Abstract
Three yeast strains (Hue-1, Hue-8, and Hue-19) with strong heavy metal tolerance were isolated from mangosteen from Hue city, Vietnam. They exhibited identical phenotype and phylogeny. Sequence analysis of the D1/D2 region of the LSU rRNA gene and the internal transcribed spacer (ITS) region demonstrated that the closest relative of these strains is Papiliotrema sp. with 2.12% and 3.55–3.7% divergence in the D1/D2 domain, and ITS domain, respectively. Based on the physiological, biochemical, and molecular data, the three strains belong to a novel species of Papiliotrema genus, for which the name Papiliotrema huenov sp. nov. is proposed. These strains are highly tolerant of heavy metals compared to other yeasts, being able to grow in the presence of 2 mM Pb (II), 2 mM Cd (II), and up to 5 mM Ni (II), but no growth was observed in the presence of 1 mM As (III).
Keywords: Papiliotrema huenov sp., heavy metal tolerance, yeast mangosteen
1. Introduction
Due to both natural processes and certain industrial enterprises, environment becomes contaminated by various pollutants, including heavy metals [1,2]. In Vietnam, most heavy metal emissions are produced by industrial activities, but mining activities and transport, as well as the spreading of fertilizer and sewage sludge also discharge heavy metals into environment [3,4]. Heavy metal pollution is becoming a serious ecological issue throughout the world because, unlike the organic wastes that can be naturally decomposed in most cases, the heavy metals accumulated in the environment usually are not removable through natural process. They accumulate in tissues throughout the food chain and potentially accumulate in the human body, becoming a serious threat to human health at levels higher than allowable limits [5]. To cope with toxic levels of heavy metal, microorganisms turn on the metal detoxification systems, which either allow heavy metals to enter the cell or remove them through the export pathway [6].
Yeast cells are adaptable to environmental metal contamination and can withstand and detoxify such metals [7,8]. This is achieved through inherent mechanisms such as transformation, crystallization, complexation, extracellular precipitation, and cell wall adsorption [9].
Heavy metal tolerant yeasts have been isolated, and their biomass used in the removal of heavy metals from industrial wastewater and/or contaminated water, for example, Rhizopus stolonifera is resistant to and can remove lead, cadmium, copper, and zinc [10]. Rhodotorula mucilaginosa [11], and Aspergillus niger [12,13] are also shown tolerance and removal activities to heavy metals, such as mercury, copper, lead, zinc, cadmium, etc.
Papiliotrema is a genus of yeast Basidiomycota, which was first described by Sampaio et al. to accommodate P. bandonii, a species which produces minute basidiocarps that are associated with pyrenomycetous ascomycetes on grass [14].
More recently, several novel Papiliotrema species in this clade have been described after isolation from leaves and flowers, such as P. phichitensis, P. siamensis, P. plantarum, P. leoncinii, and P. miconiae [15–18].
This study describes three novel yeast Papiliotrema species, isolated during a study of heavy metal absorbing yeast diversity from Vietnamese fruits and plants. Three strains were isolated from mangosteen, harvested from Hue city, located in Thua Thien Hue province. Based on phenotype and molecular analyses of D1/D2 region of the ribosomal large subunit (LSU) rRNA gene and the internal transcribed spacer (ITS) region, the three strains of the genus Papiliotrema were not assigned to any of the known species.
We propose a new monophyletic genus Papiliotrema huenov sp. nov. Strains in this study exhibited high tolerance toward Ni, Pb, and Cd, but were sensitive to As. These strains could, therefore, be used in heavy metals removal and waste treatment, following a detailed study.
2. Materials and methods
2.1. Sample collection and isolation
Thirty samples of mangosteen were collected from different farms in Hue city, Thua Thien Hue province. Samples were diced and incubated at 30 °C for 3 days. An aliquot (1.5 mL of juice) was centrifuged for 1 min at 3000 rpm in a micro-centrifuge and the supernatant was removed. The precipitate was resuspended in 200 µL of YM media (1% malt extract, 1% yeast extract, 1% peptone, and 2% of glucose). Isolation of yeasts was carried out by inoculation of serial dilutions on YMA plates (YM and 2% of agar), with 100 mg/L of chloramphenicol.
The plates were incubated at 30 °C for 3 days, and colonies with yeast-like morphology were streaked onto fresh YMA plates. The isolated yeasts were identified according to Kurtzman et al. [19]. After selection and purification, long-term storage was performed in 10 % (w/v) glycerol at −80 °C.
2.2. Physiological and biochemical characterization
Phenotypic properties of isolated yeast were described according to the standard methods [19]. Colony morphology, including colony shape and color, was established by direct macroscopic observation of yeast colonies growing on YMA plates. Images of colony morphology were captured using a Canon camera (model EOS 5D Mark IV; Canon, Tokyo, Japan).
Microscopic observations were performed using a small amount of cells from cultures or colonies fixed on a glass slide at 100× magnification to observe cell shape, presence of bud, and type of budding cells. The temperature range for optimal growth was also estimated in YM broth.
An assimilation test was carried out according to previously reported method [20]. In brief, yeast nitrogen base medium (YNB; Sigma-Aldrich, St. Louis, MO) and yeast carbon base medium (Sigma-Aldrich) were used to evaluate the assimilation of carbon and nitrogen sources by the Hue-1 strain. Cultures were grown on malt extract broth at 25 °C for 2 days, 100 µl of culture was added to a test tube containing sterilized media and incubated at 25 °C for 5 days. Test tubes were observed visually for positive or negative growth of culture on carbon and nitrogen source media.
Formation of hyphae and pseudohyphae was examined on potato dextrose agar (PDA) and corn meal agar in slide cultures for 2 weeks at 25 °C. For study of ballistoconidia formation, yeast cells were plated on PDA for 4 weeks at 15 °C based on previously reported method [19].
2.3. Phylogenetic analysis
Yeast genomic DNA of each of the isolated colonies was extracted as previously reported [21]. Strains were identified by amplification and sequencing of two rDNA including ITS1 and ITS2, and D1/D2 domain of LSU rRNA. Briefly, ITS fragments of 534 bp in size were amplified using primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3), and 560 bp DNA fragments of the 26S rDNA gene were amplified by PCR using D1/D2 region primers NL1 (5′-GCATATCAATAAGCGGGGAAAAG-3′) and the reverse primer NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) as previously described [22] at a final concentration of 0.5 μM and oneTaq Master Mix (New England biolabs, Ipswich, MA), following thermal program: preincubation for 5 min at 94 °C; 35 cycles of 94 °C for 1 min, 54 °C for 0.5 min, and 68 °C for 1.5 min, and a final extension at 68 °C for 5 min. PCR products were checked by electrophoresis in 1.5% agarose gels. Amplicons were then purified using the GenElute PCR Clean Up kit (Sigma-Aldrich). The purified amplicons were quantified based on the absorbance of DNA at 260 nm with a NanoDrop 2000 (Thermo Fisher, Waltham, MA), and sequenced by Macrogen Inc (Seoul, South Korea).
All ITS1-2 sequences of Hue 1, Hue 8, and Hue 19 were submitted to GenBank with the accession numbers MN539616, MN539618, and MN551060, respectively. Yeast strains showing nucleotide substitutions covering more than 1% of the D1/D2 domain usually are considered different species [22].
Sequences were compared pairwise using the Basic Logarithmic Alignment Search Tool (BLAST) algorithm in the National Centre for Biotechnology Information (NCBI) database (minimum 97% sequence similarity and 95% coverage). In parallel, multiple alignments were carried out using the CLUSTAL X program [23].
Evolutionary distances were calculated using Kimura’s two-parameter model [24]. Tree topologies were reconstructed using the neighbor-joining method of the MEGA 7 software package [25], with bootstrap values based on 1000 random resampling [26]. Tree topologies were recovered through a combination of the maximum-likelihood and maximum-parsimony methods.
2.4. Heavy metal tolerance of the isolated yeast
The tolerance of isolated yeasts toward heavy metals was assayed by drop test of cells onto YD medium (2% glucose, 1% yeast extract, and 2% agar) in 96-well plates, supplemented with different concentrations of heavy metals (Cd (II), Pb (II), As (III), and Ni (II)), and incubation for 5 days at 25 °C. Colony biofilms were observed and photographed by Canon camera. The effect of heavy metals on dynamic development in culture was determined by monitoring optical density measurements at 600 nm.
3. Results and discussion
3.1. Phylogenetic analysis
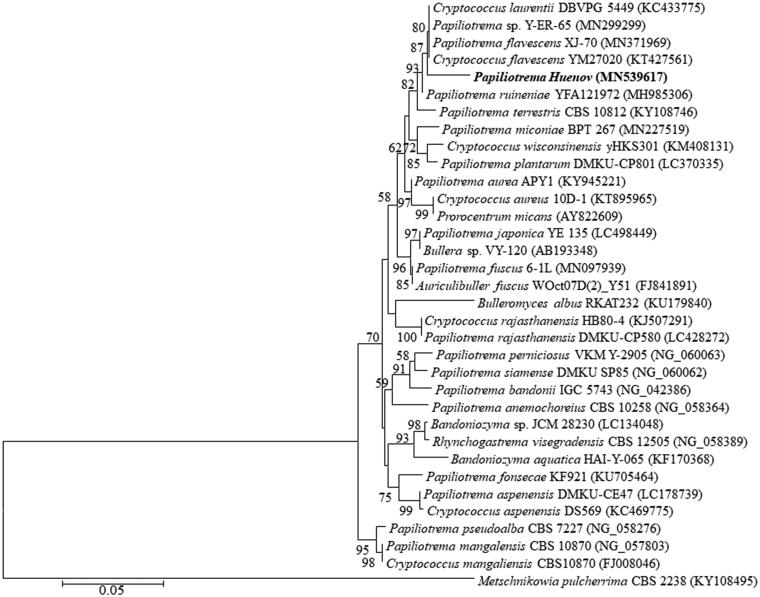
105 yeast strains were isolated from 30 mangosteen samples. Based on morphology and physiology, the isolates Hue 1, Hue 8, and Hue 19 were unique and were estimated to belong to a common group. The ITS sequences of the three strains differed slightly from each other by up to 3 nt, while the D1/D2 sequences were identical (GenBank accession number MN539617). Sequence analysis of the D1/D2 domain showed that the three strains were closely related to Papiliotrema flavescens (MN371969) and Cryptococcus flavescens (KT427561) with sequence similarity of 97.87%, but showed 2.12% nucleotide differences (12 nucleotides over 564 nts). Likewise, comparison of the ITS region revealed 3.55–3.7% nucleotide differences (17 nucleotide substitutions and 3 gaps out of 534 nts) to P. terrestris (MG251420), P. flavescens (MN371875), and Cryptococcus sp (MN077561), with 96.45–96.3% sequence similarity.
Due to the position of the new species in the genome tree, based on the D1/D2 region of the LSU rRNA gene, it is closer to Papiliotrema than to other species. A new species associated with the same genus of the Bulleromyces/Papiliotrema/Auriculibullera clade. Moreover, physiological and biochemical properties (shown in Table 1) demonstrated that the three strains are different from Cryptococcus species. In addition, several Cryptococcus species phylogenetically close to P. bandonii were proposed to be members of the genus Papiliotrema based on molecular analysis [27].
Table 1.
Differences in physiological characteristics between Papiliotrema huenov and the closest species. +, Positive; S, slowly positive; W, weak; L, latent; −, negative.
| Characteristic | Papiliotrema siamense [18] | Cryptococcus nemorosus [28] | Papiliotrema Huenov |
|---|---|---|---|
| Assimilation of carbon sources: | |||
| Maltose | − | + | W |
| Inulin | − | W | − |
| d−Ribose | W | + | S |
| N-Acetylglucosamine | + | S | + |
| Glycerol | − | S | S |
| Ethanol | − | + | − |
| Soluble starch | W | S | W |
| d-Glucuronate | − | + | − |
| l-Rhamnose | L | S | L |
| Cellobiose | − | + | − |
| Sorbose | + | W | + |
| Salicin | − | + | − |
| d-Glucitol | − | + | − |
| Galactitol | − | + | − |
| 2-Ketogluconic acid | − | + | S |
| Assimilation of nitrogen sources | |||
| Sodium nitrite | − | + | − |
| Growth without vitamins | − | − | − |
| Growth in 60% glucose | W | S | W |
In general, our morphological, physiological, and biochemical, and phylogenetic analyses clearly distinguished these strains from their close phylogenetic neighbors. Therefore, these strains (Hue 1, Hue 8, and Hue 19) represent a novel species of Papiliotrema genus. The novel species has been named Papiliotrema huenov (Figure 1).
Figure 1.
Phylogenetic tree based on sequences of the D1/D2 region of LSU rRNA gene, showing the position of Papiliotrema huenov strain among closely related taxa. The numbers at nodes indicate levels of bootstrap support (%) based on a neighbour-joining analysis of 1000 resampled datasets; only values ≥50% are given. Metschnikowia pulcherrima CBS 2238 (KY108495) was used as an outgroup. Bar, 0.05 substitutions per site.
3.2. Morphological and physiological characteristics
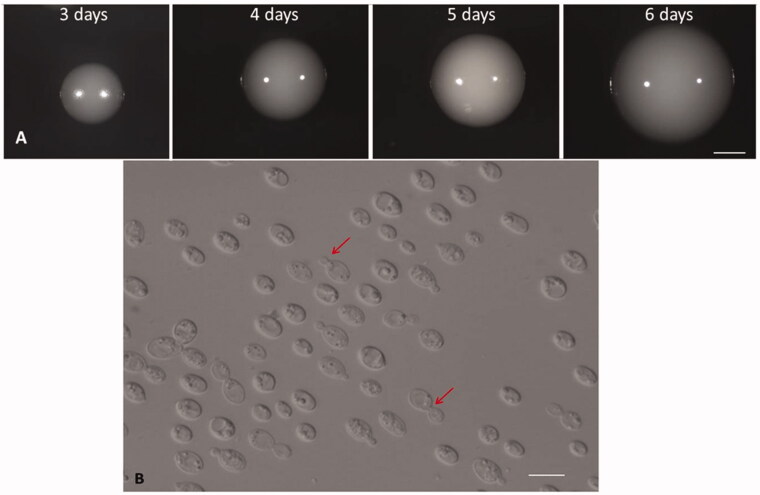
Yeast colonies were initially whitish/cream smooth at 3 days old, as the size of the colonies increased from 2 to 3 mm in diameter over 3 days (Figure 2).
Figure 2.
Colony morphology and colony development. (A) Development of colonies grown on YMA from 3 to 6 days old. Bar, 1 mm. (B) Budding cells of Papiliotrema Huenov grown in YM broth for 3 days at 25 °C. Red arrows indicate budding sites. Bar, 10 µm.
The morphology of the vegetative cells of yeast was observed after 3 days growth on YM at 25 °C. Cells exhibit globose, ovoidal shape with variations in size (2–6.5 × 2–8 µm). Pseudohyphae and hyphae were not observed after 2 weeks grown on corn meal agar and PDA agar at 25 °C. In agreement with this, ballistospores are not formed on PDA agar after culture at 15 °C for up to 4 weeks. These findings are similar to those for Papiliotrema siamense, Papiliotrema sp. [16–18] which provides further evidence that the strain belongs to the Papiliotrema clade.
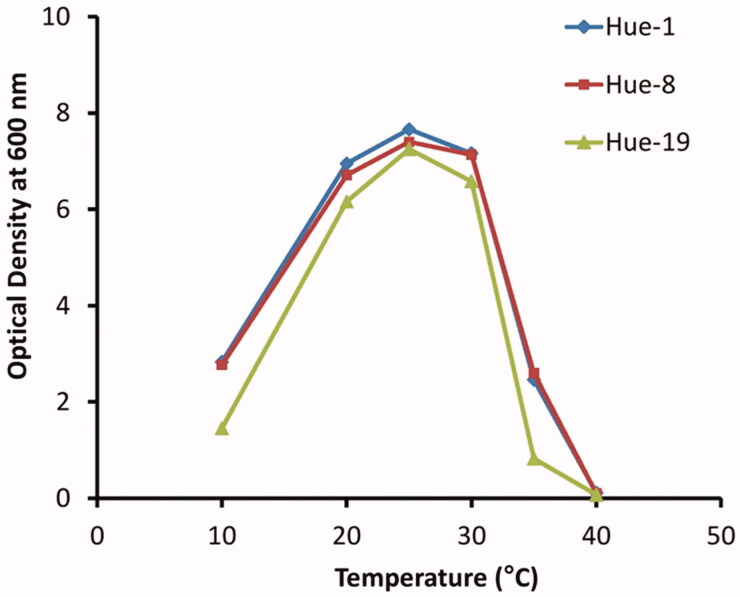
The results indicated that all yeast strains were able to grow at temperatures over a wide range from 10 °C to 35 °C. While at 25 °C is the optimal temperature for growth of the three yeast strains, poor growth was observed at 10 °C and at 35 °C, while at 40 °C, no growth was observed in all three strains (Figure 3). The current analysis also indicated that the Hue-19 strain is more sensitive to temperature compared to Hue-1 and Hue-8.
Figure 3.
Effect of temperature on dynamic growth of Papiliotrema Huenov strains. Hue-1, 8, and 19 were grown on YD agar at different temperatures (10, 20, 25, 30, 35, and 40 °C).
Results also demonstrated that isolated yeasts can utilize a wide range of carbon sources (Table 1).
Fermentation of d-glucose was negative. Nitrate, nitrite, and inulin were not assimilated. Growth was observed on 50% glucose medium but not on a medium with 10 % (w/v) NaCl plus 5 % (w/v) glucose or with 60 % glucose. Vitamins were necessary for growth.
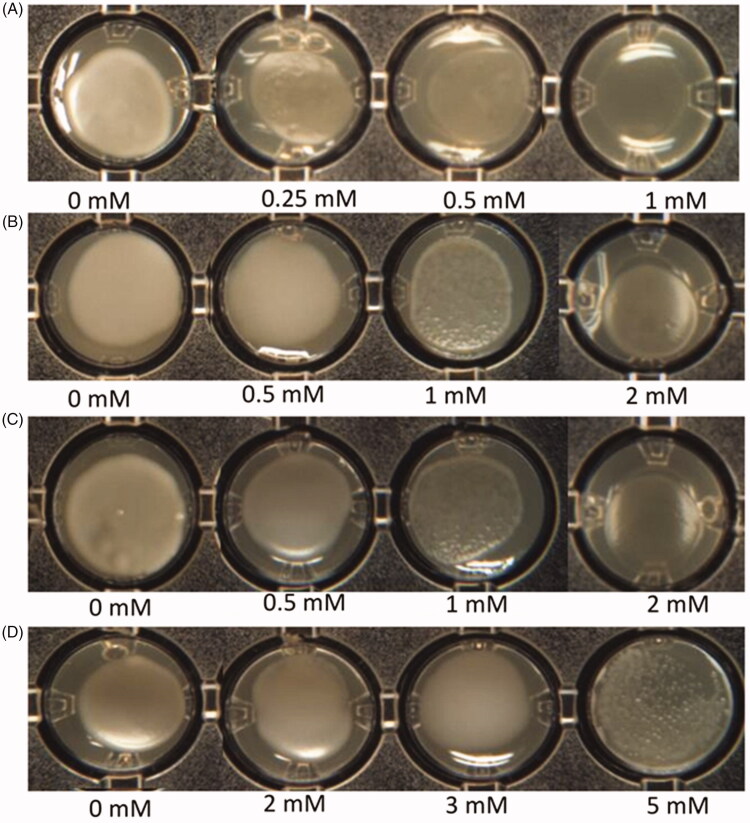
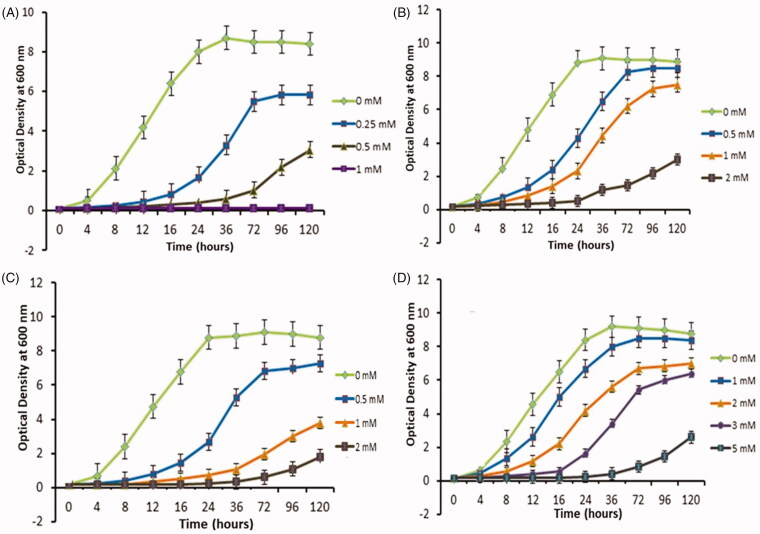
Figure 4 shows that the colonization ability and biofilm development of the Hue-1 strain, which is a representative of other strains (Hue-8 and Hue-19) varied on media, supplemented with different concentrations of Cd, Pb (II), Ni (II), and As (III). Large/giant colonies were formed on medium containing low levels of heavy metal (appox 0.5 mM), whereas there were only a few small colonies or cells could not form colonies on As media at the same concentration (Figure 4(A)). Interestingly, cells grew poorly on 2 mM Cd or Pb (Figure 4(B) and (C), respectively), while growing normally on up to 5 mM of Ni (Figure 4(D)). In agreement with the growth curve kinetics in Figure 5, the graph declined in line with increasing concentration of heavy metal. It was notable that Hue-1 exhibited high tolerance to nickel, but sensitivity to arsenic.
Figure 4.
Effect of heavy metals on Hue-1 biofilm formation on YDA solid media. (A) As. (B) Cd. (C) Pb. (D) Ni.
Figure 5.
The growth of Hue-1 in YD medium supplemented with different concentrations of heavy metals. (A) As. (B) Cd. (C) Pb. (D) Ni.
The kinetics analysis showed that, except for arsenic, low concentration (0.5 mM) of heavy metals only slightly affects Hue-1 growth. However, increasing concentration of heavy metal inhibited the growth of Hue-1 by extending the lag phase and decreasing the maximum OD600. The tolerance of Hue-1 to Pb, and Cd showed a similar pattern at low concentration (less than 0.5 mM) (Figure 5(B), (C)). However, the strain exhibited stronger tolerance to Pb than Cd. At 2 mM of Pb caused lag phase to extend to 72 h (Figure 5(C)), while at the same concentration, of Cd, cells were stressed and grew poorly and lag phase was extended to 96 h (Figure 5(B)). Similar results were obtained in yeast of the Cryptococcus sp. which displayed higher tolerance for Pb than Cd [29]. Our strains, of which Hue-1 is representative, also exhibited higher tolerance of Cd than Rhodotorula mucilaginosa, and R. aurantiaca which can tolerate a maximum of 1.5 mM [30], but higher sensitivity compared to the Rhodorula glutinis strain [31] which can tolerate at least 5 mM.
Hue-1 strain exhibits a high tolerance to Ni, but is more sensitive to arsenic. Over a range of Ni concentration (1–3 mM) (Figure 5(D)), the lag phase was not significantly extended, whereas at 0.5 mM As, lag phase was significantly prolonged to 72 h (Figure 5(A)), and the maximum OD600 dropped to lower than 3, a decrease of almost 60% compared to that at 1 mM of nickel. When As concentration rose to 1 mM, no growth was observed. This strain seems most tolerant to Ni compared to the Yarrowia lipolytica strain [32], a strain isolated from ocean that is highly tolerant of heavy metals. Moreover, none of the 70 yeast strains can tolerate more than 5 mM of nickel in according to Renata et al. [31].
The lag phase extension with increased heavy metal concentration is probably due to the stress caused by heavy metals, as reported previously [33,34]. As the concentration of heavy metal ions increased, the specific growth rate decreased and the doubling time of both yeast strains was extended. Similar results were found for other yeasts, for example, S. cerevisiae [35,36], and yeasts of the Cryptococcus sp [29].
In this study, three novel strains, named Papiliotrema huenov, were isolated from mangosteen in Hue, Vietnam. This novel species adds to overall knowledge of yeast biodiversity and represents an additional reference in its phylogenetic clade.
Isolated yeasts display significant metal tolerance, especially Ni. These results suggest the potential for application of the isolated yeast in bioremediation of heavy metals from polluted soils and waters.
Taxanomy
Type: Vietnam, Hue Prov, 16°27′59″B 107°33′29″Đ.
Yeast was isolated from mangosteen. The stock culture (Hue09072019) was deposited in the Institute of Biotechology, Hue University Vietnam.
Etymology: Papiliotrema huenov, in reference to the location of Hue city, Thua Thien Hue Province from where three strains were isolated.
Acknowledgements
The authors thank Dr. Derek Wilkinson for proofreading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Tchounwou PB, Yedjou CG, Patlolla AK, et al. . Heavy metal toxicity and the environment. Exp Suppl. 2012;101:133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song B, Zeng G, Gong J, et al. . Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals. Environ Int. 2017;105:43–55. [DOI] [PubMed] [Google Scholar]
- 3.Lasko K, Vadrevu KP, Nguyen T.. Analysis of air pollution over Hanoi, Vietnam using multi-satellite and MERRA reanalysis datasets. PLoS One. 2018;13(5):e0196629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen TTH, Zhang W, Li Z, et al. . Assessment of heavy metal pollution in Red River surface sediments, Vietnam. Mar Pollut Bull. 2016;113(1–2):513–519. [DOI] [PubMed] [Google Scholar]
- 5.Beyersmann D, Hartwig A.. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol. 2008;82(8):493–512. [DOI] [PubMed] [Google Scholar]
- 6.Wysocki R, Fortier PK, Maciaszczyk E, et al. . Transcriptional activation of metalloid tolerance genes in Saccharomyces cerevisiae requires the AP-1-like proteins Yap1p and Yap8p. Mol Biol Cell. 2004;15(5):2049–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosiner D, Gerber S, Lichtenberg FH, et al. . Impact of acute metal stress in Saccharomyces cerevisiae. PLoS One. 2014;9(1):e83330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamo GM, Brocca S, Passolunghi S, et al. . Laboratory evolution of copper tolerant yeast strains. Microb Cell Fact. 2012;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bankar A, Zinjarde S, Shinde M, et al. . Heavy metal tolerance in marine strains of Yarrowia lipolytica. Extremophiles. 2018;22(4):617–628. [DOI] [PubMed] [Google Scholar]
- 10.Fawzy EM, Abdel MFF, Elzayat SA.. Biosorption of heavy metals onto different eco-friendly substrates. J Toxicol Environ Health Sci. 2017;9(5):35–44. [Google Scholar]
- 11.Grujić S, Vasić S, Radojević I, et al. . Comparison of the Rhodotorula mucilaginosa biofilm and planktonic culture on heavy metal susceptibility and removal potential. Water Air Soil Pollut. 2017;228(2):73–18. [Google Scholar]
- 12.Acosta RI, Cardenas GJF, Rodriguez PAS, et al. . Bioremoval of different heavy metals by the resistant fungal strain Aspergillus niger. Bioinorg Chem Appl. 2018;2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Wang Q, Luo Q, et al. . Biosorption behavior of heavy metals in bioleaching process of MSWI fly ash by Aspergillus niger. Biochem Eng J. 2009;46(3):294–299. [Google Scholar]
- 14.Sampaio JP, Weiß M, Gadanho M, et al. . New taxa in the Tremellales: Bulleribasidium oberjochense gen. et sp. nov., Papiliotrema bandonii gen. et sp. nov. and Fibulobasidium murrhardtense sp. nov. Mycologia. 2002;94(5):873–887. [PubMed] [Google Scholar]
- 15.Into P, Pontes A, Jacques N, et al. . Papiliotrema plantarum sp. nov., a novel tremellaceous sexual yeast species. Int J Syst Evol Microbiol. 2018;68(6):1937–1941. [DOI] [PubMed] [Google Scholar]
- 16.Khunnamwong P, Surussawadee J, Srisuk N, et al. . Papiliotrema phichitensis f.a., sp. nov., a novel yeast species isolated from sugarcane leaf in Thailand. Antonie van Leeuwenhoek. 2018;111(12):2455–2461. [DOI] [PubMed] [Google Scholar]
- 17.Machado PD, Brandao LR, Santos AR, et al. . Papiliotrema leoncinii sp. nov. and Papiliotrema miconiae sp. nov., two tremellaceous yeast species from Brazil. Int J Syst Evol Microbiol. 2016;66(4):1799–1806. [DOI] [PubMed] [Google Scholar]
- 18.Surussawadee J, Khunnamwong P, Srisuk N, et al. . Papiliotrema siamense f.a., sp. nov., a yeast species isolated from plant leaves. Int J Syst Evol Microbiol. 2014;64(Pt 9):3058–3062. [DOI] [PubMed] [Google Scholar]
- 19.Kurtzman CP, Fell JW, Boekhout T, et al. . Methods for isolation, phenotypic characterization and maintenance of yeasts. Vol. 1 Amsterdam: Elsevier; 2011. p. 87–110. [Google Scholar]
- 20.Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. [DOI] [PubMed] [Google Scholar]
- 21.Looke M, Kristjuhan K, Kristjuhan A.. Extraction of genomic DNA from yeasts for PCR-based applications. Biotechniques. 2011;50(5):325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtzman CP, Robnett CJ.. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek. 1998;73(4):331–371. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JD, Gibson TJ, Plewniak F, et al. . The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura M. Estimation of evolutionary distances between homologous nucleotide sequences. Proc Natl Acad Sci USA. 1981;78(1):454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S, Stecher G, Tamura K.. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–791. [DOI] [PubMed] [Google Scholar]
- 27.Liu XZ, Wang QM, Goker M, et al. . Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol. 2015;81:85–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtzman C, Fell JW, Boekhout T.. The yeasts: a taxonomic study. Amsterdam: Elsevier; 2011. [Google Scholar]
- 29.Singh P, Raghukumar C, Parvatkar RR, et al. . Heavy metal tolerance in the psychrotolerant Cryptococcus sp. isolated from deep-sea sediments of the Central Indian Basin. Yeast. 2013;30(3):93–101. [DOI] [PubMed] [Google Scholar]
- 30.Balsalobre L, De Siloniz MI, Valderrama MJ, et al. . Occurrence of yeasts in municipal wastes and their behaviour in presence of cadmium, copper and zinc. J Basic Microbiol. 2003;43(3):185–193. [DOI] [PubMed] [Google Scholar]
- 31.Vadkertiova R, Slavikova E.. Metal tolerance of yeasts isolated from water, soil and plant environments. J Basic Microbiol. 2006;46(2):145–152. [DOI] [PubMed] [Google Scholar]
- 32.Bankar AV, Kumar AR, Zinjarde SS.. Environmental and industrial applications of Yarrowia lipolytica. Appl Microbiol Biotechnol. 2009;84(5):847–865. [DOI] [PubMed] [Google Scholar]
- 33.Dar N, Shakoori A.. Chromium tolerant yeast strains isolated from industrial effluents and their possible use in environmental clean-up. Bull Environ Contam Toxicol. 1999;63(6):744–750. [DOI] [PubMed] [Google Scholar]
- 34.Liu B, Wang C, Liu D, et al. . Hg tolerance and biouptake of an isolated pigmentation yeast Rhodotorula mucilaginosa. PLoS One. 2017;12(3):e0172984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, McCarthney A, Qiu X, et al. . Cd2+ impact on metabolic cells of Saccharomyces cerevisiae over an extended period and implications for bioremediation. Geomicrobiol J. 2012;29(3):199–205. [Google Scholar]
- 36.Abbas SH, Ismail IM, Mostafa TM, et al. . Biosorption of heavy metals: a review. J Chem Sci Technol. 2014;3(4):74–102. [Google Scholar]