Abstract
Dornase alfa, the recombinant form of the human DNase I enzyme, breaks down neutrophil extracellular traps (NET) that include a vast amount of DNA fragments, histones, microbicidal proteins and oxidant enzymes released from necrotic neutrophils in the highly viscous mucus of cystic fibrosis patients. Dornase alfa has been used for decades in patients with cystic fibrosis to reduce the viscoelasticity of respiratory tract secretions, to decrease the severity of respiratory tract infections, and to improve lung function. Previous studies have linked abnormal NET formations to lung diseases, especially to acute respiratory distress syndrome (ARDS). It is well known that novel coronavirus disease 2019 (COVID-19) pneumonia progresses to ARDS and even multiple organ failure. High blood neutrophil levels are an early indicator of COVID-19 and predict severe respiratory diseases. Also it is reported that mucus structure in COVID-19 is very similar to that in cystic fibrosis due to the accumulation of excessive NET in the lungs. In this study, we showed the recovery of three individuals with COVID-19 after including dornase alfa in their treatment. We followed clinical improvement in the radiological analysis (two of three cases), oxygen saturation (Spo2), respiratory rate, disappearance of dyspnoea, coughing and a decrease in NET formation and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load after the treatment. Also here, we share our preliminary results suggesting that dornase alfa has an anti-viral effect against SARS-CoV-2 infection in a green monkey kidney cell line, Vero, and a bovine kidney cell line, MDBK, without determined cytotoxicity on healthy peripheral blood mononuclear cells.
Keywords: Coronavirus disease 2019, dornase alfa, NETosis, neutrophil extracellular traps, severe acute respiratory syndrome coronavirus 2
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected more than 23 million people around the world, resulting in unprecedented health, social and economic crises. COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is manifested by flu-like symptoms and in some patients by acute respiratory distress syndrome (ARDS) and even viral pneumonia that progresses to multiple organ failure [1]. The exudative phase of ARDS is characterized by the productive and excessive immune response of pro-inflammatory cytokines and chemokines; increased neutrophil infiltration and accumulation in the alveoli; and alveolar epithelial capillary barrier disruption [2]. High blood neutrophil levels are an early indicator of SARS-CoV-2 infection and cause serious respiratory diseases. A pathogenic role has been demonstrated [3,4] for neutrophil-derived extracellular traps (neutrophil extracellular traps, NETs) in a variety of thrombo-inflammatory conditions, including sepsis [5,6], thrombosis [[6], [7], [8]] and respiratory failure [5,6]. NETs are DNA fragments, histones, microbicidal proteins and oxidant enzymes that are released by neutrophils during infection. However, in over-response, NETs have the potential to initiate and spread inflammation and thrombosis [9,10]. NETs have been reported in many lung and cardiovascular diseases in association with interleukin-1β secretion [8,[11], [12], [13], [14], [15], [16]]. Although the exact determination of COVID-19 pathophysiology requires the development of model systems, the neutrophilic infiltration relationship has been established many times in the infection sites of influenza A (H1N1) virus, SARS-CoV and Middle East respiratory syndrome coronavirus and the development of ARDS in these patients [17,18]. In vitro culturing of neutrophils from influenza-infected lungs with infected alveolar epithelial cells triggers NETosis and increased endothelial damage. Mice lacking neutrophil cells have a mild response to influenza virus [19], and higher plasma NET levels have been reported for patients with transfusion-related ARDS [20]. Similarly, recent studies confirmed the role of immune hyperactivation in the pathogenesis of COVID-19 [21]. Immune cells, especially neutrophils, infiltrate pulmonary capillaries, which causes acute fibrin deposition and extravasation into the alveolar space. This excessive accumulation of neutrophils constitutes NETs, which makes the mucus thick and viscous. The highly viscous mucus lowers the patient's respiratory function and impairs ventilation [22], suggesting that NETs may play a major role in the disease. As a mechanism, double-stranded DNA constitutes the backbone of NETs and dornase alfa promotes the clearance of NETs from plasma neutralization. Endogenous DNases, which physiologically break up this extracellular DNA, may become overwhelmed by a massive influx of NETs [23,24]. Clinically, recombinant human DNase I (rhDNase, dornase alfa) has the identical primary amino acid sequence with the native human enzyme and has been approved for the management of cystic fibrosis. Daily administration of dornase alfa is effective in the treatment of individuals with cystic fibrosis and improves pulmonary functions [25,26]. The similarity of mucus secretions in COVID-19 and cystic fibrosis patients with regard to NETs makes dornase alfa a therapeutic option in COVID-19 [27,28]. In this study, preliminary data are presented about in vitro and in vivo anti-viral and nuclease activities of dornase alfa for the clearance of SARS-CoV-2 viral load and NETs in the lungs of individuals with COVID-19.
Materials and methods
In vitro anti-viral efficiency of dornase alfa
In vitro isolation and propagation of SARS-CoV-2 from individuals diagnosed with COVID-19 are described in our previous study [29]. The median tissue culture infectious dose (TCID50) of SARS-CoV-2 was determined (see Supplementary material, Fig. S1) and was incubated with the Madin-Darby bovine kidney cell line (MDBK; ATCC CCL-22) and a 10 × TCID50 dose of the virus was incubated with the Vero cell line (CCL81; ATCC) along with dornase alfa (Pulmozyme®, Basel, Switzerland) in a dose-dependent manner (3, 30 and 100 U/mL) with 420 mg/L MgSO4 (magnesium sulphate 15%; Onfarma, Samsun, Turkey) for 8 days. The cytopathic effect (CPE) test was performed as described previously [29].
RT-PCR and gel electrophoresis
Total RNA isolation from SARS-CoV-2 was performed using Direct-zol RNA Miniprep Kits (Zymo Research, Irvine, CA, USA), and concentrations were determined using a Qubit fluorometer with the Qubit RNA HS Assay (Thermo Fisher Scientific, Waltham, MA, USA). Fifty nanograms of the SARS-CoV-2 RNA genome were incubated with none, 3 U or 10 U of dornase alfa along with 420 mg/L MgSO4 at room temperature for 20 min. Next, samples were heated at 72°C for 10 min to inactivate the dornase alfa before RT-PCR assay. SARS-CoV-2-specific RT-PCR was performed with a Bosphore Novel Coronavirus (2019-nCoV) Detection Kit (Anatolia Geneworks, Istanbul, Turkey) along with Orf1ab and E gene primers. The RT-PCR analysis was performed in a Roche Lightcycler 96.
Anti-viral effect of dornase alfa using real-time cell analysis, xCELLigence
Vero cells (25 000 cells/well) were incubated in gold microelectrode-embedded microtitre wells in xCELLigence® Real-Time Cell Analysis (RTCA) instruments (ACEA, Roche) at 37°C for 24 hours to normalize cell index with coated cells. Then, dornase alfa in a serial dilution was pre-incubated with 10 × TCID50 of SARS-CoV-2 at room temperature for 60 min. The pre-incubated mixture was inoculated into the Vero-coated cells, which were analysed in real-time for 120 hours (in total 145 hours). Cell analysis was normalized to the value of the 24th hour of culturing before incubation with dornase alfa and SARS-CoV-2 mixtures. Normalized cell index shows the proliferation and viability of the adherent cells (a higher cell index indicates higher viability and proliferation). CPE was determined by assessing cell index value in untreated control Vero cells (normalized to 1% CPE) and treated wells.
Clearance of NET formation by dornase alfa
Following the isolation of peripheral blood mononuclear cells (PBMCs) by overlaying blood on Ficoll-paque plus (GE Healthcare, Chalfont St Giles, UK), the cells were frozen at –140°C with DMSO freezing solution (40% DMSO and 60% Dextran 40). Following 24 hours of freezing, the cells were thawed and washed twice with phosphate-buffered saline. Samples were then incubated at 37°C for 2 hours in T-cell medium. (6% Human AB Serum and 1% penicillin/streptomycin, Texmacs medium) until cryopreservation and thawing-induced NET formation was detected. Next, clump-containing samples were separated into two T300 flasks with 50 mL T-cell medium and one T300 flask from each donor was treated with 100 U/mL dornase alfa (Pulmozyme®) and 420 μg/mL MgSO4 for 15 min. The supernatant from three donor samples was collected and a viable cell count was performed with Trypan blue and haemocytometer. Also, a peripheral smear was collected from the supernatant of both dornase alfa-treated and untreated control cultures stained with Wright stain as described previously [30].
Cytotoxicity of dornase alfa on PBMCs
Three healthy adult blood samples were obtained from the Acibadem Labcell Cellular Therapy Laboratory. Following the isolation of PBMCs by overlaying blood on Ficoll-paque plus (GE Healthcare), the serially diluted (300, 100, 30, 10 and 3 U/mL) dornase alfa along with 420 μg/mL MgSO4 was incubated with the PBMCs for 48 hours in T-cell medium (6% human AB Serum and 1% penicillin/streptomycin, Texmacs medium). Immune cell subtypes and their activation levels were determined by Miltenyi MACSQuant flow cytometry analysis using alfaCD3-phycoerythrin, alfaCD19-phycoerythrin.Cy7, alfaCD56-fluorescein isothiocyanate, alfaCD4-Viogreen, alfaCD8-Vioblue, alfaCD25-allophycocyanin and alfaCD107a-phycoerythrin.Cy5.5 (Miltenyi Biotec, Bergisch Gladbach, Germany). Viable cells were determined by assessing the proportion of 7-aminoactinomycin D (Miltenyi Biotec) negative cells through staining for flow cytometry.
Ethical approval and consent to participate
Individuals with COVID-19 were recruited to the clinical study within the scope of ‘Dornase Alpha for the Treatment of COVID-19’ (ClinicalTrials.gov Identifier: NCT04432987) at Acibadem Altunizade Hospital, Istanbul. Each patient was informed and approved the patient information and consent form, which is named ‘Determination of Dornase Alpha Activity in COVID-19 Treatment’. Isolation of PBMCs, SARS-CoV-2 isolation and propagation, and anti-viral in vitro tests were performed in the Acibadem Labcell Cellular Therapy Laboratory GMP and BSL-3 units. Clinical Scientific Research Studies approval (2020-04-29 T15_24_57) was acquired from the Research and Development Health Services General Directorate of the T.C. Ministry of Health.
A preliminary clinical COVID-19 case study with dornase alfa
This study involves three patients diagnosed with COVID-19 from specific PCR test and clinical/radiological findings in the Acibadem Altunizade Hospital, Istanbul. All the patients were dyspnoeic and supported by oxygen without mechanical ventilation before dornase alfa treatment. Dornase alfa was always used as add-on therapy combined with other investigational drugs for COVID-19. All patients were followed with clinical findings (oxygen saturation, i.e. Spo2, respiratory rate, fever, coughing and dyspnoea) and radiography with CT of the thorax. Dornase alfa was used daily for 3 days at a dose of 2.5 mg with a jet nebulizer at least 1 hour before chest physiotherapy, as recommended [31]. All necessary patient consents were obtained and the forms have been archived.
Quantitative RT-PCR to determine viral copy number
Nasopharyngeal and oropharyngeal specimens were collected from the three COVID-19 patients on the day before and 7 days after dornase alfa treatment. Total RNA isolations from 100 μL patient specimens in 100 μL DNA/RNA Shield solution (Zymo Research) was performed with Direct-zol RNA Miniprep Kits (Zymo Research) and isolated RNA samples were suspended in 25 μL RNAse-free water. Quantitative RT-PCR was performed with the FDA-approved QuantiVirus™ SARS-CoV-2 Test Kit (Diacarta, Richmond, CA, USA) according to the manufacturer's protocol. The quantitative RT-PCR was analysed in a Roche Lightcycler 96. Three SARS-CoV-2 genes—Orf1ab, N and E genes—were detected in the sample. SARS-CoV-2 viral copy number per mL of each patient sample was calculated based on the standard curve produced with Ct (threshold cycle) values and logarithmic viral gene copy number of the extraction control provided in the kit in a dose-dependent manner.
Statistics
In the bar graphs, independent two-tailed t-tests were performed using SPSS Statistics software (SPSS Inc., Armonk, NY, USA). No outliers were excluded in any of the statistical tests and each data point represents an independent measurement. Bar plots report the mean and standard deviation. The threshold of significance for all tests was set at p < 0.05.
Results
In vitro anti-viral efficiency of dornase alfa
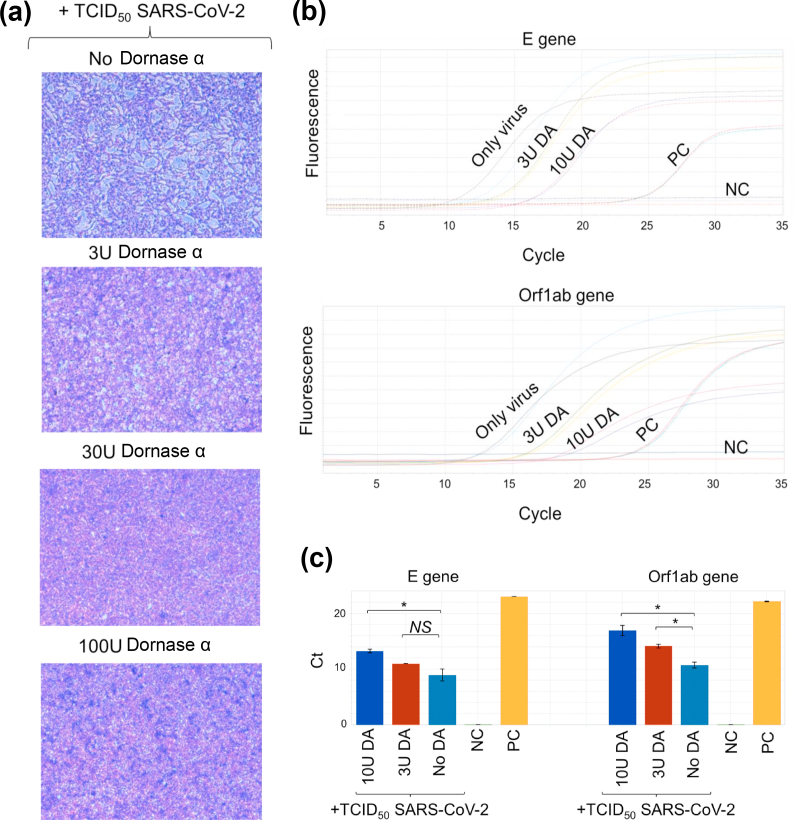
Dornase alfa degrades extracellular DNA and helps the clearance of NET that is constituted primarily from DNA fragments [32]. Although SARS-CoV-2 is a positive-strand RNA virus [33], we wanted to determine an anti-viral effect of dornase alfa co-incubated with a 1 × TCID50 dose of SARS-CoV-2 and the MDBK cell line. The results show that dornase alfa, even with a small dose (3 U/mL), efficiently inhibits the infectious capacity of SARS-CoV-2, as seen by the CPE that was lost in higher doses of dornase alfa (Fig. 1a). We then investigated whether dornase alfa can degrade the SARS-CoV-2 positive-strand RNA genome. Isolated total SARS-CoV-2 RNA was pre-incubated with dornase alfa at different doses and dornase alfa was inactivated at 72°C before RT-PCR. We detected amplifications of the E and Orf1ab genes at higher cycles than the control that included only RNA with MgSO4 (Fig. 1b). We determined that high doses of dornase alfa increased cycle threshold (Ct) of the E gene from 10 in the controls (No dornase alfa) to 14 in the 10 U dornase alfa-treated condition; a 16-fold difference (Fig. 1c). In addition, the Ct of the Orf1ab gene significantly increased from 11 in the No dornase alfa control to 17 in the 10 U dornase alfa-treated condition; a 64-fold difference (Fig. 1c). This preliminary study suggested that dornase alfa may have an anti-viral effect; although the mechanism is yet to be elucidated.
FIG. 1.
Anti-viral effect of dornase alfa against SARS-CoV-2. (a) Cytopathic effect (CPE) in MDBK cells infected with SARS-CoV-2 (10 × TCID50) along with dornase alfa in a dose-dependent manner for 8 days. (b) Detected cycle of real-time PCR fluorescence intensity of the SARS-CoV-2 RNA genome that were pre-incubated with dornase alfa at different doses. PC, positive control, synthetic E or Orf1ab genes. NC, negative control, dH2O. (c) The bar graphs showing cycle threshold (Ct) values of the dornase-alfa-treated SARS-CoV-2 samples, PC, and NC in (b). ∗p < 0.05; NS, not significant.
Anti-viral effect of dornase alfa using real-time cell analysis
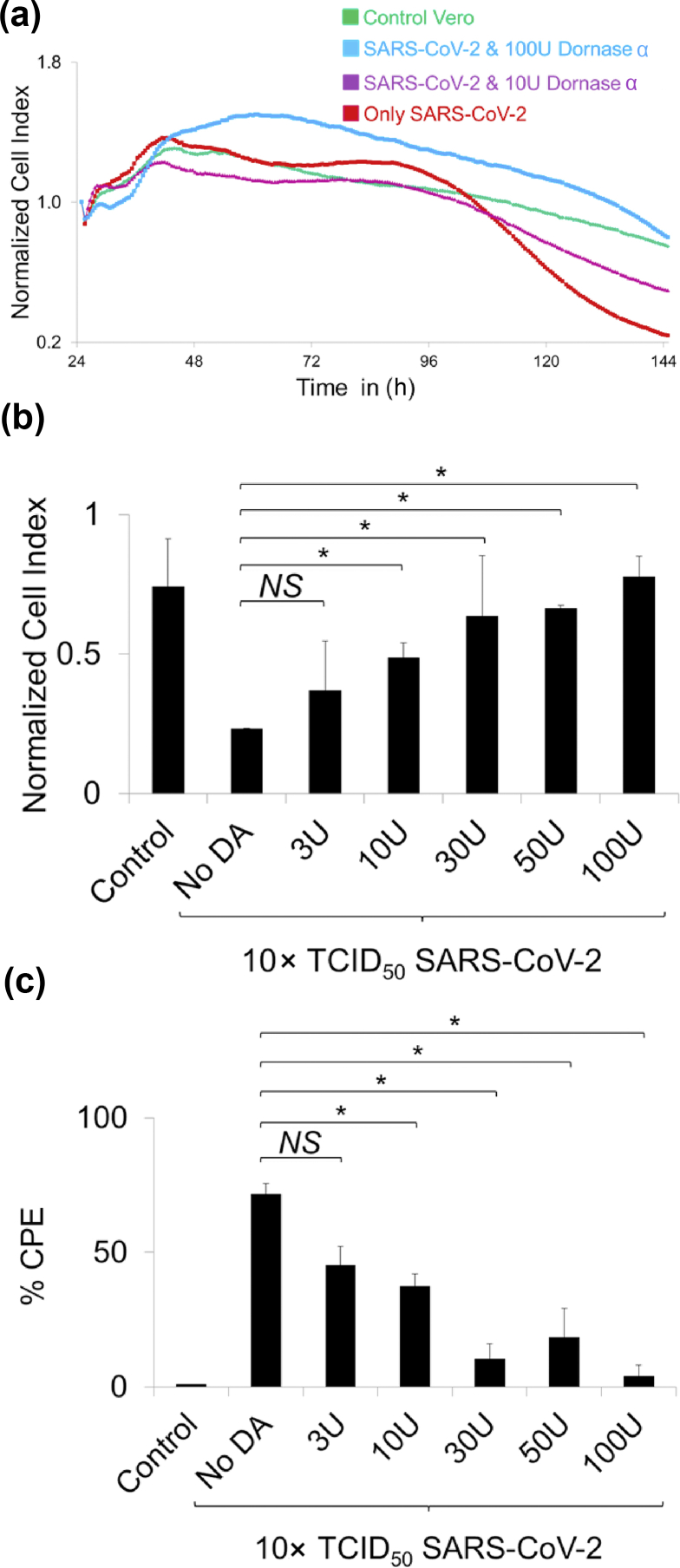
After we determined that dornase alfa may have nuclease activity against the SARS-CoV-2 genome. We asked whether dornase alfa can interfere with SARS-CoV-2 infection in a real-time cell culture system, namely xCELLigence real-time cell analysis (RTCA). A 10 × TCID50 dose of SARS-CoV-2 pre-incubated with dornase alfa at different doses—control (No dornase alfa), 3, 10, 30, 50 and 100 U along with MgSO4 for 1 hour. The different cultures were inoculated on coated Vero cells in RTCA wells and cell proliferation and viability were screened with ‘normalized cell index’ values analysed by the xCELLigence automated system. Increasing cell index meant the proliferation of viable cells. Only the SARS-CoV-2-incubated Vero cells had a significantly lowered cell index, the value recovered when SARS-CoV-2 virus was pre-incubated with dornase alfa (Fig. 2a). Furthermore, we determined that cell index value for 100 U dornase alfa plus SARS-CoV-2 was significantly higher than that of the control Vero cell condition for 120 hours of incubation (Fig. 2a). Between 120 and 144 hours, inhibition of SARS-CoV-2 propagation on the Vero cells showing CPE was more evident, showing a possible dose-dependent effect upon dornase alfa pre-treatment (Fig. 2a). Also, after 144 hours, normalized cell index analysis showed that incubation of SARS-CoV-2 with increasing doses of dornase alfa significantly increased the cell index similar to the untreated control Vero cells in a dose-dependent manner (Fig. 2b). Next, we used the same values and transformed them to CPE% by normalizing the cell index value to the CPE value (control-only Vero cell condition was normalized to 0% CPE and other cell index values were normalized with respect to that value). A lower cell index gave a higher CPE value. The CPE rate was highest in the only SARS-CoV-2-incubated condition (i.e. control No dornase alfa; Fig. 2b) whereas the value was significantly the lowest in the 100 U dornase alfa-treated SARS-CoV-2 incubated wells (Fig. 2c). This RTCA data further showed that dornase alfa may have an anti-viral effect against SARS-CoV-2. Yet, plaque assay can be perfomed to determine change in TCID50 value in dornase alfa treatment, which will be a more direct approach to show the anti-viral effect.
FIG. 2.
Cytopathic effect of SARS-CoV-2–dornase alfa incubated Vero cells in real-time cell analysis (RTCA). (a) Representative normalized cell index histogram showing proliferating viable cells that were control only Vero (green line), 100 U (blue line) or 10 U (purple line) dornase alfa-preincubated SARS-CoV-2 (blue line), and only 10 × TCID50 dose of SARS-CoV-2 (red line). Cell index was normalized based on the values at the end of 24-hour pre-culture. (b) Bar graph showing quantification of the normalized cell index value of the conditions treated with the SARS-CoV-2 along with/without dornase alfa. (c) Bar graph showing normalized cytopathic effect (% CPE) using values of normalized cell index. ∗p < 0.05; NS, not significant.
In vitro clearance of NET formation by dornase alfa
Dornase alfa has been used in the treatment of patients with cystic fibrosis for decades for the clearance of NET formation [32,34]. Several studies have shown DNase activity of dornase alfa by degrading DNA fragment-trapped NETs [26,[34], [35], [36], [37]]. In this study, we wanted to show a new approach to evaluate in vitro clearance of NET by dornase alfa as a proof of concept. We mimicked in vitro NETosis with a clump formation comprising extracellular DNA fragments, erythrocytes, lymphocytes and neutrophils [38,39]. In the test, we thawed adult human mononuclear cells. Following NET formation, we treated the cells with dornase alfa along with MgSO4 to induce nuclease activity (see Supplementary material, Fig. S2A). Similar to previous reports, a large number of single-cell mononuclear cells were freely determined after dornase alfa treatment; however, the untreated control had clumps that could be seen without a microscope (see Supplementary material, Fig. S2B). We then showed that the number and viability of the cells in the culture supernatant after dornase alfa treatment significantly increased with respect to the untreated control, which still had clumps trapping most of the cells (see Supplementary material, Fig. S2C,D) Before dornase alfa treatment there were up to 1.8 × 106 ± 1.15 × 106 in three PBMCs. However, after the treatment, the count increased to 12.9 × 106 ± 5.45 × 106 viable cells. Also, we determined that viability was not affected by dornase alfa treatment using the dye exclusion test by staining the cells with Trypan blue (see Supplementary material, Fig. S2E). This proof-of-concept study showed similar results to previous studies [26,34,36,37,40] that dornase alfa efficiently clears NETs that trapped large amount of mononuclear cells.
Cytotoxicity test of dornase alfa on PBMCs
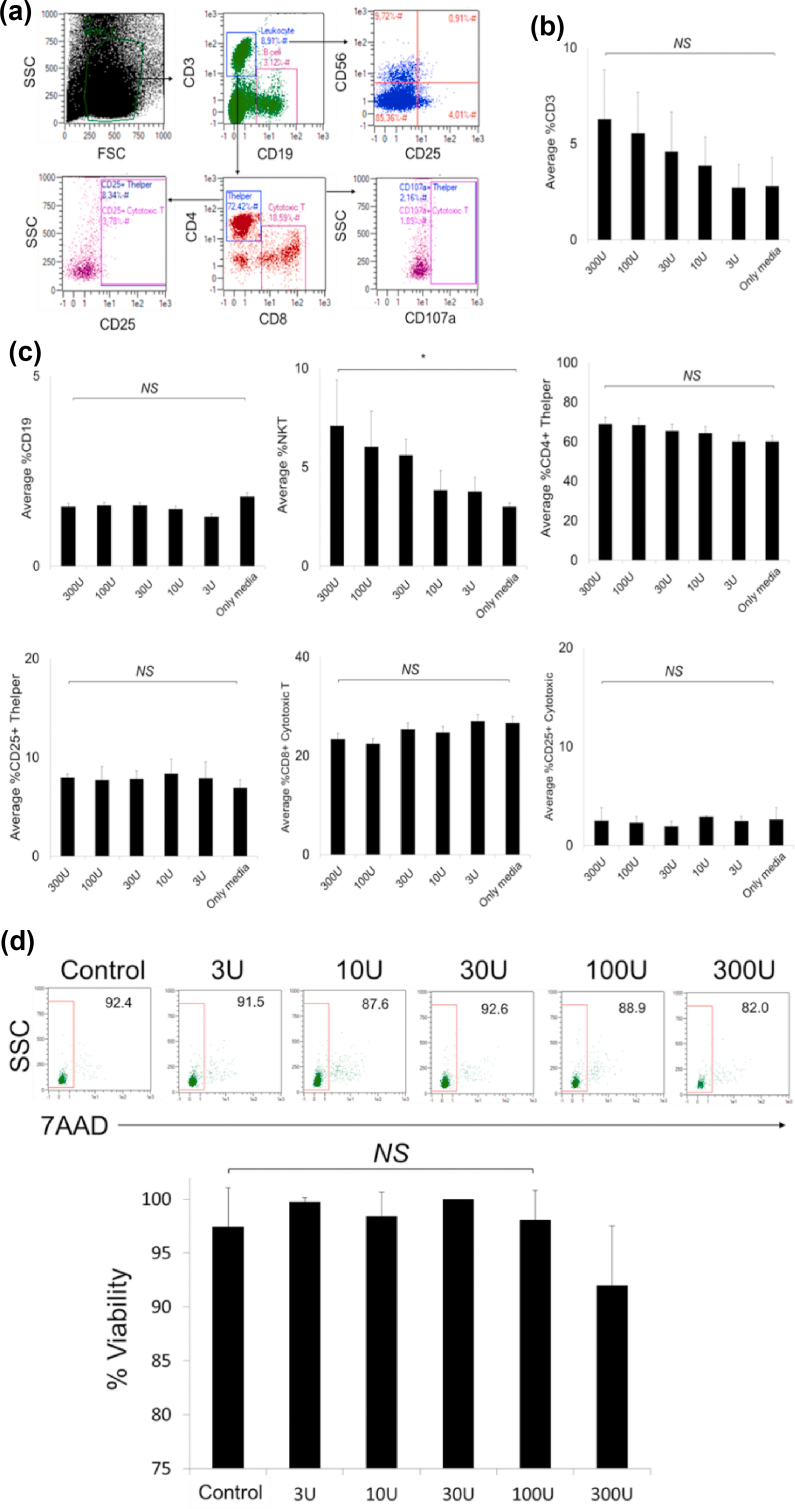
PBMCs include most of the immune cell subtypes in the human blood such as lymphocytes, neutrophils, B cells, natural killer T cells and monocytes. Healthy PBMCs are widely used in toxicology analysis of drug studies because peripheral blood is the front-line of exposure to chemicals in the body. Therefore, toxicity analysis with PBMCs is very important [39]. Although dornase alfa has been used in clinics for many years without serious adverse effect [36], we wanted to determine the cytotoxicity on healthy adult PBMCs. We incubated PBMCs with dornase alfa in a dose-dependent manner for 48 hours and assessed changes in proportions of the immune cell subtypes and up-regulation of activation markers (CD25 and CD107a) in the cells using flow cytometry as shown in Fig. 3a. We did not determine a statistically significant increase in the frequency of CD3+ T cells, especially CD3+ CD4+ T helper cells, except CD3+ CD8+ cytotoxic T cells (Fig. 3b,c). Similarly, CD19+ B-cell proportions did not change upon increasing the dornase alfa concentration (Fig. 3c). However, the proportion of CD3+ CD56+ natural killer T cells significantly increased in a dose-dependent manner (Fig. 3c). We did not determine a significant increase in CD25 and CD107a expression, suggesting that dornase alfa does not induce lymphocyte activation (Fig. 3c). Furthermore, we labelled dead cells in PBMCs that were treated with dornase alfa in a dose-dependent manner with 7-aminoactinomycin D after 72 hour of incubation (Fig. 3d) and determined that there was no significant cytotoxic effect of dornase alfa up to 100 U, although there was 10% cytotoxicity at the highest dose, 300 U (Fig. 3d). These results suggest that dornase alfa does not show cytotoxicity in PBMCs up to 100 U and it may increase the proportion of natural killer T cells, although the mechanism for this requires further study.
FIG. 3.
Drug toxicities of dornase alfa on peripheral blood mononuclear cells (PBMCs). Three healthy adult PBMC samples were inoculated with dornase alfa in a dose-dependent manner for 48 h before flow cytometric analysis. (a) Flow cytometry plots that show immune cell subtypes (CD19+ B, CD3+ CD4+ T helper, CD3+ CD8+ cytotoxic T, and CD3+ CD56+ natural killer T (NKT) cells) and their activation (with alfaCD25 and alfaCD107a). (b) The bar graph that shows the change in average frequency of total CD3+ T lymphocytes in the PBMCs after treatment. (c) The bar graphs that show average proportions of the immune cell subtypes and their activation. (d) The flow cytometry plots showing viability of the cells analysed with 7aminoactinomycin D staining in flow cytometry. The bar graph showing viable proportion of the PBMCs treated with dornase alfa. ∗p < 0.05; NS, not significant.
Preliminary case study of dornase alfa in COVID-19 patients
Case 1
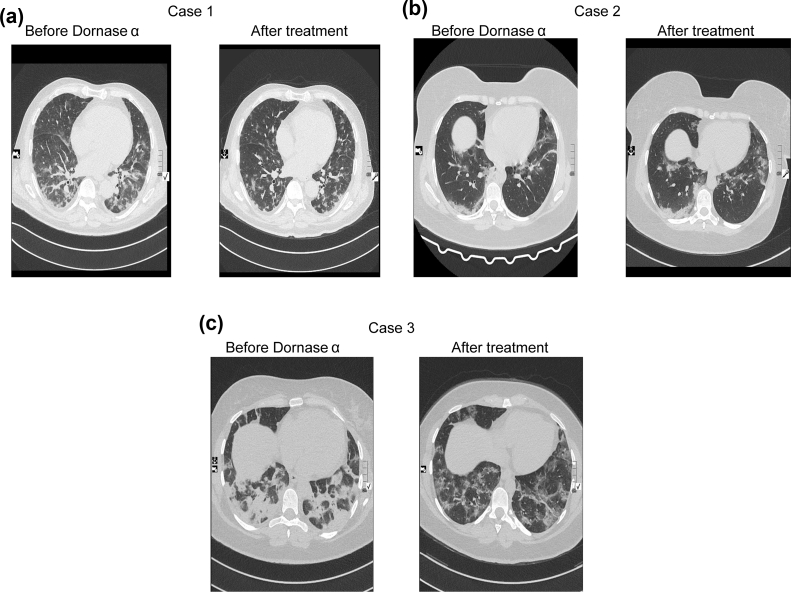
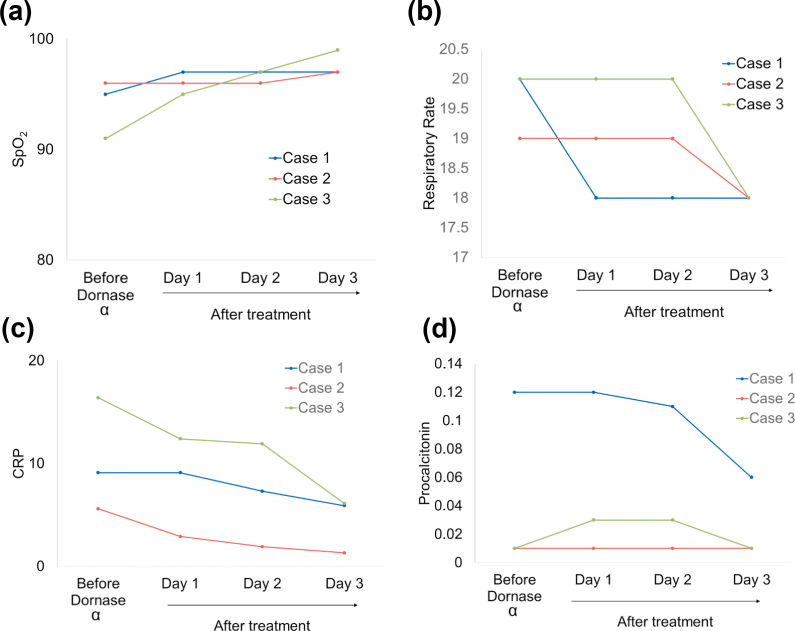
The individual was a 64-year-old man, with type 2 diabetes mellitus using daily metformin (Table 1). He had a fever and was dyspnoeic at admission. After 5 days on hydroxychloroquine therapy, he was coughing, still dyspnoeic with Spo2 97% in room air and a respiratory rate of 20 breaths/min. Favipiravir was added but after 3 days on favipiravir, he was coughing, still dyspnoeic with Spo2 decreasing to 95% in room air and respiratory rate still 20 breaths/min (Table 1). There were extensive ground-glass opacities on thorax CT. Dornase alfa was added on day 8 of the hospitalization. Twenty-four hours after the first dornase alfa administration, cough increased, dyspnoea disappeared, the respiratory rate decreased to 18 breaths/min and Spo2 increased to 97% in room air (Table 1). On the 3rd day of dornase alfa therapy, thorax CT image showed significant improvement (Fig. 4a). Also, fever, dyspnoea and coughing disappeared, Spo2 was 97% without oxygen support (Fig. 5a) with respiratory rate 18 breaths/min (Fig. 5b). Inflammatory biomarkers like C-reactive protein and procalcitonin levels decreased after 3 days of treatment (Fig. 5c, d) and (Table 1).
Table 1.
Clinical characteristics of patients and comparison of clinical and laboratory results before and after dornase alfa therapy
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Gender | M | F | F |
| Age (years) | 64 | 33 | 27 |
| PCR | Positive | Positive | Positive |
| Comorbidity | Diabetes mellitus | Breast cancer | Critical |
| Disease presentation and course | |||
| Interval between admission and dornase alfa (days) | 8 | 2 | 11 |
| Treatments | H, F | H, F | H, F |
| Clinical characteristics | |||
| Body temperature (°C) | |||
| Before dornase alfa | 36.3 | 38.3 | 37 |
| Day 1 post dornase alfa | 37.1 | 37.3 | 37.4 |
| Day 2 post dornase alfa | 36.7 | 37.2 | 36.6 |
| Day 3 post dornase alfa | 36.3 | 36 | 36.4 |
| Dyspnoea | |||
| Before dornase alfa | Yes | Yes | Yes |
| Day 1 post dornase alfa | No | No | Yes |
| Day 2 post dornase alfa | No | No | No |
| Day 3 post dornase alfa | No | No | No |
| Coughing | |||
| Before dornase alfa | Yes | Yes | Yes |
| Day 1 post dornase alfa | Yes | Yes | Yes |
| Day 2 post dornase alfa | No | Yes | No |
| Day 3 post dornase alfa | No | No | No |
| Respiratory rate | |||
| Before dornase alfa | 20 | 19 | 20 |
| Day 1 post dornase alfa | 18 | 19 | 20 |
| Day 2 post dornase alfa | 18 | 19 | 20 |
| Day 3 post dornase alfa | 18 | 18 | 18 |
| Spo2 | |||
| Before dornase alfa | 95 | 96 (with oxygen) | 91 (with oxygen) |
| Day 1 post dornase alfa | 97 | 96 | 95 (with oxygen) |
| Day 2 post dornase alfa | 97 | 96 | 97 (with oxygen) |
| Day 3 post dornase alfa | 97 | 97 | 99 (with oxygen) |
| C-reactive protein (mg/L) | |||
| Before dornase alfa | 9.1 | 5.6 | 16.4 |
| Day 1 post dornase alfa | 9.1 | 2.9 | 12.4 |
| Day 2 post dornase alfa | 7.3 | 1.9 | 11.9 |
| Day 3 post dornase alfa | 5.9 | 1.3 | 6.1 |
| Procalcitonin (ng/mL) | |||
| Before dornase alfa | 0.12 | 0.01 | 0.01 |
| Day 1 post dornase alfa | 0.12 | 0.01 | 0.03 |
| Day 2 post dornase alfa | 0.11 | 0.01 | 0.03 |
| Day 3 post dornase alfa | 0.06 | 0.01 | 0.01 |
Abbreviations: Spo2, peripheral capillary oxygen saturation; H, hydroxychloroquine; F, favipiravir.
FIG. 4.
Comparison of thorax CT images before (left) and after (right) dornase alfa therapy for all three patients. In Case 1 (a) and Case 3 (c) significant improvement occurred, in Case 2 (b) persistent lesions were detected.
FIG. 5.
Improvement in clinical outcomes following dornase alfa treatment. Temporal changes of (a) Spo2 per patient, (b) respiratory rate per patient, (c) C-reactive protein per patient and (d) procalcitonin per patient.
Case 2
The individual was a 33-year-old woman with breast cancer still receiving tamoxifen therapy (Table 1). At admission, she had a fever, was coughing and dyspnoeic, with Spo2 99% with oxygen support, and respiratory rate 19 breaths/min. After 2 days on hydroxychloroquine and favipiravir treatment, she was still dyspnoeic and coughing, respiratory rate 19 breaths/min and Spo2 decreasing to 96% with oxygen support (Table 1). She had extensive ground-glass opacities on thorax CT. Dornase alfa was added on the 2nd day of the hospitalization. After dornase alfa administration, a thorax CT image showed persistent lesions (Fig. 4b). Coughing increased, dyspnoea disappeared, Spo2 was 96% with oxygen support after 24 hours of the treatment (Fig. 5a), and respiratory rate was 19 breaths/min (Fig. 5b). On the 3rd day of dornase alfa therapy, fever, dyspnoea and coughing had disappeared, Spo2 was 97% (Fig. 5a) without oxygen support and respiratory rate was 18 breaths/min (Fig. 5b). Also, the C-reactive protein level decreased (Fig. 5c) but the procalcitonin level was stable (Fig. 5d) after 3 days of treatment (Table 1).
Case 3
This individual was a 27-year-old woman, without comorbidities (Table 1). At first admission to another centre, she had a fever, was coughing and dyspnoeic. She had extensive ground glass opacities on thorax CT and hydroxychloroquine was started. After 5 days of hydroxychloroquine therapy, she deteriorated and favipiravir was started. At admission to our centre, she was on the 3rd day of favipiravir therapy with dyspnoea and coughing, her respiratory rate was 20 breaths/min and Spo2 was 91% with oxygen support (4 L/min) (Table 1). After dornase alfa administration, a thorax CT image showed significant improvement (Fig. 4c). Twenty-four hours after first dornase alfa administration, cough increased, dyspnoea persisted, Spo2 was 95% with oxygen support (3 L/min) (Fig. 5a), and respiratory rate was 20 breaths/min (Fig. 5b). On the 3rd day of dornase alfa therapy, fever, dyspnoea and coughing had disappeared, Spo2 was 99% with oxygen support (1 L/min) (Fig. 5a) and respiratory rate was 18 breaths/min (Fig. 5b). The C-reactive protein level decreased (Fig. 5c) but the procalcitonin level was stable (Fig. 5d) after 3 days of the treatment (Table 1).
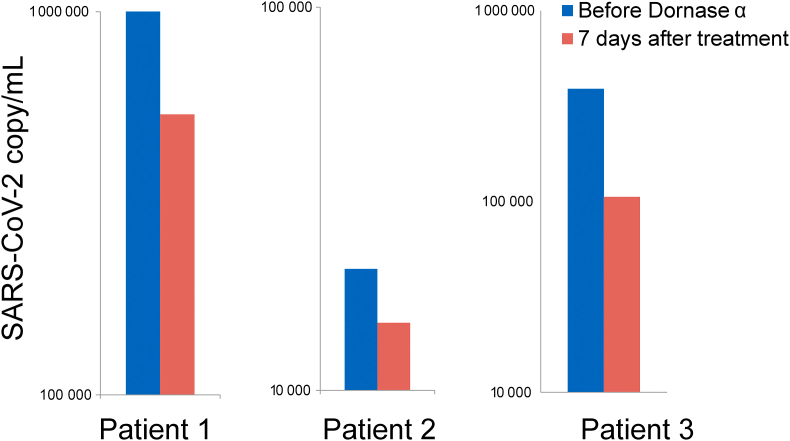
A decrease in the SARS-CoV-2 virus load in dornase-alfa-treated patient specimens
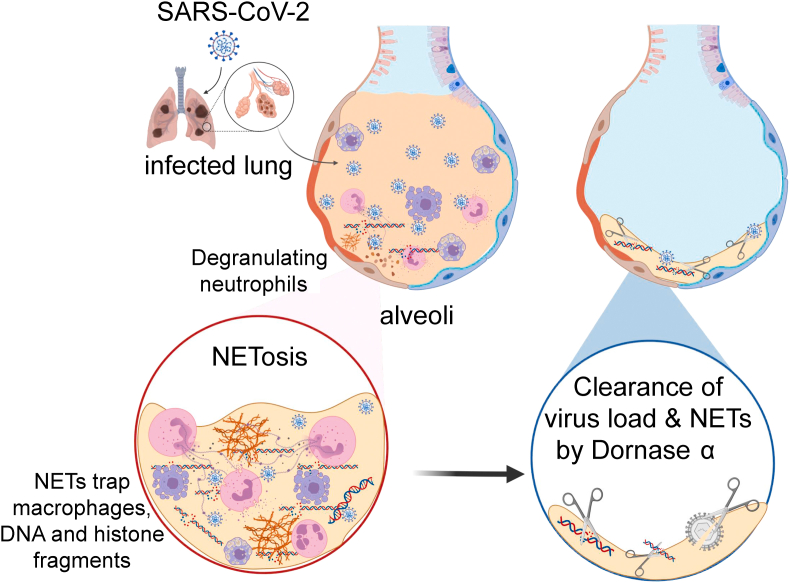
In addition to a clinical improvement in the dornase-alfa-treated patients, we also collected nasopharyngeal and oropharyngeal specimens from the patients on the day before the treatment and after 7 days of the dornase alfa treatment to assess their SARS-CoV-2 viral load using quantitative RT-PCR analysis. We determined a decrease in viral load in all three patients after dornase alfa treatment (Fig. 6). This study suggests that dornase alfa participates in the clearance of virus load and NETs in the infected lung (Fig. 7).
FIG. 6.
SARS-CoV-2 viral load in three dornase alfa-treated COVID-19 patients. The bar graphs showing SARS-CoV-2 viral copy numbers the day before the treatment and 7 days after dornase alfa treatment.
FIG. 7.
Summary of the anti-viral and nuclease activities of dornase alfa. SARS-CoV-2-infected alveoli in infected lungs that induce formation of NETs (NETosis) trapping DNA and histone fragments along with macrophages (blue) and neutrophils (pink). Dornase alfa clears SARS-CoV-2 viral load and NETs in the alveoli.
Discussion
Detection of SARS-CoV-2 RNA in COVID-19 patient samples from higher respiratory tract with low Ct values on the 4th and 7th days of the course of the disease suggests high viral loads and capability for transmission [41]. Neutrophil levels are an early indicator of SARS-CoV-2 infection and have a pathogenic role [3,4] in neutrophil-derived NETosis, which could be reversed using recombinant DNAse I. Dornase alfa has been used to resolve the highly viscous mucus of cystic fibrosis patients for decades [32,34,42]. A similar mucus structure was detected in COVID-19 patients due to the accumulation of excessive NETs in the lungs [21,22]. Here, we show our preliminary results with dornase alfa, which may have an in vitro anti-viral effect against SARS-CoV-2 infection without cytotoxicity. In this preliminary study, we also showed the effect of dornase alfa in the clearance of NET formation and SARS-CoV-2 viral load in three individuals with COVID-19 who showed clinical improvement in the radiological analysis (two out of three cases), oxygen saturation (Spo2), respiratory rate, disappearance of dyspnoea and coughing.
Dornase alfa degrades extracellular DNA fragments and promotes clearance of NET formation. In our daily-process of patient blood apheresis, we frequently use dornase alfa to clear NET-like clumps that can easily form after the cryopreservation and thawing process. Dornase alfa has been used in patients with cystic fibrosis to clear NETs in the respiratory tract. As reported in the literature [26,34,36,37,40], we recapitulated that NETs were cleared by dornase alfa in the Supplementary material (Fig. 2). This result gave rise to the thought that dornase alfa could be used in people with COVID-19, who can have NET-like formations induced by infiltrated neutrophils in their lungs. Therefore, three COVID-19 patients at different ages (64, 33 and 27 years old) were treated with dornase alfa.
We observed significant improvement in breathing by the disappearance of dyspnoea after dornase alfa therapy. All of the patients had better oxygenation after dornase alfa, shown by their Spo2 values. Increased coughing was observed after the first dornase alfa dose, most probably due to the resolving of viscous mucus, but this phenomenon was followed by the disappearance of coughing at the end of therapy. Also, after the last dornase alfa dose, decreasing respiratory rates for all patients were accepted as an improvement in pulmonary function. In addition to the improvement in the clinical data, we also observed a decrease in the inflammatory biomarkers like C-reactive protein and procalcitonin. To our knowledge, dornase alfa has no direct anti-inflammatory effects so these laboratory findings must be tested in further studies.
We detected radiological improvements in the thorax CT images that were compatible with better ventilation after dornase alfa therapy and this supported our clinical findings. Radiological improvement was significant for Case 1 and Case 3. However, persistent lesions were detected in Case 2, this also might be accepted as a response to therapy in this early phase of the disease that mostly progressed with radiological deterioration. On the other hand, we speculated that the comorbidities of cancer and COVID-19 in the same patient may have determined the difference in clinical improvement. Although it is apparent that only three patients are not enough to comment on the efficacy of dornase alfa, two independent clinical trials with dornase alfa on COVID-19 patients will evaluate its efficacy. One of them is led by the Agence Nationale de Sécurité du Médicament (EudraCT, 2020-001492-33) with the project named ‘Interest in the administration of Dornase alpha aerosol in ARDS secondary to respiratory infection by the coronavirus SARS-CoV-2/COVID-19’ that plans for 100 individuals with COVID-19 to be included. The second one is led by University College, London (ClinicalTrials.gov: NCT04359654) with the phase 2 project named ‘Nebulised Dornase Α for Treatment of COVID-19 (COVASE)’, which will recruit 50 individuals with COVID-19. These large-scale clinical trials may prove the clinical efficacy of dornase alfa in the treatment of COVID-19.
As dornase alfa is routinely taken by cystic fibrosis patients, data about the impact of COVID-19 on individuals with cystic fibrosis could be helpful to comment on the efficacy of dornase alfa. A recent study of this subject, reported very few and less severe COVID-19-positive cystic fibrosis patients across Europe [42]. These data are very interesting because they are not compatible with the reported eight times higher death rate in chronic respiratory diseases compared with the cases with no comorbidities (COVID-19 Fatality Rate by Comorbidity. www.worldometers.info). Lower severity of COVID-19 in cystic fibrosis patients could be explained by the long-term antibiotic therapy, such as azithromycin, or by the extreme efforts of families to minimize social contacts [42]. Also, dornase alfa is a regular therapy used by cystic fibrosis patients and this might be the reason for this promising result in COVID-19. Besides the patients presented in this study, we have data from two cystic fibrosis patients who recovered from COVID-19 without severe symptoms under regular dornase alfa therapy (personal communication).
All of these data gave rise to the thought that dornase alfa may have an anti-viral effect against SARS-CoV-2 infection. With this purpose, in our RTCA assay dornase alfa was co-incubated with SARS-CoV-2, and we determined that the infection and SARS-CoV-2 viral propogation on the Vero cells did not progress in increasing concentrations of dornase alfa pre-treatment. This preliminary in vitro data proposed that dornase alfa may have a possible anti-viral effect against SARS-CoV-2, which is yet to be studied in detail. Our in vitro tests have shown that the RNA genome can be degraded by pre-incubating the SARS-CoV-2 virus with dornase alfa. This result was confirmed by RTCA and cytopathic effect tests. These initial findings revealed that dornase alfa may interact directly with SARS-CoV-2.
In this study, we instantly report our preliminary data from the days of the pandemic on in vitro effects and in vivo anti-viral and nuclease activity of dornase alfa for the clearance of SARS-CoV-2 viral load and NETs in lungs, which can be added among the used drugs as a candidate for the treatment of COVID-19 patients. For these reasons, it is suggested that using dornase Α in the early period will decrease the viral load and slow down the course of the disease. Here, we also anounce that we have just started our clinical trial (ClinicalTrials.gov Identifier: NCT04432987) with early application of dornase alfa in the treatment of COVID-19 and this study involves the first case results.
Availability of data and material
The patient data used to support the findings of the study are available from the corresponding author upon request.
Funding statement
All funding for the work was supported by Acibadem Healthcare Group.
Author contributions
HKO, RZ, MK, CC and ASK performed the clinical treatments. KY, CH, MK MEY, ED, NB and NBP analysed the clinical data. CT, SD, BY, GS, DDK, SA and US performed the SARS-CoV-2 isolation, propagation, plaque assay and in vitro experiments. KY, CT and SD wrote the manuscript. EO led the clinical investigation and contributed to the design and interpretation of the data. EO was the project supervisor that developed the concept of anti-viral use of dornase alfa in COVID-19 infection. All authors have seen and approved the content and have contributed significantly to this work.
Consent for publication
This manuscript has not been submitted or accepted for publication elsewhere. The authors do not have any financial interests to report.
Conflicts of interest
The authors declare that there are no competing interests.
Acknowledgement
Figure 7 of the study was created with BioRender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2020.100756.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
FIG. S1. Assessing TCID50 of SARS-CoV-2.
FIG. S2. In vitro clearance of NET formation by dornase alfa.
References
- 1.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat Acute respiratory distress syndrome. Nat Rev Dis Prim. 2018;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potey P.M.D., Rossi A.G., Lucas C.D., Dorward D.A. Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential. J Pathol. 2019;247(5):672–685. doi: 10.1002/path.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93(3):212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 5.Iba T., Levy J.H., Raj A., Warkentin T.E. Advance in the management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Clin Med. 2019;8(5):728. doi: 10.3390/jcm8050728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward P.A., Fattahi F. New strategies for treatment of infectious sepsis. J Leukoc Biol. 2019;106(1):187–192. doi: 10.1002/JLB.4MIR1118-425R. [DOI] [PubMed] [Google Scholar]
- 7.Meng H., Yalavarthi S., Kanthi Y., Mazza L.F, Elfline M.A, Luke C.E. In vivo role of neutrophil extracellular traps in antiphospholipid antibody–mediated venous thrombosis. Arthritis Rheumatol (Hoboken, N.J.) 2017;69(3):655–667. doi: 10.1002/art.39938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav V., Chi L., Zhao R., Tourdot B.E., Yalavarthi S., Jacobs B.N. Ectonucleotidase tri(di)phosphohydrolase-1 (ENTPD-1) disrupts inflammasome/interleukin 1β-driven venous thrombosis. J Clin Invest. 2019;129(7):2872–2877. doi: 10.1172/JCI124804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twaddell S.H., Baines K.J., Grainge C., Gibson P.G. The emerging role of neutrophil extracellular traps in respiratory disease. Chest. 2019;156(4):774–782. doi: 10.1016/j.chest.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Porto B.N., Stein R.T. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberale L., Holy E.W., Akhmedov A., Bonetti N.R., Nietlispach F., Matter C.M. Interleukin-1β mediates arterial thrombus formation via NET-associated tissue factor. J Clin Med. 2019;8(12):2072. doi: 10.3390/jcm8122072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meher A.K., Spinosa M., Davis J.P., Pope N., Laubach V.E., Su G. Novel role of IL (interleukin)-1β in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018;38(4):843–853. doi: 10.1161/ATVBAHA.117.309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josefs T., Barrett T.J., Brown E.J., Quezada A., Wu X., Voisin M. Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight. 2020;5(7) doi: 10.1172/jci.insight.134796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lachowicz-Scroggins M.E., Dunican E.M., Charbit A.R., Raymond W., Looney M.R., Peters M.C. Extracellular DNA, neutrophil extracellular traps, and inflammasome activation in severe asthma. Am J Respir Crit Care Med. 2019;199(9):1076–1085. doi: 10.1164/rccm.201810-1869OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merza M., Hartman H., Rahman M., Hwaiz R., Zhang E., Renström E. Neutrophil extracellular traps induce trypsin activation, inflammation, and tissue damage in mice with severe acute pancreatitis. Gastroenterology. 2015;149(7):1920–1931. doi: 10.1053/j.gastro.2015.08.026. e8. [DOI] [PubMed] [Google Scholar]
- 16.Ridker P.M. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145–156. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol. 2005;5(12):917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blondonnet R., Constantin J.M., Sapin V., Jabaudon M. A pathophysiologic approach to biomarkers in acute respiratory distress syndrome. Dis Markers. 2016;2016:3501373. doi: 10.1155/2016/3501373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179(1):199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caudrillier A., Kessenbrock K., Gilliss B.M., Nguyen J.X., Marques M.B., Monestier M. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122(7):2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolaczkowska E., Jenne C.N., Surewaard B.G., Thanabalasuriar A., Lee W.Y., Sanz M.J. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. doi: 10.1038/ncomms7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIlroy D.J., Minahan K., Keely S., Lott N., Hansbro P., Smith D.W. Reduced deoxyribonuclease enzyme activity in response to high postinjury mitochondrial DNA concentration provides a therapeutic target for systemic inflammatory response syndrome. J Trauma Acute Care Surg. 2018;85(2):354–358. doi: 10.1097/TA.0000000000001919. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs H.J., Borowitz D.S., Christiansen D.H., Morris E.M., Nash M.L., Ramsey B.W. Effect of aerosolized recombinant human dnase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 26.McCoy K., Hamilton S., Johnson C. Effects of 12-week administration of dornase alfa in patients with advanced cystic fibrosis lung disease. Chest. 1996;110(4):889–895. doi: 10.1378/chest.110.4.889. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Alemán S.R., Campos-García L., Palma-Nicolas J.P., Hernández-Bello R., González G.M., Sánchez-González A. Understanding the entanglement: neutrophil extracellular traps (NETs) in cystic fibrosis. Front Cell Infect Microbiol. 2017;7:104. doi: 10.3389/fcimb.2017.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao Y., Lin W., Wen J., Chen G. Clinical and pathological characteristics of 2019 novel coronavirus disease ( COVID-19 ): a systematic reviews. medRxiv. 2020 doi: 10.1101/2020.02.20.20025601. [DOI] [Google Scholar]
- 29.TaŞtan C., Yurtsever B., Sir KarakuŞ G., Dİlek KanÇaĞi D., Demİr S., Abanuz S. SARS-CoV-2 isolation and propagation from Turkish COVID-19 patients. Turk J Biol. 2020;44:192–202. doi: 10.3906/biy-2004-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoppe B.R., Lassen E.D. Vol. 40. Iowa State Univ Vet; 1978. Blood smears and the use of Wright’ s stain; pp. 113–116.https://lib.dr.iastate.edu/iowastate_veterinarian [Google Scholar]
- 31.Fiel S.B., Fuchs H.J., Johnson C., Gonda I., Clark A.R. Comparison of three jet nebulizer aerosol delivery systems used to administer recombinant human DNase I to patients with cystic fibrosis. Chest. 1995;108(1):153–156. doi: 10.1378/chest.108.1.153. [DOI] [PubMed] [Google Scholar]
- 32.Papayannopoulos V., Staab D., Zychlinsky A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving dnase therapy. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci. 2020;16(10):1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratjen F., Paul K., Van Koningsbruggen S., Breitenstein S., Rietschel E., Nikolaizik W. DNA concentrations in BAL fluid of cystic fibrosis patients with early lung disease: influence of treatment with dornase alpha. Pediatr Pulmonol. 2005;39(1):1–4. doi: 10.1002/ppul.20134. [DOI] [PubMed] [Google Scholar]
- 35.Jiménez-Alcázar M., Rangaswamy C., Panda R., Bitterling J., Simsek Y.J., Long A.T. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science (New York, N.Y.) 2017;358(6367):1202–1206. doi: 10.1126/science.aam8897. [DOI] [PubMed] [Google Scholar]
- 36.Yang C., Montgomery M. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD001127.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan M.A., Ali Z.S., Sweezey N., Grasemann H., Palaniyar N. Progression of cystic fibrosis lung disease from childhood to adulthood : neutrophils, neutrophil extracellular trap (NET) formation, and NET degradation. Genes. 2019;10(3):183. doi: 10.3390/genes10030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowen K.L. Erroneous leukocyte counts and cold agglutinins. Lab Med. 1997;28:247–250. doi: 10.1093/labmed/28.4.247. [DOI] [Google Scholar]
- 39.Jeffery U. Neutrophil extracellular traps in canine immune-mediated hemolytic anemia. ProQuest Diss Theses. 2016:263. https://search.proquest.com/docview/1845054372?accountid=26646%0Ahttp://link.periodicos.capes.gov.br/sfxlcl41?url_ver=Z39.88-2004&rft_val_fmt=info:ofi/fmt:kev:mtx:dissertation&genre=dissertations+%26+theses&sid=ProQ:ProQuest+Dissertations+%26+Theses+Globa [Google Scholar]
- 40.Konstan M.W., Ratjen F. Effect of dornase alfa on in fl ammation and lung function: potential role in the early treatment of cystic fibrosis. J Cyst Fibros. 2012;11:78–83. doi: 10.1016/j.jcf.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombo C., Burgel P.R., Gartner S., van Koningsbruggen-Rietschel S., Naehrlich L., Sermet-Gaudelus I. Impact of COVID-19 on people with cystic fibrosis. Lancet Respir Med. 2020;8(5):e35–e36. doi: 10.1016/s2213-2600(20)30177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIG. S1. Assessing TCID50 of SARS-CoV-2.
FIG. S2. In vitro clearance of NET formation by dornase alfa.
Data Availability Statement
The patient data used to support the findings of the study are available from the corresponding author upon request.