ABSTRACT
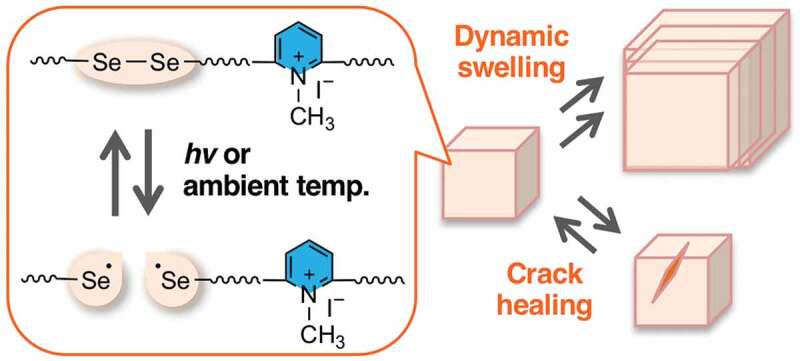
We report the dynamic behavior of diselenide-containing hydrophilic polyurethanes and hydrogels based on diselenide exchange reactions in an aqueous media. Diselenide-containing linear and cross-linked polyurethanes were synthesized via polyaddition reactions using diselenide-containing diol in combination with pyridinium diol that enhances the hydrophilicity of the polymer chains. The obtained linear polyurethanes underwent photo-induced diselenide exchange reactions with small diselenide compounds and degraded to smaller fragments, confirming the dynamicity of the obtained hydrophilic polyurethanes. The prepared hydrogels displayed characteristic large swelling behavior based on the structural reorganization through diselenide exchange either under photo-irradiation at 365 nm or even in the dark at room temperature. The diselenide-containing hydrogels also showed crack-healing behavior under the same exchanging conditions, presenting the utility of diselenide linkages as simple and useful units to offer high dynamicity to hydrogels.
KEYWORDS: Diselenide bond, dynamic covalent chemistry, self-healing, hydrogel
CLASSIFICATION: 600 Self-healing materials
Graphical Abstract
Introduction
Hydrogel is a versatile platform of biocompatible materials with its water-rich composition and softness, and the ones with stimuli-responsive functions have particularly attracted an increasing attention for the application in such as drug delivery [1,2], gene transfection [3–5], tissue engineering [6,7], and artificial muscles [8–10]. One of the powerful tools for the functionalization of hydrogels is dynamic covalent bonds (DCBs) [11–15], which can reversibly exchange under certain environmental conditions while they act as covalent bonds without those stimuli and thus more robust than common non-covalent supramolecular interactions. To date, a variety of DCBs have been demonstrated such as esters [16], olefins [17,18], Diels–Alder adducts [19,20], alkoxyamines [21–23], disulfides [24–27], imines [28,29], boronic esters [30–32], and others [33,34], and they can afford hydrogels with intelligent functions such as stimuli-induced degradation [26] and self-healing ability [27,30,35].
One of the attractive DCB candidates for the hydrogels is diselenide bonds, because of the biocompatibility of selenium analogues of various sulfur-containing biomaterials [36–40], and the intrinsic and highly dynamic properties, unlike the other common DCBs that necessitate high temperatures and/or external additives [16–23]. In contrast to the analogous disulfide bonds that generally require strong UV irradiation or high temperatures above 150°C for their exchange reactions [24,27], diselenide bonds can exchange under much milder photo and thermal treatment as pioneered by Xu and co-workers [41–44], owing to the lower dissociation energy of diselenide bonds (172 kJ mol–1) than that of disulfide bonds (240 kJ mol–1) [45]. Several studies on diselenide-containing functional polymers have subsequently been reported such as reprocessable thermosets [46,47], degradable micelles/vesicles [48,49], shape-memory polymers [50], and photo-patternable elastomers [51]. Our group has also previously investigated the damage-healing properties and photo-stability of aromatic diselenide-containing polymers [52]. However, there still have been a few reports on diselenide-containing hydrogels, and those reports are mostly limited to the ones on drug delivery and degradable materials [41,53–56]. We herein report the structural reorganization properties and crack-healing behaviors of diselenide-containing hydrogels based on diselenide exchange reactions.
Experimental
Materials
Hexamethylene diisocyanate (HDI), di-n-butyltin dilaurate (DBTDL), and tri(ethylene glycol) (TEG) were purchased from Tokyo Chemical Industry Co., Japan. Poly(ethylene glycol) (PEG) (Mn = 1,000 g mol−1) was purchased from Kanto Chemical Co., Inc., Japan. Triethylamine and all organic solvents were purchased from Wako Pure Chemical Industries, Ltd., Japan. Triethanolamine (TEA) was purchased from Sigma-Aldrich, USA. HDI was vacuum distilled and stored under N2 atmosphere at 5°C before use. 1,4-Dioxane was distilled and stored under Ar atmosphere before use. Other chemicals were used as received. Bis(2-hydroxyethyl) diselenide (DSe) [53] and 2,6-bis(hydroxymethyl)-1-methylpyridin-1-ium iodide (iPDM) [57] were prepared according to the literature procedures.
Instruments
Both 1 H and 13 C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker (USA) DPX300 spectrometer at 300 MHz (75 MHz for 13 C-NMR) in DMSO-d6. Fourier transform infrared (FT-IR) spectra were recorded on a JASCO (Japan) FT/IR-4100ST spectrophotometer on a NaCl plate for liquid samples or in KBr pellets for solid samples. Gel permeation chromatography (GPC) measurement was performed on a Malvern Panalytical (England) Viscotek GPC-1000 system at 40°C using polystyrene as a standard and DMF with 0.05 M LiBr as the eluent (0.6 mL min–1).
Synthesis of ionic linear polyurethanes (IPU)
PEG (Mn = 1,000 g mol–1) (0.623 g, 0.623 mmol) and iPDM (0.0875 g, 0.311 mmol) were added to a 30 mL Schlenk flask, and dried at 100°C under vacuum for 2 h. DSe (0.0773 g, 0.311 mmol) and DBTDL (3.0 µL, 2.50 × 10−3 mmol) were added to another Schlenk flask and freeze-dried over 1,4-dioxane. DSe and DBTDL were dissolved in dehydrated DMF (3.00 mL) under N2 atmosphere at room temperature, and the solution was transferred via a syringe to the Schlenk flask containing PEG and iPDM. Then, HDI (0.209 g, 1.25 mmol) was added to the solution. After stirring at room temperature for 24 h, the polymerization was quenched by the addition of methanol. The mixture was dropped into diethyl ether. The precipitant was purified by reprecipitation from chloroform to hexane, and dried under vacuum to give a yellow solid (IPU) (0.997 g, quantitative yield). 1 H-NMR (300 MHz, DMSO-d6): δ = 8.66 (pyridinium, 4-H), 8.00 (pyridinium, 3,5-H), 7.70 (pyridinium-CH2OCONH), 7.17 (CONH), 5.52 (pyridinium- CH2OCONH), 4.18 (SeCH2CH2O), 4.13 (N-CH3), 4.03 (PEG-CH2OCONH), 3.50 (PEG), 3.11 (SeCH2), 2.94 (CONHCH2), 1.36 (CONHCH2CH2), 1.22 (CONHCH2CH2CH2) ppm.
Synthesis of non-ionic linear polyurethanes (NIPU)
PEG (Mn = 1,000 g mol−1) (3.11 g, 3.11 mmol), DSe (0.386 g, 1.56 mmol), and 2,6-bis(hydroxymethyl)pyridine (PDM, 0.228 g, 1.56 mmol) were added to a 50 mL two-neck flask, followed by freeze-drying over 1,4-dioxane. Dehydrated DMF (13.8 mL) and HDI (1.05 g, 6.23 mmol) were added to the flask under N2 atmosphere, followed by the addition of 0.75 mL (1.25 × 10–2 mmol) of DBTDL diluted in dehydrated tetrahydrofuran (THF) (10.0 µL DBTDL in 1.00 mL THF). After stirring at room temperature for 24 h, the polymerization was quenched by the addition of methanol. The mixture was dropped into a saturated NaCl aqueous solution. The precipitant was dissolved in chloroform, dried over anhydrous Na2SO4, and dropped into hexane. The precipitant in hexane was dried under vacuum to give a light yellow solid (NIPU) (3.54 g, 81% yield). 1 H-NMR (300 MHz, DMSO-d6): δ = 7.70 (pyridine, 4-H), 7.29 (pyridine, 3,5-H), 5.19 (pyridine-CH2OCONH), 4.96 (CONH), 4.33 (SeCH2CH2O), 4.20 (PEG-CH2OCONH), 3.64 (PEG), 3.16 (CONHCH2 and SeCH2), 1.49 (CONHCH2CH2), 1.33 (CONHCH2CH2CH2) ppm.
Diselenide bond exchange reaction between IPU and DSe
IPU (10 mg) and excess amount of DSe (20 mg, 8.1 × 10–2 mmol) were dissolved in water (10.0 mL) and stirred under the conditions as follows: i) irradiated by 9 W handy UV lamp at 365 nm from a distance of 8 cm, and ii) stored in a brown glass vial at room temperature. After stirring for 24 h, water was removed by freeze-drying. The products were purified by reprecipitation from methanol to diethyl ether and dried under vacuum.
Synthesis of cross-linked polyurethanes (CLPU)
PEG (Mn = 1,000 g mol–1) (0.623 g, 0.623 mmol) and iPDM (0.0875 g, 0.311 mmol) were added to a 30 mL Schlenk flask, and dried at 100°C under vacuum for 2 h. DSe (0.0773 g, 0.311 mmol), TEA (0.0619 g, 0.415 mmol), and DBTDL (4.5 µL, 3.75 × 10–3 mmol) were added to another Schlenk flask and freeze-dried over 1,4-dioxane. DSe, TEA, and DBTDL were dissolved in dehydrated DMF (3.70 mL) under N2 atmosphere at room temperature, and the solution was transferred via a syringe to the Schlenk flask containing PEG and iPDM. Then, HDI (0.314 g, 1.88 mmol) was added to the solution. After vigorous stirring for about 1 minute, the mixture was transferred to a PTFE Petri dish and stored under N2 atmosphere at room temperature for 5 days to give an orange transparent cross-linked polyurethane gel. The obtained gel was immersed in diethyl ether for 5 h, chloroform for 12 h, and hexane for 5 h, and finally dried under vacuum to give a bulk cross-linked polymer (1.16 g, quantitative yield). The obtained CLPU bulk sample was cut to 5 × 5 × 1 mm3 pieces and stored in a brown glass vial. FT-IR (KBr): 3405, 2925, 2869, 2373, 2339, 1712, 1632, 1536, 1459, 1352, 1252, 1106, 949, 849 cm–1.
A control polymer was synthesized in a similar manner. PEG (Mn = 1,000 g mol−1) (0.623 g, 0.623 mmol) and iPDM (0.0875 g, 0.311 mmol) were added to a 30 mL Schlenk flask, and dried at 100°C under vacuum for 2 h. TEG (0.0467 g, 0.311 mmol), TEA (0.0619 g, 0.415 mmol), and DBTDL (4.5 µL, 3.75 × 10−3 mmol) were added to another Schlenk flask and freeze-dried over 1,4-dioxane. TEG, TEA, and DBTDL were dissolved in dehydrated DMF (3.70 mL) under N2 atmosphere at room temperature, and the solution was transferred via a syringe to the Schlenk flask containing PEG and iPDM. Then, HDI (0.314 g, 1.88 mmol) was added to the solution. After vigorous stirring for about 1 minute, the mixture was transferred to a PTFE Petri dish and stored under N2 atmosphere for 5 days to give an orange transparent cross-linked polyurethane gel. The obtained gel was then immersed in diethyl ether for 5 h, chloroform for 12 h, and hexane for 5 h, and finally dried under vacuum to give a bulk cross-linked polymer (1.16 g, quantitative yield). The obtained CLPUctrl bulk sample was cut to 5 × 5 × 1 mm3 pieces and stored in a brown glass vial. FT-IR (KBr): 3430, 2924, 2869, 2373, 2341, 1707, 1653, 1540, 1463, 1353, 1254, 1104, 950, 846 cm–1.
Water-swelling behavior of bulk CLPUs
The bulk CLPU samples (5 × 5 × 1 mm3, 50 mg) were immersed in deionized (DI) water (20 mL) and put under the conditions as follows: i) irradiated by 9 W handy UV lamp at 365 nm from a distance of 8 cm at room temperature, ii) stored in a brown glass vial at room temperature, and iii) stored in a brown glass vial and put in a refrigerator at 5°C.
Preparation and self-healing behavior of CLPU hydrogels
The as-prepared CLPU gels before drying were immersed in DI water for 5 h. Then, the water was changed to fresh DI water. The procedure was repeated 5 times to give CLPU hydrogels. The hydrogels were scratched by a surgical knife, the healing experiment was performed under the conditions as follows: i) irradiated by 9 W handy UV lamp at 365 nm from a distance of 8 cm at room temperature, ii) stored in a brown glass vial at room temperature, and iii) stored in a brown glass vial and put in a refrigerator at 5°C. The healing behavior of the scratched samples was observed by optical microscopy.
Results and discussion
Synthesis and hydrophilicity of ionized linear polyurethanes (IPU)
As a model polymer of diselenide-containing hydrogel, we first prepared a hydrophilic linear polyurethane (IPU) that contains diselenide bonds and pyridinium units in the main chain. The polyaddition reaction was carried out using poly(ethylene glycol) (PEG) (Mn = 1,000 g mol–1), bis(2-hydroxyethyl) diselenide (DSe), (2,6-bis(hydroxymethyl)-1-methylpyridin-1-ium iodide (iPDM), and hexamethylene diisocyanate (HDI) in the presence of di-n-butyltin dilaurate (DBTDL) as the catalyst (Scheme 1(a)). We prepared the three types of IPUs with different DSe and iPDM ratios to investigate the proper composition (Table 1) for both sufficient dynamicity and hydrophilicity. We also prepared non-ionized polyurethane (NIPU, Scheme 1(b)) as a control sample following the same manner as IPU using PDM instead of iPDM. The structures of the obtained polyurethanes were characterized by 1 H NMR (Figure 1), and GPC measurement confirmed the formation of polymer products.
Scheme 1.
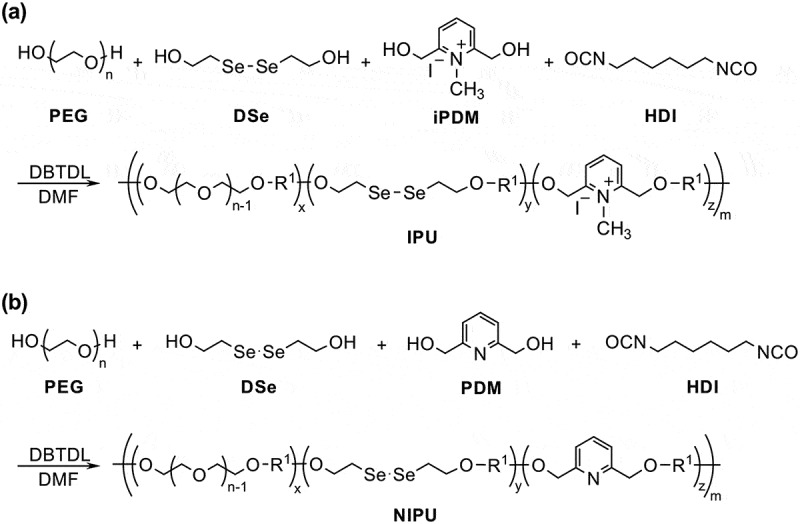
Synthesis of (a) ionic and (b) non-ionic polyurethanes with diselenide linkages.
Table 1.
Results of the synthesis of diselenide-containing linear polyurethanes.
| Sample | DSe (mol%)a |
iPDM (mol%)a |
PDM (mol%)a |
Yield (%) |
Mnb | Mw/Mnb |
|---|---|---|---|---|---|---|
| IPU1 | 6.0 | 13 | – | Quant. | 27,600 | 1.33 |
| IPU2 | 8.5 | 9.0 | – | Quant. | 21,600 | 1.31 |
| IPU3 | 14 | 5.0 | – | 91 | 20,300 | 1.24 |
| NIPU | 13 | – | 12 | 81 | 17,500 | 2.60 |
a Calculated from 1H NMR spectra.
bEstimated by GPC measurements.
Figure 1.
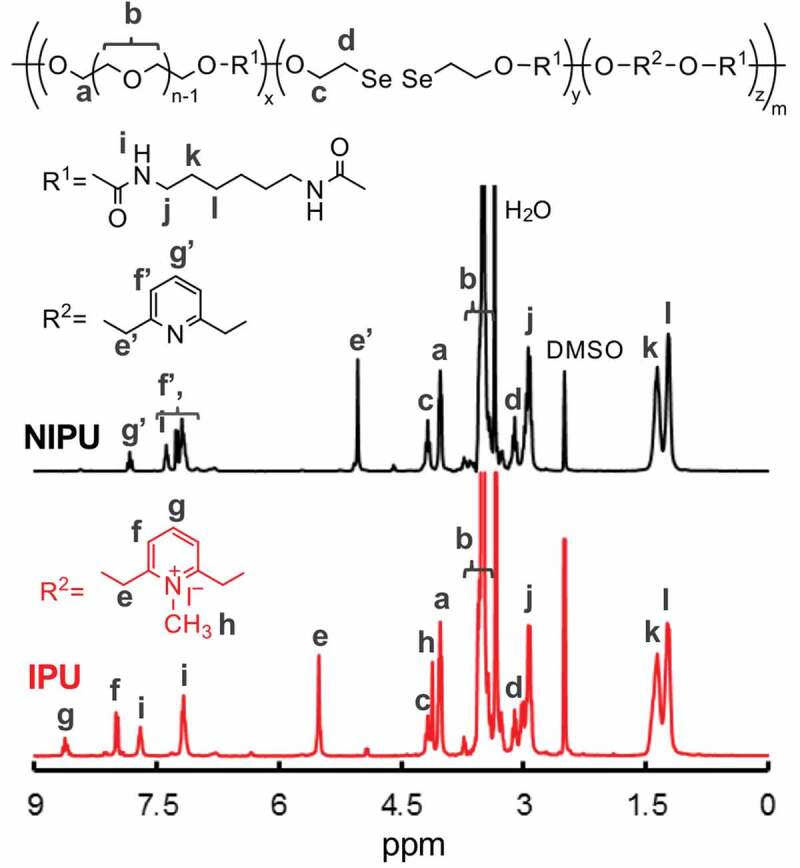
1 H NMR spectra of NIPU and IPU (300 MHz, DMSO-d6).
We then investigated the hydrophilicity of IPUs with different iPDM ratios by adding these polymers into deionized water (1.0 mg mL–1). IPU1 and IPU2 with larger pyridinium ratios completely dissolved in water to afford transparent solutions, while the aqueous solution of IPU3 with the least iPDM ratio resulted in a slight suspension (Figure 2). These results suggested that at least 9 mol% pyridinium unit ratio endow sufficient hydrophilicity with the herein designed diselenide-containing polyurethanes.
Figure 2.

Photographs of the aqueous solutions of IPUs and NIPU (1.0 mg mL–1).
Degradation of IPU by diselenide bond exchange reaction with DSe
The dynamic property of the diselenide-containing linear polyurethanes in water was investigated using IPU2. A photo-induced diselenide exchange reaction between IPU2 and an excess amount of DSe (15 folds of the Se-Se bond in IPU2) was performed in water under photo-irradiation at 365 nm, and the reaction was monitored by GPC measurement. After 24 hours of the irradiation at room temperature, the GPC curves shifted to lower molecular weight region with a decrease of Mw from 12,200 to 7,200 (Figure 3). Although the molecular weight decrease was not significant due to the smaller composition ratio of diselenide bond (ca. 1.2 moieties per chain) as determined by water solubility test, this result indicated that the diselenide linkages in the polymer chain exchanged with the low molecular diselenide (DSe), confirming the photo-induced exchanging ability of diselenide bonds in the designed hydrophilic polyurethanes in water.
Figure 3.
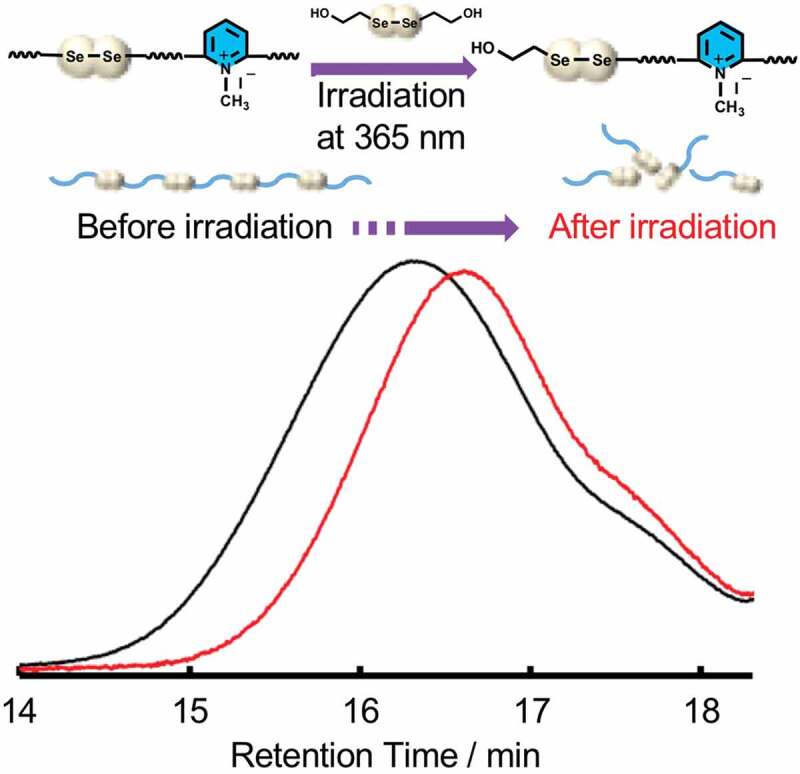
GPC curves of IPU2 before (black) and after (red) the exchange reaction with DSe.
Preparation of cross-linked polyurethanes (CLPUs)
The dynamic properties of diselenide bonds in hydrogel networks were then investigated using diselenide-containing hydrophilic cross-linked polyurethanes (CLPUs), which would reflect the dynamic nature of diselenide bonds much clearer than the IPUs with a few diselenide compositions. CLPUs were prepared in a similar manner as IPUs, where triethanolamine (TEA) was used as a cross-linker in addition to PEG (Mn = 1,000 g mol–1), DSe, iPDM, and HDI (Scheme 2(a)). As a control sample, chemically cross-linked hydrophilic polyurethane was concurrently synthesized by using tri(ethylene glycol) (TEG) instead of DSe (Scheme 2(b)). The feed compositions of each CLPU are summarized in Table 2. After 5 days of polymerization, the CLPUs were obtained as transparent gels swollen with DMF. The crude gels were purified by immersion in diethyl ether, chloroform, and hexane in sequence, and then dried under vacuum to obtain bulk samples of CLPUs. FT-IR spectroscopy of the obtained CLPUs showed the disappearance of the peak corresponding to isocyanate groups at around 2270 cm–1 and the emergence of the peaks corresponding to urethane groups at around 1712 cm–1 and 1661 cm–1 (Figure 4), indicating that HDI had completely reacted with the OH groups. All the CLPUs were obtained quantitatively, indicating the quantitative conversion of the fed monomers into the cross-linked networks.
Scheme 2.
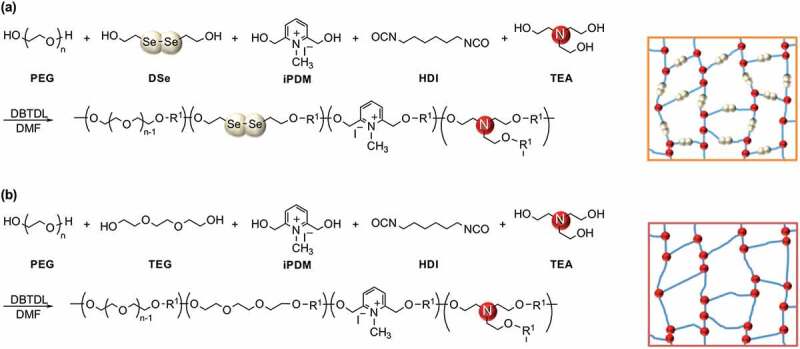
Synthesis of hydrophilic cross-linked polyurethanes (CLPUs) (a) with diselenide bonds and (b) without diselenide bonds.
Table 2.
Compositions of the CLPUs.
| Sample | Feed molar ratioa |
||||
|---|---|---|---|---|---|
| PEG | DSe | TEG | iPDM | TEA | |
| CLPU1 | 0.50 | 0.16 | – | 0.34 | 0.34 |
| CLPU2 | 0.50 | 0.25 | – | 0.25 | 0.34 |
| CLPU-ctrl | 0.50 | – | 0.25 | 0.25 | 0.34 |
aAll the products were obtained quantitatively.
Figure 4.
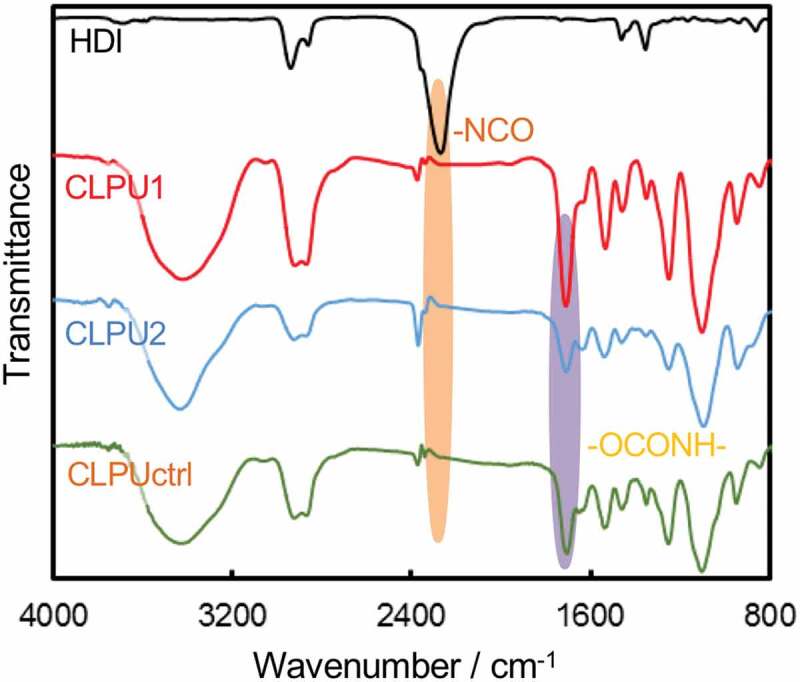
FT-IR spectra of CLPUs in comparison with HDI.
Water-swelling behavior of bulk CLPUs
The dynamic property of the obtained CLPUs was first evaluated by water-swelling tests under the photo-irradiation at 365 nm, in the dark at 25°C, and in the dark at 5°C, each of which should offer different dynamic nature to the diselenide bonds and thus different swelling behaviors. In general, chemically cross-linked polymers swell in their good or θ solvents and reach swelling equilibrium states, where the solvation-induced stretching of the polymer chains and elastic force of the stretched chains are equaled [58,59]. On the other hand, the dynamic networks can relax the stretched polymer chains via structural reorganization induced by bond exchange reactions, which lead to a decrease of the crosslink density and higher swelling degree [60]. The swelling tests were performed with immersing a square-cut specimen of each CLPU in deionized water. The swelling degree (Q) was defined as the following equation:
| (1) |
where W0 and Wt are the weight of the bulk sample at the initial and swollen state, respectively. When subjected to swelling under photo-irradiation at 365 nm (Figure 5(a), diamond plots), CLPU1 once reached a maximum swelling degree up to Q ≈ 63 after 2 days (point B), and then the swelling degree gradually got smaller and finally resulted in complete dissolution of the gel to afford a yellow transparent solution after 5 days (point C) (Figure 5(b)). In contrast, the swelling degree of CLPU1 was drastically suppressed up to Q ≈ 5.4 in the dark at 5°C (Figure 5(a), filled circle plots), and this is similar to that of CLPU-ctrl without diselenide bond, which showed almost the same swelling behavior with Q = 5.1 ~ 7.6 after 6 days in the employed conditions (Figure 5(b)). These results indicate that the large swelling of CLPU1 under photo-irradiation was based on the structural reorganization of the network induced by diselenide exchange reactions. Since the swelling test was carried out in the presence of a large amount of water, depolymerization of the network became favorable under bond-exchanging conditions in an entropy-driven manner, leading to the dissolution of the hydrogels in water (Figure 5(e)) [60]. One can think of the possibility of force-induced diselenide dissociation by the stretching force during the swelling, but it is very unlikely considering the other dynamic networks with weaker dynamic covalent bonds that showed the large swelling only under the bond-exchanging conditions [60,61]. The suppressed swelling of CLPU1 in the dark at 5°C should derive from the fixed diselenide bonds without photo- and thermal stimuli. Interestingly, CLPU1 in the dark at room temperature also showed large swelling up to Q ≈ 63 after 4 days and maintained nearly constant swelling degree (Figure 5(a), square plots), suggesting that the thermal diselenide exchange reaction occurred. This is supported by the result of CLPU2, which dissolved in water even in the dark at room temperature after 6 days (Figure 5(d), square plots). These results well demonstrate that the diselenide bonds in the hydrogel networks possess highly dynamic properties and can autonomously exchange even in an ambient environment.
Figure 5.
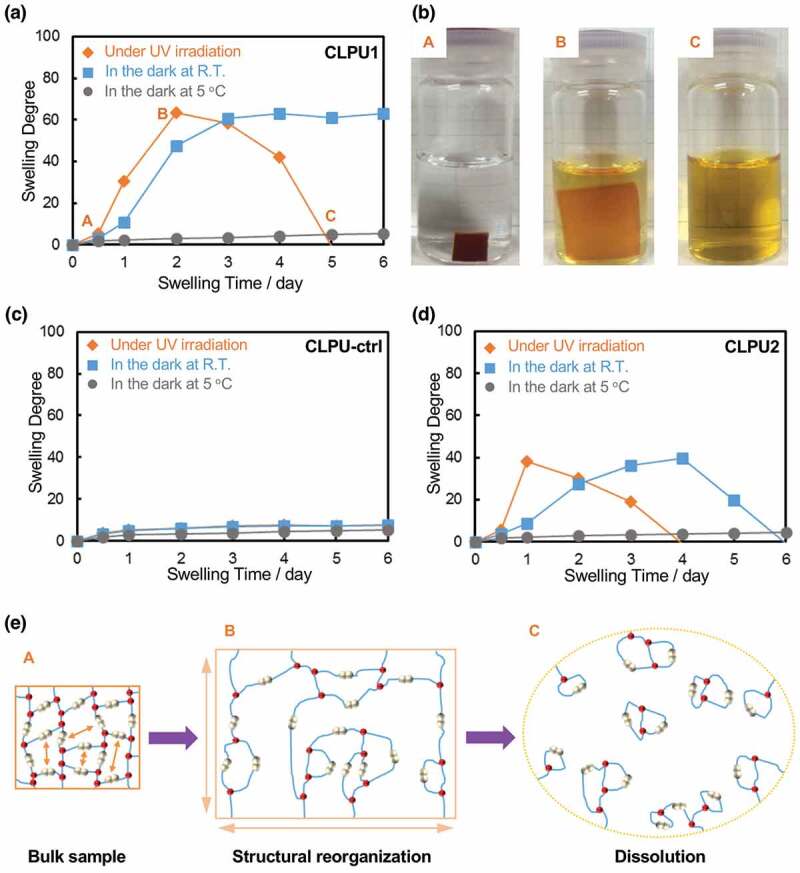
(a) Swelling behavior of CLPU1. (b) Photographs of the specimen of CLPU1 at different swelling states. (c) Swelling behavior of CLPU-ctrl and (d) CLPU2. (e) Illustration of the structural reorganization and entropy-driven cyclization depolymerization of diselenide-containing hydrogels.
Crack-healing behavior of CLPU hydrogels
The dynamic properties of the diselenide-containing hydrogels were also investigated by crack-healing tests. Each specimen of the CLPU hydrogels was scratched by a surgical knife and subjected to certain conditions for 24 h, and the crack part was observed by optical microscopy. The results of the healing tests of CLPU1 hydrogels are shown in Figure 6. It can be seen that the scratched area became almost homogeneous with a tiny remnant of the original crack both under photo-irradiation at 365 nm and in the dark at room temperature after 24 h, while no healing was observed with the sample kept in the dark at 5°C. These results indicate that the diselenide exchange reaction occurred and reconstruct the gel network at the crack surface under photo-irradiation and in the dark at room temperature. Since the diselenide exchange reaction dramatically suppressed in the dark at 5°C, the specimen in that condition showed little change with the crack. These results are well consistent with the ones of the previously conducted swelling tests. The test using CLPU2 with a higher diselenide ratio than CLPU1 resulted in more homogeneous surface after photo-irradiation for 24 h (Figure 7(a), left), plausibly due to the diselenide-rich composition that induces the more diselenide exchange reactions and thus crack-healing properties. On the other hand, CLPU2 in the dark at room temperature showed a bit poorer or comparable healing behavior to CPLU1 (Figure 7(a), right). Considering the crack-healing mechanism that first requires the contact of each damaged surface followed by homogenization of the network by structural reorganization [62], the chain mobility is another important factor. The effect of chain mobility besides the bond exchangeability to the healing performances had actually been observed in our previous study on the diselenide-containing bulk networks [52]. Taking into account the preceding reports on the photo-induced plasticization of the photo-exchangeable polymer networks [63], the particularly better healing of CLPU2 under photo-irradiation presumably derived from the photo-induced increase of the inner chain mobility that derives from the diselenide-rich composition. As a control experiment, we also carried out the same test using CLPU-ctrl without diselenide bonds, which resulted in little change with their crack after subjected to either photo-irradiation or placed in the dark at room temperature (Figure 7(b)). These results demonstrate the availability of diselenide bonds as an effective functional unit to endow hydrogels with various dynamic properties that can be triggered by mild conditions.
Figure 6.
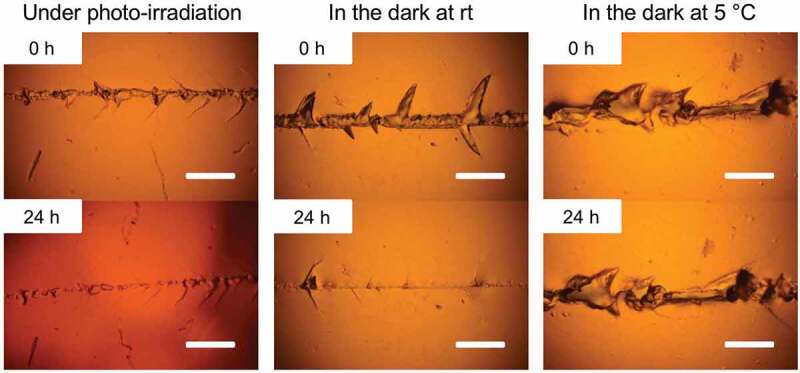
Photographs of the scratches on CLPU1 before and after placed under photo-irradiation at 365 nm in the dark at room temperature, and in the dark at 5°C (scale bar: 0.2 mm).
Figure 7.
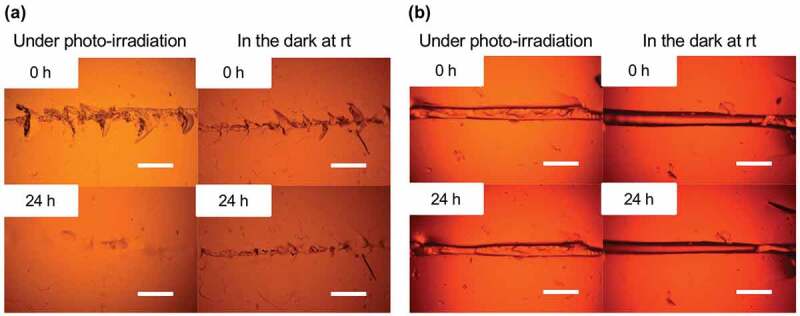
Photographs of the scratches on (a) CLPU2 and (b) CLPU-ctrl before and after placed under photo-irradiation at 365 nm and in the dark at room temperature (scale bar: 0.2 mm).
Conclusions
In this work, we investigated the dynamic properties of diselenide-containing hydrogels with dynamic diselenide linkages and hydrophilic pyridinium units. The model study using linear polyurethanes incorporated with these units confirmed the photo-induced exchangeability of the herein designed polymers. Diselenide-containing crosslinked polyurethane was then synthesized, and the dynamic properties in the hydrogel state were evaluated by water-swelling and crack-healing tests. The water-swelling test revealed the characteristic large swelling and dissolution behavior of the diselenide-containing hydrogels under photo-irradiation at 365 nm and also even in the dark at ambient temperature, presenting the highly dynamic properties of diselenide bonds in the hydrogel networks. Such dynamic properties were also confirmed in the crack-healing test, in which CLPU2 with diselenide-rich composition showed the most efficient healing based on the network reorganization by photo-induced diselenide exchange reaction. The herein obtained knowledge would contribute to the further development of dynamic diselenide chemistry particularly in the field of the soft matters such as biocompatible materials as the appropriate area of the mild stimuli-inducible dynamic nature of diselenide bonds.
Funding Statement
This work was supported by KAKENHI grant [17H01205] from the Japan Society for the Promotion of Science (JSPS), JST CREST grant number [JPMJCR1991], Japan, and the Mirai Program (JST, Japan) grant number [JPMJMI18A2].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Dai H, Xu J, Qin H, et al. A temperature-responsive copolymer hydrogel in controlled drug delivery. Macromolecules. 2006;39:6584–6589. [Google Scholar]
- [2].Hu X, Liu W, Liu L, et al. Cyclodextrin-cross-linked diaminotriazine-based hydrogen bonding strengthened hydrogels for drug and reverse gene delivery. J Biomater Sci Polym Ed. 2013;24:1869–1882. [DOI] [PubMed] [Google Scholar]
- [3].Zhang P, Liu W, Li W, et al. Cationic polymer brush grafted-nanodiamond via atom transfer radical polymerization for enhanced gene delivery and bioimaging. J Mater Chem. 2011;21:7755–7764. [Google Scholar]
- [4].Tang L, Liu W, Bai T, et al. Robust MeO2MA/vinyl-4,6-diamino-1,3,5-triazine copolymer hydrogels-mediated reverse gene transfection and thermo-induced cell detachment. Biomaterials. 2011;32:1943–1949. [DOI] [PubMed] [Google Scholar]
- [5].Wang N, Liu W, Zhang Y, et al. High-strength photoresponsive hydrogels enable surface-mediated gene delivery and light-induced reversible cell adhesion/detachment. Langmuir. 2014;30:11823–11832. [DOI] [PubMed] [Google Scholar]
- [6].Sun L, Wang W, Zhang J, et al. Fenton reaction-initiated formation of biocompatible injectable hydrogels for cell encapsulation. J Mater Chem B. 2013;1:3932–3939. [DOI] [PubMed] [Google Scholar]
- [7].Nan W, Liu W, Gao H, et al. Fabrication of a shape memory hydrogel based on imidazole–zinc ion coordination for potential cell-encapsulating tubular scaffold application. Soft Matter. 2013;9:132–137. [Google Scholar]
- [8].Moschou EA, Daunert S, Bachas LG, et al. Artificial muscle material with fast electroactuation under neutral pH conditions. Chem Mater. 2004;16:2499–2502. [Google Scholar]
- [9].Moschou EA, Daunert S, Bachas LG, et al. Voltage-switchable artificial muscles actuating at near neutral pH. Sens Actuators B. 2006;115:379–383. [Google Scholar]
- [10].Takashima Y, Harada A, Otsubo M, et al. Expansion-contraction of photoresponsive artificial muscle regulated by host-guest interactions. Nat Commun. 2012;3:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lehn J-M. Dynamic combinatorial chemistry and virtual combinatorial libraries. Chem Eur J. 1999;5:2455–2463. [Google Scholar]
- [12].Rowan SJ, Stoddart JF, Cousins GRL, et al. Dynamic covalent chemistry. Angew Chem Int Ed. 2002;41:898–952. [DOI] [PubMed] [Google Scholar]
- [13].Wojtecki RJ, Meador MA, Rowan SJ. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat Mater. 2011;10:14–27. [DOI] [PubMed] [Google Scholar]
- [14].Otto S. Dynamic molecular networks: from synthetic receptors to self-replicators. Acc Chem Res. 2012;45:2200–2210. [DOI] [PubMed] [Google Scholar]
- [15].Mondal M, Hirsch AK. Dynamic combinatorial chemistry: a tool to facilitate the identification of inhibitors for protein targets. Chem Soc Rev. 2015;44:2455–2488. [DOI] [PubMed] [Google Scholar]
- [16].Montarnal D, Leibler L, Tournilhac F, et al. Silica-like malleable materials from permanent organic networks. Science. 2011;334:965–968. [DOI] [PubMed] [Google Scholar]
- [17].Otsuka H, Takahara A, Sakada M, et al. Scrambling reaction between polymers prepared by step-growth and chain-growth polymerizations: macromolecular cross-metathesis between 1,4-polybutadiene and olefin-containing polyester. Chem Commun. 2009;9:1073–1075. [DOI] [PubMed] [Google Scholar]
- [18].Lu Y-X, Guan Z, Leibler L, et al. Making insoluble polymer networks malleable via olefin metathesis. J Am Chem Soc. 2012;134:8424–8427. [DOI] [PubMed] [Google Scholar]
- [19].Chen X, Wudl FA, Ono K, et al. Thermally re-mendable cross-linked polymeric material. Science. 2002;295:1698–1702. [DOI] [PubMed] [Google Scholar]
- [20].Ishida K, Yoshie N. Synthesis of readily recyclable biobased plastics by Diels–Alder reaction. Macromol Biosci. 2008;8:916–922. [DOI] [PubMed] [Google Scholar]
- [21].Otsuka H, Takahara A, Higaki Y, et al. Polymer scrambling: macromolecular radical crossover reaction between the main chains of alkoxyamine-based dynamic covalent polymers. J Am Chem Soc. 2003;25:4064–4065. [DOI] [PubMed] [Google Scholar]
- [22].Zhang ZP, Yuan C, Zhang MQ, et al. Alkoxyamine with reduced homolysis temperature and its application in repeated autonomous self-healing of stiff polymers. Polym Chem. 2013;4:4648–4657. [Google Scholar]
- [23].Otsuka H. Reorganization of polymer structures based on dynamic covalent chemistry: polymer reactions by dynamic covalent exchanges of alkoxyamine units. Polym J. 2013;45:879–891. [Google Scholar]
- [24].Otsuka H, Nagano S, Kobashi Y, et al. A dynamic covalent polymer driven by disulfide metathesis under photoirradiation. Chem Commun. 2010;46:1150–1152. [DOI] [PubMed] [Google Scholar]
- [25].Fairbanks BD, Anseth KS, Bowman CN, et al. Photodegradable, photoadaptable hydrogels via radical-mediated disulfide fragmentation reaction. Macromolecules. 2011;44:2444–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Deng G, Chen Y, Yu H, et al. Dynamic hydrogels with an environmental adaptive self-healing ability and dual responsive sol–gel transitions. ACS Macro Lett. 2012;1:275–279. [DOI] [PubMed] [Google Scholar]
- [27].Michal BT, Rowan SJ, Spencer EJ, et al. Inherently photohealable and thermal shape-memory polydisulfide networks. ACS Macro Lett. 2013;2:694–699. [DOI] [PubMed] [Google Scholar]
- [28].Ulrich S, Lehn J-M. Reversible switching between macrocyclic and polymeric states by morphological control in a constitutional dynamic system. Angew Chem Int Ed. 2008;47:2240–2243. [DOI] [PubMed] [Google Scholar]
- [29].Belowich ME, Stoddart JF. Dynamic imine chemistry. Chem Soc Rev. 2012;41:2003–2024. [DOI] [PubMed] [Google Scholar]
- [30].Deng CC, Sumerlin BS, Abboud KA, et al. Boronic acid-based hydrogels undergo self-healing at neutral and acidic pH. ACS Macro Lett. 2015;4:220–224. [DOI] [PubMed] [Google Scholar]
- [31].Cromwell OR, Chung J, Guan Z. Malleable and self-healing covalent polymer networks through tunable dynamic boronic ester bonds. J Am Chem Soc. 2015;137:6492–6495. [DOI] [PubMed] [Google Scholar]
- [32].Röttger M, Leibler L, van der Weegen R, et al. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science. 2017;356:62–65. [DOI] [PubMed] [Google Scholar]
- [33].Imato K, Otsuka H. Self-healing of chemical gels cross-linked by diarylbibenzofuranone-based trigger-free dynamic covalent bonds at room temperature. Angew Chem Int Ed. 2012;51:1138–1142. [DOI] [PubMed] [Google Scholar]
- [34].Ying H, Zhang Y, Cheng J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat Commun. 2014;5:3218–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wei Z, Chen YM, Zhou J, et al. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem Soc Rev. 2014;43:8114–8131. [DOI] [PubMed] [Google Scholar]
- [36].Xu H, Cao W, Zhang X. Selenium-containing polymers: promising biomaterials for controlled release and enzyme mimics. Acc Chem Res. 2013;46:1647–1658. [DOI] [PubMed] [Google Scholar]
- [37].Miao X, Yang Z, Zheng W, et al. Switchable catalytic activity: selenium-containing peptides with redox-controllable self-assembly properties. Angew Chem Int Ed. 2013;52:7781–7785. [DOI] [PubMed] [Google Scholar]
- [38].Pillai R, Uyehara-Lock JH, Bellinger FP. Selenium and selenoprotein function in brain disorders. IUBMB Life. 2014;66:229–239. [DOI] [PubMed] [Google Scholar]
- [39].Zeng X, Xue W, Li M, et al. Redox poly(ethylene glycol)-b-poly(l-lactide) micelles containing diselenide bonds for effective drug delivery. J Mater Sci Mater Med. 2015;26:234. [DOI] [PubMed] [Google Scholar]
- [40].Schoenmakers E, Chatterjee K, Agostini M, et al. Mutation in human selenocysteine transfer RNA selectively disrupts selenoprotein synthesis. J Clin Invest. 2016;126:992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cao W, Xu H, Miao X, et al. γ-Ray-responsive supramolecular hydrogel based on a diselenide-containing polymer and a peptide. Angew Chem Int Ed. 2013;52:6233–6237. [DOI] [PubMed] [Google Scholar]
- [42].Ji S, Xu H, Yu Y, et al. Dynamic diselenide bonds: exchange reaction induced by visible light without catalysis. Angew Chem Int Ed. 2014;53:6781–6785. [DOI] [PubMed] [Google Scholar]
- [43].Ji S, Xu H, Yu Y, et al. Visible-light-induced self-healing diselenide-containing polyurethane elastomer. Adv Mater. 2015;27:7740–7745. [DOI] [PubMed] [Google Scholar]
- [44].Xia J, Ji S, Xu H. Diselenide covalent chemistry at the interface: stabilizing an asymmetric diselenide-containing polymer via micelle formation. Polym Chem. 2016;7:6708–6713. [Google Scholar]
- [45].Kildahl NK. Bond energy data summarized. J Chem Educ. 1995;72:423–424. [Google Scholar]
- [46].An X, Zhu J, Irusta L, et al. Aromatic diselenide crosslinkers to enhance the reprocessability and self-healing of polyurethane thermosets. Polym Chem. 2017;8:3641–3646. [Google Scholar]
- [47].Pan X, Du FE P, Zhu X, et al. Selenolactone as a building block toward dynamic diselenide-containing polymer architectures with controllable topology. ACS Macro Lett. 2017;6:89–92. [DOI] [PubMed] [Google Scholar]
- [48].Wenjing L, Lijuan Z, Wen L, et al. Mesoscopic simulations of drug-loaded diselenide crosslinked micelles: stability, drug loading and release properties. Colloid Surf B. 2019;182:110313. [DOI] [PubMed] [Google Scholar]
- [49].Xia J, Xu H, Pan S, et al. Diselenide-containing polymeric vesicles with osmotic pressure response. ACS Macro Lett. 2019;8:629–633. [DOI] [PubMed] [Google Scholar]
- [50].Ji S, Xu H, Sun C, et al. Visible light-Induced plasticity of shape memory polymers. ACS Appl Mater Interfaces. 2017;9:33169–33175. [DOI] [PubMed] [Google Scholar]
- [51].Fan F, Xu H, Wang S, et al. Swelling-induced 3D photopatterning on a diselenide-containing elastomer. J Mater Chem C. 2019;7:10777–10782. [Google Scholar]
- [52].Suzuki N, Otsuka H, Ohishi T, et al. Enhancement of the stimuli-responsiveness and photo-stability of dynamic diselenide bonds and diselenide-containing polymers by neighboring aromatic groups. Polymer. 2018;154:281–290. [Google Scholar]
- [53].Cheng X, Li H, Sun T, et al. Oxidation- and thermo-responsive poly(N-isopropylacrylamide-co-2-hydroxyethyl acrylate) hydrogels cross-linked via diselenides for controlled drug delivery. RSC Adv. 2015;5:4162–4170. [Google Scholar]
- [54].Gong C, Wu G, Li B, et al. Injectable dual redox responsive diselenide-containing poly(ethylene glycol) hydrogel. J Biomed Mater Res A. 2017;105A:2451–2460. [DOI] [PubMed] [Google Scholar]
- [55].Gao Y, Dong CM. Triple redox/temperature responsive diselenide-containing homopolypeptide micelles and supramolecular hydrogels thereof. J Polym Sci Part A: Polym Chem. 2018;56:1067–1077. [Google Scholar]
- [56].Anugraha DSB, Lim KT, Kim M, et al. Near-infrared light-responsive alginate hydrogels based on diselenide-containing cross-linkage for on demand degradation and drug release. Carbohydr Polym. 2019;223:115070. [DOI] [PubMed] [Google Scholar]
- [57].Laschat S, Bach M, Southan A, et al. Synthesis of pyridine acrylates and acrylamides and their corresponding pyridinium ions as versatile cross-linkers for tunable hydrogels. Synthesis. 2014;46:1243–1253. [Google Scholar]
- [58].Flory PJ, Rehner J. Statistical mechanics of cross-linked polymer networks i. rubberlike elasticity. J Chem Phys. 1943;11:512–520. [Google Scholar]
- [59].Ono T, Sada K, Shinkai S, et al. Lipophilic polyelectrolyte gels as super-absorbent polymers for nonpolar organic solvents. Nat Mater. 2007;6:429–433. [DOI] [PubMed] [Google Scholar]
- [60].Imato K, Otsuka H, Nishihara M, et al. Network reorganization of dynamic covalent polymer gels with exchangeable diarylbibenzofuranone at ambient temperature. J Am Chem Soc. 2014;136:11839–11845. [DOI] [PubMed] [Google Scholar]
- [61].Takahashi A, Goseki R, Otsuka H. Thermally adjustable dynamic disulfide linkages mediated by highly air-stable 2,2,6,6-tetramethylpiperidine-1-sulfanyl (TEMPS) radicals. Angew Chem Int Ed. 2017;56:2016–2021. [DOI] [PubMed] [Google Scholar]
- [62].Döhler D, Michael P, Binder WH. Principles of self-healing polymers. In: Binder WH, editor. Self-healing polymers: from principles to applications. Weinheim (Germany): Wiley-VCH; 2013. p. 7–14. [Google Scholar]
- [63].Scott TF, Bowman CN, Cook WD, et al. Photoinduced plasticity in cross-linked polymers. Science. 2005;308:1615–1617. [DOI] [PubMed] [Google Scholar]


