Abstract
Objectives
To describe the fraction of asymptomatic health-care workers (HCWs) in two designated hospitals for coronavirus disease 2019 (COVID-19) treatment in Wuhan and explore the factors associated with asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Methods
All HCWs in Wuhan Union Hospital and Wuhan Red Cross Hospital with either positive SARS-CoV-2 nucleic acid or positive antibody test before 18 April 2020 were included. Exposure, epidemiological and demographic information were retrospectively collected by a structured questionnaire. Medical records were also reviewed for clinical characteristics and CT images of HCWs.
Results
As of 18 April 2020, a total of 424 HCWs were identified. Among them, 276 (65.1%) were symptomatic and 148 (34.9%) were asymptomatic. Fifty-five (19.9%) families of the symptomatic HCWs and 16 (10.8%) families of the asymptomatic HCWs were infected with SARS-CoV-2. HCWs with infected family members tended to be symptomatic (OR 2.053, 95% CI 1.130–3.730; p 0.018). Multivariable logistic regression analysis exhibited that performing tracheal intubation or extubation (OR 4.057, 95% CI 1.183–13.909; p 0.026) was associated with an increased likelihood of symptomatic SARS-CoV-2 infection, whereas consistent use of N95 respirators (OR 0.369, 95% CI 0.201–0.680; p 0.001) and eye protection (OR 0.217, 95% CI 0.116–0.404; p < 0.001) were associated with an increased likelihood of asymptomatic SARS-CoV-2 infection.
Conclusions
Asymptomatic SARS-CoV-2 infection in HCWs comprised a considerable proportion of HCW infections during the pandemic of COVID-19. Those who performed tracheal intubation or extubation were most likely to develop related symptoms, whereas those taking aggressive measures, including consistent use of N95 masks and eye protection, tended to be asymptomatic cases.
Keywords: Asymptomatic infection, Eye protection, N95 respirators, Severe acute respiratory syndrome coronavirus 2
Introduction
The ongoing outbreak of 2019 novel coronavirus disease (COVID-19) was first reported in Wuhan, China and spread rapidly across the world [1,2]. Health-care workers (HCWs) have been at high risk for developing COVID-19 through nosocomial infection during the pandemic. Until 15 April 2020, a total of 6764 asymptomatic patients with COVID-19 had been reported in China [3]. The percentage of asymptomatic infections among confirmed COVID-19 patients were reported to be 7.9%–87.8%, which occupied a considerable proportion [[4], [5], [6]]. A previous study also demonstrated that among HCWs with COVID-19, 8/19 (42.1%) were asymptomatic [7]. Clinical characteristics of patients with asymptomatic versus symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have been previously compared [8]. However, the factors associated with symptomatic and asymptomatic SARS-CoV-2 infection are still unknown. In this study, we investigated 148 asymptomatic and 276 symptomatic HCWs who tested positive for either SARS-CoV-2 nucleic acid or antibody. By comparing the differences in epidemiological, demographic and clinical features between asymptomatic and symptomatic HCWs, we hope to give new insights into the transmission of SARS-CoV-2 among HCWs and offer some indirect evidence that symptom severity may be associated with the initial infectious dose of SARS-CoV-2.
Methods
Hospital setting and participants
This multicentre, retrospective, cohort study was conducted at Wuhan Union Hospital and Wuhan Red Cross Hospital. This study was approved by the Institutional Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (20200036) and the requirement for informed consent was waived by the Ethics Committee. From 31 December 2019 to 18 April 2020, a total of 7733 and 820 HCWs had been working in Wuhan Union Hospital and Wuhan Red Cross Hospital, respectively. Since 18 March 2020, all HCWs have been requested to receive SARS-CoV-2 nucleic acid and antibody detection tests before they return to their original work. Those HCWs who had been working in high-risk departments or had close contact with suspected or confirmed COVID-19 patients should also be subjected to chest CT scan. Until 18 April 2020, a total of 5839 HCWs in Wuhan Union Hospital and 634 workers in Wuhan Red Cross Hospital were subjected to SARS-CoV-2 nucleic acid and antibody tests.
RNA extraction, RT-PCR assay and antibodies test
The RNA extraction, RT-PCR assay and antibodies test were performed according to the previous study [9]. Total RNA was extracted from nasopharyngeal and/or oropharyngeal swab samples of HCWs within 2 hours using the respiratory sample RNA isolation kit (Xi'an Tianlong Science and Technology Co. Ltd, Xi'an, China and Guangzhou HEAS BioTech Co., Ltd, Guangzhou, China). RT-PCR assay was performed using a detection kit for SARS-CoV-2 RNA (Wuhan Easydiagnosis Biomedicine Co., Ltd, Wuhan, China and Daan Gene Co., Ltd of Sun Yat-sen University, Guangzhou, China). The serum SARS-CoV-2 antibodies were detected using the diagnostic kit for IgM/IgG antibody to SARS-CoV-2 (Zhuhai Livzon Diagnostics Inc., Zhuhai, China).
Definitions
Isolation wards, fever outpatient clinics, emergency departments, intensive care units, medical laboratories and radiology departments were defined as high-risk departments, given that HCWs in these departments inevitably contacted both probable and confirmed COVID-19 patients and their specimens and were always at high risk of exposure to SARS-CoV-2. The co-morbidities in our study included hypertension, cardiovascular disease, diabetes, cerebrovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, chronic liver disease, rheumatic diseases and malignancy.
Data collection
Exposure, epidemiological and demographic information were retrospectively collected by a structured questionnaire. All HCWs except one (who had died) turned in their surveys and for the deceased HCW, his colleagues were asked to help with the questionnaire. Information about the frequency of hand washing after making contact with confirmed or suspected COVID-19 patients and the frequency of using different types of personal protective equipment (never, sometimes, most of the time, all of the time) was requested from each HCW. Medical records were also reviewed for clinical characteristics and CT images of HCWs. On 27 January 2020, we initiated the follow up of infected HCWs for the exposure history. Since then, once an infected HCW is reported, his/her contact and exposure history are established by interview and recorded immediately.
Statistical analyses
To avoid overfitting in our multivariable model, we calculated the numbers of variables allowed to be enrolled in our logistic regression model based on a previous study for guidance on sample size requirements for prediction models [10]. Considering a total number of 424 HCWs (with 148 asymptomatic HCWs), five variables could be enrolled in the multivariable analyses. The events per candidate predictor parameter was 29.6, the model could explain <15% of the variability with mean absolute percentage error <0.05.
Variables included in the multivariable logistic regression model were selected based on the least absolute shrinkage and selection operator (LASSO) regression and the previous evidence [11]. In the LASSO regression, the performance of the model was augmented with ten-fold cross-validation, and the covariates were selected by minimum (λ min). Among 16 variables (including age, sex, job category, co-morbidities, tracheal intubation or extubation, conduct of oropharyngeal or nasopharyngeal examination, sputum suction, cardiopulmonary resuscitation, hand washing, isolation gown, surgical mask or N95 respirators, N95 respirators, gloves, hair cover, and eye protection) entered into the LASSO regression model, seven variables (age, job category, co-morbidities, tracheal intubation or extubation, N95 respirators, hair cover and eye protection) were available for the next step. A previous study had shown that younger age was associated with asymptomatic SARS-CoV-2 infection [8]. Another study showed that age and co-morbidities were predictors for symptom development in the initially asymptomatic carriers at admission [12]. Two studies showed that tracheal intubation procedure was related to increased risk of transmission for both SARS-CoV-1 and SARS-CoV-2 to HCWs [13,14]. Face mask and eye protection, especially N95 respirators, were most consistently linked to reduced COVID-19 infection among HCWs [14,15]. Face mask was also reported to be associated with asymptomatic SARS-CoV-2 infection based on several uncontrolled reports [16]. Therefore, we chose age, co-morbidities, tracheal intubation or extubation, N95 respirators and eye protection as the five variables for our multivariable logistic regression model and these were adjusted using an enter approach.
In the analysis, frequency of using personal protective equipment and frequency of hand hygiene practice were coded into two categories: used inconsistently (i.e. ‘never or sometimes used’) or used consistently (‘used most or all of the time’). Continuous and categorical variables were presented in median and interquartile range, or numbers and percentage, respectively. Continuous variables were compared using an unpaired t test. Differences between proportions of categorical variables were assessed using χ2 tests or Fisher's exact tests. Logistic regression model was used to calculate OR and 95% CI for asymptomatic infection. A two-sided p value < 0.05 was considered significant. The RStudio (version 1.2.5033) package ‘glmnet’ was used to perform the LASSO regression. Other statistical analyses were performed using SAS software package (version 9.4) (SAS Inc., Cary, NC, USA).
Results
Laboratory test of SARS-CoV-2
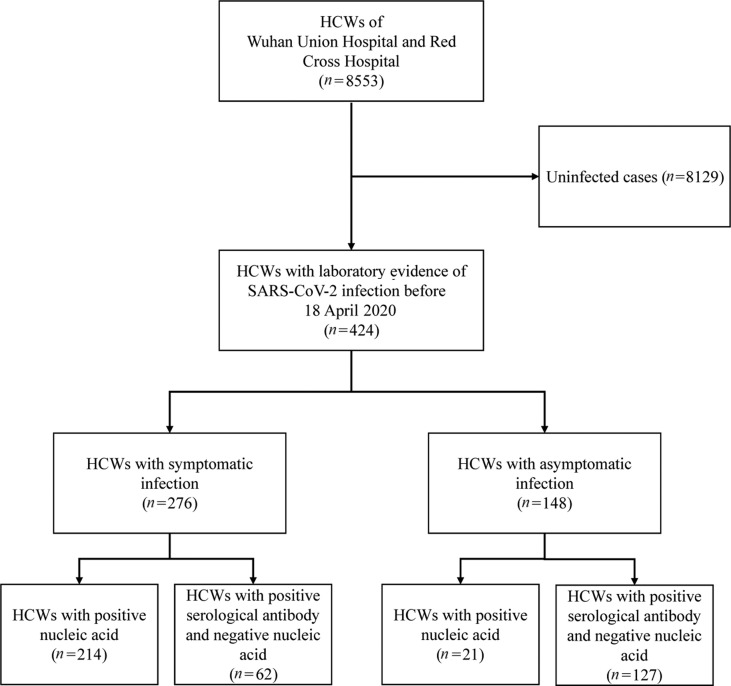
After the outbreak of COVID-19, 424/8553 (5.0%) HCWs had laboratory evidence of SARS-CoV-2 infection before 18 April 2020, with 326/7733 (4.2%) and 98/820 (12.0%) HCWs in Wuhan Union Hospital and Wuhan Red Cross Hospital, respectively. Among them, 276 HCWs were symptomatic and 148 HCWs were asymptomatic. The laboratory tests of the 424 HCWs are shown in Table 1 . Of the 276 symptomatic HCWs, 236 had a positive SARS-CoV-2 nucleic acid test and 62 were specifically positive for SARS-CoV-2 antibody but negative for SARS-CoV-2 nucleic acid. Among the 148 asymptomatic HCWs, 21 had positive SARS-CoV-2 nucleic acid test, the remaining 127 HCWs were specifically positive for SARS-CoV-2 antibody but negative for SARS-CoV-2 nucleic acid (Fig. 1 ).
Table 1.
Laboratory testing for SARS-CoV-2 in 424 HCWs with laboratory evidence of SARS-CoV-2 infection
| Laboratory testing for SARS-CoV-2 | Symptomatic HCWs (n = 276) | Asymptomatic HCWs (n = 148) |
|---|---|---|
| Positive RT-PCR SARS-CoV-2 nucleic acid | 214 (77.5%) | 21 (14.2%) |
| Serological SARS-CoV-2 antibody test | 275 (99.6%) | 148 (100%) |
| Positive SARS-CoV-2 antibody test | 233 (84.7%) | 146 (98.6%) |
| Positive SARS-CoV-2 nucleic acid with positive antibody test | 171 (62.0%) | 19 (12.8%) |
| Positive SARS-CoV-2 nucleic acid with negative antibody test | 42 (15.2%) | 2 (1.4%) |
| Positive SARS-CoV-2 nucleic acid without antibody test | 1 (0.4%) a | 0 |
| Negative SARS-CoV-2 nucleic acid with positive antibody test | 62 (22.5%) | 127 (85.8%) |
Abbreviations: HCW, health-care worker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Fig. 1.
Study flow.
Demographic and clinical characteristics
The demographic and clinical characteristics are presented in Table 2 and in the Supplementary material (Table S1). Median age of the 424 HCWs was 35 years (interquartile range 30.0–42.0 years) and 286 (67.5%) were women. No significant differences were seen in age and sex between symptomatic and asymptomatic HCWs. Among the 424 HCWs, 136 (32.1%) worked in internal medicine, 112 (26.4%) in the surgery department, 15 (3.5%) in the paediatrics department, 30 (7.1%) in the departments of obstetrics and gynaecology and 131 (30.9%) in other departments. Of the 424 HCWs, physicians were more likely to be symptomatic (OR 1.697, 95% CI 1.065–2.704; p 0.026) whereas health-care assistants were more likely to be asymptomatic (OR 0.594, 95% CI 0.366–0.964; p 0.035). Chest CT results are shown in Table 2. Among the 142 asymptomatic HCWs undergoing CT scan, only 33 (23.2%) showed abnormal CT images, whereas 257 (93.1%) of the symptomatic HCWs showed abnormal chest CT images. More symptomatic HCWs showed bilateral pulmonary infiltration than asymptomatic HCWs (180/257 versus 16/33, p 0.013). Compared with families of asymptomatic HCWs, more families of symptomatic HCWs were infected with SARS-CoV-2 (55/276 versus 16/148, p 0.017). HCWs with infected family members tended to be symptomatic cases (OR 2.053, 95% CI 1.130–3.730; p 0.018).
Table 2.
Demographic and clinical characteristics of symptomatic and asymptomatic HCWs with laboratory evidence of SARS-CoV-2 infection
| Characteristics | Symptomatic HCWs (n = 276) |
Asymptomatic HCWs (n = 148) |
p value | |
|---|---|---|---|---|
| Male sex | 96 (34.8%) | 49 (33.1%) | 0.729b | |
| Age (years) | Mean (SD) | 36.1 (8.2) | 36.4 (8.5) | 0.768c |
| ≤29 | 57 (20.7%) | 36 (24.3%) | ||
| 30–39 | 137 (49.6%) | 62 (41.9%) | ||
| 40–49 | 61 (22.1%) | 36 (24.3%) | ||
| ≥50 | 21 (7.6%) | 14 (9.5%) | ||
| Job category | Nurses | 141 (51.1%) | 78 (52.7%) | 0.751b |
| Physicians | 88 (31.9%) | 32 (21.6%) | 0.025b | |
| Health-care assistants | 47 (17.0%) | 38 (25.7%) | 0.034b | |
| Department | Internal medicine | 99 (35.9%) | 37 (25.0%) | 0.022b |
| Surgery | 79 (28.6%) | 33 (22.3%) | 0.159b | |
| Obstetrics and gynaecology | 16 (5.8%) | 14 (9.5%) | 0.161b | |
| Paediatrics | 10 (3.6%) | 5 (3.4%) | 0.897b | |
| Others | 72 (26.1%) | 59 (39.9%) | 0.003b | |
| High-risk departments | 73 (26.4%) | 72 (50.6%) | <0.001b | |
| Chest CT scan | Total | 276 (100%) | 142 (95.9%) | |
| Abnormal images | 257 (93.1%) | 33 (23.2%) | <0.001b | |
| Bilateral pulmonary infiltration | 180 (70.0%) | 16 (48.5%) | 0.013b | |
| Patchy and ground-glass opacity | 243 (94.6%) | 31 (93.9%) | 0.885b | |
| Consolidation | 64 (24.9%) | 7 (21.2%) | 0.643b | |
| Hospitalized | 193 (69.9%) | 2 (1.4%) a | <0.001d | |
| Co-morbidities | 22 (8.0%) | 6 (4.1%) | 0.122b | |
| Infected families | 55 (19.9%) | 16 (10.8%) | 0.017b | |
Abbreviations: HCW, health-care worker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
The two asymptomatic HCWs were admitted to the hospital for further examination with recurrence of positive SARS-CoV-2 nucleic acid in March 2020.
Calculated using the χ2 test.
Calculated using the unpaired t test.
Calculated using the Fisher's exact test.
Univariate and multivariable analyses
The comparison in high-risk procedures and infection protective measures between asymptomatic HCWs and symptomatic HCWs are shown in Table 3 . No significant differences were seen in any high-risk procedures between asymptomatic HCWs and symptomatic HCWs. Compared with symptomatic HCWs, asymptomatic HCWs more consistently used hand washing, isolation gowns, eye protection, N95 respirators, gloves and hair covers for protection (p < 0.001).
Table 3.
Risk procedures and protective measures in symptomatic and asymptomatic HCWs with laboratory evidence of SARS-CoV-2 infection
| Characteristic | Symptomatic HCWs (n = 276) |
Asymptomatic HCWs (n = 148) |
p value | |
|---|---|---|---|---|
| Tracheal intubation or extubation | 17 (6.2%) | 4 (2.7%) | 0.159a | |
| Oropharyngeal or nasopharyngeal examination | 31 (11.2%) | 21 (14.2%) | 0.376b | |
| Sputum suction | 30 (10.9%) | 16 (10.8%) | 0.985b | |
| Cardiopulmonary resuscitation | 18 (6.5%) | 10 (6.8%) | 0.926b | |
| Hand washing | Consistently | 193 (69.9%) | 126 (85.1%) | 0.001b |
| Isolation gown | Consistently | 59 (21.4%) | 74 (50.0%) | <0.001b |
| Surgical mask or N95 respirators | Consistently | 217 (78.6%) | 125 (84.5%) | 0.147b |
| N95 respirators | Consistently | 67 (24.3%) | 98 (66.2%) | <0.001b |
| Gloves | Consistently | 150 (57.0%) | 123 (83.1%) | <0.001b |
| Hair cover | Consistently | 138 (50.0%) | 124 (83.8%) | <0.001b |
| Eye protection | Consistently | 44 (15.9%) | 90 (60.8%) | <0.001b |
Abbreviations: HCW, health-care worker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Calculated using the Fisher's exact test.
Calculated using the χ2 test.
We also analysed the factors associated with asymptomatic infection among HCWs by using a logistic regression model. The univariable logistic regression analysis showed that working in high-risk departments, hand washing consistently and consistent use of isolation gown, N95 respirators, gloves, hair cover, and eye protection were associated with an increased likelihood of asymptomatic infection (see Supplementary material, Table S1). Although univariable analyses showed no significant association between tracheal intubation or extubation and asymptomatic SARS-CoV-2 infection (OR 2.363, 95% CI 0.780–7.156; p 0.128). In multivariable analyses, tracheal intubation or extubation (OR 4.057, 95% CI 1.183–13.909; p 0.026) was associated with an increased likelihood of symptomatic SARS-CoV-2 infection. Consistent use of N95 respirators (OR 0.369, 95% CI 0.201–0.680; p 0.001) and eye protection (OR 0.217, 95% CI 0.116–0.404; p < 0.001) were associated with decreased likelihood of symptomatic SARS-CoV-2 infection (Table 4 ).
Table 4.
Multivariable logistic regression model in factors associated with symptomatic SARS-CoV-2 infection
| Factors | Multivariable OR (95% CI) | p value |
|---|---|---|
| Age (years)a | 0.975 (0.948–1.003) | 0.077 |
| Co-morbidities present (yes vs no) | 2.368 (0.844–6.640) | 0.101 |
| Tracheal intubation or extubation (yes vs no) | 4.057 (1.183–13.909) | 0.026 |
| N95 respirators (consistent use vs inconsistent use) | 0.369 (0.201–0.680) | 0.001 |
| Eye protection (consistent use vs inconsistent use) | 0.217 (0.116–0.404) | <0.001 |
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Per 1-unit increase.
Discussion
Asymptomatic carriers of SARS-CoV-2 have been reported in the general population by several reports [[3], [4], [5], [6]]. However, evidence about the differences between symptomatic and asymptomatic individuals with SARS-CoV-2 infection is limited. Here, we described the incidence of asymptomatic infection of SARS-CoV-2 during a full screening in a hospital setting. Tracheal intubation or extubation was found to be associated with an increased likelihood of symptomatic SARS-CoV-2 infection, whereas consistent use of N95 respirators and eye protection tended to be linked to asymptomatic infection. All these findings suggested a potential relationship between initial infectious dose and disease severity.
The incidence of asymptomatic carriers in the general population in China was announced by China's National Health Commission on 1 April 2020, that 130 (78%) of 166 positive cases were asymptomatic [17]. Two studies reported the occurrence of asymptomatic infection in relatively confined spaces; information from the Diamond Princess cruise ship and charter flights estimated the incidence to be 17.9% and 30.8%, respectively [18,19]. These relatively high incidences of asymptomatic cases in the general population put the public on the alert for the presence of asymptomatic HCWs. With respect to this concern, 1270 HCWs were screened for SARS-CoV-2 in a large UK teaching hospital, showing that 31 (2.4%) tested positive for SARS-CoV-2 nucleic acid, comparable to the 148/6473 (2.3%) in our study [20]. Despite the fact that our proportion from a general HCW screening may be close to the actual incidence rate, these data should be interpreted by contextualizing them in specific hospital settings when taking into account multiple factors, including the supply of medical resources, SARS-CoV-2 virulence and the number of infected patients hospitalized.
The clinical spectrum of COVID-19 can be very heterogeneous, ranging from asymptomatic infection to respiratory failure [2]. The mechanisms affecting the interactions between the virus and the host thereby determining such variable clinical manifestations have yet to be fully characterized. So far, most research into understanding the wide clinical range of COVID-19 has been focused on the host immune status including age, co-morbidities, and circulating B- and T-cell responses [[21], [22], [23], [24]]. However, in our hospital setting, age and co-morbidities were not found to be associated with symptomatic infection. This difference might be related to the fact that HCWs in our study comprised a young population (median age 35 years) and few underlying diseases.
Viral load of SARS-CoV-2 was reported to be associated with severe clinical outcomes in patients with COVID-19 [25]. However, the roles of other virological properties, including initial infectious dose, evolution and virulence, on clinical outcomes are still unknown. In 12 African green monkeys exposed to different target doses of aerosolized Middle East respiratory syndrome coronavirus, a dose-dependent increase of respiratory disease signs was observed [26]. In our study, we first showed that tracheal intubation or extubation was associated with an increased likelihood of symptomatic SARS-CoV-2 infection. One possible explanation could be that a relatively high initial virus exposure might lead to a high viral load in the respiratory tract, thereby overcoming the barriers of the immune responses and causing symptomatic infection or even unfavourable disease outcome. N95 respirators and eye protection have been reported to be associated with a much lower risk of infection in a recent systematic review of 172 observational studies [15]. It has also been reported that face coverings and masks were useful to filter out the majority of viral particles, but not all [27,28]. Hence, some HCWs with the protection of eye protection and N95 respirators still became infected but tended to be asymptomatic. This finding supported the hypothesis that universal masking reduces the ‘inoculum’ or dose of the virus for the mask-wearer, leading to milder and asymptomatic infection manifestations [16]. All the factors associated with asymptomatic SARS-CoV-2 infection are tied to increased or decreased initial infectious dose.
Our study had several limitations. First, recall bias, especially in those HCWs with asymptomatic infection, added to the complexity of the study and might affect the results in our study. Second, until 18 April 2020, as not all HCWs in the two hospitals had tested SARS-CoV-2 nucleic acid and serological antibody, the number of actual asymptomatic HCWs in our study might be underestimated. Nevertheless, most of the HCWs who had a high-risk exposure history were subjected to SARS-CoV-2 nucleic acid and serological antibody tests.
In summary, this study reported a large number of asymptomatic HCWs with laboratory evidence of SARS-CoV-2 in Wuhan, China. Among HCWs with laboratory evidence of SARS-CoV-2, those who had performed tracheal intubation or extubation were more likely to develop related symptoms, whereas those taking aggressive measures including consistent use of N95 respirators and eye protection tended to be asymptomatic cases. These results suggest that the initial infectious dose of SARS-CoV-2 plays an important role in symptom severity of COVID-19.
Author's contributions
YJ designed the study, FW, NX, LD, YM, LC and HO collected the epidemiological and clinical data. SZ, MG and ZW summarized all the data. SZ and MG drafted the manuscript, and all authors revised the final manuscript.
Transparency declaration
YJ, SZ, MG, FW, NX, YM, ZW, LD, LC and HO report no conflicts of interest. This work was supported by the National Natural Science Foundation of China for SARS-CoV-2 (no. 82041018), the National Natural Science Foundation of China (no. 81770096 and no. 81700091), the Independent Innovation Research Fund for Huazhong University of Science and Technology (2020kfyXGYJ) and the State Project for Essential Drug Research and Development, China (no. 2019ZX09301001).
Acknowledgements
We thank all the HCWs fighting COVID-19 in the front-line. They are putting their lives on the line and have shown great courage during this unexpected crisis.
Editor: A. Huttner
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.08.038.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Univariable logistic regression model of factors associated with symptomatic SARS-CoV-2 infection.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Hu Y., liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Health Commission of the People’s Republic of China FAQs on the prevention and control of the spread of COVID-19 caused by asymptomatic infected persons. http://www.nhc.gov.cn/xcs/yqfkdt/202003/718c79c96f3e46409dd49303d41a00ef.shtml Available from:
- 4.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020:M20–M3012. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goh Y.Y., Feaster M. High proportion of asymptomatic SARS-CoV-2 infections in 9 long-term care facilities, Pasadena, California, USA, April 2020. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2610.202694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mei X., Zhang Y., Zhu H., Ling Y., Zou Y., Zhang Z. Observations about symptomatic and asymptomatic infections of 494 patients with COVID-19 in Shanghai,China. Am J Infect Control. 2020 doi: 10.1016/j.ajic.2020.06.221. S0196–6553(20)30646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stubblefield W.B., Talbot H.K., Feldstein L., Tenforde M.W., Rasheed M.A.U., Mills L. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients—Nashville, Tennessee. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa936. ciaa936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang R., Gui X., Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa461. ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley R.D., Ensor J., Snell K.I.E., Harrell F.E.J., Martin G.P., Reitsma J.B. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. doi: 10.1136/bmj.m441. [DOI] [PubMed] [Google Scholar]
- 11.Leisman D.E., Harhay M.O., Lederer D.J., Abramson M., Adjei A.A., Bakker J. Development and reporting of prediction models: guidance for authors from editors of respiratory, sleep, and critical care journals. Crit Care Med. 2020;48:623–633. doi: 10.1097/CCM.0000000000004246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C., Zhou M., Liu Y., Guo T., Ou C., Yang L. Characteristics of asymptomatic COVID-19 infection and progression: a multicenter, retrospective study. Characteristics of asymptomatic COVID-19 infection and progression: a multicenter, retrospective study. Virulence. 2020 doi: 10.1080/21505594.2020.1802194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou R., Dana T., Buckley D.I., Selph S., Fu R., Totten A.M. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173:120–136. doi: 10.7326/M20-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;S0140-6736(20):31142–31149. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi M., Beyrer C., Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020 doi: 10.1007/s11606-020-06067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;369:m1375. doi: 10.1136/bmj.m1375. [DOI] [PubMed] [Google Scholar]
- 18.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiura H., Kobayahi T., Miyama T., Suzuki A., Jung S.M., Hayashi K. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivett L., Sridhar S., Sparkes D., Routledge M., Jones N.K., Forrest S. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9 doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S., Guo M., Duan L., Wu F., Hu G., Wang Z. Development and validation of a risk-factor-based system to predict short-term survival in adult hospitalized patients with COVID-19: a multicenter, retrospective, cohort study. Crit Care. 2020;24:438. doi: 10.1186/s13054-020-03123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuri-Cervantes L., Pampena M.B., Meng W., Rosenfeld A.M., Ittner C.A.G., Weisman A.R. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultheiß C., Paschold L., Simnica D., Mohme M., Willscher E., Wenserski L.V. Next-generation sequencing of T and B cell receptor repertoires from COVID-19 patients showed signatures associated with severity of disease. Immunity. 2020;S1074-7613(20):30279–X. doi: 10.1016/j.immuni.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Yan L., Wan L., Xiang T., Le A., Liu J. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Totura A., Livingston V., Frick O., Dyer D., Nichols D., Nalca A. Small particle aerosol exposure of African green monkeys to MERS-CoV as a model for highly pathogenic coronavirus infection. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2612.201664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smereka J., Ruetzler K., Szarpak L., Filipiak K.J., Jaguszewski M. Role of mask/respirator protection against SARS-CoV-2. Anesth Analg. 2020 doi: 10.1213/ANE.0000000000004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahl P., Bhattacharjee S., Silva C.D., Chughtai A.A., Doolan C., MacIntyre C.R. Face coverings and mask to minimise droplet dispersion and aerosolisation: a video case study. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215748. thoraxjnl-2020-215748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariable logistic regression model of factors associated with symptomatic SARS-CoV-2 infection.