Abstract
Antimicrobial resistance (AMR) continues to be a global problem and continues to be addressed through national strategies to improve diagnostics, develop new antimicrobials and promote antimicrobial stewardship. Patients who attend general (ambulatory) practice with symptoms of respiratory tract infections (RTIs) are invariably assessed by some sort of clinical decision rule (CDR). However, CDRs rely on a cluster of non-specific clinical observations. A narrative review of the literature was undertaken to ascertain the value of C reactive protein (CRP) point-of-care testing (POCT) to guide antibacterial prescribing in adult patients presenting to general practitioner (GP) practices with symptoms of RTI. Studies that were included were Cochrane reviews, systematic reviews, randomised controlled trials, cluster randomised trials, controlled before and after studies, cohort studies and economic evaluations. An overwhelming number of studies demonstrated that the use of CRP tests in patients presenting with RTI symptoms reduces index antibacterial prescribing. GPs and patients report a good acceptability for a CRP POCT and economic evaluations show cost-effectiveness of CRP POCT over existing RTI management in primary care. POCTs increase diagnostic precision for GPs in the better management of patients with RTI. With the rapid development of artificial intelligence, patients will expect greater precision in diagnosing and managing their illnesses. Adopting systems that markedly reduce antibiotic consumption is a no-brainer for governments that are struggling to address the rise in AMR.
Keywords: bacterial infection, respiratory infection
Background
Antimicrobial resistance (AMR) continues to be a high priority global issue1 2 and there is a direct relationship between the prescribing of antimicrobials and the development of AMR.3
Respiratory tract infections (RTIs) are among the most common acute conditions leading to general practitioner (GP) consultations and to antibiotic prescribing in primary care, even though 70% are viral, and many others are minor self-limiting bacterial infections.4 Thus, the use of antibiotics in such situations is deemed to be mostly inappropriate. There is concern that a lack of new antibiotics will threaten global efforts to contain AMR infections and hence we should strive to preserve existing agents and not use them indiscriminately.5 In the treatment of infections in the community (primary care) there has been some progress in reducing antimicrobial use in some countries but not in others, and there is a wide range of prescribing between individual European countries (figure 1).6 In the UK around 50% of all general practice consultations for RTIs result in an antibiotic prescription, however there is a wide range of such prescribing (20% to 80%),7 but in the Netherlands only 22.5% of RTI episodes in 2010 were treated with antibiotics.8
Figure 1.
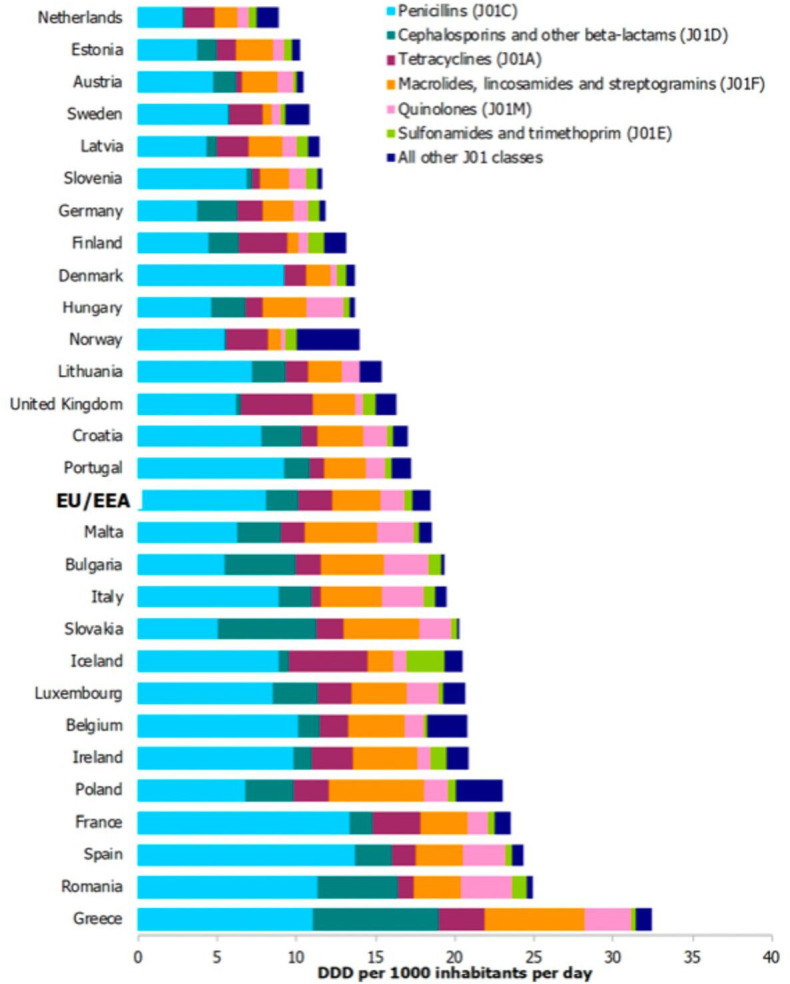
Consumption of antibacterials for systemic use (ATC group J01) by country and ATC group level 3 in the community in EU/EEA countries In 2018 (expressed as DDD per 1000 inhabitants per day) (adapted from the Annual epidemiological report for 2018. Stockholm: ECDC [6]).ATC, Anatomical Therapeutic Chemical, DDD, Defined Daily Dose; DUR, Drug Utilization Research EU/EEE, European Union/European Economic Area.
Objective
This paper seeks to review the evidence for the use of CRP point-of-care testing (POCT) in reducing antimicrobial prescribing in primary care by prescribers who see patients presenting with symptoms of RTI. CRP-POCT has been adopted by the National Institute for Health and Clinical Excellence (NICE)9 and PHE10 and there have been many new insights and repetitive reviews in the last 5 years. However, there has been little adoption of these recommendations in clinical practice. While the growth of antibiotic resistance has continued it ‘has not captured the sustained focus of national leaders and country-level actors, including care providers’.11
Prescribing antibacterials in primary care has mainly been empirical and based on non-specific clinical signs and symptoms rather than more precise diagnostic tools that are readily available in secondary care, for example, radiology, immunology, microbiology and chemical pathology testing. While some of these empirical clinical assessments have been objective, for example, temperature, blood pressure and respiratory rate, others are more subjective, for example, pain, inflammation and general malaise. A number of these have been incorporated into clinical decision rules (CDRs) and assessed for both negative and positive prognostic utility.
CDRs in RTIs in primary care
The literature is abundant with work on CDRs: pharyngitis identifying patients with group A streptococcus infection,12 and three are most widely known: The Centor Study is probably the most frequently used worldwide for adults.13 The McIsaac rule was derived for children.14 The DESCARTE Study (Decision rule for the Symptoms and Complications of Acute Red Throat in Everyday practice) in adults presenting with sore throat of less than 2 weeks duration used the FeverPAIN Score.15 NICE guidelines suggest that scores of 0 or 1 are unlikely to benefit from antibiotics, while scores of ≥4 should be considered for an antibiotic prescription.16
Sinus puncture, the reference standard for acute bacterial rhinosinusitis was used to compare with a CDR from a study in Swedish emergency room patients with symptoms lasting less than 3 months, identifying four criteria: purulent rhinorrhoea with unilateral predominance, local pain with unilateral predominance, bilateral purulent rhinorrhoea and presence of pus in the nasal cavity. In this algorithm, two or more of four positive findings are 95% sensitive and 77% specific for acute bacterial rhinosinusitis.17 A CDR based on the Williams criteria was recommended by the Canadian Sinusitis Symposium guidelines.18
In the largest prospective cohort of primary care patients with LRTI, serious adverse outcomes (death, hospital admission or late-onset pneumonia) occurred in 1.1% of the patients.19 Patients radiographed within 1 week of the index consultation, showed four independent predictors of radiograph-confirmed pneumonia: temperature >37.8°C, crackles on auscultation, oxygen saturation <95% and pulse >100 beats/minute.20 The CRB-65 rule was developed to predict the risk of admission to the intensive care unit and mortality in hospitalised patients with community-acquired pneumonia.21 CRB-65 has superseded the complex Pneumonia Severity Index, which used 20 different clinical variables and the CURB-65 that includes the measurement of urea, which limits is timeliness and value in primary care. However, in a meta-analysis of validation studies, CRB-65 overpredicted the probability of 30-day mortality in the community setting across all strata of predicted risk, low, intermediate and high.22 Modified scoring systems have been studied with similar results, like the CORB75 (similar to the classical CRB-65, but adding oxygen saturation and increasing the age to 75 years).23 The STARWAVe clinical algorithm is a clinical rule to improve antibiotic use in children presenting with acute respiratory tract infection (ARTI) and cough.24 A study that examined all published sign and symptom models for prediction of pneumonia in primary care were externally validated by individual patient data of previously performed diagnostic studies.25 A recent meta-analysis of studies evaluating the diagnostic value of clinical features to identify radiographic pneumonia among adults in the primary care setting, the clinical variable with the best pooled positive likelihood ratio (PLR) was the respiratory rate ≥20 breaths/minute (PLR 3.47), followed by temperature ≥38°C, and pulse rate >100 beats/minute. Cough, sputum, crackles, dyspnoea and chest pain were limited as single predictors for the diagnosis of pneumonia and none of these criteria reached the PLR of 2.5. Laboratory tests showed the highest pooled PLRs with C reactive protein (CRP) >20 mg/L associated with a PLR of 3.76.26
COPD—acute exacerbations
For many years, guidelines have recommended the use of antibiotics for acute exacerbations of chronic obstructive pulmonary disease (COPD) based on the Anthonisen Study, in a randomised placebo-controlled trial in patients with exacerbations of severe COPD, comprising three patient-reported items: increased dyspnoea, increased sputum volume and increased sputum purulence.27 Antibiotics showed a significant benefit that was largely accounted for by patients with type 1 exacerbations (those with the three criteria), whereas there was no significant difference between antibiotic and placebo in patients who only had one of the defined symptoms.
UK Guidelines for treating RTI in Primary Care include:
Cough (acute): antimicrobial prescribing NICE guideline,28 NICE Clinical guideline for LRTI, using CRB659 and PHE guidance on Managing Infections in Primary Care.10
Biomarkers in RTI in primary care
CRP POCT had been implemented in Scandinavian countries for about three decades but without proper guidelines on indications and cut-off values to be used. This had resulted in certain degrees of overuse of CRP testing (particularly when used in upper RTI).29 Without guidelines antibacterials can be prescribed following poor interpretation of CRP levels.30 However, CRP POCT was introduced in the Netherlands with guideline-based cut-offs and indications and (in most cases) individual training and instructions programmes (in collaboration with ISO-accredited diagnostic centres and laboratories).31 The use of POCT in Denmark increased by 45.8% during 2004–2013. CRP tests increased by 132%, and bacteriological cultures by 101.7%. POCT preceded 28% of antibiotic prescriptions in 2004 increasing to 44% in 2013. The use of POCT varied more than fivefold among individual practices in 2013.32
The influence of biomarkers on antimicrobial prescribing rates in patients presenting in primary care with symptoms of RTI
A 2015 survey of countries that employed CRP POCT as a diagnostic and/or prognostic tool in general practice showed those countries that used CRP POCT to some or a wide extent were: Finland, Netherlands, Denmark, Norway, Sweden, Germany, Czech Republic, Hungary, Austria, Slovenia, Latvia and Estonia.33 34 Interestingly, these countries are the lowest 12 prescribers of antibacterials in the latest ESAC survey (figure 1).35 We have sought to review the evidence for the use of point-of-care (POC) biomarkers in reducing antimicrobial prescribing in primary care by prescribers who see patients presenting with symptoms of RTI. We felt that an update of our 2016 paper was timely and that, with the addition of all relevant publications since 2015, represents the current status of CRP POCT for patients presenting in primary care with symptoms of RTI.36
Methods for review
We undertook a narrative review of the evidence on CRP POCT for adults presenting to GPs with symptoms of RTI in order to:
Determine whether CRP POCT can reduce antibacterial prescribing.
Ascertain the safety and acceptability of CRP POCT for patients and GPs.
Determine the cost-effectiveness of CRP POCT in a National Health Service (NHS) setting.
Papers reviewed were in English and published between March 2017 and December 2019.
Search terms and string were: CRP or C-reactive protein or biomarkers or procalcitonin and infections or infection respiratory tract or antibiotics or antimicrobials and primary care and point of care testing. Databases searched were: EMBASE, Excerpta Medica, (Ovid), Journals@Ovid Full Text, (Ovid), PubMed, MEDLINE (Ovid), SpringerLink, Wiley Interscience Journals, NHS Evidence, The Cochrane Collaboration. All bibliographies for selected articles were searched for further articles to include within the review. The subsequent evaluation and reporting was undertaken in line with current guidance for literature review and a narrative, or semisystematic methodology, was adopted as papers were considered that had either quantitative or qualitative design.37
Results
English (199).
English and humans (177).
Published in the last 5 years (147).
Review (34).
The 21 most relevant studies included systematic reviews of randomised controlled trials, cluster randomised controlled trials, and observational and economic evaluations as shown in online supplementary table 1.
bmjresp-2020-000624supp001.pdf (98.4KB, pdf)
The most comprehensive evidence of the value of CRP POCT in patients presenting with symptoms of RTI in primary care in reducing antimicrobial prescribing is reported in a systematic review (Cochrane Review) that concluded ‘Performing a point-of-care CRP test in ambulatory care accompanied by clinical guidance on interpretation reduces the immediate antibiotic prescribing in both adults and children’.38 Nineteen studies used CRP POCT and included 11 RCTs and 8 non-randomised studies reporting on 16 064 patients in total. The Forest plots from this study show highly significant difference towards CRP POCT for antibiotic prescribing at index consultation for all patients, RCTs; all patients, non-randomised studies, RCTs; adults only, if cut-off guidance applied; and RCTs, children only, if cut-off guidance applied.
There was no available evidence to suggest an effect on other patient outcomes or healthcare processes. This is a pivotal publication and included studies until March 2017.
A systematic review of studies reporting reduction of antibiotic prescriptions for ARTIs in primary care found that communication skills training and POCT were the most effective interventions and that trials with initially lower prescription rates were less likely to be successful.39 A narrative review of what factors affect antibiotic prescribing for ARTIs in primary care concluded that widespread adoption of successful strategies in primary care is imperative.40
A cluster randomised to usual care, internet-based training on CRP POCT, internet-based training on enhanced communication skills and interactive booklet, or both interventions combined. Internet-based training in enhanced communication skills remained effective in the longer term for reducing antibiotic prescribing. The early improvement seen with CRP training wanes, and this training becomes ineffective for lower RTIs, the only current indication for using CRP testing. However, due to a less intensive follow-up there was very poor take-up of booklets and POCT in the second phase of the study.41
Using CRP POCT in LRTI a multicentre, open-label, randomised, controlled trial in participants aged at least 1 year with a documented fever or a chief complaint of fever resulted in a modest but significant reduction in antibiotic prescribing, with patients with high CRP being more likely to be prescribed an antibiotic, and no evidence of a difference in clinical outcomes.42
A thematic analysis of data from preintervention and postintervention patients and healthcare workers found widespread positive attitudes towards CRP POCT among patients and healthcare workers. Patients’ views were influenced by an understanding of CRP POCT as a comprehensive blood test that provides specific diagnosis that corresponds to notions of good care. Healthcare workers use the test to support their negotiations with patients and to legitimise ethical decisions in an increasingly restrictive antibiotic policy environment.43
In an audit-based study aimed at assessing GPs’ reliance on patient history, examination findings and the influence of the utilisation of POCTs in antibiotic prescribing for sore throat and LRTI, a negative POCT result was negatively associated with antibiotic prescribing and GPs using POCTs attached less weight to clinical criteria.44
In a small feasibility study, patients who would have received antibiotics for RTI were referred by a GP practice to a local pharmacy for CRP POCT. Patients who had a CRP of less than 100 were given a leaflet and told to visit the GP if symptoms did not resolve within 3 weeks. Sixty-three per cent of patients had a CRP value of <5 mg/L and were deemed to have self-limiting illness and not requiring an antibiotic. Ten per cent of the patients had a CRP over 100 mg/L and were recommended to receive an antibiotic. Most CRP tests took an additional 5–10 min from the initial consultation with the GP to the patient’s total consultation time. Almost all patients found the test useful and would recommend it as it provided reassurance that the symptoms were not serious.45
In another pilot study to investigate CRP POCT in a community pharmacy patients accessed the scheme by either referral from GPs, pharmacy staff or self-referral. This study showed high degrees of patient satisfaction with concurrent reduction in unnecessary antibiotic prescribing by 86%.46
In a prospective observational study, Dutch GPs were surveyed about specific antibiotic prescribing following Dutch College of General Practitioners (DCGP) guidance on the use of CRP POCT. The largest variation in prescribing occurred in patients who presented with CRP values between 20 mg/L and 100 mg/L. Most GPs followed the DCGP guidelines and used low CRP values as a negative indicator not to prescribe an antibiotic.47
Paediatrics
In a study to assess whether use of POC CRP by the GP reduces antibiotic prescriptions, children with suspected non-serious LRTI were included and randomised to either use of POC CRP or usual care. Antibiotic prescription rates were measured and compared between groups using generalising estimating equations. The study did not reach the required number of patients and while a small reduction of antibiotics was found, statistical significance was not reached.48
In another study in children with non-severe acute infections, CRP POCT did not influence antibiotic prescribing concluding that systematic CRP POCT without guidance is not an effective strategy to reduce antibiotic prescribing for non-severe acute infections in children in primary care. Eliciting parental concern and providing a safety net without POC CRP testing conversely increased antibiotic prescribing. GPs possibly need more training in handling parental concern without inappropriately prescribing antibiotics.49
In a qualitative study, Dutch GPs' perceptions of the addition of point-of-care CRP testing to the diagnostic process in children with suspected LRTI differed from their perceptions of this in adults. GPs noted that they used POCT CRP in adults for diagnostic certainty, and as a tool to communicate a non-prescription decision. GPs indicated they seldom used POCT CRP in children to convince parents that antibiotics were not necessary. Themes identified included: patient characteristics; vulnerability of the child; clinical presentation; availability of evidence; the impact of the procedure; and use of point-of-care CRP testing as a communication tool.50
These studies are at variance with findings that CRP POCT in children is feasible in primary care and is likely to be acceptable.38 51 However, it will not reduce antibiotic prescribing and hospital referrals until GPs accept its diagnostic value in children.52
Chronic obstructive pulmonary disease
A multicentre, open-label, randomised, controlled trial involving patients with a diagnosis of COPD CRP-guided prescribing of antibiotics for exacerbations of COPD in primary care clinics resulted in a lower percentage of patients who reported antibiotic use and who received antibiotic prescriptions from clinicians, with no evidence of harm.53
Health economics
Using a decision-analytical model to estimate the cost-effectiveness of testing, compared with standard care, in adults presenting in primary care with symptoms of ARTI, POC CRP testing as implemented in routine practice was less cost-effective than when adhering to clinical guidelines.54 A budget impact model calculated that CRP POCT was more expensive than usual care.55 However, in both studies there were no estimates of the implications for antibiotic resistance nor for Clostridium difficile infection.
Barriers and facilitators to CRP POCT adoption
A qualitative study explored the views of general practice staff on the use of CRP POCT for the management of lower RTIs in England who felt the test could help general practice staff improve patient care and education if incorporated into routine care, but this would need enthusiasts with dedicated POCT instruments or smaller, cheaper, more portable instruments.56
A mathematical model for designing networks of CRP POCT could optimise the cost and travel distance for patients to access testing across a given region.57
A mixed-methods UK study with CRP POCT confirmed costs and funding as important barriers in addition to physical and operational constraints and cited training and the value of a local champion as enablers.58
In a US study to ascertain which POCTs would be most beneficial to add to clinical practice, incorporating CRP POCT with clinical guidelines was felt to strengthen the utility of this test, when there is diagnostic uncertainty.59 A qualitative study highlighted reimbursement and incentivisation, quality control and training, laboratory services, practitioner attitudes and experiences, effects on clinic flow and workload, use in pharmacy and gaps in evidence as barriers to implementation.60 In a South African study, clinicians saw POCTs as potentially useful for positively addressing both clinical and social drivers of the overprescribing of broad-spectrum antibiotics.61
Governmental reviews
The use of CRP POCT may reduce unnecessary antibiotic prescribing (which carries a risk of adverse effects and the development of antibiotic resistance), but seems unlikely in the absence of a funded implementation programme.4
HTA assessments in EU and Ireland have confirmed the value of CRP POCT in helping to reduce antibiotic prescribing in primary care that might address the AMR crisis.62
Discussion
The accurate and rapid assessment of whether patients need antibiotics when they present to primary care with symptoms of RTI continues to challenge primary care clinicians and researchers. On the one hand there are advocates of using simple non-invasive non-specific clinical observations grouped into CDRs. On the other hand, there are protagonists that advocate bringing more accurate and specific but slightly more invasive tests into clinical practice to improve the prognostic accuracy at primary care clinicians’ fingertips and particularly, tests that will produce results within 5 min. Blood tests now offer precision for the diagnosis and management of many diseases that were previously the province of the hospital clinical specialist but are now included in the General Medical Service contracts. These include; diabetes, hypercholesterolaemia, endocrine disorders, (HbA1c, cholesterol, FBC, calcium, glucose, renal and liver function, thyroid function tests, serum vitamin B12 and folate, lithium).63
Improving diagnostics to address AMR was the subject of a special report commissioned by the UK government in 2015 that concluded ‘For material progress to happen over the next 5 years healthcare systems need to leapfrog to using rapid diagnostics wherever possible, before using an antibiotic’.64
The European Joint Programming Initiative on Antimicrobial Resistance Transnational Working Group ‘Antimicrobial Resistance - Rapid Diagnostic Tests’ is proposing to consider a ‘mix-and-match’ package for the implementation of POCT. It constitutes a multisectoral collaboration between medical, technological and industrial opinion leaders involved in in vitro diagnostics development, medical microbiology and clinical infectious diseases. The mix-and-match implementation package is designed to encourage the implementation of rapid infectious disease and AMR POCT in transnational medical environments for use in the fight against increasing AMR.65
There has been plenty of advocacy but action is now needed as ‘………antimicrobial stewardship efforts and improving access to diagnostics are vital to avert the antimicrobial crisis……’.66 There is overwhelming evidence that CRP POCT can offer a significant strengthening of primary care clinicians’ diagnostic precision in addressing whether or not a patient presenting with symptoms of RTI needs antibiotics or not. The main barriers appear to be financial constraints from the central government and the reluctance of, or lack of incentives for, primary care clinicians to adopt new diagnostic processes. At the moment there is much emphasis from worldwide leaders (many microbiologists and infectious diseases specialists) on the development of new tests for aetiology. This may be helpful, but most antibacterials are prescribed in general practice. What is particularly needed in general practice is a simple, rapid test that separates seriously ill from non-seriously ill patients, despite the actual aetiological agent. So, the emphasis should be on the implementation of available POCTs that have proven to be helpful in this respect.
With the rapid development of artificial intelligence, patients will expect greater precision in diagnosing and managing their illnesses. Adopting systems that markedly reduce antibiotic consumption is a no-brainer for governments that are struggling to address the rise in AMR.
Limitations of the study
This paper is an update of a narrative review that was published in 2015.36 A full systematic review was not undertaken as a number of the references reviewed used a qualitative methodology. A pivotal systematic review was published in 2019,38 however this systematic review had a publication cut-off in March 2017, whereas our paper included published papers till December 2019.
Footnotes
Contributors: JC is the lead author and all the other coauthors have contributed to the development of this work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JC has chaired, presented and received honoraria at meetings supported by Astellas, Cubist, Abbott, LumiraDx and HHI. CL has received research grants from Abbott Diagnostics. CB has received advisory board fees from Roche Molecular Systems and grant support from Roche Molecular Diagnostics. RH received a grant from Alere for a study on the value of point-of-care CRP measurement in children and speaker fees from Alere for lectures on antibiotic resistance.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. Freely available.
References
- 1.World Health Organisation (WHO) Global action plan on antimicrobial resistance, 2015. [Google Scholar]
- 2.O'Neill J. Tackling drug-resistant infections globally: final report and recommendations. London: HM Government and Wellcome Trust, 2016. [Google Scholar]
- 3.Goossens H, Ferech M, Vander Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005;365:579–87. 10.1016/S0140-6736(05)70799-6 [DOI] [PubMed] [Google Scholar]
- 4.Drug and Therapeutics Bulletin Point-of-care CRP testing in the diagnosis of pneumonia in adults. Drug Ther Bull 2016;54:117–20. 10.1136/dtb.2016.10.0432 [DOI] [PubMed] [Google Scholar]
- 5.WHO Lack of new antibiotics threatens global efforts to contain drug-resistant infections, 2020. Available: https://www.who.int/news-room/detail/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections
- 6.European Centre for Disease Prevention and Control Antimicrobial consumption in the EU/EEA : Annual epidemiological report for 2018. Stockholm: ECDC, 2019. [Google Scholar]
- 7.Gulliford MC, Dregan A, Moore MV, et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014;4:e006245. 10.1136/bmjopen-2014-006245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Broek d'Obrenan J, Verheij TJM, Numans ME, et al. Antibiotic use in Dutch primary care: relation between diagnosis, consultation and treatment. J Antimicrob Chemother 2014;69:1701–7. 10.1093/jac/dku005 [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Clinical Excellence (NICE) Pneumonia in adults: diagnosis and management, 2019. Available: https://www.nice.org.uk/guidance/cg191 [PubMed]
- 10.Public Health England Management and treatment of common infections antibiotic guidance for primary care: for consultation and local adaptation. London: Public Health England, 2017. [Google Scholar]
- 11.Laxminarayan R, Van Boeckel T, Frost I, et al. The Lancet infectious diseases Commission on antimicrobial resistance: 6 years later. Lancet Infect Dis 2020;20:e51–60. 10.1016/S1473-3099(20)30003-7 [DOI] [PubMed] [Google Scholar]
- 12.Le Marechal F, Martinot A, Duhamel A, et al. Streptococcal pharyngitis in children: a meta-analysis of clinical decision rules and their clinical variables. BMJ Open 2013;3. 10.1136/bmjopen-2012-001482. [Epub ahead of print: 09 Mar 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centor RM, Witherspoon JM, Dalton HP, et al. The diagnosis of strep throat in adults in the emergency room. Med Decis Making 1981;1:239–46. 10.1177/0272989X8100100304 [DOI] [PubMed] [Google Scholar]
- 14.McIsaac WJ, White D, Tannenbaum D, et al. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ 1998;158:75–83. [PMC free article] [PubMed] [Google Scholar]
- 15.Little P, Moore M, Hobbs FDR, et al. Primary care streptococcal management (PriSM) study: identifying clinical variables associated with Lancefield group A β-haemolytic streptococci and Lancefield non-group a streptococcal throat infections from two cohorts of patients presenting with an acute sore throat. BMJ Open 2013;3:e003943. 10.1136/bmjopen-2013-003943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Clinical Excellence Sore throat (acute):antimicrobial prescribing. London: National Institute for Health and Clinical Excellence, 2018. [Google Scholar]
- 17.Berg O, Carenfelt C. Analysis of symptoms and clinical signs in the maxillary sinus empyema. Acta Otolaryngol 1988;105:343–9. 10.3109/00016488809097017 [DOI] [PubMed] [Google Scholar]
- 18.Williams JW, Simel DL, Roberts L, et al. Clinical evaluation for sinusitis. making the diagnosis by history and physical examination. Ann Intern Med 1992;117:705–10. 10.7326/0003-4819-117-9-705 [DOI] [PubMed] [Google Scholar]
- 19.Moore M, Stuart B, Lown M, et al. Predictors of adverse outcomes in uncomplicated lower respiratory tract infections. Ann Fam Med 2019;17:231–8. 10.1370/afm.2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore M, Stuart B, Little P, et al. Predictors of pneumonia in lower respiratory tract infections: 3C prospective cough complication cohort study. Eur Respir J 2017;50. 10.1183/13993003.00434-2017. [Epub ahead of print: 22 Nov 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax 2000;55:219–23. 10.1136/thorax.55.3.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNally M, Curtain J, O'Brien KK, et al. Validity of British thoracic Society guidance (the CRB-65 rule) for predicting the severity of pneumonia in general practice: systematic review and meta-analysis. Br J Gen Pract 2010;60:e423–33. 10.3399/bjgp10X532422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, et al. Validation of the CORB75 (confusion, oxygen saturation, respiratory rate, blood pressure, and age ≥ 75 years) as a simpler pneumonia severity rule. Infection 2014;42:371–8. 10.1007/s15010-013-0565-1 [DOI] [PubMed] [Google Scholar]
- 24.Hay AD, Redmond NM, Turnbull S, et al. Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med 2016;4:902–10. 10.1016/S2213-2600(16)30223-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schierenberg A, Minnaard MC, Hopstaken RM, et al. External validation of prediction models for pneumonia in primary care patients with lower respiratory tract infection: an individual patient data meta-analysis. PLoS One 2016;11:e0149895. 10.1371/journal.pone.0149895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Htun TP, Sun Y, Chua HL, et al. Clinical features for diagnosis of pneumonia among adults in primary care setting: a systematic and meta-review. Sci Rep 2019;9:7600. 10.1038/s41598-019-44145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196–204. 10.7326/0003-4819-106-2-196 [DOI] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellance Cough (acute): antimicrobial prescribing, 2019. Available: https://www.nice.org.uk/guidance/ng120/chapter/Recommendations
- 29.André M, Schwan A, Odenholt I, et al. The use of CRP tests in patients with respiratory tract infections in primary care in Sweden can be questioned. Scand J Infect Dis 2004;36:192–7. 10.1080/00365540410019372 [DOI] [PubMed] [Google Scholar]
- 30.Engström S, Mölstad S, Lindström K, et al. Excessive use of rapid tests in respiratory tract infections in Swedish primary health care. Scand J Infect Dis 2004;36:213–8. 10.1080/00365540310018842 [DOI] [PubMed] [Google Scholar]
- 31.Minnaard MC, van de Pol AC, Hopstaken RM, et al. C-reactive protein point-of-care testing and associated antibiotic prescribing. Fam Pract 2016;33:408–13. 10.1093/fampra/cmw039 [DOI] [PubMed] [Google Scholar]
- 32.Haldrup S, Thomsen RW, Bro F, et al. Microbiological point of care testing before antibiotic prescribing in primary care: considerable variations between practices. BMC Fam Pract 2017;18:9. 10.1186/s12875-016-0576-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooke J, et al. Straight to the point - ensuring the rational use of antibiotics in primary care using C-reactive protein testing a consensus report. Munro and Forster, 2015. [Google Scholar]
- 34.Cooke J. C-reactive Protein (CRP) as a point of care test (POCT) to assist in the management of patients presenting with symptoms of respiratory tract infection (RTI) - a new role for community pharmacists? Pharm Manag 2016;32:25–9. [Google Scholar]
- 35.European Centre for Disease Prevention and Control Surveillance of antimicrobial resistance in Europe 2018. European Centre for Disease Prevention and Control, 2019. [Google Scholar]
- 36.Cooke J, Butler C, Hopstaken R, et al. Narrative review of primary care point-of-care testing (POCT) and antibacterial use in respiratory tract infection (RTI). BMJ Open Respir Res 2015;2:e000086–10. 10.1136/bmjresp-2015-000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder H. Literature review as a research methodology: an overview and guidelines. J Bus Res 2019;104:333–9. 10.1016/j.jbusres.2019.07.039 [DOI] [Google Scholar]
- 38.Verbakel JY, Lee JJ, Goyder C, et al. Impact of point-of-care C reactive protein in ambulatory care: a systematic review and meta-analysis. BMJ Open 2019;9:e025036–11. 10.1136/bmjopen-2018-025036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Köchling A, Löffler C, Reinsch S, et al. Reduction of antibiotic prescriptions for acute respiratory tract infections in primary care: a systematic review. Implement Sci 2018;13:47. 10.1186/s13012-018-0732-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Connor R, O'Doherty J, O'Regan A, et al. Antibiotic use for acute respiratory tract infections (ARTI) in primary care; what factors affect prescribing and why is it important? A narrative review. Ir J Med Sci 2018;187:969–86. 10.1007/s11845-018-1774-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Little P, Stuart B, Francis N, et al. Antibiotic prescribing for acute respiratory tract infections 12 months after communication and CRP training: a randomized trial. Ann Fam Med 2019;17:125–32. 10.1370/afm.2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Althaus T, Greer RC, Swe MMM, et al. Effect of point-of-care C-reactive protein testing on antibiotic prescription in febrile patients attending primary care in Thailand and Myanmar: an open-label, randomised, controlled trial. Lancet Glob Health 2019;7:e119–31. 10.1016/S2214-109X(18)30444-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haenssgen MJ, Charoenboon N, Althaus T, et al. The social role of C-reactive protein point-of-care testing to guide antibiotic prescription in northern Thailand. Soc Sci Med 2018;202:1–12. 10.1016/j.socscimed.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Llor C, Molero JM, Moragas A, et al. Use of point-of-care tests and antibiotic prescribing in sore throat and lower respiratory infections by general practitioners. Enferm Infecc Microbiol Clin 2020;38:21–4. 10.1016/j.eimc.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 45.Cooke J, Sheraz M, Hill J, et al. Community pharmacists working with GPs reduce antibiotic prescribing for RTIs using CRP point-of-care-testing. J Pharm Pharmacol 2019;71:9–10. [Google Scholar]
- 46.Wakeman M, Cork T. A pilot study investigating point of care C-reactive protein (POC CRP) testing in community pharmacy to deliver appropriate interventions in respiratory tract infections (RTIs). J Pharm Pharmacol 2019;71:11–12. [Google Scholar]
- 47.Schuijt TJ, Boss DS, Musson REA, et al. Influence of point-of-care C-reactive protein testing on antibiotic prescription habits in primary care in the Netherlands. Fam Pract 2018;35:179–85. 10.1093/fampra/cmx081 [DOI] [PubMed] [Google Scholar]
- 48.Schot MJ, Van den Bruel A, Broekhuizen BD, et al. Point-of-care C-reactive protein to assist in primary care management of children with suspected non-serious lower respiratory tract infection: a randomised controlled trial. BJGP Open 2018;2:bjgpopen18X101600. 10.3399/bjgpopen18X101600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemiengre MB, Verbakel JY, Colman R, et al. Reducing inappropriate antibiotic prescribing for children in primary care: a cluster randomised controlled trial of two interventions. Br J Gen Pract 2018;68:e204–10. 10.3399/bjgp18X695033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schot MJ, Broekhuizen BD, Cals JW, et al. C-reactive protein point-of-care testing in children with cough: qualitative study of GPs' perceptions. BJGP Open 2018;1:bjgpopen17X101193. 10.3399/bjgpopen17X101193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verbakel JY, Lemiengre MB, De Burghgraeve T, et al. Should all acutely ill children in primary care be tested with point-of-care CRP: a cluster randomised trial. BMC Med 2016;14:131. 10.1186/s12916-016-0679-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van den Bruel A, Jones C, Thompson M, et al. C-reactive protein point-of-care testing in acutely ill children: a mixed methods study in primary care. Arch Dis Child 2016;101:382–6. 10.1136/archdischild-2015-309228 [DOI] [PubMed] [Google Scholar]
- 53.Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med 2019;381:111–20. 10.1056/NEJMoa1803185 [DOI] [PubMed] [Google Scholar]
- 54.Holmes EAF, Harris SD, Hughes A, et al. Cost-effectiveness analysis of the use of point-of-care C-reactive protein testing to reduce antibiotic prescribing in primary care. Antibiotics 2018;7. 10.3390/antibiotics7040106. [Epub ahead of print: 07 Dec 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fawsitt CG, Lucey D, Harrington P, et al. PMD18 a cost-effectiveness and budget impact analysis of C-reactive protein point-of-care testing to guide antibiotic prescribing for respiratory tract infections in primary care settings in Ireland. Value in Health 2019;22:S672 10.1016/j.jval.2019.09.1431 [DOI] [PubMed] [Google Scholar]
- 56.Eley CV, Sharma A, Lecky DM, et al. Qualitative study to explore the views of general practice staff on the use of point-of-care C reactive protein testing for the management of lower respiratory tract infections in routine general practice in England. BMJ Open 2018;8:e023925. 10.1136/bmjopen-2018-023925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamas-Fernandez C, Hayward G, Moore M, et al. A mathematical model for designing networks of C-reactive protein point of care testing. PLoS One 2019;14:e0222676. 10.1371/journal.pone.0222676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson M, Cross L, Sandison N, et al. Funding and policy incentives to encourage implementation of point-of-care C-reactive protein testing for lower respiratory tract infection in NHS primary care: a mixed-methods evaluation. BMJ Open 2018;8:e024558. 10.1136/bmjopen-2018-024558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardy V, Thompson M, Keppel GA, et al. Qualitative study of primary care clinicians' views on point-of-care testing for C-reactive protein for acute respiratory tract infections in family medicine. BMJ Open 2017;7:e012503. 10.1136/bmjopen-2016-012503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huddy JR, Ni MZ, Barlow J, et al. Point-of-care C reactive protein for the diagnosis of lower respiratory tract infection in NHS primary care: a qualitative study of barriers and facilitators to adoption. BMJ Open 2016;6:e009959. 10.1136/bmjopen-2015-009959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Hecke O, Butler C, Mendelson M, et al. Introducing new point-of-care tests for common infections in publicly funded clinics in South Africa: a qualitative study with primary care clinicians. BMJ Open 2019;9:e029260. 10.1136/bmjopen-2019-029260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Brien K, Gloeckner L, Jordan K. C-reactive protein point-of-care testing (CRP POCT) to guide antibiotic prescribing in primary care settings for acute respiratory tract infections (RTIs). EUnetHTA, 2019. [Google Scholar]
- 63.NHS England and The British Medical Association A five-year framework for GP contract reform to implement the NHS long term plan, 2019. [Google Scholar]
- 64.O'Neill Rapid diagnostics: stopping unnecessary use of antibiotics. London: HM Government and Wellcome Trust, 2015. [Google Scholar]
- 65.Hays JP, Mitsakakis K, Luz S, et al. The successful uptake and sustainability of rapid infectious disease and antimicrobial resistance point-of-care testing requires a complex 'mix-and-match' implementation package. Eur J Clin Microbiol Infect Dis 2019;38:1015–22. 10.1007/s10096-019-03492-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The Lancet The antimicrobial crisis: enough advocacy, more action. Lancet 2020;395:247. 10.1016/S0140-6736(20)30119-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2020-000624supp001.pdf (98.4KB, pdf)


