs) (1). Such a phenomenon, coupled with deeper understanding of its indolent biology (2), has led to judicious pivots in diagnosis, staging, and treatment (3,4). One such de-escalatory approach, active surveillance, entails dynamic monitoring with thyroidectomy only for those cancers that progress (5–8).
Despite its feasibility, active surveillance to date has seen limited adoption (9). Challenges have included physician reluctance, patient anxiety, and the need for well-validated selection criteria. Trial outcomes have been based on arbitrary parameters of size and progression (i.e., 1.0 cm size limit, 3 mm growth limit) that are necessarily conservative to establish safety—yet such thresholds may not translate into true mortality risk. Moreover, while active surveillance data suggest that older patients are at much lower risk for progression (10), it is paradoxically recognized that such patients exhibit more aggressive disease and greater mortality (11–15). Thus, much remains unknown about which patients represent the ideal active surveillance candidates.
To date, no thyroid active surveillance patient has died from cancer progression (5–8). Analysis of deaths caused by thyroid cancer in patients managed nonoperatively, although not a direct parallel to active surveillance, may nonetheless offer unique insight into setting reasonable inclusion criteria and perhaps expanding them. Herein, we investigated the effects of progressive age and size on survival using a continuous recursive multivariable model, comparing nonsurgical and surgical outcomes via a large U.S. cancer registry.
Materials and Methods
Data source
All data regarding demographic characteristics and cancer incidence were obtained from the Surveillance, Epidemiology, and End Results (SEER) Program, derived from 18 cancer registries across the United States, covering ∼28% of incident cases in the United States (http://seer.cancer.gov). The SEER-18 Regs Custom Data with additional treatment fields (Nov 2017 submission) was utilized spanning 1984 through 2015 and weaned with SEER*Stat v8.3.5. This study was deemed exempt by the Cedars-Sinai Institutional Review Board.
Patient selection
Patients with biopsy-proven well-differentiated PTC were evaluated (Supplementary Fig. S1). ICD-03 histologies comprised 8050 (PTC NOS), 8260 (PTC), 8340 (follicular variant of PTC), 8341 (PTC, microcarcinoma), and 8343 (PTC, encapsulated). To mirror active surveillance criteria, patients were required to be N0 and M0 based on clinical or pathological criteria. Tumors >10 cm in size, incomplete tumor size, staging, treatment, or follow-up data (<2 months) were excluded. Those who received external beam radiation therapy (EBRT) or systemic therapy, either as palliative (nonsurgical cohort) or adjuvant (surgical cohort), were eliminated. For the nonsurgical cohort, those who received any intervention (e.g., radioactive iodine [RAI], EBRT, or systemic therapy) were excluded. Patients who planned to have surgery but died before it was performed were omitted. For the surgical cohort, those documented to have an incomplete surgery were excluded, to avoid confounding effects of subtherapeutic regimens. Based on the American Joint Committee on Cancer 8th Edition (AJCC 8E) cutoffs, age groups were stratified as younger (14–55 years), middle (56–75 years), and older (>75 years). Size groups were stratified as small (0–4 cm), medium (4.1–6 cm), and large (>6 cm).
Statistical methods
Bivariate analysis between surgical and nonsurgical patients was examined with the Welch t-test and the Pearson chi-squared test for continuous and categorical variables as appropriate. Median follow-up was calculated using the reverse Kaplan–Meier method (16). Univariate and multivariable competing risk analyses were carried out using a Fine and Gray subdistribution hazards model with thyroid cancer-specific mortality as the primary outcome and other cause mortality as the competing risk (17). Cumulative incidence curves were compared using Gray's test (18). Survival analysis were carried out using the Cox proportional hazard models and survival curves compared using the log-rank test (19,20). Multivariable analyses were performed using a stepwise variable selection procedure based on the Akaike information criterion (AIC) (21). Multivariable models with the lowest AIC values were selected as final models. The proportional hazards assumption was assessed graphically and analytically with scaled Schoenfeld residuals (22).
To further balance for measurable confounders, propensity scores were estimated for each patient using a multivariable logistic regression model (23) adjusting for age, sex, race/ethnicity, marriage, geographic region, and RAI. The estimated propensity scores were then matched using the nearest neighbor method to create a 1:1 matched cohort with a caliper of 0.2. Propensity score-matched cohorts were further assessed for the quality of matching using the standardized mean differences before and after matching and visually by the distribution of the propensity scores. A standardized mean difference of >0.1 was considered indicative of a significant imbalance.
To allow for a nonlinear association with thyroid cancer-specific mortality, age and size were modeled as a restricted cubic spline function with three knots placed at 39, 62, and 81 years for age and 2, 11, and 35 mm for size corresponding to the fixed 10th, 50th, and 90th percentiles (24). Estimated effects were illustrated with a smoothed restricted cubic spline plots of the adjusted hazard ratio (HR) versus size and age. Subdistribution HR was estimated with the Fine and Gray model, adjusted for sex, race/ethnicity, marriage, geographic region, size (three knots), surgery, age (three knots), and RAI. A relative hazard threshold was delineated at 0.15. Change points for age and size were further estimated with a piecewise linear regression models (25).
Statistical analyses were performed using R (version 3.5.1; R Foundation, Vienna, Austria) with two-sided tests and a significance level of 0.05.
Results
Patient cohorts
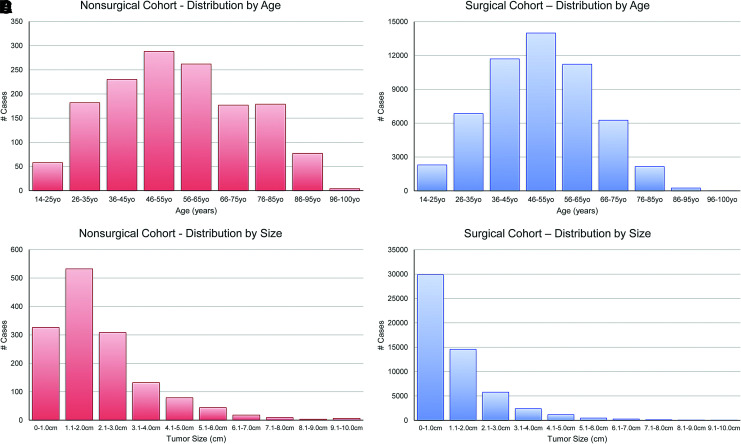
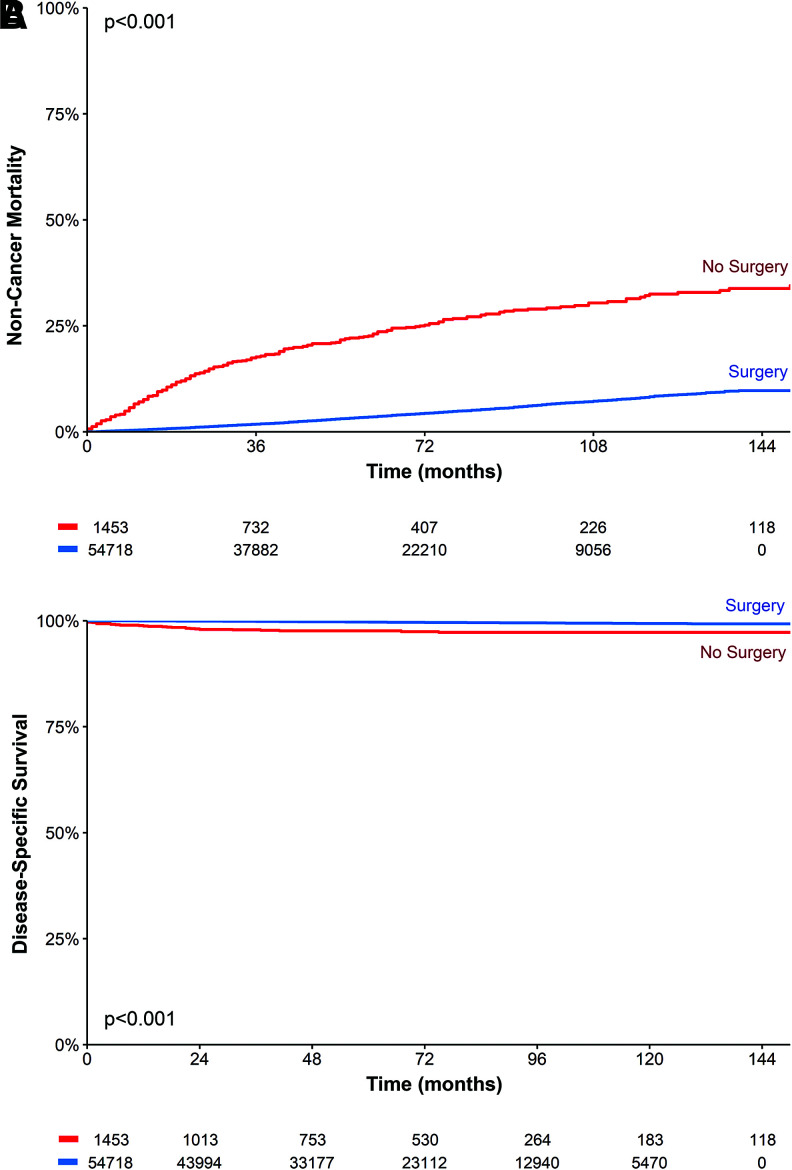
A total of 1453 nonoperative patients and 54,718 surgical patients met the inclusion criteria (Supplementary Fig. S1). Mean age at diagnosis was 55.4 years (SD ±18.3) and 50.3 years (SD ±14.5); average thyroid nodule size measured 2.2 cm (SD ±1.5) and 1.3 cm (SD ±1.7); median follow-up time was 51.0 months (95% confidence interval [CI] 48–54) and 61.0 months (CI 61–62), respectively (Table 1 and Fig. 1). In these cohorts, 2.2% of the nonoperative group and 0.3% of the surgical group died of thyroid cancer. The 10-year disease-specific survival (DSS) was 97.2% versus 99.3%. The 10-year other-cause mortality (i.e., noncancer mortality) was 32.5% versus 8.3% (Fig. 2), suggesting that the nonsurgical patients had a much higher rate of death from other causes. For the nonoperative patients, 60.4% (878/1453) were not recommended to have surgery. The remainder (39.6%) were recommended to have surgery, but either refused or failed to for unknown reasons.
Table 1.
Baseline Characteristics Stratified by Surgical and Nonsurgical Management
| Characteristic | Overall | Nonsurgical | Surgical | p |
|---|---|---|---|---|
| n = 56,171 | n = 1453 | n = 54,718 | ||
| Age at diagnosis, years, mean (SD) | 50.4 (14.7) | 55.4 (18.3) | 50.3 (14.5) | <0.001 |
| 14–55 | 35,590 (63.4%) | 755 (52.0%) | 34,835 (63.7%) | <0.001 |
| 56–75 | 17,925 (31.9%) | 439 (30.2%) | 17,486 (32.0%) | |
| >75 | 2656 (4.73%) | 259 (17.8%) | 2397 (4.38%) | |
| Sex | ||||
| Male | 11,144 (19.8%) | 391 (26.9%) | 10,753 (19.7%) | <0.001 |
| Female | 45,027 (80.2%) | 1062 (73.1%) | 43,965 (80.3%) | |
| Race/ethnicity | ||||
| White | 38,542 (68.6%) | 846 (58.2%) | 37,696 (68.9%) | <0.001 |
| Black | 3470 (6.18%) | 83 (5.71%) | 3387 (6.19%) | |
| Asian | 5737 (10.2%) | 218 (15.0%) | 5519 (10.1%) | |
| Hispanic | 7643 (13.6%) | 262 (18.0%) | 7381 (13.5%) | |
| Other | 779 (1.39%) | 44 (3.03%) | 735 (1.34%) | |
| Marital status | ||||
| Not married | 20,526 (36.5%) | 737 (50.7%) | 19,789 (36.2%) | <0.001 |
| Married | 35,645 (63.5%) | 716 (49.3%) | 34,929 (63.8%) | |
| Region | ||||
| West | 25,086 (44.7%) | 801 (55.1%) | 24,285 (44.4%) | <0.001 |
| Midwest | 4713 (8.39%) | 91 (6.26%) | 4622 (8.45%) | |
| Southwest | 3589 (6.39%) | 76 (5.23%) | 3513 (6.42%) | |
| East | 22,783 (40.6%) | 485 (33.4%) | 22,298 (40.8%) | |
| Size, cm, mean (SD) | 13.6 (17.3) | 21.8 (14.8) | 13.4 (17.3) | <0.001 |
| 0–1.0 | 30,197 (53.8%) | 326 (22.4%) | 29,871 (54.6%) | <0.001 |
| 1.1–4.0 | 23,688 (42.2%) | 972 (66.9%) | 22,716 (41.5%) | |
| >4.0 | 2286 (4.07%) | 155 (10.7%) | 2131 (3.89%) | |
| T classification | ||||
| T1 | 41,912 (74.6%) | 821 (56.5%) | 41,091 (75.1%) | <0.001 |
| T2 | 6981 (12.4%) | 451 (31.0%) | 6530 (11.9%) | |
| T3 | 6434 (11.5%) | 154 (10.6%) | 6280 (11.5%) | |
| T4 | 844 (1.50%) | 27 (1.86%) | 817 (1.49%) | |
| Surgery extent | ||||
| No surgery | 54,718 (97.4%) | 0 (0.00%) | 54,718 (100%) | <0.001 |
| Surgery | 1453 (2.59%) | 1453 (100%) | 0 (0.00%) | |
| Radioactive iodine | ||||
| Not given | 36,886 (65.7%) | 1453 (100%) | 35,458 (64.8%) | <0.001 |
| Given | 19,285 (34.3%) | 0 (0.00%) | 19,260 (35.2%) | |
| EBRT | ||||
| No | 55,802 (99.3%) | 1453 (100%) | 54,349 (99.3%) | 0.003 |
| Yes | 369 (0.66%) | 0 (0.00%) | 369 (0.67%) | |
| ETE | ||||
| No | 50,658 (90.2%) | 1421 (97.8%) | 49,237 (90.0%) | <0.001 |
| Yes | 5513 (9.81%) | 32 (2.20%) | 5481 (10.0%) | |
EBRT, external beam radiation therapy; ETE, extrathyroidal extension.
FIG. 1.
Histogram of cohort distribution. (A) Nonsurgical cohort by age. (B) Nonsurgical cohort by size. (C) Surgical cohort by age. (D) Surgical cohort by size. Color images are available online.
FIG. 2.
Kaplan–Meier survival curves for nonsurgical versus surgical cohorts comparing (A) noncancer mortality and (B) disease-specific survival. Color images are available online.
Association of surgery with survival
In univariate analysis, surgery was strongly associated with improved DSS (HR 0.157 [CI 0.105–0.236], p < 0.001). After adjustment for covariates in a multivariable model, surgery remained predictive of improved DSS (HR 0.555 [CI 0.364–0.847], p = 0.006) (Table 2). Similarly, older age and increased size independently conferred increased cancer-specific mortality risk (p < 0.001).
Table 2.
Univariate and Multivariable Analyses of Disease-Specific Survival, Stratified by Surgical and Nonsurgical Management
| Characteristic | Univariate | p | Multivariable | p |
|---|---|---|---|---|
| Age at diagnosis | 1.091 (1.080–1.102) | <0.001 | a | <0.001 |
| Sex | ||||
| Male | 1.000 | — | 1.000 | — |
| Female | 0.413 (0.313–0.545) | <0.001 | 0.598 (0.449–0.797) | <0.001 |
| Race/ethnicity | ||||
| White | 1.000 | — | b | |
| Black | 1.162 (0.671–2.012) | 0.593 | ||
| Asian | 1.033 (0.647–1.650) | 0.892 | ||
| Hispanic | 1.388 (0.959–2.009) | 0.082 | ||
| Other | 0.830 (0.206–3.351) | 0.793 | ||
| Marital status | ||||
| Not married | 1.000 | — | 1.000 | — |
| Married | 0.585 (0.447–0.766) | <0.001 | 0.694 (0.523–0.922) | 0.012 |
| Region | ||||
| West | 1.000 | — | b | |
| Midwest | 0.836 (0.508–1.376) | 0.481 | ||
| Southwest | 0.800 (0.450–1.422) | 0.447 | ||
| East | 0.710 (0.527–0.956) | 0.024 | ||
| Size, cm | ||||
| 0–1.0 | 1.000 | — | a | <0.001 |
| 1.1–4.0 | 4.061 (2.766–5.961) | <0.001 | ||
| >4.0 | 24.942 (16.506–37.691) | <0.001 | ||
| T classification | ||||
| T1 | 1.000 | — | b | |
| T2 | 3.614 (2.364–5.525) | <0.001 | ||
| T3 | 7.049 (4.882–10.179) | <0.001 | ||
| T4 | 51.968 (36.235–74.534) | <0.001 | ||
| Radioactive iodine | ||||
| Not given | 1.000 | — | b | |
| Given | 1.268 (0.966–1.664) | 0.087 | ||
| Surgery | ||||
| No | 1.000 | — | 1.000 | — |
| Yes | 0.157 (0.105–0.236) | <0.001 | 0.555 (0.364–0.847) | 0.006 |
Age and tumor size were modeled continuously using a restricted cubic spline function with three knots at age 39, 62, 81, and size 2, 11, 35, respectively.
Variables dropped from multivariable model through stepwise variable selection.
Effects of increasing age and size on survival differences
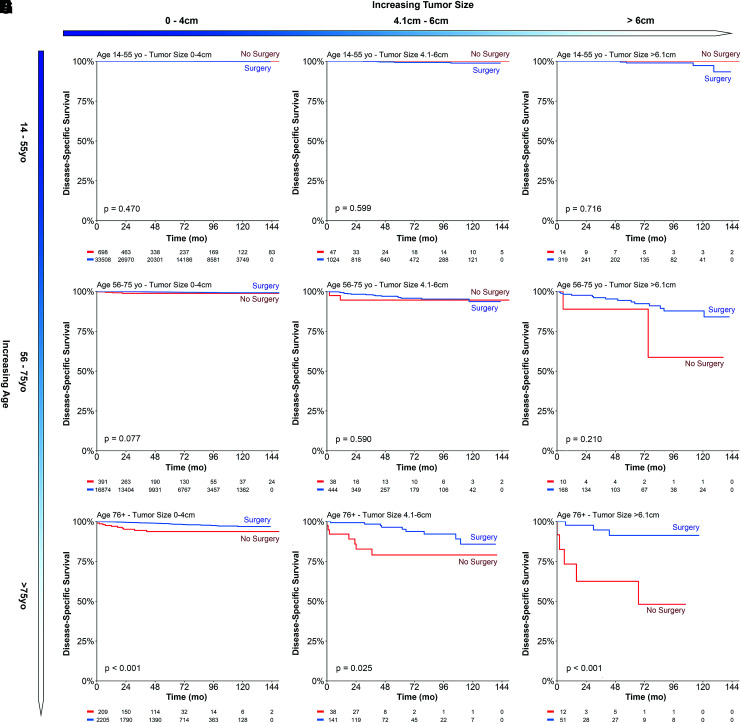
Given the importance of age and size in thyroid cancer staging, subgroup analysis was performed comparing the nonoperative and surgery cohorts. Using different age (14–55, 55–75, and >75 years) and size (0–4, 4.1–6, and >6 cm) thresholds, a 3 × 3 Kaplan–Meier survival matrix was created to visualize mortality trends (Fig. 3). Collectively, enlarging size and older age led to progressively greater differences in thyroid cancer-related mortality between the nonoperative and surgical patients.
FIG. 3.
Kaplan–Meier survival curve matrix of disease-specific survival comparing surgery with nonoperative cohorts, with increasing size and age. (A) Age 14–55 years, tumor size 0–4 cm. (B) Age 14–55 years, tumor size 4.1–6 cm. (C) Age 14–55 years, tumor size >6 cm. (D) Age 56–75 years, tumor size 0–4 cm. (E) Age 56–75 years, tumor size 4.1–6 cm. (F) Age 56–75 years, tumor size >6 cm. (G) Age >75 years, tumor size 0–4 cm. (H) Age >75 years, size 4.1–6 cm. (I) Age >75 years, tumor size >6 cm. Color images are available online.
For the younger-age group, surgery compared with no-surgery was not associated with any difference in the 10-year DSS in any size group (Fig. 3A–C). Although not statistically significant, the middle-age group showed a greater separation in DSS relative to the younger-age group (Fig. 3D, E). All older-age cohorts showed a significant survival advantage with surgery, with larger tumors (>75 years, >6 cm) demonstrating the greatest difference in thyroid cancer-related mortality (91.3% vs. 48.1%, p < 0.001) (Fig. 3G–I).
Because the surgical and nonsurgical cohorts had disparate baseline characteristics, propensity score matching was used to account for unbalanced covariates. After propensity score matching (Supplementary Fig. S2), all younger-age cohorts (14–55 years) and middle-age cohorts (56–75 years) continued to show no difference in DSS with surgery versus nonoperative management. In contrast, all older-age cohorts (>75 years) continued to show survival improvements with surgery regardless of size, with statistically significant improvements observed among 0–4 and >6 cm tumors.
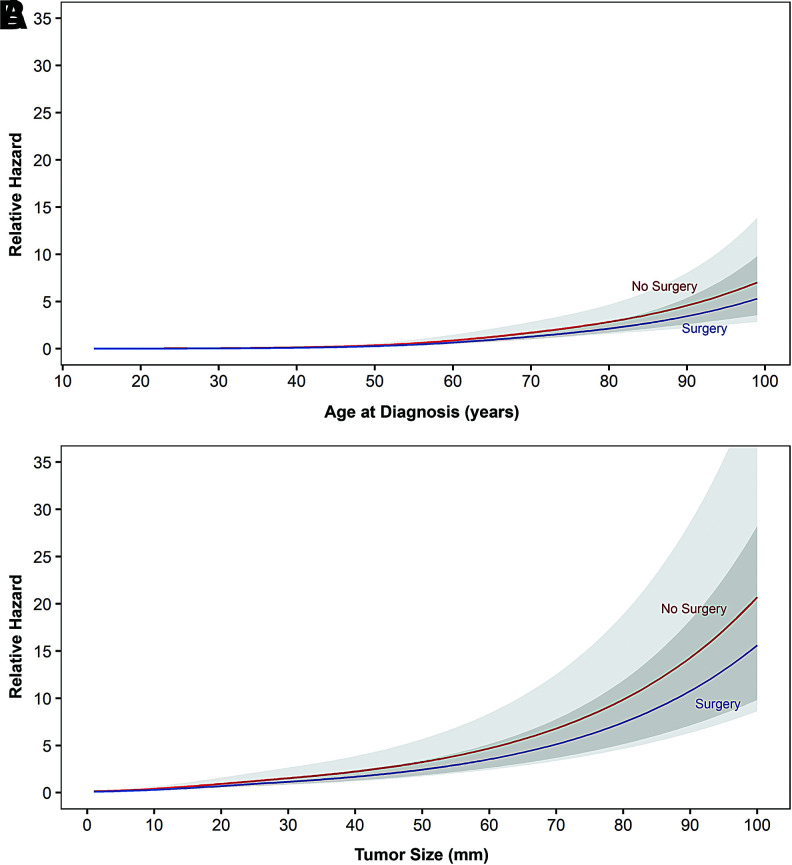
Restricted cubic spline plots
Given their nonlinear relationships, multivariable restricted cubic spline functions were generated to model the relationship of age and size with DSS in nonoperative patients, controlling for covariates included in the multivariable model. The difference in relative hazard increased slowly over advancing size and age (Fig. 4). At the 60 years threshold, the difference measured 0.08. Similarly, at the 2 cm threshold, the relative hazard difference measured 0.12. Mortality risk did not appear clinically meaningful below these thresholds, and in fact demonstrated overlapping 95% CIs throughout the entire measured range. The magnitude of hazard was much less pronounced for age compared with size.
FIG. 4.
Restricted cubic spline plot exhibiting relative hazard from disease-specific mortality risk in papillary thyroid carcinoma patients managed nonoperatively. (A) Escalating mortality risk with increasing age (three knots at 39, 62, and 81 years), controlled for sex, race/ethnicity, marriage, geographic region, size (three knots at 2, 11, and 35 mm), surgery, and RAI. Linear change points are identified at 50, 72, and 88 years of age. (B) Escalating mortality risk with increasing size (three knots at 2, 11, and 35 mm), controlled for sex, race/ethnicity, marriage, geographic region, surgery, age (three knots at 39, 62, and 81 years), and RAI. Linear change points are identified at 4.6, 6.9, and 8.7 cm. RAI, radioactive iodine. Color images are available online.
For age, a change point was identified at the 72 years threshold, an inflection point beyond which relative hazard was escalated in slope for each additional year. For size, change points were identified at the 4.5 and 6.8 cm thresholds, where relative hazard was accelerated in slope beyond each change point in parabolic manner.
Discussion
In this study, we examined the collective impact of age and size on thyroid cancer survival on a large cohort of patients managed nonoperatively. We focused on these two factors given their relevance in decision-making for active surveillance. In comparison to surgical patients, we demonstrate that nonsurgical patients exhibited roughly equivalent disease-specific mortality at younger ages and increased risk of thyroid cancer-related death at older ages. Using a continuous multivariable approach with spline plots, we found that increasing age independently escalates cancer-specific mortality risk without surgery, with a difference in survival hazard detected past 60 years of age. Increasing size was also independently associated with cancer-specific mortality hazard beyond 2.0 cm, exhibiting a comparatively steeper slope and greater magnitude of hazard.
Surgery has remained the central pillar of treatment for PTC for decades, especially for tumors with aggressive histology and regional nodal metastasis. Nevertheless, for the cohort studied here, it is compelling that the relative benefit is not meaningful until patients older or tumors larger than previously studied are considered. The indolent nature of PTC suggests that survival differences may be difficult to discriminate in younger patients or smaller tumors or that much longer follow-up would be needed to perceive any distinction. At levels beyond the initial inflection points, the magnitude of hazard may be technically apparent but not clinically meaningful. What risk a patient will tolerate is thus beyond the scope of a population-scale analysis: it instead entails individual decisions that weigh mortality risk from cancer against benefits from active monitoring, such as avoiding surgical complications and the need for lifelong hormone substitution (26).
This analysis encompasses the largest nonsurgical cohort to date, capturing a spectrum of age and size ranges not previously examined. Like active surveillance trials, we restricted our analysis to cases without regional or distant metastatic spread: advanced-stage patients would be expected to do far worse without treatment. Notably, nonsurgical patients had favorable outcomes despite presumably not undergoing modern active surveillance protocols; similar or better outcomes are likely with strict monitoring and clear parameters to trigger surgery.
With precedent in other disciplines such as prostate cancer, active surveillance has emerged as a viable alternative to surgery for papillary thyroid microcarcinomas (<1 cm), with more than 2000 patients studied across Japan, South Korea, and the United States (5–8). To date, no patients undergoing an active surveillance paradigm have died of thyroid cancer. Yet while prostate active surveillance has been widely adopted in practice for selected patients, the same has not yet occurred in thyroid malignancies (9,27,28). Our data support broad clinical implications regarding this management paradigm in low-risk PTC: it corroborates findings from those single-arm studies, compares favorably against matched surgical cohorts over long-term follow-up, and suggests that larger size thresholds than previously considered may be reasonable for active surveillance. Our data suggest that there is no dramatic difference in hazard between a 1.0 or 2.0 cm cancer, and that hazard accumulates incrementally with growth yet does not accelerate in risk until 4.5 cm. Similarly, risk with age climbs slowly after age 60 years and does not escalate in slope until after age 72 years. We expect that spline plots for true active surveillance cohorts would mirror or improve upon what was observed for our nonoperative cohort. By evaluating mortality risk rather than disease progression [known to be divergent in thyroid cancer (29)], our results bolster promising trial results and alleviate concerns that untreated or unchecked clinical progression may disproportionately worsen survival.
Older age has long been associated with higher risk PTC and remains a prognostic covariate that is unique to thyroid cancer staging systems (30). Explanations proposed for this relationship include decreased uptake or response to RAI, increased levels of thyrotropin, declining immune systems, and higher rates of BRAFV600E mutations (13,31–33). All are noted to have increased prevalence in the elderly patients, and either diminish treatment impact or facilitate cancer progression.
Our age results differ from prior active surveillance studies, which have suggested that older thyroid cancer patients are in fact less likely to progress. Based on a Japanese model of monitored patients, the estimated lifetime probability of disease progression appears to fall with age (60.3% for patients in their 20s, compared with 3.5% for patients in their 70s) (10). Other active surveillance trials have upheld these findings on multivariable analysis (7). To be sure, the populations studied may differ demographically: active surveillance patients self-select into closely monitored trials with very-low-risk cancers and undergo curative surgery with progression. In contrast, SEER nonoperative patients may have been too frail to undergo surgery amid a range of larger tumors considered higher risk.
A more probable explanation for the age discrepancy is what constitutes an event: 3 mm size growth or development of nodal disease may describe progression but not translate into mortality risk. Trial patients were also limited to ≤1.0 cm thyroid cancers, which selects out aggressive disease and favors indolent tumors [previously described as a subclinical reservoir (2)]. Such cancers are likely more prevalent in older populations, who accumulate larger reservoirs over their natural lifespan (34). Given that our data set encompasses all sizes and measures cancer-specific deaths rather than 3 mm growth, we would expect our findings to better align with widely acknowledged mortality data establishing older age as a major independent risk factor in thyroid malignancies (11–15). As such, we would not suggest denying active surveillance to older patients: its appeal lies in dynamic monitoring, which would presumably reveal a cancer's aggressiveness over time and allow for appropriate intervention if needed. It is empirically clear that many older patients, especially with smaller thyroid cancers, do not progress and if carefully triaged are otherwise eligible for monitoring. The individual acceptance of risk (from cancer or from surgery) thus remains a matter of informed consensus between patient and clinician.
Several caveats to this analysis deserve mention, including its retrospective nature, possible coding errors inherent in large registries, inability to confirm histology or N0 status in nonoperative patients, and absence of detail on growth kinetics involving local, regional, or distant spread. Small survival differences between surgical and nonsurgical approaches may exist at younger ages or smaller tumors, yet require larger cohorts, more events, or longer follow-up to appreciate. An alternative explanation to worsening mortality in nonoperative patients involves attribution bias, where patients diagnosed with thyroid cancer may be misattributed to die from it. Older patients, who are more likely to harbor comorbidities that lead to noncancer-related deaths, may in particular be prone to erroneous coding, as has been seen in prostate cancer (35). To test this theory, we examined the patients with nonoperative papillary thyroid microcarcinomas (<1 cm) (rarely if ever lethal): none was found to have died from their cancer, suggesting minimal if any effect from this potential bias.
Another potential confounder that may explain the lack of survival difference in certain groups is the registry recording interval: as SEER only tracks whether surgery was performed for 1 year after diagnosis, some nonoperative cases may conceivably have undergone surgery after this period (36). Survival differences could potentially converge if surgery was later performed in the nonoperative groups. However, this scenario is dubious given the clear differences in noncancer mortality (Fig. 2) between the nonoperative and surgical cohorts: a markedly higher incidence of death from other causes is seen in the nonoperative group. Such variance supports two distinct populations, rather than staggered surgical groups distinguished by a 1-year lag in thyroidectomy.
In summary, we describe the spectrum of age and size thresholds that confer mortality risk in nonoperative thyroid cancers. Our findings illustrate the escalating gradients at which a nonsurgical approach may confer hazard and help reconcile prior findings regarding age and size. A nonsurgical approach as an alternative to surgery may be reasonable in larger tumors than previously considered, particularly for younger patients. As overdiagnosis and overtreatment emerge as increasingly germane across cancer disciplines (37), the relative impact of intervention becomes considerably more valuable to gauge (38,39). Conveying such a continuum of risk would be an effective means to articulate patient prognosis and tailor shared decision-making.
Supplementary Material
Author Disclosure Statement
Z.S.Z. was on the external advisory board for the Scripps Proton Therapy Center and has been a paid consultant for EMD Serono. For all other authors, no competing financial interests exist.
Funding Information
Donna and Jesse Garber Award for Cancer Research (A.S.H.).
Supplementary Material
References
- 1. Davies L, Welch HG. 2014. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140:317–322 [DOI] [PubMed] [Google Scholar]
- 2. Ho AS, Davies L, Nixon IJ, Palmer FL, Wang LY, Patel SG, Ganly I, Wong RJ, Tuttle RM, Morris LG. 2015. Increasing diagnosis of subclinical thyroid cancers leads to spurious improvements in survival rates. Cancer 121:1793–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, Epling JW, Jr, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Siu AL, Tseng CW. 2017. Screening for thyroid cancer: US Preventive Services Task Force Recommendation Statement. JAMA 317:1882–1887 [DOI] [PubMed] [Google Scholar]
- 4. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, Tomoda C, Takamura Y, Kobayashi K, Miya A. 2010. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 34:28–35 [DOI] [PubMed] [Google Scholar]
- 6. Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. 2010. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 34:1222–1231 [DOI] [PubMed] [Google Scholar]
- 7. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, Untch B, Ganly I, Shaha AR, Shah JP, Pace M, Li D, Bach A, Lin O, Whiting A, Ghossein R, Landa I, Sabra M, Boucai L, Fish S, Morris LGT. 2017. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg 143:1015–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwon H, Oh HS, Kim M, Park S, Jeon MJ, Kim WG, Kim WB, Shong YK, Song DE, Baek JH, Chung KW, Kim TY. 2017. Active surveillance for patients with papillary thyroid microcarcinoma: a Single Center's Experience in Korea. J Clin Endocrinol Metab 102:1917–1925 [DOI] [PubMed] [Google Scholar]
- 9. Ho AS, Daskivich T, Sacks WL, Zumsteg ZS. 2019. Parallels between low-risk prostate and thyroid cancer: a review. JAMA Oncol 5:556–564 [DOI] [PubMed] [Google Scholar]
- 10. Miyauchi A, Kudo T, Ito Y, Oda H, Sasai H, Higashiyama T, Fukushima M, Masuoka H, Kihara M, Miya A. 2018. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery 163:48–52 [DOI] [PubMed] [Google Scholar]
- 11. 2017. MD MBA (ed) AJCC Cancer Staging Manual. 8th ed. Springer, New York [Google Scholar]
- 12. Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, Morris L, Vaisman F, Corbo R, Momesso D, Vaisman M, Carvalho A, Learoyd D, Leslie WD, Nason RW, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gönen M, Pathak KA, Shen WT, Sywak M, Kowalski L, Freeman J, Perrier N, Shah JP. 2016. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid 26:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. 2016. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol 34:4415–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nixon IJ, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gönen M, Shah JP. 2016. Defining a valid age cutoff in staging of well-differentiated thyroid cancer. Ann Surg Oncol 23:410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, Sywak M, Eskander AE, Freeman JL, Campbell MJ, Shen WT, Vaisman F, Momesso D, Corbo R, Vaisman M, Shaha A, Tuttle RM, Shah JP, Patel SG. 2015. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid 25:1106–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schemper M, Smith TL. 1996. A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343–346 [DOI] [PubMed] [Google Scholar]
- 17. Fine JP, Gray RJ. 1999. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509 [Google Scholar]
- 18. Gray RJ. 1988. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154 [Google Scholar]
- 19. Cox DR, Oakes D. 1984. Analysis of Survival Data. Taylor & Francis, New York [Google Scholar]
- 20. Harrington DP, Fleming TR. 1982. A class of rank test procedures for censored survival data. Biometrika 69:553–566 [Google Scholar]
- 21. Venables WN. 2002. Modern applied statistics with S. Ripley BD, Venables WN, Masw SP. (eds). Springer, New York [Google Scholar]
- 22. Grambsch PM, Therneau TM. 1994. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81:515–526 [Google Scholar]
- 23. Austin PC. 2011. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46:399–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrell F. 2001. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. 2nd ed. Springer, New York [Google Scholar]
- 25. Muggeo VMR. 2003. Estimating regression models with unknown break-points. Stat Med 22:3055–3071 [DOI] [PubMed] [Google Scholar]
- 26. D'Agostino TA, Shuk E, Maloney EK, Zeuren R, Tuttle RM, Bylund CL. 2018. Treatment decision making in early-stage papillary thyroid cancer. Psychooncology 27:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cooperberg MR, Carroll PR. 2015. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA 314:80–82 [DOI] [PubMed] [Google Scholar]
- 28. Lowenstein LM, Basourakos SP, Williams MD, Troncoso P, Gregg JR, Thompson TC, Kim J. 2019. Active surveillance for prostate and thyroid cancers: evolution in clinical paradigms and lessons learned. Nat Rev Clin Oncol 16:168–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Links TP, van Tol KM, Jager PL, Plukker JT, Piers DA, Boezen HM, Dullaart RP, de Vries EG, Sluiter WJ. 2005. Life expectancy in differentiated thyroid cancer: a novel approach to survival analysis. Endocr Relat Cancer 12:273–280 [DOI] [PubMed] [Google Scholar]
- 30. Tuttle M, Morris LF, Haugen B, Shah J, Sosa JA, Rohren E, Subramaniam RM Hunt JL, Perrier ND 2017. Thyroid-differentiated and anaplastic carcinoma. In: Amin MB, et al. (ed). AJCC Cancer Staging Manual, 8th ed. Springer International Publishing, New York [Google Scholar]
- 31. Haymart MR. 2009. Understanding the relationship between age and thyroid cancer. Oncologist 14:216–221 [DOI] [PubMed] [Google Scholar]
- 32. Shen X, Zhu G, Liu R, Viola D, Elisei R, Puxeddu E, Fugazzola L, Colombo C, Jarzab B, Czarniecka A, Lam AK, Mian C, Vianello F, Yip L, Riesco-Eizaguirre G, Santisteban P, O'Neill CJ, Sywak MS, Clifton-Bligh R, Bendlova B, Sýkorová V, Xing M. 2018. Patient age-associated mortality risk is differentiated by BRAF V600E status in papillary thyroid cancer. J Clin Oncol 36:438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marti JL, Morris LGT, Ho AS. 2018. Selective use of radioactive iodine (RAI) in thyroid cancer: no longer “one size fits all”. Eur J Surg Oncol 44:348–356 [DOI] [PubMed] [Google Scholar]
- 34. Ho AS, Chen I, Melany M, Sacks WL. 2018. Evolving management considerations in active surveillance for micropapillary thyroid carcinoma. Curr Opin Endocrinol Diabetes Obes 25:353–359 [DOI] [PubMed] [Google Scholar]
- 35. Quinn M, Babb P. 2002. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part II: individual countries. BJU Int 90:174–184 [DOI] [PubMed] [Google Scholar]
- 36. Davies L, Welch HG. 2010. Thyroid cancer survival in the United States: observational data from 1973 to 2005. Arch Otolaryngol Head Neck Surg 136:440–444 [DOI] [PubMed] [Google Scholar]
- 37. Esserman LJ, Thompson IM, Reid B, Nelson P, Ransohoff DF, Welch HG, Hwang S, Berry DA, Kinzler KW, Black WC, Bissell M, Parnes H, Srivastava S. 2014. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol 15:e234–e242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tuttle RM, Zhang L, Shaha A. 2018. A clinical framework to facilitate selection of patients with differentiated thyroid cancer for active surveillance or less aggressive initial surgical management. Expert Rev Endocrinol Metab 13:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brito JP, Ito Y, Miyauchi A, Tuttle RM. 2016. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid 26:144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.