Abstract
Objective
To review and critically appraise the ocular manifestation and the presence of SARS-CoV-2 through PCR positivity from ocular samples in COVID-19-related patients. Moreover, to evaluate the time and severity association of ocular manifestation to systemic disease of COVID-19.
Methods and analysis
A systematic literature search from PubMed, ScienceDirect and Google Scholar databases was performed using standardised Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline. Selected keywords were related to COVID-19, ocular manifestation and PCR testing of SARS-CoV-2. Studies were assessed for their validity, and the data were extracted by two independent reviewers. Observational, case series and case report studies were included if they met the selection criteria. Meta-analysis was performed to estimate the pooled prevalence of ocular manifestations and PCR positivity from tears.
Results
Thirty-one articles were qualitatively reviewed, and 14 studies were included in the meta-analysis. The pooled prevalence of ocular manifestation among COVID-19-related patients was 0.05 (95% CI 0.02% to 0.08). The overall PCR from tears samples positivity rate from COVID-19-related patients presenting with ocular manifestation was 0.38 (95% CI 0.14% to 0.65). Ocular manifestation could precede systemic manifestation in about 0.28 (95% CI 0.05% to 0.58) of COVID-19-related patients with ocular manifestations. Besides, ocular manifestation was not associated with a severe form of COVID-19.
Conclusion
Although the overall number of ocular manifestation and SARS-CoV-2 PCR positivity rate from ocular samples was very low, around a quarter of COVID-19-related patients with ocular manifestation presented their ocular manifestation earlier than the systemic manifestation regardless of the severity. Interestingly, SARS-CoV-2 PCR was positive from one-third of ocular samples, which could potentially be the source of infection to the respiratory tract and the environment, although the infectivity is yet to be determined.
Keywords: tears, infection, microbiology, ocular surface
Key messages.
What is already known about this subject?
SARS-CoV-2 can infect and replicate in the eyes through angiotensin receptor enzyme-2 receptor found in conjunctiva and cornea.
Conjunctivitis among patients with COVID-19 had been reported.
The nasolacrimal duct can transmit the virus from the eyes to the nasopharynx.
What are the new findings?
The overall prevalence of ocular manifestation among patients with COVID-19 is 5%.
Around a quarter of ocular manifestation could precede systemic manifestations among COVID-19-related patients with ocular manifestation.
SARS-CoV-2 PCR was positive from one-third of ocular samples among COVID-19-related patients with ocular manifestation.
How might these results change the focus of research or clinical practice?
Conjunctivitis in patients suspected to have COVID-19 could potentially be the source of infection due to SARS-COV-2.
Detecting SARS-CoV-2 from ocular samples is difficult. Yet, ophthalmologists or general practitioners facing conjunctivitis in patients highly suspected to have COVID-19 or in areas with high transmission of COVID-19 should wear adequate personal protective equipment, including mask and goggles/face shield.
Introduction
The 2019 novel coronavirus (2019-nCoV) or SARS-CoV-2, a single-stranded positive-sense RNA virus, belongs to the family of Coronaviridae. COVID-19, the disease caused by SARS-CoV-2 infection, can range from being asymptomatic to critically ill, leading to death.1 2 In December 2019, SARS-CoV-2 started to spread, and COVID-19 had become a global pandemic. By 14 May 2020, it was estimated that more than four million people being infected and 294 046 deaths worldwide were caused by COVID-19.3 WHO issued the first set of personal protective equipment guideline in March 2020 based on the previous experience in managing Middle Eastern respiratory syndrome coronavirus and SARS-CoV in 2004.4 In their recommendation, wearing goggles or face shield was included as a protection against SARS-CoV-2 transmission, even though there was still lack of published studies reporting eye infection caused by COVID-19 at that time. From the previous SARS-CoV experience, coronavirus could be found in tears, based on PCR positivity.5 Human-to-human aerosol transmission has been described mainly via the respiratory tract through droplets.1 2 However, the possibility of SARS-CoV-2 transmission through the ocular surface in the population is maybe often overlooked.
Lu et al6 warned that ocular manifestation may have appeared earlier than predicted. Their argument was based on the report of Guangfa Wang, a member of the national expert panel who inspected Wuhan and then got infected by SARS-CoV-2 at the beginning of the COVID-19 spread. Wang described that his red eyes had started several days before respiratory symptoms of COVID-19 appeared. As it may appear earlier, the ocular manifestation may be under-reported. Guan et al,7 in their first report of a COVID-19 patient in China, found that ocular manifestation only contributed in around 1% of patients. Subsequently, Wu et al8 reported that ocular manifestation might be as high as 30% among patients with COVID-19. In comparison to lung and bronchial tissue cultures, the highest viral replication of SARS-CoV-2 at 48 hours was found in conjunctival tissue culture based on the recent ex vivo study.9 Moreover, SARS-CoV-2 PCR from tears can be still positive up to 3 weeks from the onset of systemic symptom even though the nasopharyngeal swab result is already negative.10 As SARS-CoV-2 replication in ocular surface may continue for a relatively long period and the virus can be transported to nasopharyngeal mucosa through the nasolacrimal duct,11 there is a possibility that ocular manifestation can affect systemic COVID-19. With the growing number of recently published studies, we performed a systematic review and meta-analysis to elaborate the possibility of the eye as an infection source for systemic COVID-19 by looking at the ocular manifestation and the presence of SARS-CoV-2 in the eye. We also aimed to describe the ocular clinical manifestation and the onset of ocular symptoms of COVID-19 with their relation to the presenting systemic manifestation along with its severity level.
Methods
Literature search strategy
We performed a systematic review according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.12 Literature search was conducted from 4 to 9 June 2020 using three electronic databases: PubMed, ScienceDirect and Google Scholar. The literature search would be expanded using a snowballing method to the references of retrieved papers. Articles were identified with search strategy: “SARS-CoV-2 [supplementary concept]” OR “2019-nCoV” OR “COVID-19 [supplementary concept]” AND “conjunctiv*” OR “eye manifestations (MeSH)” OR “cornea (MeSH)” OR “ocular surface” OR “dry eye syndromes (MeSH)”. This study was registered to the International Prospective Register of Systematic Reviews (PROSPERO registration number CRD42020194245).
Eligibility criteria
The enrolment date of studies was restricted from December 2019 to 1 June 2020. Records were managed by Mendeley software to exclude duplicates. Articles in English and human subjects were obtained. The inclusion criteria were (1) peer-reviewed observational case series and case report studies of COVID-19-related patients, including confirmed and suspected cases; (2) studies providing ocular manifestations; (3) if available, studies reporting PCR positivity in either ocular, elsewhere samples or both. Studies that reported ocular involvement but did not describe the ocular manifestations or reported only subjective ocular complaints were excluded. If it is necessary, the original author of each study was contacted by email to request further information.
Data extraction
Three authors (RLDN, IS and DFK) independently reviewed titles and abstracts generated by the search. A standardised data abstraction table was designed to capture all relevant information required for analysis. For all included studies, we recorded the following information: author, date of publication, study design, PCR positivity in nasal swab and tears, the onset of ocular manifestation, description of ocular and systemic manifestations, and disease severity. Ocular symptom duration and treatment given by the healthcare providers were also noted whenever the data are available.
Quality assessment
Two authors independently assessed the quality of included studies (DFK and ASR). The risk of bias and quality of primary studies or systematic reviews were assessed using the Newcastle-Ottawa Scale for longitudinal and cross-sectional studies.13 The quality levels then were graded as good, fair or poor. For case series and case report studies, the quality was assessed using Murad et al’s14 set of criteria and graded as poor, moderate r good quality. Discrepancies and disagreements were resolved by consensus, and/or resolution of the conflict was performed by a third reviewer (IP) if necessary.
Patient involvement
Patients were not directly involved in the design of this study.
Statistical analysis
We undertook an initial descriptive analysis of the studies. The heterogeneity between estimates was assessed using the I2 statistic. For studies with calculable prevalence for each item, the meta-analysis was performed using a random-effects model conducted using the MetaXL 5.3 (www.epigear.com) add-in for Microsoft Excel Professional Plus 2013. A pooled prevalence figure was calculated with a 95% CI. The pooled OR for the association of ocular manifestation with COVID-19 severity was calculated using a random model effect. The Mantel-Haenszel method was used to weight the studies. This statistical approach was performed using Review Manager V.5.4.
Results
Study selection
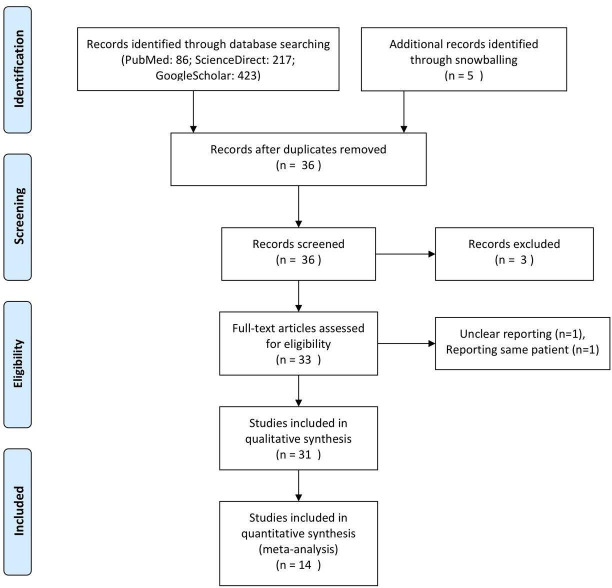
Our initial search identified 31 studies to be analysed qualitatively in our systematic review. There were 5 longitudinal, 9 cross-sectional, 5 case series, and 12 case report studies being reviewed for analysis. For quantitative analysis, 14 studies could be included in the pooled meta-analysis of prevalence. The flowchart of study selection is illustrated in figure 1. All articles were assessed for validity (online supplemental file). Tables 1 and 2 summarise the characteristics and main findings of the observational and case report studies, respectively.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart for the study selection process.
Table 1.
Characteristics of observational studies
| Study | Design | Patients (n) | PCR positivity rate | Treatment for ocular manifestation | ||||
| All | Ocular manifestation | Systemic manifestation | PCR tears/ocular manifestation | PCR tears/all COVID-19-related patients | PCR positivity rate other than tears sample | |||
| Guan et al7 | Cross-sectional | 1099 | 9 | 1099 | n/a | n/a | 100% NP+; 4/1099 (0.36%) stool+; 4/1099 (0.36%) rectal+ | n/a |
| Wu et al8 | Cross-sectional | 38 | 12 | 38 | 0.17 | 0.05 | 28/38 NP+ | n/a |
| Xia et al43 | Prospective observational | 30 | 1 | 30 | 1.00 | 0.03 | 30/30 NP+ | n/a |
| Zhang et al15 | Cross-sectional | 102 | 2 | n/a | 1.00 | 0.02 | 72/102 NP+ | Ganciclovir eye drops |
| Xie et al44 | Retrospective | 33 | 0 | 33 | n/a | 0.06 | 100% NP+ | n/a |
| Chen et al45 | Cross-sectional | 535 | 27 | 535 | n/a | n/a | 343/535 NP+ | Ofloxacin, tobramycin, ganciclovir eye drops, artificial tear |
| Kumar et al46 | Cross-sectional | 45 | 0 | 31 | n/a | 0.02 | 100% NP+ | n/a |
| Zhou et al47 | Cross-sectional | 121 | 8 | 121 | 0.13 | 0.02 | 100% NP+ | n/a |
| Deng et al41 | Cross-sectional | 114 | 0 | 114 | n/a | 0 | 90/114 NP + | n/a |
| Karimi et al48 | Cross-sectional | 43 | 1 | 43 | 1.00 | 0.07 | 30/43 NP + | n/a |
| Grimaud et al49 | Retrospective | 20 | 6 | 20 | n/a | n/a | 10 NP+ (and 15 serology+) | n/a |
| Valente et al30 | Prospective observational | 27 | 4 | 23 | 0.25 | 0.11 | 100% NP+ | n/a |
| Seah et al24 | Prospective observational | 17 | 1 | 14 | 0 | 0 | 17/17 (100%) NP+ | n/a |
| Fang et al50 | Cross-sectional | 32 | 0 | 28 | n/a | 0.16 | 32/32 (100%) NP+, 25/32 (78,1%) saliva+ | n/a |
n/a, not applicable; NP, nasopharyngeal.
Table 2.
Characteristics and details of case series and case report studies
| Study | Design | Patients with ocular involvement (n) | Systemic manifestation | Ocular manifestation | ||||
| Description | Severity | Onset (in relation to systemic manifestation) | Description | Duration | Treatment | |||
| Chen et al23 | Case report | 1 | Sore throat, diarrhoea | Non-severe | Day 13 after | Redness foreign body sensation and tearing. Physical examination: bilateral conjunctival injection, watery discharge, inferior bilateral conjunctival follicles, preauricular lymph node | 5 days | Ribavirin eye drops |
| Cheema et al20 | Case report | 1 | Rhinorrhea, cough, nasal congestion | Non-severe | Parallel | Conjunctivitis, photophobia, watery discharge Physical examination: pseudodendritic epithelial defect, subepithelial infiltrate, conjunctival follicles |
n/a | Valacyclovir and moxifloxacin eye drops |
| Daruich et al51 | Case report | 1 | Fever, headache, cough, severe dyspnoea | Severe | 3 hours after | Foreign body sensation, conjunctival hyperaemia, eyelid oedema | 11 days | Topical antibiotic and corticosteroid |
| Chiotos et al52 | Case series | 2 | Case 3: fever, diarrhoea, periumbilical pain, hypovolaemic shock, respiratory failure, extremity oedema, fissured lips, strawberry tongue Case 4: fever, fissured lips, nuchal rigidity, morbilliform rash, emesis, diarrhoea, swollen hand, respiratory failure |
Case 3: severe Case 4: severe | Case 3: day 5 after Case 4: parallel |
n/a | n/a | n/a |
| Scalinci et al22 | Case series | 5 | No systemic manifestation | n/a | n/a | Chemosis, epiphora, photophobia | n/a | Moxifloxacin eye drops |
| Hu et al17 | Case report | 1 | Fever, fatigue, cough, sputum CT scan: ground-glass opacity in lung |
Severe | n/a | None | None | None |
| Navel et al25 | Case report | 1 | Cough, headache, nausea, myalgia, dyspnoea | Severe | Day 17 after | Conjunctival hyperaemia, clear secretion, follicles, petechiae, tarsal haemorrhagic, chemosis, pseudomembrane Fluorescein test: keratitis punctate PCR tears negative |
10 days | Azithromycin eye drops and a low dose of dexamethasone, daily debridement |
| Ying et al53 | Case report | 1 | No systemic manifestation | None | n/a | Both eyes redness, watery eye, mild eyelids swelling | 4 days | n/a |
| Salducci and La Torre18 | Case report | 1 | No systemic manifestation | None | n/a | Both eyes red, irritated and swollen, pseudomembranes, conjunctival chemosis, serous secretions, periauricular and submaxilaries lymph node enlargement | 7 days | Gancyclovir, artificial tears, and cold compress |
| Wu et al54 | Case report | 1 | No symptoms and physical examination finding, but laboratory: lymphopaenia and myocardial damage | Non-severe | Day 7 after | Conjunctivitis and eyelid dermatitis | n/a | n/a |
| Khavandi et al19 | Case report | 1 | Fever, dry cough, short breath, CT scan of lungs showed ground-glass opacities | Moderate | Day 4 before | Burning sensation in the unilateral eye, mucoid discharge and follicular conjunctivitis. | n/a | Symptomatic treatment with artificial tear |
| Wolfler et al55 | Case series | 1 | 5/9 cardiac injury, mild to moderate, fever, GI symptoms, 3/9 rash, 2/9 respiratory failure | 3/9 severe | n/a | Non-exudative conjunctivtis | n/a | n/a |
| Colavita et al10 | Case report | 1 | Non-productive cough, sore throat, coryza (first-day hospitalisation, onset 1 day) fever (38°C), nausea, vomiting (day 4) | n/a | Parallel | Bilateral conjunctivitis (onset 1 day) persistent, improved at day 15, resolved at day 20 Day 3: severe conjunctival hyperaemia, chemosis, epiphora Day 9: severe conjunctival hyperaemia, epiphora Day 13: moderate conjunctival hyperaemia, epiphora Day 16: mild conjunctival hyperaemia Day 21: normal |
16 days | n/a |
| Xuejie et al21 | Case series | 2 | Case 1: core throat and discomfort with cough and low fever up to 38.5°C Chest CT images showed typical COVID-19 findings Case 2: mild cough (day 1) sore throat, dizziness and headache (day 5) |
n/a | Case 1: day 4 before Case 2: parallel |
Case 1: viscous secretion and mild hyperaemia in the conjunctival sac conjunctival congestion and thin mucous secretions by slit lamp Case 2: eye itching (day 1), mild conjunctival congestion slight foreign body sensation, thin water secretion |
Case 1: 1 week Case 2: 1 week |
Case 1: ganciclovir eye drops and sodium hyaluronate eye drops were administered in both eyes four times per day during home isolation Case 2: local ganciclovir and sodium hyaluronate eye drops |
| Ya et al56 | Case series | 3 | Case 1: Mild dry cough and fatigue Case 2: Fever, cough, and fatigue Case 3: n/a |
n/a | Case 1: Day two after Case 2: Day three before Case 3: Day three after |
Case 1: hyperaemia and itching, conjunctival congestion in both eyes. Thin, mucous secretions were seen in the conjunctival sac. The lens was slightly cloudy, and the vitreous was turbid. Case 2: no obvious incentive of hyperaemia, itching and blurred vision Ophthalmic examination results: Visual acuity: right 0.8, left 0.8; intraocular pressure: right 17 mm Hg,left 18 mm Hg; hyperaemia in both eyes, mild chemosis, white mucous secretions can be seen in the conjunctival sac Case 3: hyperaemia, itching and foreign body sensation; more secretion in the conjunctival sac Ophthalmic examination results: conjunctival congestion in both eyes, thin watery secretions in the conjunctival sac |
Case 1: n/a (died due to COVID-19) Case 2: n/a Case 3: 1 week |
Case 1: Ganciclovir eye drops for both eyes, 4 times/day; Levofloxacin eye drops, 6 times/day; ganciclovir was applied to the eyes with gel once per night Case 2: the treatment of conjunctivitis is the same as case 1 Case 3: the treatment is the same as case 1 |
| Casalino et al57 | Case report | 1 | Intermittent dry cough and mild fever; the temperature was 38°C and the oxygen saturation level was 97% (SpO2); chest X-ray: increased bronchovascular marking with no definite signs suggestive of pneumonia | n/a | Day 2 before | Redness and watery discharge in the RE associated with foreign body sensation, viral conjunctivitis in the RE | Improved in 3 days | Topical tobramycin–dexamethasone |
| Lu et al6 | Case report | 1 | Pneumonia | n/a | Several days before | Redness of the eyes | n/a | n/a |
GI, gastrointestinal; n/a, not applicable; RE, right eye.
bmjophth-2020-000563supp001.pdf (108.8KB, pdf)
Ocular manifestation and PCR positivity rate for SARS-CoV-2 among COVID-19-related patients
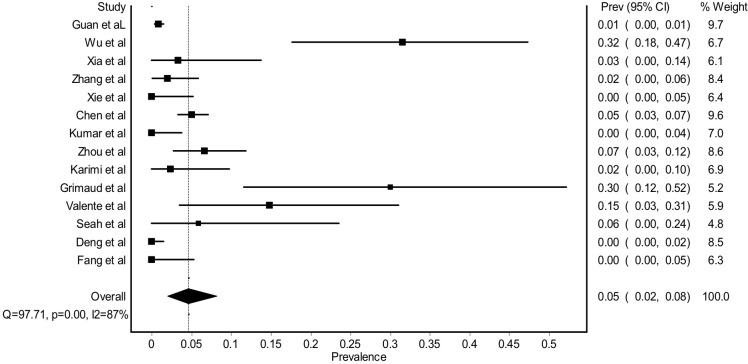
A meta-analysis to determine the estimation of ocular manifestation among COVID-19-related patients was performed. The pooled prevalence of ocular manifestation among COVID-19-related patients was 0.05 (0.02%–0.08 95% CI). The I2 for heterogeneity test was 86%, suggesting a high level of heterogeneity (figure 2). Most of the studies with a large number of patients (Guan et al,7 Wu et al8 and Zhang et al15) included patients already admitted to the hospital. In addition, most of the studies included in this study reported only ocular complaints from their subjects without any further ocular examination.
Figure 2.
Forest plot estimating the pooled prevalence of ocular manifestation among COVID-19-related patients.
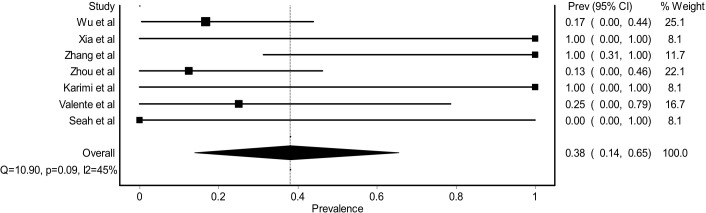
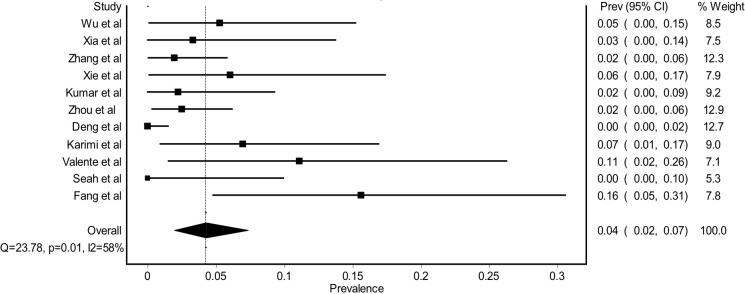
From COVID-19-related patients with ocular manifestations, overall PCR positivity from tears samples was 0.38 (95% CI 0.14% to 0.65, figure 3). The pooled PCR positivity from tears was higher compared with general PCR tears positivity from all COVID-19-related patients. If all available data of PCR positivity from tears were combined regardless of the presence of ocular manifestation, the pooled PCR positivity was only 0.04 (95% CI 0.02% to 0.07, figure 4).
Figure 3.
Forest plot estimating the pooled prevalence of PCR tears (+) among COVID-19-related patients with ocular manifestation.
Figure 4.
Forest plot estimating the pooled prevalence of PCR tears (+) among all COVID-19-related patients.
Relationship between the onset of ocular symptoms with the systemic manifestation of COVID-19
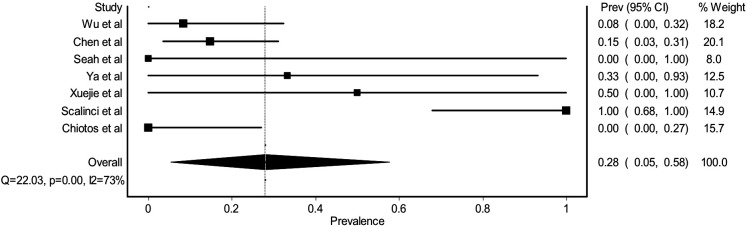
Several studies had reported the onset of ocular symptoms in relation to the presence of any systemic manifestation. From the pooled analysis, 0.28 (95% CI 0.05% to 0.58, figure 5) of ocular manifestation appeared without any systemic manifestations noticed by the patients. Frequent touching of the eyes with hands,16 elderly (age >60 years),17–19 immunosuppression,17 lacrimal duct abnormalities,17 swimming20 and being healthcare workers6 21 were reported to be possible predisposing factors for ocular manifestation of COVID-19.
Figure 5.
Forest plot estimating the pooled prevalence of ocular manifestation before any systemic manifestation among COVID-19 related patients with ocular manifestation.
Ocular manifestation can occur before, parallel or after the presence of systemic manifestation. Based on Scalinci et al’s22 report, conjunctivitis could be the only manifestation of COVID-19 without any subsequent systemic manifestation. Interestingly, they found that the nasopharyngeal swab yielded a positive result in all of these patients.22 In another study, Cheema et al20 found that when the ocular and systemic manifestation occurred at the same time in the early stage of the disease, PCR samples from both tears and nasopharyngeal mucosa could be positive. Moreover, they found that the cycle threshold for tears sample had a weaker signal compared with a nasopharyngeal swab.20 From the case series and case report studies, we found that the method and cycle threshold for PCR testing might influence the positivity rate of tear samples. Chen et al23 and Hu et al17 took tear samples through putting sterile synthetic fibre or cotton swabs into the lower fornix of each eye with or without topical anaesthesia and found that this yielded a positive result, whereas Seah et al24 took the tears samples with Schirmer strips and found that none of the samples was positive for SARS-CoV-2 even in the patient with conjunctivitis.
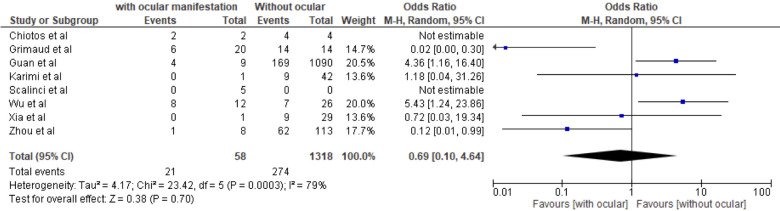
Severe COVID-19 was not associated with the presence of ocular manifestation (figure 6). A study by Navel et al25 found haemorrhagic conjunctivitis in an intubated patient with COVID-19 after several days of hospitalisation. However, PCR testing yield negative for SARS-CoV-2 even after excluding other potential microorganisms that could contribute to the ocular manifestation.25
Figure 6.
Forest plot estimating the OR of ocular manifestation in relation to severe COVID-19.
Discussion
Our systematic review and meta-analysis found a low prevalence of ocular manifestation in COVID-19-related patients. We also found that general PCR positivity from ocular samples was very low. However, when patients with COVID-19 had ocular manifestation, the positivity rate increased. The most common ocular manifestation being reported was epiphora, conjunctival injection and chemosis, similar to the other form of viral conjunctivitis with follicular reaction.
Our findings were in accordance with the previous systematic review by Aiello et al26 that available earlier. Most of the studies included in their review were also included in our current study. In addition, we further performed a meta-analysis and elaborate on the association of ocular manifestation with the systemic manifestation of COVID-19. Also, we added the data from case series and reports of individual patients with COVID-19 that explain the time course and disease severity of COVID-19 among those presenting with ocular manifestation.
The ocular surface can be the window of SARS-CoV-2 entry to the human body as the ACE-2 receptor found in conjunctiva and cornea. The TMPRSS2 protease activity also appeared in the ocular surface.9 27 Recent work by Zhou et al28 and Collin et al29 found that ACE-2 receptor and TMPRSS2 are highly found in the ocular surface epithelium, which can be the entry portal of coronavirus. Inflammation milieu could potentiate upregulation of ACE-2 and TMPRSS2.29 As the virus may replicate after the ocular surface is being infected by the SARS-CoV-2, the virus can cause ocular manifestation prior to any systemic symptoms. Although most of the ocular manifestation being reported was associated with red eyes, a case report by Hu et al17 found that SARS-CoV-2 could be found in tears of asymptomatic eyes. In this case, further evaluation revealed a nasolacrimal duct obstruction. Even after the nasopharyngeal swab had turned negative, the tears were still found to be positive with low signal.17 Another report among paediatric patients with COVID-19 asymptomatic of ocular manifestation showed that their tears could also be positive for SARS-CoV-2.30 Moreover, it was noted that patients with only ocular manifestation could have nasopharyngeal mucosa positive for SARS-CoV-2.22 It raises the possibility that infection of COVID-19 in the eyes could lead to systemic manifestation via either nasolacrimal duct or touching the nose by hands contaminated with the virus from tears. Second, it may implicate that patients with ocular manifestation presenting with viral conjunctivitis-like symptoms can be a source of transmission of SARS-CoV-2 to the population or unaware healthcare professionals. The possibility of the virus being transported to the nasopharynx was previously described, and it can occur experimentally in N95 respirator use without eye protection.11 31
We found that the ocular manifestation was not associated with a severe form of COVID-19. However, subtle laboratory values may differ between patients with and without ocular manifestation. Two previous initial meta-analyses found the discrepancy of the evidence whether ocular manifestation could attribute to a severe form of COVID-19.32 33 Loffredo et al33 found that conjunctivitis among patients with COVID-19 was significantly associated with severe COVID-19. However, the conclusion was based only on three studies. As our study added more available data, we found the severity of COVID-19 was not associated with the ocular manifestation. From Wu et al’s8 study, patients with COVID-19 presenting with ocular involvement had higher white blood cells, procalcitonin, C reactive protein and lactate dehydrogenase. Further evaluation needs to be done to confirm this finding. The severity of COVID-19 was probably mainly attributed to other systemic risk factors and the age of the patients.
Although we found that there was no significant ocular manifestation related to posterior segment abnormalities, the possible retinal involvement had been reported in patients with COVID-19. The ACE-2 receptor had also been reported in the human retina.34 Based on retinal biopsy specimens from 12 patients with COVID-19 who died, three specimens showed positive SARS-CoV-2 RNA with a weak signal. There was no report about any previous ocular manifestations until these patients died. However, the viral replication was not performed from these samples.35 Interestingly, Marinho et al36 reported recent ocular coherence tomography examination of COVID-19-related patients without any ocular manifestation. They found a subclinical change in ganglion cells and inner plexiform layers among these patients.36 Thus, ophthalmologists should be careful if they found this subtle change in OCT unintentionally or if they face patients with visual deterioration without typical of ocular surface manifestation of COVID-19-related patients.
Collecting adequate tears specimen for PCR testing may be a challenge in determining the true magnitude of ocular manifestation directly caused by SARS-CoV-2. We found a discrepancy in the result of PCR positivity of SARS-CoV-2 in tears by the methods of tear sampling. Seah et al24 found Schirmer strip to obtain the virus and could not prove any positive result, even in patients with COVID-19 with conjunctivitis. Because of the high cycle threshold for detecting the presence of SARS-CoV-2 in tears compared with nasopharyngeal swab,10 the sampling method could potentially determine the positivity rate. Previously, Satpathy et al37 and Ma et al 38 found that scrapping specimen yielded a better PCR positivity rate compared with tears specimen based on the analysis of ocular herpes simplex cases. As described in a study evaluating nasal swab and aspirates, the quality of the samples for optimal virological diagnosis should be rich in cells. The more cells obtained, the more accurate the diagnosis. Even if there is no standardised minimum amount of cells for virological detection, this might apply in ocular samples for SARS-CoV-2 detection.39
Based on our findings, ophthalmologists should be aware of the possibility that patients may present only with viral conjunctivitis due to SARS-CoV-2 infection and may develop systemic manifestation several days later. Around one-third of patients who presented with ocular manifestation had signs and symptoms before they develop systemic COVID-19 manifestation. Moreover, around a third of COVID-19 related patients with ocular manifestations had SARS-CoV-2 PCR positive from the eye samples which could be infectious, although the infectivity of ocular samples yet to be determined.40 For hospitalised COVID-19 patient, any red eyes should be suspicious for the ocular manifestation of COVID-19 until proven otherwise. However, to our knowledge, there was no sight-threatening ocular condition found among COVID-19 patients that directly attributed to SARS-CoV-2 infection in the eyes. Moreover, the treatment option was varied across studies, ranging from artificial tears to antiviral eyedrops (tables 1 and 2). The ocular manifestation could improve in all studies without any complication. Urgent consultation with an ophthalmologist may not be needed as long as the ocular manifestation does not get worse during the treatment course. For the ophthalmologist facing viral conjunctivitis-like patients without any systemic manifestation but high risk of being infected with SARS-CoV-2, it is reasonable if the tears specimen for PCR testing could be obtained in a repeated manner or in combination with the nasopharyngeal swab. Performing routine PCR testing for SARS-CoV-2 among patients with COVID-19 without ocular manifestation may yield a very low positivity rate, as previously reported by Deng et al.41 Eye protection and mask should be worn in managing patients with red eyes in daily clinical practice. A recent systematic review found that using eye protection or face mask, in addition to physical distancing and using respirators, could further reduce the risk of being infected by SARS-CoV-2.42
This study had several limitations. First, the general positivity rate of ocular samples for SARS-CoV-2 estimated mostly by studies conducting in hospital settings. This value can be overestimated. Second, the description of onset and ocular manifestation varies between studies included. Also, most of the studies did not describe in detail how to obtain tear samples. As we previously mentioned, the technique possibly influences the positivity rate. Subgroup analysis of pooled prevalence could not be performed further as the data for ocular manifestations were limited. Language bias may also be of relevance in this systematic review.
Conclusions
After carefully reviewing the available literature, we found that ocular manifestation can be the first presenting symptom of COVID-19. Eye infection of SARS-CoV-2 could potentially lead to systemic manifestation, although its evidence needs further investigation. Patients with COVID-19 with ocular manifestation were not associated with severe COVID-19. Detecting SARS-CoV-2 from tears yielded a relatively low positivity rate. However, we acknowledged the possibility of SARS-CoV-2 transmission from patients with COVID-19 presenting with only ocular manifestation.
Footnotes
Contributors: All authors contributed significantly to this study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article.
References
- 1.Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med 2020;288:192–206. 10.1111/joim.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singhal T. A review of coronavirus Disease-2019 (COVID-19). Indian J Pediatr 2020;87:281–6. 10.1007/s12098-020-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Coronavirus disease 2019 (COVID-19) situation report-115; 2020. https://apps.who.int/iris/bitstream/handle/10665/332090/nCoVsitrep14May2020-eng.pdf?sequence=1&isAllowed=y [Accessed 06/17/2020].
- 4.World Health Organization Infection prevention and control during health care when COVID-19 is suspected; 2020. https://www.who.int/publications-detail-redirect/10665-331495 [Accessed 06/17/2020].
- 5.Loon S-C, Teoh SCB, Oon LLE, et al. The severe acute respiratory syndrome coronavirus in tears. Br J Ophthalmol 2004;88:861–3. 10.1136/bjo.2003.035931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.C-W L, Liu X-F, Jia Z-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet 2020:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol 2020;138:575–8. 10.1001/jamaophthalmol.2020.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui KPY, Cheung M-C, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med 2020;8:687–95. 10.1016/S2213-2600(20)30193-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colavita F, Lapa D, Carletti F, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med 2020;173:242–3. 10.7326/M20-1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belser JA, Rota PA, Tumpey TM. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev 2013;77:144–56. 10.1128/MMBR.00058-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G, Shea B, O’Connel D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] 2019.
- 14.Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60–3. 10.1136/bmjebm-2017-110853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Chen X, Chen L, et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf 2020;18:360–2. 10.1016/j.jtos.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong N, Yu W, Xia J, et al. Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol 2020. 10.1111/aos.14445. [Epub ahead of print: 26 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y, Chen T, Liu M, et al. Positive detection of SARS-CoV-2 combined HSV1 and HHV6B virus nucleic acid in tear and conjunctival secretions of a non-conjunctivitis COVID-19 patient with obstruction of common lacrimal duct. Acta Ophthalmol 2020. 10.1111/aos.14456. [Epub ahead of print: 14 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salducci M, La Torre G. COVID-19 emergency in the cruise's ship: a case report of conjunctivitis. Clin Ter 2020;171:e189–91. 10.7417/CT.2020.2212 [DOI] [PubMed] [Google Scholar]
- 19.Khavandi S, Tabibzadeh E, Naderan M, et al. Corona virus disease-19 (COVID-19) presenting as conjunctivitis: atypically high-risk during a pandemic. Cont Lens Anterior Eye 2020;43:211–2. 10.1016/j.clae.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheema M, Aghazadeh H, Nazarali S, et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can J Ophthalmol 2020;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xuejie L, Ming W, Jing D, et al. Novel coronavirus disease with conjunctivitis and conjunctivitis as first symptom: two cases report. CJEO 2020. [Google Scholar]
- 22.Scalinci SZ, Trovato Battagliola E. Conjunctivitis can be the only presenting sign and symptom of COVID-19. IDCases 2020;20:e00774. 10.1016/j.idcr.2020.e00774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Liu M, Zhang Z, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol 2020;104:748–51. 10.1136/bjophthalmol-2020-316304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology 2020;127:977–9. 10.1016/j.ophtha.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navel V, Chiambaretta F, Dutheil F. Haemorrhagic conjunctivitis with pseudomembranous related to SARS-CoV-2. Am J Ophthalmol Case Rep 2020;19:100735. 10.1016/j.ajoc.2020.100735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiello F, Gallo Afflitto G, Mancino R, et al. Coronavirus disease 2019 (SARS-CoV-2) and colonization of ocular tissues and secretions: a systematic review. Eye 2020;34:1206–11. 10.1038/s41433-020-0926-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26:681–7. 10.1038/s41591-020-0868-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou L, Xu Z, Castiglione GM, et al. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf 2020;18:537–44. 10.1016/j.jtos.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collin J, Queen R, Zerti D, et al. Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface. Ocul Surf 2020. 10.1016/j.jtos.2020.05.013. [Epub ahead of print: 03 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valente P, Iarossi G, Federici M, et al. Ocular manifestations and viral shedding in tears of pediatric patients with coronavirus disease 2019: a preliminary report. J Aapos 2020. 10.1016/j.jaapos.2020.05.002. [Epub ahead of print: 09 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bischoff WE, Reid T, Russell GB, et al. Transocular entry of seasonal influenza-attenuated virus aerosols and the efficacy of n95 respirators, surgical masks, and eye protection in humans. J Infect Dis 2011;204:193–9. 10.1093/infdis/jir238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Dai C, Lv X, et al. Letter to the editor: are severe COVID-19 patients more susceptible to conjunctivitis? J Med Virol 2020. 10.1002/jmv.26084. [Epub ahead of print: 29 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loffredo L, Pacella F, Pacella E, et al. Conjunctivitis and COVID-19: a meta-analysis. J Med Virol 2020. 10.1002/jmv.25938. [Epub ahead of print: 24 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senanayake PdeS, Drazba J, Shadrach K, et al. Angiotensin II and its receptor subtypes in the human retina. Invest Ophthalmol Vis Sci 2007;48:3301–11. 10.1167/iovs.06-1024 [DOI] [PubMed] [Google Scholar]
- 35.Casagrande M, Fitzek A, Püschel K, et al. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm 2020;28:721–5. 10.1080/09273948.2020.1770301 [DOI] [PubMed] [Google Scholar]
- 36.Marinho PM, Marcos AAA, Romano AC, et al. Retinal findings in patients with COVID-19. Lancet 2020;395:1610. 10.1016/S0140-6736(20)31014-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satpathy G, Mishra AK, Tandon R, et al. Evaluation of tear samples for herpes simplex virus 1 (HSV) detection in suspected cases of viral keratitis using PCR assay and conventional laboratory diagnostic tools. Br J Ophthalmol 2011;95:415–8. 10.1136/bjo.2010.191049 [DOI] [PubMed] [Google Scholar]
- 38.Ma J-X, Wang L-N, Zhou R-X, et al. Real-time polymerase chain reaction for the diagnosis of necrotizing herpes stromal keratitis. Int J Ophthalmol 2016;9:682–6. 10.18240/ijo.2016.05.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnin P, Miszczak F, Kin N, et al. Study and interest of cellular load in respiratory samples for the optimization of molecular virological diagnosis in clinical practice. BMC Infect Dis 2016;16:384. 10.1186/s12879-016-1730-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun C-B, Wang Y-Y, Liu G-H, et al. Role of the eye in transmitting human coronavirus: what we know and what we do not know. Front Public Health 2020;8:155. 10.3389/fpubh.2020.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng C, Yang Y, Chen H, et al. Low risk of SARS‐CoV‐2 transmission through the ocular surface. Acta Ophthalmol 2020;395 10.1111/aos.14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 2020;395:1973–87. 10.1016/S0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol 2020;92:589–94. 10.1002/jmv.25725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie H-T, Jiang S-Y, Xu K-K, et al. SARS-CoV-2 in the ocular surface of COVID-19 patients. Eye Vis 2020;7:23 10.1186/s40662-020-00189-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L, Deng C, Chen X, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: a cross-sectional study. Acta Ophthalmol 2020. 10.1111/aos.14472. [Epub ahead of print: 18 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar K, Prakash AA, Gangasagara SB, et al. Presence of viral RNA of SARS-CoV-2 in conjunctival swab specimens of COVID-19 patients. Indian J Ophthalmol 2020;68:1015–7. 10.4103/ijo.IJO_1287_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y, Duan C, Zeng Y, et al. Ocular findings and proportion with conjunctival SARS-COV-2 in COVID-19 patients. Ophthalmology 2020;127:982–3. 10.1016/j.ophtha.2020.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karimi S, Arabi A, Shahraki T, et al. Detection of severe acute respiratory syndrome Coronavirus-2 in the tears of patients with coronavirus disease 2019. Eye 2020;34:1220–3. 10.1038/s41433-020-0965-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grimaud M, Starck J, Levy M, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care 2020;10:69. 10.1186/s13613-020-00690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang Z, Zhang Y, Hang C, et al. Comparisons of viral shedding time of SARS-CoV-2 of different samples in ICU and non-ICU patients. J Infect 2020;81:147–78. 10.1016/j.jinf.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daruich A, Martin D, Bremond-Gignac D. Ocular manifestation as first sign of coronavirus disease 2019 (COVID-19): interest of telemedicine during the pandemic context. J Fr Ophtalmol 2020;43:389–91. 10.1016/j.jfo.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc 2020;9:393–8. 10.1093/jpids/piaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ying NY, Idris NS, Muhamad R, et al. Coronavirus disease 2019 presenting as conjunctivitis. Korean J Fam Med 2020. 10.4082/kjfm.20.0090. [Epub ahead of print: 01 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu P, Liang L, Chen C, et al. A child confirmed COVID-19 with only symptoms of conjunctivitis and eyelid dermatitis. Graefes Arch Clin Exp Ophthalmol 2020;258:1565–6. 10.1007/s00417-020-04708-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolfler A, Mannarino S, Giacomet V, et al. Acute myocardial injury: a novel clinical pattern in children with COVID-19. Lancet Child Adolesc Health 2020;4:e26–7. 10.1016/S2352-4642(20)30168-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ya Y, Yanping S, Ming Y, et al. Novel coronavirus pneumonia combined with conjunctivitis: three cases report. CJEO 2020. [Google Scholar]
- 57.Casalino G, Monaco G, Di Sarro PP, et al. Coronavirus disease 2019 presenting with conjunctivitis as the first symptom. Eye 2020;34:1235–6. 10.1038/s41433-020-0909-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2020-000563supp001.pdf (108.8KB, pdf)