Abstract
Human genetic variation in the gene CACNA1C, which codes for the alpha-1c subunit of Cav1.2 L-type calcium channels (LTCCs), has been broadly associated with enhanced risk for neuropsychiatric disorders including major depression, bipolar and schizophrenia. Little is known about the specific neural circuits through which CACNA1C and Cav1.2 LTCCs impact disease etiology. However, serotonin (5-HT) neurotransmission has been consistently implicated in these neuropsychiatric disorders and Cav1.2 LTCCs may influence 5-HT neuron activity during relevant behavioral states such as stress. We utilized a temporally controlled and 5-HT neuron specific Cacna1c knockout mouse model to assess stress-coping behavior using the forced swim test and dorsal raphe (DR) 5-HT neuron Fos activation. Furthermore, we assessed 5-HT1A receptor function and feedback inhibition of the DR following administration of the 5-HT1A antagonist WAY-100635. We find that 5-HT neuron Cacna1c knockout disrupts active-coping behavior in the forced swim test and that this behavioral effect is rescued by blocking 5-HT1A receptors. Moreover, Cacna1c knockout mice display enhanced Fos expression in caudal DR 5-HT neurons and an enhanced response to a 5-HT1A receptor antagonist in rostral DR 5-HT neurons, indicating that loss of Cacna1c disrupts both 5-HT neuron activation and 5-HT1A dependent feedback inhibition across the caudal to rostral DR. Collectively, these results reveal an important role for 5-HT neuron Cav1.2 LTCCs in stress-coping behavior and 5-HT1A receptor function. This suggests that alterations in CACNA1C function or expression could influence the development or treatment of neuropsychiatric disorder through serotonergic mechanisms.
Keywords: Serotonin, Dorsal Raphe, Cacna1c, L-type calcium channels, Cav1.2, psychiatric disorders
1. Introduction
Polymorphisms in the gene CACNA1C are strongly and consistently associated with increased risk for the development of neuropsychiatric conditions including schizophrenia, bipolar disorder and major depression (Green et al. 2010; Wray et al. 2012; Ferreira et al. 2008; Cross-Disorder Group of the Psychiatric Genomics Consortium 2013)]. CACNA1C codes for the pore-forming alpha-1c subunit of Cav1.2 voltage-gated L-type calcium channels (LTCCs), which are expressed in the majority of CNS neurons (Sinnegger-Brauns 2009). Cav1.2 LTCCs open with fast kinetics following neuronal excitation above action potential threshold to dramatically increase calcium influx, yet have exceptionally slow inactivation kinetics, and therefore Cav1.2 LTCCs play a predominant role in the coupling neuronal excitation to long-term changes in calcium-dependent gene expression and functional activity (Wheeler et al. 2012). As the most consistently observed risk-associated polymorphism of CACNA1C is in a non-coding region, regionally specific increases or decreases in overall Cav1.2 LTCC expression are likely responsible for its behavioral impact (Heyes et al. 2015). The identification of specific neural circuits through which CACNA1C and Cav1.2 LTCCs impact behaviors that are relevant to the etiology and treatment of neuropsychiatric conditions would greatly inform the development of novel therapeutics.
Forebrain projecting serotonin (5-hydroxytryptamine; 5-HT) neurons have been consistently implicated in behaviors relevant to the etiology of neuropsychiatric conditions including the response to stress (Michelsen et al. 2007). The largest group of forebrain projecting 5-HT neurons arises from the dorsal raphe nucleus (DR) of the midbrain and prepontine hindbrain, and the role of CACNA1C and the Cav1.2 LTCCs expressed on these neurons has yet to be explored. Regionally specific innervation of the forebrain arises from distinct structural and functional subgroups of DR 5-HT neurons separated along the rostral (cell-group B7) to caudal (cell-group B6) axis of the DR (Commons 2016). Activation of these 5-HT neuron subgroups, as assessed by expression of the immediately early gene product Fos, changes under behavioral states such as acute stress. Since different subgroups of 5-HT neurons preferentially innervate different forebrain targets, this differential activation has the potential to generate region-specific increases and decreases in extracellular serotonin (Kirby and Lucki, 1997). Furthermore, there is evidence that feedback inhibitory mechanisms mediated by 5-HT1A receptors may help shape the selective activation of different subgroups of 5-HT neurons (Sperling and Commons, 2011; Bang et al., 2012). Due to these features of selective activation and their topographic patterns of projections, there is the emerging understanding that subgroups of serotonin neurons may be functionally distinct.
Cav1.2 LTCCs could potentially influence the regional pattern of DR 5-HT neuron activation, in part because they have been implicated in feedback inhibition. Specifically, somatodendritic release of 5-HT containing vesicles within the DR is dependent on calcium influx through LTCCs. This local release of serotonin has the capacity to engage 5-HT1A receptors and promote feedback inhibition (Chazal and Ralston 1987; Colgan et al. 2012). It is also possible that different groups of 5-HT neurons may be differentially sensitive to Cav1.2 LTCC function because of developmental differences between different groups of 5-HT neurons. The role of LTCC in regulating 5-HT neuron function may be particularly relevant during stress, since LTCC are prominent targets of corticosteroid hormones in the limbic forebrain (Joels and Karst, 2012). Taken together, these observations raise the possibility that Cav1.2 LTCCs could influence both overall activation and 5-HT1A dependent feedback inhibition of DR 5-HT neurons during behavioral states such as acute stress and these effects may be subregionally organized.
Importantly, 5-HT neurons express multiple subtypes of LTCCs and whether CACNA1C, encoding the Cav1.2 isoform, specifically contributes to DR 5-HT neuron function has not been directly assessed. We have recently studied aspects of 5-HT system structure and function in a mouse model of the neurodevelopmental disorder Timothy Syndrome (TS2-neo) that contains a brain-wide gain of function mutation in Cacna1c resulting in delayed Cav1.2 LTCC inactivation (Bader et al. 2011). Intriguingly, TS2-neo mice exhibit an enhanced active coping response during acute stress, increased Fos expression in 5-HT neurons of the caudal DR and enhanced 5-HT1A dependent feedback-inhibition of the rostral DR (Ehlinger and Commons 2017). These results raise the possibility that 5-HT neuron Cacna1c could directly regulate both stress coping behavior and the activation of ascending 5-HT neuron subgroups. However, Cacna1c might also act indirectly within efferent brain regions to modify both behavior and 5-HT neuron function (Dao et al 2010; Moosmang et al. 2005). Thus, whether Cacna1c acts directly on the DR 5-HT neuron system remains unknown.
In the present study, we assessed the direct impact of Cacna1c on stress coping behavior, DRN 5-HT neuron immediate early gene expression and 5-HT1A dependent feedback inhibition using a conditional and cell-type specific Cacna1c knockout mouse model. This study reveals for the first time that 5-HT neuron Cacna1c regulates the activation of DR 5-HT neuron subpopulations and the neurobehavioral response to acute stress.
2. Materials and Methods
2.1. Mice
All procedures involving mice were approved by the Boston Children’s Hospital Institutional Animal Care and Use Committee, and followed the National Institutes of Health guide for the care and use of laboratory animals and the additional institutional guidelines of Boston Children’s Hospital and Harvard Medical School.
Original breeding pairs were purchased from Jackson Laboratory (Jax; Bar Harbor, ME). All transgenic and mutant strains were initially crossed to Jax #000664 C57BL/6J mice, including Jax #007914 homozygous B6;Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (Ai14tdTomato), Jax #016584 hemizygous STOCK Tg(Tph2-icre/ERT2)6Gloss/J (Tph2iCre), and Jax #024714 homozygous STOCK Cacna1ctm3Hfm/J (Cacna1cflox/flox). Initial crosses between these strains yielded breeders used for the generation of mice for subsequent experiments. Specifically, the breeding of 1) mice homozygous for a Cacna1cflox/flox transmutation and hemizygous for a tamoxifen (TAM) inducible Tph2-creERT2 transgene (Tph2icre) with 2) mice homozygous for a Cacna1cflox/flox transmutation and heterozygous for the Cre-dependent tdTomato fluorescent reporter (Cacna1cflox/floxAi14tdTomato) produced all experimental and control groups used in the following experiments. Previous characterization of Cacna1cflox/flox allele shows that with cre-recombination leads to about 80% loss of dihydropuridine sensitive LTCC currents in hippocampal neurons with residual currents likely dependent on Cav1.3 (Moosemang et al., 2005).
2.2. Tamoxifen administration protocol
In our mice, TAM administration induces a recombination event to disrupt Cacna1c expression within 5-HT neurons, allowing for specificity and temporal control in TAM treated Tph2iCre mice. From postnatal day (P)35–40, mice received either 1mg of 10mg/ml TAM (Sigma-Aldrich #T5648) in sunflower oil (Sigma-Aldrich #S5007) or sunflower oil as vehicle (VEH) twice per day intraperitoneally (10 total injections), an administration protocol previously shown to exert efficient recombination in Tph2iCre mice (Weber et al. 2008; Weber et al. 2015). The two main groups of experimental mice were 1) TAM treated Tph2iCreCacna1c−/− , in which 5-HT neuron Cacna1c expression is disrupted and 2) TAM treated Cacna1c+/+ , with unaltered Cacna1c expression. Initial behavioral characterization yielded no sex differences in the examined behaviors, therefore subsequent experiments were sex balanced but underpowered to determine additional sex differences. Two additional control groups were administered VEH rather than TAM: 1) VEH-Tph2icreCacna1c+/+ mice, used to assess the impact of Tph2iCre transgene expression alone and 2) VEH-Cacna1c+/+ mice, used to assess the impact of TAM treatment alone.
2.3. Behavioral testing
Experiments began approximately 4 weeks after the final TAM or VEH injection (P68±2) to avoid potential acute neurobehavioral effects of TAM (Vogt et al. 2008) (Figure 1A). Locomotor activity was assessed using an open-field apparatus (Photobeam Activity System, San Diego Instruments, San Diego, CA). Within clear plexiglass cages (10”×19”×8”h), accumulated beam breaks in Tph2iCreCacna1c−/− (n = 13) and Cacna1c+/+ (n =15), mice were counted, including ambulatory, fine and rearing movements over three 5-minute time bins for a total of 15 minutes. Movement measures were totaled within each time bin to acquire total locomotor activity scores. The percentage of time spent in the center rectangle (5” × 9.5”) of the open-field was calculated, with less time in the center compartment indicating avoidance of the more aversive portion of the open-field.
Figure 1.
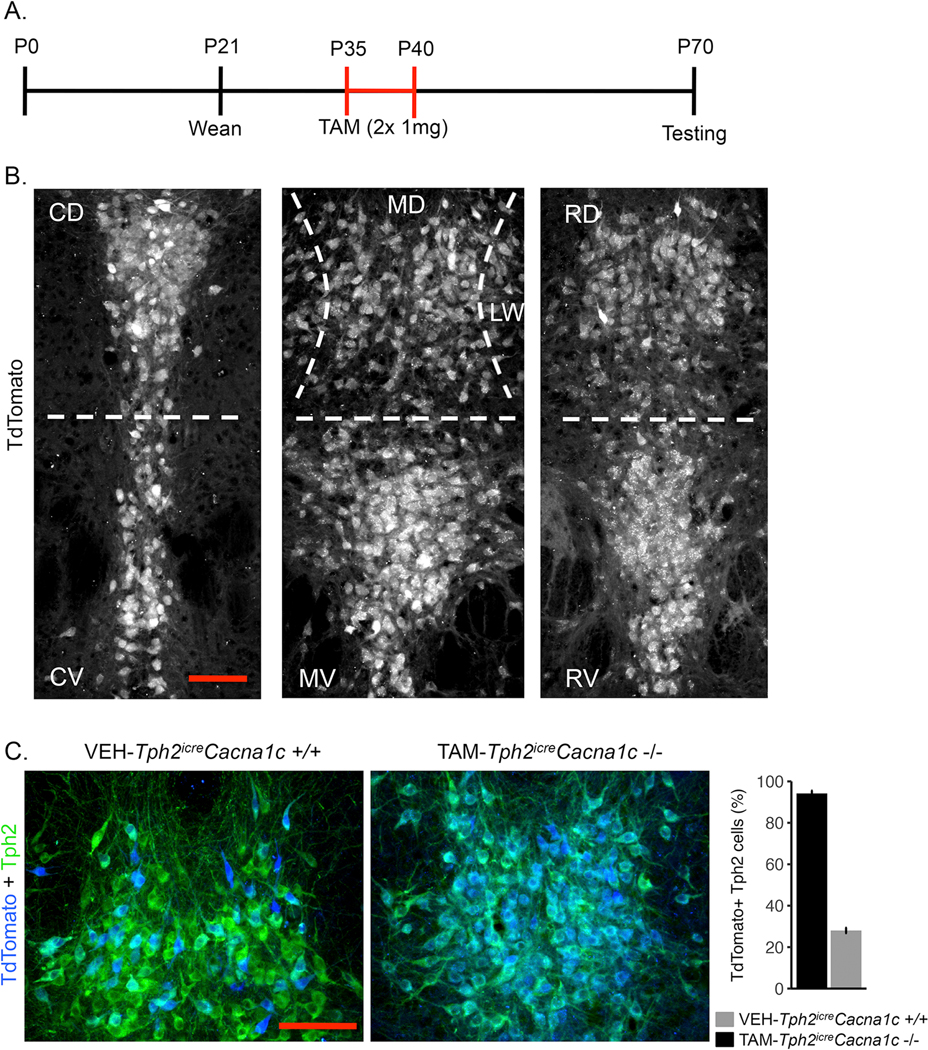
Experimental timeline and TAM-induced recombination in 5-HT neuron Cacna1c knockout mice. A) Timeline (postnatal day; P) of TAM administration and behavioral/biological testing. B) TAMinduced recombination indicated by TdTomato expression in Ai14TdTomatoTph2icreCacna1c−/− mice is exhibited throughout the caudal-rostral (left to right) and dorsal-ventral extent of the dorsal raphe nucleus. Dorsal raphe subregions abbreviated as CD = caudal-dorsal, CV = caudal-ventral, MD = medial-dorsal, MV = medial-ventral, LW = lateral wings, RD = rostral-dorsal, & RV = rostral-ventral. Scale bar (red) = 100μm. C) Left, TdTomato expression (blue) within serotonergic neurons (Tph2 immunopositive, green) of VEH treatedTph2icreCacna1c−/− compared to TAM treatedTph2icreCacna1c−/− mice. Scale bar (red) = 100μm. Right, recombination rate (percentage of TdTomato+ serotonergic neurons) following TAM administration compared to VEH administration.
A forced swim test (FST) was used to assess stress-coping behavior and for subsequent analysis of DR 5-HT neuron Fos expression. Immediately following locomotor activity testing, experimental groups were injected subcutaneously with 0.9% NaCl (Saline) and returned to their home-cage for 10-minutes. As an additional behavioral control for the effect of Tph2iCre transgene expression and TAM administration alone in the FST, a set of VEH-Tph2iCreCacna1c+/+ (n = 8) and VEH-Cacna1c+/+ (n = 13) groups were subjected to the same procedure. Mice were then placed into a cylindrical glass tank (46cm high × 20cm diameter) filled 20cm deep with 25±1° C water for 15 minutes while being videotaped for behavioral analysis, a length of time that allows for a deeper characterization of the adaptation from active (swimming; movement through the tank with coordinated movement of both hindpaws) to passive (immobility; no movement through the tank and only minor movements of hindpaws to stay afloat) coping strategy during the forced swim stress and produces a robust elevation in DR 5-HT neuron Fos activation (Commons 2008; Ehlinger and Commons 2017). Coping strategy was scored as either active or passive using the 5-second time interval sampling method, in which mice are counted as active when swimming behavior was exhibited for the majority of each 5-second interval (Cryan et al. 2005). The number of swim counts was summed into three 5-minute time bins, making a total of 60 possible swim counts per 5-minute time bin. Thus active coping and passive coping counts sum to 60, and one measure can be derived from the other. To facilitate comparison with other work where passive coping is reported, a passive coping score totaling counts over the last 4-minutes of the first 6-minutes of the FST is also reported (Crawley et al. 2007). Immediately after the forced swim, mice were removed from the tank, quickly towel dried and returned to their home cage.
To examine the role of 5-HT1A receptors, a separate group of Tph2iCreCacna1c−/− (n = 11) and Cacna1c+/+ (n = 11) mice were administered the 5-HT1A antagonist WAY-100635 (WAY; 0.4 mg/kg; Sigma Aldrich #W108) 10-minutes prior to the FST and behavior was recorded as previously described. To determine whether FST behavioral effects observed following WAY administration reflected an altered state of locomotor activity, a final group of Tph2iCreCacna1c−/− (n = 9) and Cacna1c+/+ (n = 12) mice were administered WAY (0.4 mg/kg) 10-minutes prior to placement in the open-field, with locomotor activity and percentage center time recorded as previously described.
Statistical assesment of behavior was performed in R. For active coping behavior, mixed-ANOVA with time as a repeated measure and genotype (Tph2iCre or Tph2+/+), treatment (TAM or VEH) and drug (saline or WAY) as between-subjects factors was used. The Greenhouse-Geisser correction for degrees of freedom (df) was applied following a significant (p <.05) Mauchly’s Test for Sphericity (corrected degrees of freedom denoted throughout the text by a subscript “G” prior to dfvalues). Following significant interaction with time (p < .05), ANOVA was performed within individual time bins followed by bonferroni corrected post-hoc t-tests to compare unique genotype, treatment and drug groups (significance at p < .016). For passive coping behavior, ANOVA with genotype, treatment and drug as between-subjects factors was used, followed by Bonferroni corrected post-hoc t-tests between unique groups (p < .016).
2.4. Immunofluorescence
To examine the role of Cacna1c on activation of DR 5-HT neuron subregions, a subset of the mice used in FST experiments were used for immunofluorescence experiments assessing the impact of Cacna1c knockout on expression of the immediately early gene product Fos within 5-HT neurons, as well as to assess recombination efficiency in Tph2iCre mice. Fos was not measured in unstressed mice because Fos levels tend to be very low in 5-HT neurons in the absence of stress (Commons, 2008). 120 minutes following the FST, a time-point that allows for translation of the Fos protein a subset of Tph2iCreCacna1c−/− (n = 8), Cacna1c+/+ (n = 8), VEH-Tph2iCreCacna1c+/+ (n = 7), and VEH-Cacna1c+/+ (n = 9), as well as WAY exposed Tph2iCreCacna1c−/− (n = 9) and Cacna1c+/+ (n = 10) mice were perfused intracardially with 4.0% paraformaldahyde (PFA) in 0.1 M phosphate buffer. Brains were stored in the same 4.0% PFA solution overnight then equilibrated in a solution of 30.0% sucrose in 0.1 M phosphate buffer at 4.0° C. 40 μm thick coronal sections were made through the whole brain, storing every third section from the DR in separate containers. Immunofluorescence processing was performed on floating sections. 5-HT neuron and Fos co-labeling was detected by incubating sections in primary antisera for tryptophan hydroxlase 2 (TPH2; synthesizing enzyme for 5-HT) raised in rabbit (Novus Biologicals, #NB100–74555) diluted 1:1000 and Fos raised in goat (Santa Cruz, #SC-52) diluted 1:500 in PBS-BSA-TA at room temperature for 24 hours, followed by incubation in Alexa 488 anti-rabbit and Alexa 647 anti-goat raised in donkey (Invitrogen) diluted 1:500 at room temperature for 90 minutes. Sections were rinsed in 0.05 M phosphate buffer, mounted, dried, and cover-slipped with a glycerol-based mounting medium.
Every 3rd section throughout the rostral-caudal and dorsal-ventral extent of the DR (Paxinos and Franklin 2001) was imaged using an Olympus IX-81 spinning disk confocal fluorescence microscope with 20x objective, Hamamatsu Orca ER camera, Slidebook software (3i) and filter cubes selective for Alexa 488, tdtomato, and Alexa 647. For each channel of every 1344 × 1024 pixel (216 × 165μm) image, a 20 μm z-stack with 1 μm step-size was obtained while keeping imaging parameters identical for all samples. Max projection images were made for each channel.
Recombination efficiency was assessed in a subset of both TAM exposed Tph2iCreCacna1c−/− mice (n =6) and VEH exposed VEH-Tph2iCreCacna1c+/+ (n = 5) mice that were also heterozygous for a Cre-dependent fluorescent reporter (Ai14tdTomato). After observing robust TdTomato expression in all rostral-caudal, dorsal-ventral, and lateral wings DRN subregions of Tph2iCreCacna1c−/− mice (Figure 1B), two 50μm × 50μm regions per mouse were selected from medial DRN sections with a high density of Tph2-positive neurons, and cells dually immunolabeled for Tph2 and tdTomato were visualized on individual and merged images of each channel in ImageJ and manually enumerated using the cell-counter plugin in FIJI (Figure 1C). The percentage of cells exhibiting recombination was calculated as the number of dually labeled cells divided by the total number of Tph2-positive cells and compared between groups using an independent samples t-test. As expected, TAM treatment induced an extremely robust Cre-mediated recombination exclusively in Tph2 neurons (Figure 1B, 1C). TdTomato expression was observed in a significantly greater percentage of Tph2 positive neurons from TAM treated Tph2iCre mice compared to VEH treated Tph2iCre mice (t[9] = 35.96, p = 4.92 × 10−11) (Figure 1C, 1D). Nonetheless, recombination was also observed in some 5-HT neurons (~28%) from VEH treated Tph2iCre mice, suggesting leakiness in this tamoxifen-inducible CreER driver line. While this observation represents a limitation of the current study, it underscores the importance of utilizing multiple control groups that either express or do no express the Tph2iCre transgene in our assessments of behavior and 5-HT neuron activation.
To quantitatively assess 5-HT neuron activation across DR subpopulations, cells dually labeled for Tph2 and Fos were quantified within 7 distinct subregions of the DR delineated across the rostral-caudal axis (3 regions: rostral, −4.16 to −4.24mm; middle, −4.36 to −4.84mm; caudal, −4.96 to −5.20), dorsal-ventral axis (2 regions: defined by characteristic separation of immunolabeled 5-HT neurons), and the lateral wings (Sperling and Commons 2011) using the cell-counter plugin in FIJI. This yielded two to three sections per region per animal, and counts were averaged to yield a single per section per region mean value for each animal. For statistical analysis, a mixed-ANOVA with within-subjects factor of DRN subregion and between-subjects factors of genotype (Tph2iCre or Tph2+/+), treatment (TAM or VEH) and drug (saline or WAY) was performed (with Greenhouse-Geisser correction for df as previously described). Following significant interaction with subregion, individual ANOVAs within DR subregion were performed and post-hoc comparisons between individual genotype, treatment and drug conditions via independent-samples t-tests using a false-discovery rate (FDR) of 5% based on the seven DR subregions examined (pFDR < .05) (Benjamani and Hochberg 1995).
3. Results
First, we examined the neurobehavioral role of 5-HT neuron Cacna1c during acute stress by exposing Tph2iCreCacna1c−/−, TAM-Cacna1c+/+ and both VEH control groups to a 15-minute FST and measuring both active and passive coping behavior. A genotype by treatment interaction (F(1,45) = 4.503, p = .039) and a trend towards a time by genotype interaction (FG(1.66, 74.76) = 3.235, p = .054) was observed on active coping behavior, with the most pronounced behavioral effect observed during the first 5-minutes of the forced swim (F(1,45) = 7.219, p = .010) (Figure 2A). Specifically, Tph2iCreCacna1c−/− mice displayed lower active coping behavior compared to Cacna1c+/+ mice during the first and second five minutes of the FST, which normalizes by the third five minutes of testing. Similarly, a genotype by treatment interaction was observed on passive coping behavior, in which Tph2iCreCacna1c−/− mice exhibited increased immobility compared to Cacna1c+/+ mice during the initial portion of the FST. In contrast, coping behavior in VEH-Tph2iCreCacna1c+/+ and VEH-Cacna1c+/+ was similar to that of Cacna1c+/+ mice, suggesting that the alterations in coping behavior were not simply a result of transgene expression or TAM exposure alone, and required the extensive recombination evident seen after TAM treatment and baseline recombination in VEH-Tph2iCreCacna1c+/+ mice was insufficient to generate an altered behavioral response. No differences in general locomotor activity were observed between Tph2iCreCacna1c−/− and Cacna1c+/+ mice in the open-field test (Figure 2B), however Tph2iCreCacna1c−/− mice spent less time in the open-field center compared to Cacna1c+/+ mice (t[26] = 2.312, p = .029), suggesting that 5-HT neuron Cacna1c knockout also impacted behavior within this comparatively less stressful context.
Figure 2.
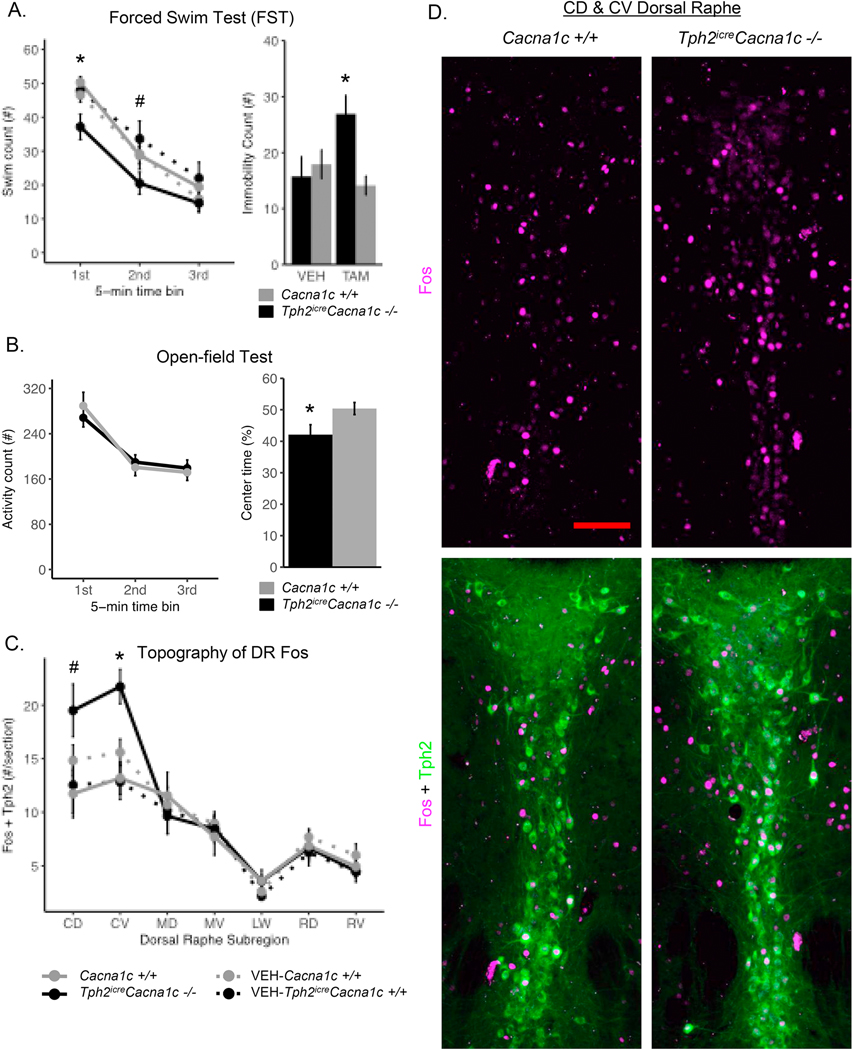
Neurobehavioral effect of 5-HT neuron Cacna1c knockout. A) Forced-swim test swim count (active-coping behavior) measured across 5-minute time bins (right) or expressed as passive coping score over the last 4-minutes of the first 6-minutes (left), ±SEM. *p < .02 and #p < .05 between TAM treated groups. B) Left, Open-field test beam-breaks (locomotor activity count) measured across 5minute time bins, ±SEM. Right, Percentage of time spent in the central rectangle of the open-field test, ±SEM. *p< .05. C) Average number of serotonergic (Tph2-immunopositive) cells dually-labeled for Fos per tissue section examined, across distinct caudal-rostral, dorsal-ventral and lateral wings DR subregions, ±SEM. *pFDR < .05 and #p < .05 between TAM treated groups. D) Representative images of the caudal DR from experimental mice. Immunofluorescent labeling of Fos (magenta; top) and cells dually labeled for Fos and Tph2 (green; bottom). Individual 216 × 165μm confocal images were merged using the pairwise stitching plugin of FIJI to display both CD and CV subregions on single representative sections. Scale bar (red) = 100μm.
The rostral and caudal DR are both anatomically and functionally distinct, as their activation level appears differentially regulated during acute stress (Commons 2008). We suspected that disrupting 5-HT neuron Cacna1c would influence the overall pattern of 5-HT neuron activation that occurs across DR 5-HT neuron subregions, and we examined this possibility by quantifying Fos expression within DR 5-HT neurons following acute stress to allow for the direct assessment of DR 5HT neuron activation across all unique rostral to caudal subregions within each subject. Through immunofluorescent labeling of Tph2 and Fos, we observed a subregion by genotype by treatment interaction (FG(3.53,98.97) = 5.863, p = .0003) that reflected a dramatically elevated number of Fos expressing 5-HT neurons in the caudal DR subregions (caudal-dorsal, F(1,28) = 5.293, p = .029; caudal-ventral, F(1,28) = 14.233, p = .0007) of Tph2iCreCacna1c−/− compared to Cacna1c+/+ or either VEH control group (Figure 2D). These results reveal that 5-HT neuron Cacna1c has a regionally-specific influence on Fos expression within DR 5-HT neurons.
The activation of DR 5-HT neurons is tightly regulated by 5-HT1A dependent feedback inhibition via local 5-HT neuron interconnections and local release of 5-HT. Given the regionally specific alteration in Fos expression and a known role for 5-HT neuron LTCCs in 5-HT1A dependent feedback-inhibition (Colgan et al. 2012), we then tested if altered coping behavior and the observed pattern of DR 5-HT neuron Fos expression persisted in Tph2iCreCacna1c−/− mice when 5-HT1A receptors were blocked. To explore this possibility, we administered the 5-HT1A antagonist WAY100635 (WAY; 0.4mg/kg) prior to the FST in a separate group of experimental mice. For active coping behavior in the FST, interactions between genotype and drug (F(1, 45) = 12.554, p = .0009), genotype and time (FG(1.72, 77.66) = 9.685, p = .0004 ), and drug and time (FG(1.72, 77.66) = 9.043, p = .0006) were observed. During the first 5 minutes of the FST, WAY treatment rescued active coping behavior in Tph2iCreCacna1c−/− mice while it slightly decreased active coping behavior in Cacna1c+/+ mice (F(1, 45) = 8.410, p = .006). Similarly, WAY treatment completely normalized the increased passive-coping behavior previously observed in Tph2iCreCacna1c−/− mice yet had no effect on Cacna1c+/+ mice (Figure 3A). Although active-coping behavior in Cacna1c+/+ mice returned to normal for the remainder of the FST, Tph2iCreCacna1c−/− mice displayed a sustained elevation in active coping behavior throughout the second (F(1, 45) = 8.687, p = .005) and third (F(1, 45) = 13.013, p = .0008) five-minutes of the FST. These selective effects of WAY within Tph2iCreCacna1c−/− mice is specific to coping behaviors in the FST, as WAY administered to a separate group of experimental mice prior to open-field testing revealed no differences in either general locomotor activity nor center time (Figure 3B).
Figure 3.

Neurobehavioral effect of the 5-HT1A receptor antagonist WAY-100635 (WAY) following 5HT neuron Cacna1c knockout. A) Forced-swim test swim count (active-coping behavior) measured across 5-minute time bins (right) or expressed as passive coping score over the last 4-minutes of the first 6-minutes (left), ±SEM. *p < .02 and #p < .05 for the effect of WAY in Tph2icreCacna1c−/− mice. B) Left, Open-field test beam-breaks (locomotor activity count) measured across 5-minute time bins, ±SEM. Right, Percentage of time spent in the central rectangle of the open-field test, ±SEM. C) Average number of serotonergic (Tph2-immunopositive) cells dually-labeled for Fos per tissue section examined, across distinct caudal-rostral, dorsal-ventral and lateral wings DR subregions, ±SEM. *pFDR < .05 between WAY treated groups. D) Representative images of the rostral-dorsal (RD) DR from WAY-exposed experimental mice. Immunofluorescent labeling of Fos (magenta; top) and cells dually labeled for Fos and Tph2 (green; bottom). Scale bar (red) = 100μm. E) Representative images of the lateral wings (LW; one hemisphere only) of the DR from WAY-exposed experimental mice. Immunofluorescent labeling of Fos (magenta; top) and cells dually labeled for Fos and Tph2 (green; bottom). Scale bar (red) = 100μm.
Given these unique neurobehavioral and pharmacological effects in Tph2iCreCacna1c−/− mice, we finally assessed whether the activation of DR 5-HT neuron subregions is also altered when 5HT1A receptors were systemically blocked. WAY increased Fos expression in 5-HT neurons across all DR subregions of both Tph2iCreCacna1c−/− and Cacna1c+/+ (F(1, 32) = 83.436, p = 1.98 × 10−10), suggesting that the 5-HT1A antagonist effectively disengaged feedback inhibition in both groups (Figure 3C). However, a subregion by genotype by drug interaction (FG(4.32, 138.24) = 4.044, p = .0008) was revealed reflecting a larger increase of 5-HT neuron Fos expression in both the lateral wings (F(1, 32) = 7.244, p = .011) and rostral-dorsal (F(1, 32) = 6.257, p = .018) subregions of WAY exposed Tph2iCreCacna1c−/− mice compared to their Cacna1c+/+ counterparts (Figure 3D, 3E). Collectively, these results suggest that loss of 5-HT neuron Cacna1c expression has region-dependent effects on both Fos expression and 5-HT1A-dependent feedback inhibition of DR 5-HT neurons.
4. Discussion
Polymorphism of CACNA1C, which encodes the pore-forming subunit of Cav1.2 LTCCs, is one of the most consistently identified genetic risk factors for schizophrenia, bipolar disorder and major depressive disorder, and it is critical to understand how this gene and its protein product impacts the neurobehavioral circuits most relevant for the etiology and treatment of these disorders. In the present study, we have identified a role for 5-HT neuron Cacna1c in regulating acute stress coping behavior, 5-HT neuron activation and 5-HT1A dependent feedback inhibition across the rostral to caudal extent of the DR. Given the roles of both stress and 5-HT system structure/function in neuropsychiatric disorder, the present results reveal a potential biochemical mechanism through which 5-HT neuron Cav1.2 LTCCs are poised to contribute to the neurobehaviorial stress response.
Acute stress is a primary risk factor for the development of a broad range of neuropsychiatric conditions (McLaughlin et al. 2010) and a disrupted neurobehavioral response to acute stress could either contribute to or be indicative of disease state progression (McEwen 2004). In animal models, disrupting the neural circuits that are active during acute stress can help elucidate the biochemical mechanisms that contribute to human conditions. We find that 5-HT neuron Cacna1c knockout reduces active coping behavior in the FST, with additional small but significant disruptions in the comparatively less stressful context of the open-field, indicating that 5-HT neuron Cav1.2 LTCCs provide critical control over normal stress-coping behavior. While this is the first direct neurobehavioral assessment of DR 5-HT neuron Cacna1c, previous research has characterized the behavioral impact of other regionally specific disruptions (Bhat et al. 2012). For example, conditional Cacna1c knockout in the hippocampus disrupts spatial memory (Moosmang et al. 2005; White et al. 2008) while conditional knockout in the prefrontal cortex causes avoidance of the aversive portion of the environment in the elevated plus maze (Lee et al. 2012). Most recently, it was shown that conditional knockout in the nucleus accumbens increases susceptibility to the negative impact of social defeat stress and increases anxiety-like behavior (Terrillion et al. 2017). Thus, the present results identify a new region of interest in the 5-HT system and a novel role for 5-HT neuron Cacna1c in the regulation of stress-coping behavior.
Known risk factors for neuropsychiatric disorder, such as stress, produce behavioral impairments in the FST while clinically effective treatments, such as anti-depressants, rescue behavioral impairments in the FST (Castagné et al. 2011; Lucki 1997). While the present results raise the possibility that disrupted CACNA1C function or expression within 5-HT neurons could contribute to the etiology of neuropsychiatric disorder, the most consistently identified risk-increasing polymorphism in CACNA1C is in a non-coding region of the gene and has often been shown to increase mRNA expression in both induced human neurons and in human brain tissue (Bigos et al. 2010; Yoshimizu et al. 2015). Interestingly, the effect of risk-associated CACNA1C polymorphisms on Cav1.2 expression may be regionally dependent, as decreased expression has been observed in the human cerebellum of risk-allele carriers (Gershon et al. 2014). How the human risk allele impacts Cav1.2 function or expression specifically within the DR, let alone in 5-HT neurons, is currently unknown. Importantly, previous results in mouse models would suggest that the brain-wide pattern of changes in Cav1.2 expression or function is most relevant when assessing altered neurobehavioral responses such as those revealed in the present study. That is, both whole brain Cav1.2 LTCC haploinsufficiency and alternatively a gain of function mutation in Cacna1c appear to increase active coping behavior in the FST (Ehlinger and Commons 2017; Dao et al. 2010), yet when Cacna1c knockout is isolated to 5-HT neurons we reveal decreased active coping-behavior. These results underscore the importance of considering circuit-specific versus brain-wide alterations in gene function or expression when assessing animal behavior models such as the FST and any potential relationship to neuropsychiatric disorder.
Although the genetic approach used in this study would impact all 5-HT neurons, the largest group of forebrain-projecting 5-HT neurons arise from the DR and these are associated with regulating affective and emotive processes. Given the capacity of 5-HT neuron Cacna1c to influence stress-coping behavior we predicted that DR 5-HT neurons would display altered Fos-activation following Cacna1c knockout. Our results show increased Fos expression exclusively within the caudal DR, raising the possibility that caudal DR 5-HT neurons are particularly relevant for the observed changes in stress-coping behavioral responses in the FST. There is a consistent association of the caudal DR activity with stressful, aversive conditions as well as helplessness (Guo and Commons, 2017, Ehlinger and Commons, 2017; Hammack et al. 2002). However, Fos expression in the caudal DR 5-HT neurons doesn’t correlate simply with coping behavior in the FST. Indeed, 5-HT neurotransmission plays a unique role in emotion and mood, which may not have a linear relationship to motor behavior or coping strategy. However, the selective changes seen in the caudal DR are intriguing given several lines of evidence implicating dysfunction of the caudal DR in the etiology of major depressive disorder. In humans, increased expression of Tph2 and loss of 5-HT1A receptors has been identified specifically in the caudal DR of depressed suicides (Bach-Mizrachi et al. 2008; Bodrini et al. 2008), while both afferents (orbitofrontal cortex, cingulate cortex) and an efferent target (hippocampus) of the caudal DR display reduced volume in patients with depression (Arnone et al. 2013; Wise et al. 2017). Taken together, the present results further identify the caudal DR (B6) as an integral component of the neural networks relevant to stress-coping behavior and indicate Cacna1c knockout disrupts both normal Fos activation of this area in conjunction with behavior.
Blocking 5-HT1A receptors completely reversed the behavioral deficit in the FST caused by disruption of 5-HT neuron Cacna1c. At the same time, the 5-HT1A antagonist WAY had no detectable effect in the relevant litter-mate control mice, although WAY sometimes has effects in other wild-type mouse lines depending on secondary experimental conditions including age and genetic background (O’Neill and Conway 2001; Ehlinger & Commons, 2017). Thus the present results suggest that increased 5-HT1A receptor activation following 5-HT neuron Cacna1c knockout is necessary for the behavioral deficit in stress-coping behavior. Like pharmacotherapies, in this study WAY was administered systemically such that the cellular or circuit mechanism is poorly resolved. However, this result may be particularly relevant for understanding previous reports on the combined treatment efficacy of 5-HT and LTCC acting pharmacotherapies, in which some clinical trials utilizing serotonin reuptake inhibitors in combination with LTCC antagonists have shown improved treatment outcomes (Taragano et al. 2001; Taragano et al. 2005). Furthermore, patient genotype with respect to CACNA1C may be highly relevant for the pharmacological treatment of psychiatric disorders, as a recent report suggests that mixed bipolar and major depression patients with risk-associated polymorphism in CACNA1C exhibit sustained cognitive impairments that would typically recover following 6-weeks of SSRI pharmacotherapy in patients without the risk allele (Lin et al. 2017). As genotype and cell-type specific targeting of neurotherapeutics becomes increasingly viable, it is possible that a combined targeting of Cav1.2 LTCCs in conjunction with traditional 5-HT system therapeutics would produce a sustained or more complete treatment response in certain populations.
In addition to rescuing coping behavior in the FST, blocking 5-HT1A receptors revealed additional differences in the topography of 5-HT neuron Fos activation in Cacna1c knockout mice. Although the 5-HT1A antagonist elevated Fos expression throughout the rostral to caudal extent of the DR in both groups and activation of the caudal DR was similar between genotypes, substantially elevated Fos expression appeared in several rostral DR subregions of Cacna1c KO mice. This finding implicates a change in feedback regulation of rostral DR neurons in Cacna1c KO mice either by direct or indirect multisynaptic mechanisms. The rostral two-thirds (B7 cell-group) and caudal one third (B6 cell-group) of the nucleus are organized into two distinct structural network domains with distinct overall patterns of input and output connectivity (Commons 2016; Commons 2015). These areas may differentially function under different behavior conditions as we have previously found differences between the rostral and caudal DR following other behavioral and/or genetic manipulations [Ehlinger and Commons 2017; Sperling and Commons 2011). Furthermore, these areas may influence each other via 5-HT1A dependent feedback inhibition, as blocking 5-HT1A receptors reciprocally influences the rostral and caudal poles of the DR (Sperling and Commons 2011). The present results further support the notion that the rostral and caudal DR may also represent distinct functional network domains.
A parsimonious explanation of the Fos studies and the behavioral analysis together is that Cacna1c knockout in serotonin neurons alters the balance of excitation and feedback inhibition that exists between different groups of serotonin neurons and consequently disrupts behavior. Blocking 5HT1A receptors and 5-HT1A-receptor-dependent feedback inhibition restores behavior, possibly by compensating for altered topographic patterns of activity. It is tempting to speculate that serotonin neurons in the rostral and caudal poles of the DR reciprocally regulate each other, as the interconnectivity of 5-HT neurons within the DR has been previously proposed as an important mechanism for regionally dependent 5-HT1A dependent feedback inhibition (Bang et al. 2012). However, it’s important to point out that there are many potential mechanisms for the differential response by neurons in different DR subregions. Neurons in these different areas are developmentally distinct (Alonso et al. 2013) and variation in expressed genes within individual 5-HT neurons of might make them respond differently to loss Cacna1c (Kast et al. 2017; Okaty et al. 2015).
In conclusion, we show that 5-HT neuron Cacna1c knockout disrupts the behavioral response to an acute stressor and that this behavioral deficit is rescued by blocking 5-HT1A receptors, revealing an important role for 5-HT neuron Cav1.2 LTCCs in the regulation of stress-coping behavior. This is consistent with literature associating LTCC as important mediators of the response to stress (Joels and Karst, 2012). Moreover, this manipulation regionally enhances 5-HT neuron Fos expression while altering 5-HT1A dependent feedback inhibition across the rostral to caudal extent of the DR, raising the possibility that Cav1.2 LTCCs directly impact the regional balance of DR 5-HT system activation to influence behavior. Understanding the mechanisms through which 5-HT neuron Cacna1c, and LTCCs more broadly, impact DR 5-HT system function will be important areas for future study, as well as the potential relationship between CACNA1C genotype and altered 5-HT system functional activity in clinical populations.
Highlights.
Loss of the L-type calcium channel subunit Cav1.2 selectively in serotonin neurons disrupts normal behavioral response to stress.
Blocking 5-HT1A receptors restores normal behavior.
There is evidence for altered balance of activation and feedback inhibition between different subgroups of serotonin neurons when Cav1.2 is lacking.
Highlights.
Serotonin (5-HT) neuron Cacna1c knockout disrupts active coping behavior
5-HT neuron Cacna1c knockout enhances caudal dorsal raphe (DR) Fos expression
Blocking 5-HT1A receptors rescues coping behavior and enhances rostral DR response
Acknowledgements
We would like to acknowledge Richard Tenpenney and Christopher Panzini for assistance with data collection, analysis and animal care.
Funding and Disclosure
Funding was provided by the National Institutes of Health grants DA021801 and HD036379 (KGC), the Brain and Behavior Foundation NARSAD Independent Investigator Award (KGC), and the Anesthesia Research Distinguished Trailblazer Award (DGE). DGE reports no biomedical financial interests and no potential conflicts of interests. KGC reports that she has received compensation from Zogenix, Inc, for professional services unrelated to the contents of this manuscript.
Abbreviations
- 5-HT
Serotonin
- 5-HT1A
Serotonin type 1A receptor
- DR
Dorsal raphe
- LTCC
L-type calcium channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso A, Merchán P, Sandoval JE, Sánchez-Arrones L, Garcia-Cazorla A, Artuch R, et al. (2013): Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains. Brain Struct Funct. 218: 1229–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Juhasz G, Thomas EJ, Downey D, et al. (2013): State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry. 18: 1265–1272. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V (2008): Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 13: 507–513, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader PL, Faizi M, Kim LH, Owen SF, Tadross MR, Alfa RW, et al. (2011): Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proc Natl Acad Sci USA. 108: 15432–15437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG (2012): Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 35: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 57: 289–300. [Google Scholar]
- Bhat S, Dao DT, Terrillion CE, Arad M, Smith RJ, Soldatov NM, Gould TD (2012): CACNA1C (Cav1.2) in the pathophysiology of psychiatric disease. Prog Neurobiol. 99: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, et al. (2010): Genetic Variation in CACNA1C Affects Brain Circuitries Related to Mental Illness. Arch Gen Psychiatry. 67: 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V (2008): Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 42: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagné V, Moser P, Roux S, Porsolt RD (2011): Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. Chapter 8: Unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Chazal G, Ralston HJ (1987): Serotonin-containing structures in the nucleus raphe dorsalis of the cat: an ultrastructural analysis of dendrites, presynaptic dendrites, and axon terminals. J Comp Neurol. 259: 317–329. [DOI] [PubMed] [Google Scholar]
- Colgan LA, Cavolo SL, Commons KG, Levitan ES (2012): Action potential-independent and pharmacologically unique vesicular serotonin release from dendrites. J Neurosci. 32: 15737–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG (2008): Evidence for topographically organized endogenous 5-HT-1A receptordependent feedback inhibition of the ascending serotonin system. Eur J Neurosci. 27: 2611–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG (2015): Two major network domains in the dorsal raphe nucleus. J Comp Neurol. 523: 1488–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG (2016): Ascending serotonin neuron diversity under two umbrellas. Brain Struct Funct. 221: 3347–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Gerfen CR, Rogawski RA, Sibley DR, Skolnick P, Wray S, editors. Short protocols in neuroscience: systems and behavioral methods. Hoboken (NJ): John Wiley & Sons, Inc.; 2007. [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium (2013): Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 381: 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I (2005): Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test Neuroscience & Biobehavioral Reviews, Animal Models of Depression and Antidepressant Activity; 29: 547–569. [DOI] [PubMed] [Google Scholar]
- Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, et al. (2010): Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry. 68: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlinger DG, Commons KG (2017): Altered Cav1.2 function in the Timothy syndrome mouse model produces ascending serotonergic abnormalities. Eur J Neurosci. 46: 2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MAR, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. (2008): Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 40: 1056–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon ES, Grennan K, Busnello J, Badner JA, Ovsiew F, Memon S, et al. (2014): A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol Psychiatry. 19: 890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, et al. (2010): The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Molecular Psychiatry. 15: 1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YP, Commons KG. (2017). Serotonin neuron abnormalities in the BTBR mouse model of autism. Autism Research. 10(1):66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF (2002): The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 22: 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes S, Pratt WS, Rees E, Dahimene S, Ferron L, Owen MJ, Dolphin AC (2015): Genetic disruption of voltage-gated calcium channels in psychiatric and neurological disorders. Prog Neurobiol. 134: 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Karst H. (2012): Corticosteroid effects on calcium signaling in limbic neurons. Cell Calcium. 51(3–4):277–83. [DOI] [PubMed] [Google Scholar]
- Kast RJ, Wu H-H, Williams P, Gaspar P, Levitt P (2017): Specific Connectivity and Unique Molecular Identity of MET Receptor Tyrosine Kinase Expressing Serotonergic Neurons in the Caudal Dorsal Raphe Nuclei. ACS Chem Neurosci. 8: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Lucki I (1997): Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J Pharmacol Exp Ther. 282: 967–976. [PubMed] [Google Scholar]
- Lee AS, Ra S, Rajadhyaksha AM, Britt JK, De Jesus-Cortes H, Gonzales KL, et al. (2012): Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol Psychiatry. 17: 1054–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. (2007): Genome-wide atlas of gene expression in the adult mouse brain. Nature. 445: 168–176. [DOI] [PubMed] [Google Scholar]
- Lin K, Xu G, Shi L, Lu W, Guan L, Ouyang H et al. (2017): CACNA1C polymorphisms Impact Cognitive Recovery in Patients with Bipolar Disorder in a Six-week Open-label Trial. Sci Rep. 7(1): 7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I (1997): The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 8: 523–532. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Hiroi R, Mackenzie SM, Robin NC, Cohn A, Kim JJ, Neumaier JF (2011): Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior. Biol Psychiatry. 69: 780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2004): Protection and Damage from Acute and Chronic Stress: Allostasis and Allostatic Overload and Relevance to the Pathophysiology of Psychiatric Disorders. Annals of the New York Academy of Sciences. 1032: 1–7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Conron KJ, Koenen KC, Gilman SE (2010): Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med. 40: 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HWM (2007): The dorsal raphe nucleus--from silver stainings to a role in depression. Brain Res Rev. 55: 329–342. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Müller J, et al. (2005): Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 25: 9883–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty BW, Freret ME, Rood BD, Brust RD, Hennessy ML, deBairos D, et al. (2015): Multi-Scale Molecular Deconstruction of the Serotonin Neuron System. Neuron. 88: 774–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MF, Conway MW (2001): Role of 5-HT(1A) and 5-HT(1B) receptors in the mediation of behavior in the forced swim test in mice. Neuropsychopharmacology. 24: 391–398. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K (2012): The Mouse Brain in Stereotaxic Coordinates, 4th ed Academic Press. [Google Scholar]
- Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, et al. (2009): Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol. 75: 407–414. [DOI] [PubMed] [Google Scholar]
- Sperling R, Commons KG (2011): Shifting topographic activation and 5-HT1A receptor-mediated inhibition of dorsal raphe serotonin neurons produced by nicotine exposure and withdrawal. European Journal of Neuroscience. 33: 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taragano FE, Allegri R, Vicario A, Bagnatti P, Lyketsos CG (2001): A double blind, randomized clinical trial assessing the efficacy and safety of augmenting standard antidepressant therapy with nimodipine in the treatment of “vascular depression.” Int J Geriatr Psychiatry. 16: 254–260. [DOI] [PubMed] [Google Scholar]
- Taragano FE, Bagnatti P, Allegri RF (2005): A double-blind, randomized clinical trial to assess the augmentation with nimodipine of antidepressant therapy in the treatment of “vascular depression.” Int Psychogeriatr. 17: 487–498. [DOI] [PubMed] [Google Scholar]
- Terrillion CE, Francis TC, Puche AC, Lobo MK, Gould TD (2017): Decreased Nucleus Accumbens Expression of Psychiatric Disorder Risk Gene Cacna1c Promotes Susceptibility to Social Stress. Int J Neuropsychopharmacol. 20: 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt MA, Chourbaji S, Brandwein C, Dormann C, Sprengel R, Gass P (2008): Suitability of tamoxifen-induced mutagenesis for behavioral phenotyping. Exp Neurol. 211: 25–33. [DOI] [PubMed] [Google Scholar]
- Weber T, Böhm G, Hermann E, Schütz G, Schönig K, Bartsch D (2009): Inducible gene manipulations in serotonergic neurons. Front Mol Neurosci. 2: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Vogt MA, Gartside SE, Berger SM, Lujan R, Lau T, et al. (2015): Adult AMPA GLUA1 receptor subunit loss in 5-HT neurons results in a specific anxiety-phenotype with evidence for dysregulation of 5-HT neuronal activity. Neuropsychopharmacology. 40: 1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L (2014): Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron. 83: 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, Tsien RW (2012): Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 149: 1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG (2008): Conditional forebrain deletion of the L-type calcium channel Ca V 1.2 disrupts remote spatial memories in mice. Learn Mem. 15: 1–5. [DOI] [PubMed] [Google Scholar]
- Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. (2017): Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Molecular Psychiatry. 22: 1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Pergadia ML, Blackwood DHR, Penninx BWJH, Gordon SD, Nyholt DR, et al. (2012): Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry. 17: 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


