Abstract
The COVID-19 pandemic has magnified U.S. health disparities. Though disparities in COVID-19 hospitalization by race-ethnicity are large, disparities by income and education have not been studied. Using an index based on preexisting health conditions and age, we estimate disparities in vulnerability to hospitalization from COVID-19 by income, education, and race-ethnicity for U.S. adults. The index uses estimates of health condition and age effects on hospitalization for respiratory distress prior to the pandemic validated on COVID-19 hospitalizations. We find vulnerability arising from preexisting conditions is nearly three times higher for bottom versus top income quartile adults and 60 % higher for those with a high-school degree relative to a college degree. Though non-Hispanic Blacks are more vulnerable than non-Hispanic Whites at comparable ages, among all adults the groups are equally vulnerable because non-Hispanic Blacks are younger. Hispanics are the least vulnerable. Results suggest that income and education disparities in hospitalization are likely large and should be examined directly to further understand the unequal impact of the pandemic.
Keywords: COVID-19, Health disparities, Preexisting health conditions
1. Introduction
The COVID-19 pandemic is not the great equalizer. Vulnerability to COVID-19 based on preexisting health conditions collides with long-standing U.S. health disparities by income, education, and race-ethnicity, especially in midlife (Azar et al., 2020; Case & Deaton, 2015; Center for Disease Control & Prevention, 2020a; Cummings et al., 2020; Hertz, Unger, Cornell, & Saunders, 2005; Hicken, Lee, Morenoff, House, & Williams, 2013; National Center for Health Statistics, 2019; Petrilli et al., 2020; Richardson et al., 2020). Blacks and socioeconomically disadvantaged groups are more likely to have at least one health condition that increases vulnerability to COVID-19 (Adams, Grandpre, & Katz, 2020; Raifman & Raifman, 2020; Selden & Berdahl, 2020). COVID-19 hospitalization rates are higher for Blacks and Hispanics than non-Hispanic Whites (Center for Disease Control & Prevention, 2020b) but data on hospitalization by education and household income are limited and not available at the national level.
This brief provides U.S. nationally representative estimates of disparities in vulnerability to severe complications from COVID-19 arising from preexisting conditions across socioeconomic status and race-ethnicity. To translate disparities in preexisting health conditions into vulnerability to hospitalization from COVID-19, we use estimates of the effects of health conditions and age on the likelihood of hospitalization for respiratory conditions before the COVID-19 pandemic validated against patients hospitalized due to COVID-19 (DeCaprio et al., 2020). We combine these estimated effects with data on the prevalence of preexisting health conditions reported in the Panel Study of Income Dynamics (PSID) for nationally representative estimates of disparities in vulnerability to hospitalization for COVID-19 arising from preexisting conditions for U.S. adults.
Individual-level information on socioeconomic characteristics in the PSID expands knowledge of unequal impacts of COVID-19 across education and income beyond studies that used aggregate census data to infer COVID-19 disparities by socioeconomic status (Azar et al., 2020; Price-Haywood, Burton, Fort, & Seonane, 2020). Our results complement existing work on race-ethnic disparities in vulnerability to COVID-19. We show that gaps in vulnerability by socioeconomic status and race-ethnicity emerge early in life when they may intersect with disparities in exposure through work (Mongey, Pilossoph, & Weinberg, 2020).
2. Study data and methods
2.1. Panel Study of Income Dynamics
The PSID is a U.S. national longitudinal survey that began in 1968. The original sample included oversamples of Black and low-income families. With the later addition of samples of more recent immigrants, the 2017 wave includes 26,455 individuals representative of the national population when weighted (PSID, 2019). We use the 2017 data on adults ages 25 and older, a sample of 13,831 household heads and spouses/cohabiting partners. Observations with missing data on age, educational attainment, household income, race-ethnicity, or preexisting health conditions were dropped for an analytic sample size of 13,150.
Education is categorized as no more than 12 years (high school/GED or less), 13–15 years (some college), and at least 16 years (BA degree or more). For household income we classify each adult according to the income quartile of their household (<$29,096; $29,096-$56,760; $56,761-$101,820; >$56,761 - $101,820 in 2016 dollars). Race-ethnicity is coded as Hispanic, non-Hispanic Black [NH Black], non-Hispanic White [NH White], and non-Hispanic other. Sample size is too small to report estimates for non-Hispanic other race. Estimates of income (McGonagle, Schoeni, Sastry, & Freedman, 2012) and the distribution of gender, age, education, and race-ethnicity from the PSID (Appendix Tables A-1 and A-2) match estimates from the U.S. survey used for official poverty and income data.
The PSID asks whether a person was ever told by a health professional they have health conditions including asthma, diabetes, heart disease, heart attack, hypertension, lung disease, neurological conditions, cancer, stroke, and kidney disease. We create an indicator of severe obesity (BMI ≥ 40) from reported height and weight. The PSID also asks whether respondents were hospitalized in the previous year. Appendix B reports question wording and how PSID estimates of health condition prevalence compare to other national data sources.
2.2. Relative vulnerability index
To translate differences in health conditions and age into predictions about the likelihood of serious complications from COVID-19 requires information on the size of effects of complications from different preexisting conditions and on the interactions of these effects with age, but evidence on the size of the effects of individual preexisting conditions on severe illness from COVID-19 is limited (Center for Disease Control & Prevention, 2020c). We use estimated effects of preexisting conditions and age from a model, developed by DeCaprio et al. (2020), predicting pre-pandemic in-patient hospitalizations associated with acute respiratory distress syndrome, pneumonia (not caused by tuberculosis), inflenza, acute bronchitis and other upper respiratory infections. Based on medical claims for over 2 million persons, the model uses multivariable logistic regression to predict hospitalizations from these respiratory infections with individual-level data on preexisting health conditions and recent hospitalization (risk factors), gender, and age. The model was validated on approximately 14,000 patients hospitalized due to COVID-19. We apply the coefficient estimates from the regression to the PSID data to create for each sample member a value for the relative vulnerability index, , which is the odds that individual i has severe illness relative to that for a 30-year-old female with no risk factors. Details of the method are in Appendix B.
The index connects individuals’ age and health conditions to an externally validated measure of the risk of hospitalization for severe complications of COVID-19. The index provides a more complete measure of vulnerability than counts of conditions because the index allows certain conditions to increase vulnerability to COVID-19 more than others and for these effects to vary with age (Appendix Fig. B-2).
2.3. Statistical analyses
The analysis describes disparities in the prevalence of risk factors to inform the analysis of group differences in relative vulnerability to severe complications. We calculate the prevalence of each risk factor by age, education, income, and race-ethnicity. We test whether differences are statistically significant from the base category (age 25−44; BA or more; top income quartile; NH White). We tabulate the percent of the population with 0, 1, 2 and 3+ risk factors by age group and by age group within education, income quartile, and race-ethnicity.
Second, we calculate median values of the relative vulnerability index by age group and number of risk factors and by age group within education, income quartile, and race-ethnicity. Analyses use 2017 PSID cross-sectional weights.
3. Study results
3.1. Disparities in health-related risk factors
Table 1 shows the unequal distribution of health-related risk factors for COVID-19 by educational attainment, income, and race-ethnicity. Adults with no more than a high school education have higher rates for almost all risk factors (except cancer and chronic kidney disease) compared to adults with a college degree. Adults in the bottom quartile of household income have higher prevalence for every risk factor compared to those in the top quartile. Compared to NH Whites, NH Blacks have higher prevalence of most risk factors, with nearly all of these differences being statistically significant. Prevalence rates for most risk factors are the same or lower for Hispanics than NH Whites (or NH Blacks), except for diabetes. Differences by education and income in nearly all conditions emerge in early adulthood (Appendix Tables C-2 and C-3).
Table 1.
Prevalence (%) of Health-Related Risk Factors by Age, Education, Income, and, Race-Ethnicity.
| Age |
Education |
Household Income |
Race-ethnicity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 25−44 | 45−64 | 65+ | HS or less | BA or more | Bottom Quartile | Top Quartile | NH White | NH Black | Hispanic | |
| Risk factors: | |||||||||||
| Hypertension | 33 | 14 | 35*** | 59*** | 39*** | 26 | 46*** | 25 | 33 | 44*** | 23*** |
| Diabetes | 12 | 4 | 14*** | 23*** | 15*** | 9 | 18*** | 8 | 11 | 16*** | 14* |
| Asthma | 11 | 12 | 10** | 9*** | 11* | 10 | 15*** | 9 | 11 | 13** | 9** |
| Hospitalization | 10 | 7 | 9** | 17*** | 12*** | 7 | 18*** | 6 | 10 | 12* | 9 |
| Cancer | 9 | 2 | 7*** | 21*** | 9 | 9 | 11** | 8 | 10 | 6*** | 5*** |
| Lung disease | 6 | 3 | 6*** | 11*** | 9*** | 3 | 12*** | 2 | 6 | 7 | 3*** |
| Heart disease | 6 | 1 | 5*** | 14*** | 7*** | 4 | 11*** | 3 | 6 | 7 | 2*** |
| Severe obesity | 5 | 5 | 6 | 4* | 6*** | 3 | 7*** | 3 | 4 | 9*** | 5 |
| Heart attack | 4 | 1 | 4*** | 10*** | 6*** | 2 | 7*** | 2 | 5 | 5 | 2*** |
| Stroke | 3 | 1 | 3*** | 8*** | 4*** | 2 | 7*** | 2 | 3 | 6*** | 2* |
| Neurological conditions | 3 | 1 | 2*** | 5*** | 4*** | 1 | 6*** | 0 | 3 | 2** | 2*** |
| Chronic Kidney Disorder | 1 | <1 | 1** | 1*** | 1 | 1 | 1 | 0 | 1 | 1 | <1* |
| Number of Risk Factors: | |||||||||||
| 0 | 47 % | 64 % | 46 % | 20 % | 42 % | 54 % | 30 % | 56 % | 45 % | 40 % | 57 % |
| 1 | 27 % | 25 % | 28 % | 29 % | 26 % | 28 % | 27 % | 28 % | 28 % | 26 % | 24 % |
| 2 | 13 % | 7% | 14 % | 22 % | 15 % | 11 % | 19 % | 10 % | 13 % | 16 % | 12 % |
| 3+ | 13 % | 3% | 12 % | 28 % | 17 % | 7% | 24 % | 6% | 13 % | 18 % | 7% |
| N | 13,150 | 6,808 | 4,392 | 1,950 | 5,262 | 4,406 | 2,550 | 3,774 | 7,082 | 4,158 | 1,464 |
Data Source: 2017 Wave, PSID. Sample: PSID heads and spouses, 25+ years old. Weights: PSID individual cross-section weights. Notes: Household income quartiles calculated with family weights. Asterisks (*) are for tests of risk factors of a subgroup being significantly different from base group: * P-value ≤ 0.10; ** P-value ≤ 0.05; *** P-value ≤ 0.01. Base groups are 25−44, BA or more, top income quartile, NH White.
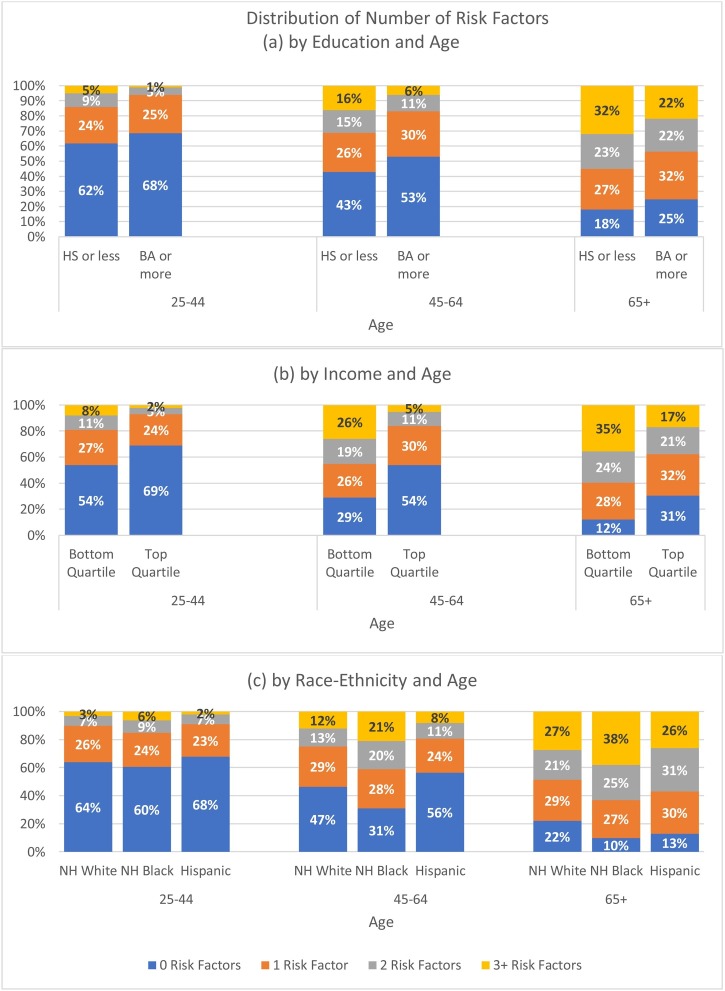
There are also socioeconomic status and race-ethnic disparities in the number of conditions. Adults with 12 years of schooling or less are more than twice as likely to have 3+ risk factors compared those with a college degree. Adults in the bottom household income quartile are four times more likely to have 3+ risk factors compared to those in the top quartile. NH Blacks are more likely to have 3+ risk factors compared to NH Whites while Hispanics are less likely to have 3+ risk factors. Risk factors accumulate at earlier ages for those with 12 years of schooling or less, those in the bottom quartile of income, and for NH Blacks (Fig. 1 (a) – (c)). Fewer adults age 45−64 with household incomes in the top income quartile have 2+ conditions (16 %) than those age 25−44 in the bottom income quartile (19 %), and fewer adults in the oldest age group with household incomes in the top quartile have 2+ conditions (38 %) than those in middle age (45−64) with incomes in the bottom quartile (45 %). These differences are statistically significant.
Fig. 1.
Distribution of Number of Risk Factorsby Education and Age, Income and Age, and Race-ethnicity and Age.
Notes: Data Source: 2017 Wave, PSID. Sample: PSID heads and spouses, 25 years and older. Weights: PSID individual cross-section weights.
3.2. Disparities in relative vulnerability to severe illness from COVID-19
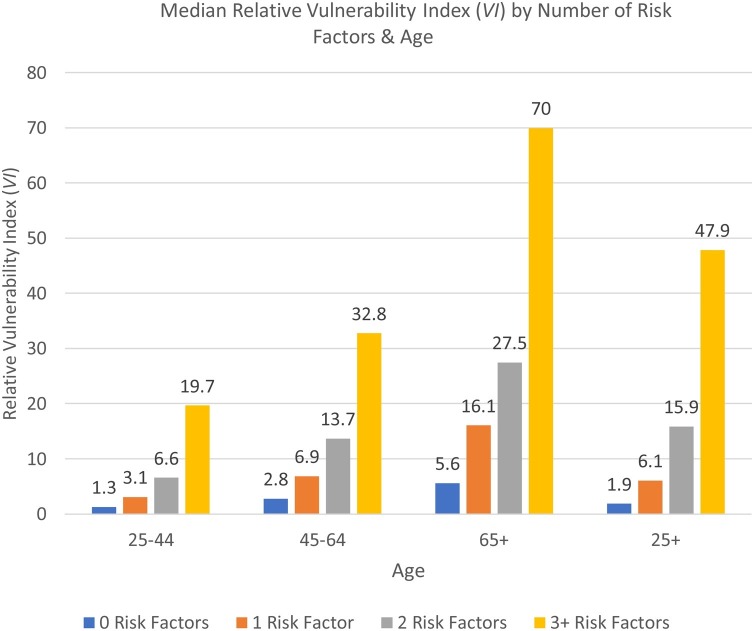
Relative vulnerability varies substantially by number of risk factors and by age (Fig. 2 ), indicating that counts of preexisting conditions show only part of the picture of disparities in COVID-19 complications. The median relative vulnerability is 4.08 (not shown). Among all ages, those with no risk factors are 1.9 times more vulnerable compared to a 30 year-old female with no risk factors but the median risk of individuals with 3+ risk factors is 47.9 times higher. Age increases vulnerability even before age 65; adults in middle age with 3+ risk factors are more vulnerability (32.8) than older adults with two risk factors (27.5).
Fig. 2.
Median Relative Vulnerability Index (VI) by Number of Risk Factors & Age.
Notes: Data Source: 2017 Wave, PSID. Sample: PSID heads and spouses, 25 years and older. Weights: PSID individual cross-section weights.
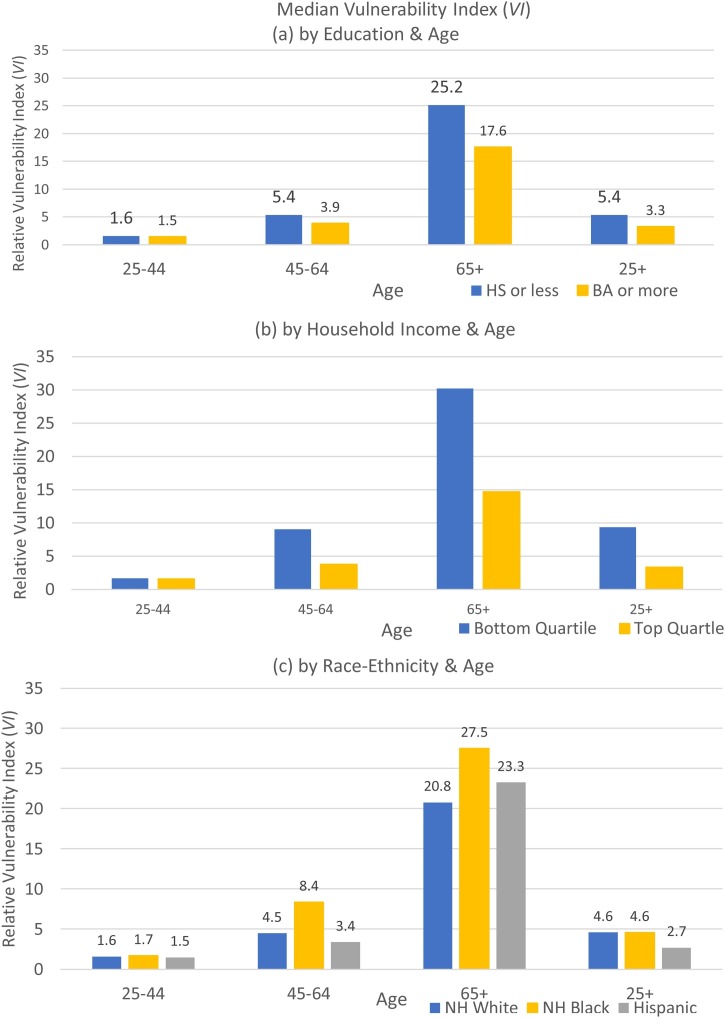
Disparities in vulnerability by socioeconomic status are very large. On average, relative vulnerability is 60 % higher for those with high school or less (5.4) than for those with a college degree (3.3). This gap emerges in midlife (Fig. 3 (a)). Among all adults, there is nearly a 3-fold difference in relative vulnerability by income, with those in the bottom income quartile having 9.4 higher odds of severe illness compared to the reference person, while the odds are only 3.4 times higher for those in the top income quartile (Fig. 3(b)). This gap by income emerges in midlife. Education and income gaps in specific preexisting conditions are magnified by the vulnerability index, which reflects the greater impact on hospitalization of diseases like hypertension, severe obesity, and lung disease – more common among those with low levels of schooling and income – and the older ages of these groups (see Table 1 and Appendix Fig. B-2).
Fig. 3.
Median Vulnerability Index (VI) by Education & Age, Income and Age, and Race-ethnicity and Age.
Notes: Data Source: 2017 Wave, PSID. Sample: PSID heads and spouses, 25 years and older. Weights: PSID individual cross-section weights.
By age 45, sizeable differences in relative vulnerability emerge between NH Blacks and NH Whites. These gaps remain large at older ages (Fig. 3(c)). Hispanics have lower relative vulnerability at younger ages and in midlife. Across the whole sample, the difference in relative vulnerability between NH Blacks and NH Whites is muted because those who are age 65 and older are a higher percentage of the NH White than NH Black population (Appendix Table A-3). Hispanics have lower relative vulnerability at younger ages and they are younger than Blacks and Whites (Appendix Table A-3). Both factors contribute to lower vulnerability for Hispanics than NH Whites among all adults.
3.3. Comparisons with COVID-19 hospitalizations
Hospitalization is influenced by two channels: exposure and susceptibility to infection and its complications. The CDC warns that susceptibility to complications depends heavily on preexisting conditions, but exposure to COVID-19 also depends on other factors. Separating these channels empirically is beyond the scope of available data. Instead we compare the model’s predictions for disparities in vulnerability with disparities among those who have been hospitalized with COVID-19. The comparison sheds light on the likely channel through which disparities in hospitalizations may operate.
Similar to Selden and Berdahl (2020), we conclude that NH Black – NH White and Hispanic – NH White disparities in hospitalization from COVID-19 (Center for Disease Control & Prevention, 2020b) are larger than would be predicted by preexisting health conditions. Among those age 65+, NH Blacks are 3.8 times more likely than NH Whites to be hospitalized with COVID-19 (Center for Disease Control & Prevention, 2020b) but Fig. 3c shows that they are only 32 % more vulnerable to complications requiring hospitalization based on pre-existing health conditions. Preexisting health conditions and age make Hispanics less vulnerable than NH Whites. Differences in exposure either through family members or work likely explain the large race-ethnic disparities in hospitalizations (Selden & Berdahl, 2020).
Data on education and household income are not available in hospital surveillance data. Yet Fig. 3a and 3b shows that the socioeconomic disparities in preexisting conditions would predict even larger disparities in hospitalizations than we find by race-ethnicity and that these disparities emerge in midlife. Appendix Table C-3 shows that in midlife, disparities by education and income exist even within race-ethnic groups. Disparities in vulnerability from preexisting health conditions are likely to be compounded in actual COVID-19 hospitalizations because low-income workers and those without a college degree are less likely to be able to socially distance at work or work from home (Mongey et al., 2020). Because education and income are not collected in the surveillance data, we must rely on other methods, such as ours, to estimate disparities in likely outcomes.
4. Conclusion
Using a model validated on COVID-19 hospitalizations, we show large disparities across education and income in the prevalence of conditions associated with adverse outcomes, and in the overall risk of severe complications. These disparities emerge early in life, prior to age 65. Our approach highlights how new models of vulnerability to COVID-19 can be combined with existing nationally representative data to illuminate the likely effects of the pandemic, especially when surveillance data are limited.
The large socioeconomic disadvantages in COVID-19 vulnerability we document may exacerbate the already higher mortality of those without a college education that Case and Deaton (2015) call deaths of despair. A strength of our study is the application of a model of health condition effects validated on COVID-19 hospitalizations to individual-level information on health, socioeconomic status, and race-ethnicity measured in the same source. But the large differences we find may still understate disparities in vulnerability from preexisting health conditions because disadvantaged populations have higher rates of undiagnosed diseases, socioeconomic status and race-ethnicity affect hospitalization regardless of preexisting conditions, and among those diagnosed with a disease, effective control for some diseases is lower for disadvantaged populations (Barcellos, Goldman, & Smith, 2012; Chatterji, Joo, & Lahiri, 2012; Chobanian, 2009; Lowcock, Rosella, Foisy, McGeer, & Crowcroft, 2012; Pleasants, Riley, & Mannino, 2016; Smith, 2007). Future research should examine the social factors that magnify disparities in risk from COVID-19.
Acknowledgments
This paper was prepared with support, in part, from the Aging Studies Institute and the Center for Aging and Policy Studies at Syracuse University which receives core support (P30AG066583) from the National Institute on Aging, the Duke Center for Population Health and Aging, which receives core support (P30AG034424) from the National Institute on Aging, and by the California Center for Population Research at the University of California at Los Angeles, which receives core support (P2C-HD041022) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The collection of data used in this study was partly supported by the National Institutes of Health under grant number R01 HD069609 and R01 AG040213, and the National Science Foundation under award numbers SES 1157698 and 1623684.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.rssm.2020.100553.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adams M.L., Grandpre J., Katz D.L. medRxiv; 2020. Updated estimates of comorbidities associated with risk for COVID-19 complications based on US data. 2020.2005.2002.20088781. [DOI] [Google Scholar]
- Azar K.M.J., Shen Z., Romanelli R.J., Lockhart S.H., Smits K., Robinson S. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Affairs. 2020;39(7):1253–1262. doi: 10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- Barcellos S.H., Goldman D.P., Smith J.P. Undiagnosed disease, especially diabetes, casts doubt on some of reported health ‘advantage’ of recent Mexican immigrants. Health Affairs. 2012;31:2727–2737. doi: 10.1377/hlthaff.2011.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A., Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. PNAS. 2015;112:15078–15083. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention . 2020. COVID-19 laboratory-confirmed hospitalization.https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html Preliminary data as of August 15, 2020. [Google Scholar]
- Center for Disease Control and Prevention . 2020. COVIDView, a weekly surveillance summary of U.S. COVID-19 activity, week 33 ending.https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html August 15, 2020. [Google Scholar]
- Center for Disease Control and Prevention . 2020. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html Accessed 21 August 2020. [PubMed] [Google Scholar]
- Chatterji P., Joo H., Lahiri K. Racial/ethnic- and education-related Disparities in the Control of Risk Factors for Cardiovascular disease among individuals with diabetes. Diabetes Care. 2012;35:305. doi: 10.2337/dc11-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian A.V. The hypertension paradox — More uncontrolled disease despite improved therapy. NEJM. 2009;361:878–887. doi: 10.1056/NEJMsa0903829. [DOI] [PubMed] [Google Scholar]
- Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. The Lancet. 2020;395(10239):P1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio D., Gartner J., McCall C.J., Burgess T., Garcia K., Kothari S. Vol. 2003. arXiv; 2020. p. 2003.07347. (Building a COVID-19 vulnerability index). [Google Scholar]
- Hertz R.P., Unger A.N., Cornell J.A., Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Archives of Internal Medicine. 2005;165:2098–2104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- Hicken M.T., Lee H., Morenoff J.S., House J.S., Williams D.R. Racial/ethnic disparities in hypertension prevalence: Reconsidering the role of chronic stress. AJPH. 2013;104:117–123. doi: 10.2105/AJPH.2013.301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowcock E.C., Rosella L.C., Foisy J., McGeer A., Crowcroft N. The social determinants of health and pandemic H1N1 2009 influenza severity. AJPH. 2012;102:e51–e58. doi: 10.2105/AJPH.2012.300814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle K.A., Schoeni R.F., Sastry N., Freedman V.A. The Panel Study of Income Dynamics: Overview, recent innovations, and potential for life course research. Longitudinal and Life Course Studies. 2012;3(2) doi: 10.14301/llcs.v3i2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongey S., Pilossoph L., Weinberg A. National bureau of economic research working paper series No. 27085. 2020. Which workers bear the burden of social distancing policies? [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics . National Center for Health Statistics; US: 2019. Health, United States, 2018. [PubMed] [Google Scholar]
- Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasants R., Riley I., Mannino D. Defining and targeting health disparities in chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease. 2016;11:2475–2496. doi: 10.2147/COPD.S79077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Haywood E.G., Burton J., Fort D., Seonane L. Hospitalization and mortality among black patients and white patients with COVID-19. NEJM. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PSID . Institute for Social Research, University of Michigan; 2019. PSID main interview user manual: Release 2019. [Google Scholar]
- Raifman M.A., Raifman J.R. Disparities in the population at risk of severe illness from COVID-19 by race/ethnicity and income. American Journal of Preventive Medicine. 2020;59(1):P137–139. doi: 10.1016/j.amepre.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden T.M., Berdahl T.A. COVID-19 and racial/ethnic disparities in health risk, employment, and household composition. Health Affairs. 2020 doi: 10.1377/hlthaff.2020.00897. [DOI] [PubMed] [Google Scholar]
- Smith J.P. Nature and causes of trends in male diabetes prevalence, undiagnosed diabetes, and the socioeconomic status health gradient. PNAS. 2007;104:13225–13231. doi: 10.1073/pnas.0611234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.