Abstract
Background
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2, has caused a major pandemic. Patients with cancer are at higher risk of severe COVID-19. We aimed to describe and compare the immunological features of cancer patients hospitalised for COVID-19 or other concomitant, cancer-related illness.
Methods
In this prospective study, the clinical and immunological characteristics of 11 cancer patients with COVID-19 and 11 non–COVID-19 cancer patients hospitalised in the same unit at the same period for other medical issues were analysed. We also used 10 healthy volunteers as controls. Peripheral immune parameters were analysed using multiparametric flow cytometry.
Results
The median age of COVID-19–positive cancer patients was 71.1 years, and 66.4 years for controls. Compared with non–COVID-19 cancer patients, COVID-19–positive cancer patients had more extensive lymphopenia and hypoalbuminemia, with higher levels of C-reactive protein. In COVID-19 patients, elevated procalcitonin was associated with a higher risk of death. By phenotypic analysis, COVID-19–positive patients presented CD3 lymphopenia, with inversion of the CD4/CD8 ratio and modification of monocyte activation, with accumulation of mMDSC (monocytic Myeloid-Derived Suppressor Cells) -like cells and a decrease in activated monocytes. Analysis of the T-cell compartment revealed a T-dependent inflammatory response with accumulation of Th17 cells and cytotoxic CD8 T cells producing TNFα, a decrease in HLA-DR (Human Leukocyte Antigen – DR isotype)-positive CD8 T cells and Treg/CD8 ratio.
Conclusion
SARS-CoV-2 infection in cancer patients is associated with CD4 T-cell lymphopenia with induction of an inflammatory T-cell response, accumulation of IFNγ+ TNFα+ CD8 T and Th17 cells, and a concomitant modification of monocyte activation status.
Keywords: COVID-19, Cancer, Immunomonitoring
1. Introduction
Since early 2020, the coronavirus disease 2019 (COVID-19) pandemic has affected millions of people worldwide. Although harmless for 85% of the population, COVID-19 can be life-threatening in vulnerable (e.g. older, immunosuppressed or comorbid) patients. Estimated mortality from COVID-19 is 1–3%, i.e. 10 to 30 times higher than the death rate from seasonal influenza. The causative agent of COVID-19 is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1].The virus spreads via small droplets projected from the mouth and nose. Analysis of data on the propagation of SARS-CoV-2 in China suggests that close contact with an infected person is necessary. In fact, propagation is mainly limited to family members, health professionals and other persons in close contact with the infected individual [2]. This suggests that quarantine is the best means to contain the epidemic. To this end, lockdown was implemented in many countries around the world starting in March 2020.
Many comorbidities, such as older age, obesity, or diabetes, pathologies that affect the immune system, and cancer can all incur an increased risk of severe forms of COVID-19 infection [3]. Accordingly, emerging data from China, Italy and North America indicate that severe disease is more frequent and the death rate higher in patients with cancer who contract COVID-19 [[4], [5], [6], [7]]. Recent data from the British National Health System (NHS) show that organ transplant, immunosuppression status, presence of cancer or haematological malignancy are associated with a high risk of death [8].
Lymphopenia, increased pro-inflammatory markers and cytokines, and potential blood hypercoagulability characterise severe COVID-19 cases, with features reminiscent of cytokine-release syndromes. Although immunosuppression and cancer could enhance the risk of severe COVID-19, one might also suspect that such patients would develop an unusual form of immune response after exposure to COVID-19. Therefore, we decided to explore this question by performing peripheral blood immunomonitoring on patients with cancer hospitalised in our cancer centre for mild to severe COVID-19 infection over a one-month period. We further compared them to cancer patients hospitalised for intercurrent non–COVID-19 medical problems during the same period and also to healthy volunteers (HVs).
2. Material and methods
2.1. Study participants
From March 30th to May 9th, 2020, 11 consecutive patients with positive diagnosis of SARS-CoV-2 during the care of their cancer disease were hospitalised in Georges Francois Leclerc centre. Concomitantly 11 matched cancer patients without SARS-CoV-2 infection were hospitalised in Georges Francois Leclerc centre during the same period for another intercurrent complication during the care of their cancer disease. We used as control group of healthy blood donors from Etablissement Francais du Sang. The study was declared as an ancillary study of the NCT02281214 and NCT02840604. The study was approved by the CNIL (Commission Nationale de l'Informatique et des Libertés) (national commission for data privacy) and the local ethics committee, and was performed according to the Helsinki declaration and in compliance with the European reglementation.
We reprospectively obtained the medical history, physical examination, and haematological, biochemical, radiological, and microbiological data. The data collection forms were reviewed independently by two researchers.
2.2. Laboratory measurements
Real-time reverse transcription polymerase chain reaction (RT-PCR) assays were done for SARS-CoV-2. Respiratory specimens were collected by the local CDC (Centers for Disease Control) and then shipped to designated authoritative laboratories to detect SARS-CoV-2. The presence of SARS-CoV-2 in respiratory specimens was detected by real-time reverse transcription (RT-PCR) methods. The SARS-CoV-2 VIASURE Real-Time PCR Detection kit (supplied by CERTEST Biotec) containing the primers and probe targeting the CoV envelope gene were used. The use of this kit for the targeting of SARS-CoV-2 gene has been validated by the CNR (Centre National de Référence) virology respiratory laboratory in Lyon. Conditions for the amplifications were 45 °C for 15 min, 95 °C for 2 min, followed by 45 cycles of 95 °C for 10 s and 60 °C for 50 s.
2.3. Clinical laboratory measurements
Initial clinical laboratory investigations included a complete blood count, serum biochemical test (including liver and renal function, creatine kinase, low-density lipoprotein and electrolytes) and coagulation profile.
2.4. Evaluation of peripheral blood immunological indicators
2.4.1. Leucocyte population identification and numeration
2.4.1.1. Liquid reagents
Antibody clones CD127-Brilliant Violet 605 (clone A019D5), CCR7-Brilliant Violet 650 (clone G043H7) and CD45RA-Brilliant Violet 785 (cloneHI100) were purchased from BioLegend.
Dried format reagents: Using Beckman Coulter's custom design service and its dry coating technology, custom tubes containing CD16-FITC (clone 3G8), CD56-PE (clone N901), CD25-ECD (clone B1.49.9), HLA-DR-PE-Cyanine5.5 (Clone Immu-357), CD14-PE-Cyanine7 (clone RMO52), CD4-APC (Clone 13B8.2), CD8-Alexa Fluor 700 (Clone B9.11), CD3-AA750 (Clone UCTH1), CD15-PacBlue (Clone 80H5) and CD45-Krome Orange (Clone J.33) were produced.
2.4.1.2. Staining protocol
100 μL of total heparinized blood was added in DURAClone tube containing liquid antibodies, vortexed immediately for 15s and incubated for 15 min at room temperature in the dark. Two millilitre of red blood lysis solution (VersaLyse, Beckman Coulter) containing 50 μL of the fixative agent (IOTest Solution, Beckman Coulter) was added, inverted and incubated for 15 min in the dark. Before acquisition on CytoFLEX cytometer (Beckman Coulter), 100 μL of Flow-Count Fluorospheres was added to DURAClone tubes to allow the count of leucocyte population. This labelling has allowed us to study the proportions and the number of CD15+ neutrophils, CD14+ monocytes, CD3+ T, CD4+ T, CD8+ NK, NKT, Tγδ, Treg cells among others and the expression of cell surface markers as well as CD45RA, CCR7 and HLA-DR expression. The gating strategy is described in Supplementary Fig. 6.
2.4.2. Lymphocyte function analysis
2.4.2.1. Liquid reagents
Antibody clone IL-2-Brilliant Violet 605 (clone MQ1-17H12) was purchased from BioLegend.
2.4.2.2. Dried format reagents
Using Beckman Coulter's custom design service and its dry coating technology, custom tubes containing IFNγ-FITC (Clone 45.15), CD25-PE (Clone B1.49.9), CD4-PE-Cyanine5.5 (Clone 13B8.2), IL-4-PE-Cyanine7 (Clone MP4-25D2), Foxp3-AF647 (Clone 259D), TNFα-AF700 (Clone IPM2 (188)), CD3-AA750 (Clone UCHT1), IL-17A-PacBlue (Clone B168) and CD8-KromeOrange (B9.11) were produced.
2.4.2.3. Staining protocol
50 μL of total heparinized blood was added in DURActiv 1 dry tube (Beckman Coulter) containing Phorbol myristate acetate (PMA), ionomycin and brefeldin A for 3 h at 37 °C. After activation, 25 μL of PerFix-NC R1 buffer (PerFix-NC kit, Beckman Coulter) was added on vortex and incubated for 15 min at room temperature. Then, 2 mL of PBS 1X was added, and after centrifugation the pellet was resuspended in 25 μL of foetal bovine serum (FBS, Dutscher) and 300 μL of PerFix-NC R2 buffer was added. A 325 μL aliquot was transferred to a DURAClone tube containing the liquid antibody, vortexed immediately for 15s and incubated for 1 h at room temperature in the dark. PBS 1 × (3 mL) was added to the tubes, incubated for 5 min at room temperature in the dark before centrifugation for 5 min at 500g. After supernatant removal, the cells were resuspended in 3 mL of 1X PerFix-NC R3 buffer before another 5-min centrifugation at 500g. The pellet was dried and resuspended in 300 μL of 1X R3 buffer. Acquisition was done on CytoFLEX cytometer. The proportion of IFNγ+, TNFα+, IL-2+, IL-17A+ or IL-4+ expression by CD4+ T or CD8+ T cells and the proportion of CD25+ Foxp3+ CD4+ or CD8+ cells were studied with this labelling procedure. The gating strategy is described in Supplementary Fig. 7.
All analyses were done with Kaluza 1.3 software (Beckman Coulter).
2.5. Statistics
Continuous variables are expressed as median (IQR, InterQuartile Range) and compared with the Mann Whitney U test. Categorical variables are expressed as number (%) and compared by χ2 test or Fisher's exact test between COVID and non-COVID patients. A two-sided α of less than 0.05 was considered statistically significant. Statistical analyses were done using PRISM software.
3. Results
3.1. Patient demographics and baseline characteristics of COVID-19 and non–COVID-19 cancer hospitalised patients
Between March 30th and May 9th, 2020, a total of 22 cancer patients were admitted to our cancer center for suspicion of SARS-CoV-2 infection. The diagnosis was confirmed for 11 patients, including five with positive PCR, and six with negative PCR but compatible CT scan images and no other plausible aetiology. Among the 11 COVID-19–positive patients, median age was 71.1 years, and few comorbidities were reported. Nine of the 11 had metastatic disease, and four had received more than two lines of therapy. All 11 patients received antibiotic therapy, nine required oxygen therapy and eight had fever. At baseline, mean oxygen saturation (SpO2) was 90% (58–95). Table 1 summarises the characteristics of the patients, and Supplementary Table 1 represents individual data for each patient.
Table 1.
Baseline demographics.
| COVID-19–positive |
COVID-19–negative |
|
|---|---|---|
| Characteristic | n = 11 | n = 11 |
| Median age | 77.1 () | 66.4 () |
| Caucasian | 11 (100%) | 11 (100%) |
| Cancer type | ||
| Lung cancer | 4 (36.4%) | 5 (45.5%) |
| Breast cancer | 1 (9.1%) | 1 (9.1%) |
| Sarcoma | 3 (27.3%) | 0 |
| Head and neck cancer | 1 (9.1%) | 1 (9.1%) |
| Biliary/pancreatic cancer | 0 | 2 (18.2%) |
| Skin cancer | 0 | 1 (9.1%) |
| Stomach cancer | 0 | 1 (9.1%) |
| Endometrial cancer | 1 (9.1%) | 0 |
| Hepatocarcinoma | 1 (9.1%) | 0 |
| Stage | ||
| Localised | 1 (9.1%) | 1 (9.1%) |
| Locally advanced/metastatic | 10 | 10 |
| Cancer therapy | ||
| 1st line | 7 (63.6%) | 4 (36.4%) |
| 2nd line | 1 (9.1%) | 5 (45.5%) |
| >2nd line | 3 (27.3%) | 2 (18.2%) |
| ECOG | ||
| 0 | 0 | 1 (9.1%) |
| 1 | 1 (9.1%) | 2 (18.2%) |
| 2 | 7 (63.6%) | 7 (63.6%) |
| 3 | 1 (9.1%) | 1 (9.1%) |
| 4 | 2 (18.2%) | 0 |
| Comorbidity | ||
| High blood pressure | 4 (36.4%) | 6 (54.6%) |
| Diabetes | 2 (18.2%) | 2 (18.2%) |
| Asthma | 0 | 2 (18.2%) |
| Smoking | 6 (54.6%) | 5 (45.5%) |
| PCR | ||
| Positive | 5 (45.5%) | 0 |
| Negative | 6 (54.6%) | 11 (100%) |
| CT scan | ||
| Positive | 7 (63.6%) | 0 |
| Negative | 0 | 5 (45.5%) |
| Unperformed | 4 (36.4%) | 6 (54.6%) |
| Symptoms | ||
| Fever | 8 (72.8%) | 6 (54.6%) |
| Pulmonary symptoms | 10 (90.9%) | 4 (36.4%) |
| O2 required | 9 (81.9%) | 3 (27.3%) |
| Antibiotherapy | 11 (100%) | 5 (45.5%) |
| Amox-ac clavulanique | 4 (36.4%) | 3 (27.3%) |
| C3G (Céphalosporines orales de 3ème Génération) | 1 (9.1%) | 0 |
| Tazocillin | 6 (54.6%) | 1 (9.1%) |
| Vancomycin | 1 (9.1%) | |
| No antibiotherapy | 0 | 6 (54.6%) |
| Biological features (median) | ||
| Albumin | 22.5 (14–31) | 30 (19–34) |
| CRP (C-Reactive Protein) | 153.5 (47–552) | 71.5 (1–136) |
| Procalcitonin | 0.36 (0.14–29.07) | 0.16 (0.08–1.57) |
CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; PCR, polymerase chain reaction.
Among the 11 non-COVID cancer patients, median age was 66.4 years. This group was comparable with the COVID-19–positive patients, with similar metastatic status, cancer type and comorbidity status (Table 1). As a second control data set, we also used data from 10 HVs with a mean age of 60.
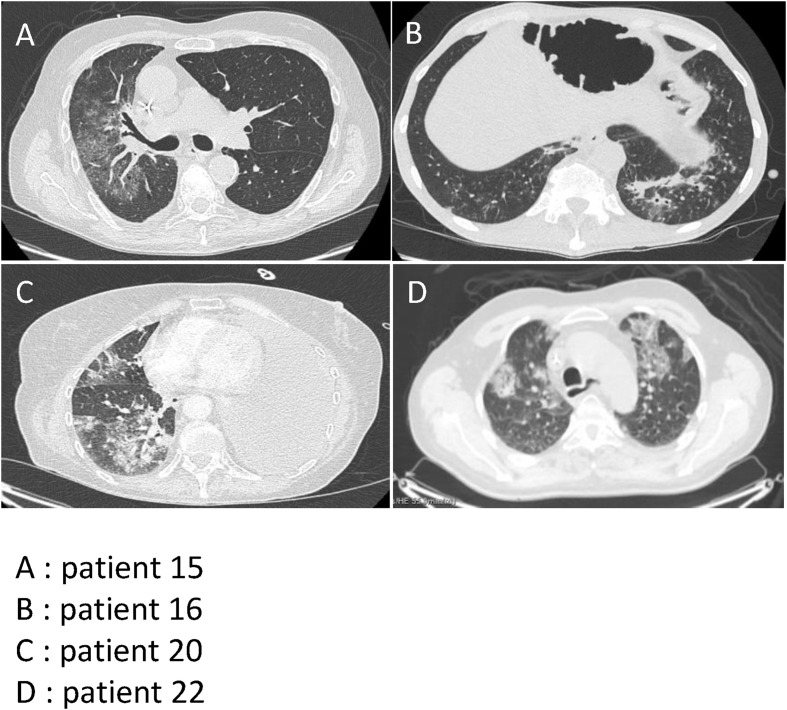
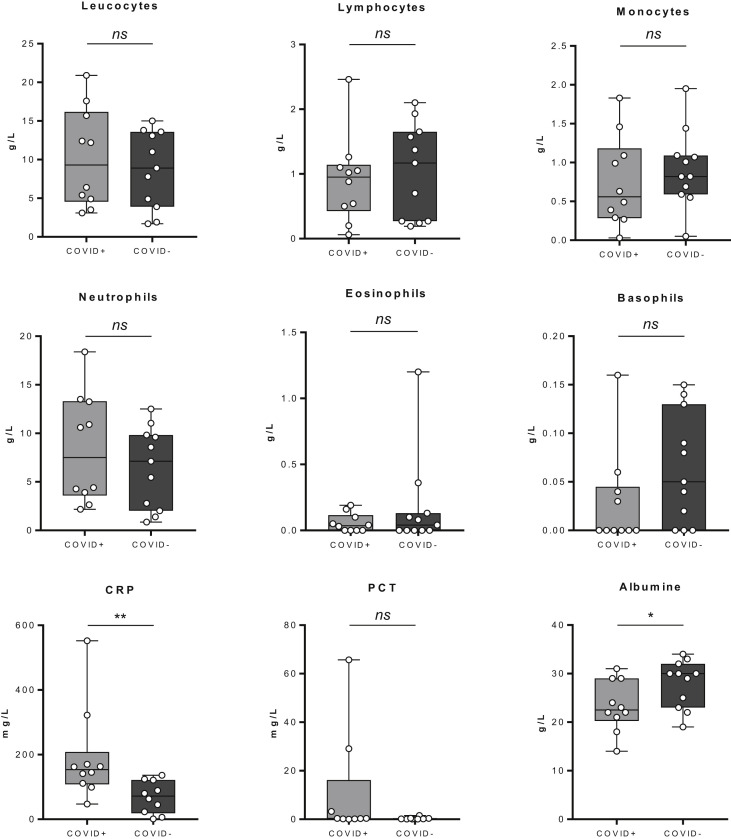
Among the 11 COVID-19–positive patients, interstitial lung abnormalities were observed on chest image analysis. Typical findings were bilateral ground-glass opacities and subsegmental areas of high density (Fig. 1 ). At the time of admission, laboratory analysis showed a non-significant trend towards lymphopenia compared with the non-COVID cancer patients. Patients who died from COVID-19 were classified as severe COVID-19. Severe COVID-19 cases had increased WBC counts, but lower lymphocyte counts (Fig. 2 ). Further biological tests showed a significant increase in C-reactive protein in COVID-19–positive patients (153.5 versus 71.5, p = 0.0029). Procalcitonin elevation trended to be associated a higher risk of death (Fisher test p = 0.16). Albumin concentrations were significantly lower in COVID-19–positive patients than in non-COVID cancer patients (Fig. 2).
Fig. 1.
Positive CT scan in COVID-19–positive patients. A: patient 15 with unilateral ground-glass opacities. B: patient 16 with consolidation. C: patient 20 with consolidation and ground-glass opacity. D: patient 22 with bilateral ground-glass opacities.
Fig. 2.
The box plot of routine laboratory biological analysis between COVID-19–positive and –negative patients. The data presented constitute the analyses performed on 11 COVID-19–positive patients and 11 COVID-19–negative patients. Statistical difference is determined by a Mann–Whitney test. ns = no significant, ∗ = p < 0.05, ∗∗ = p < 0.01.
At the date of database lockout (25 May 2020), six severe COVID-19–positive patients died (four men and two women), at a median age of 62.2 years (33.5–81.7). All had locally advanced or metastatic cancer, and all had pulmonary symptoms. Onlytwo of the six had a comorbidity (high blood pressure).
3.2. Analysis of blood phenotypic immune parameters
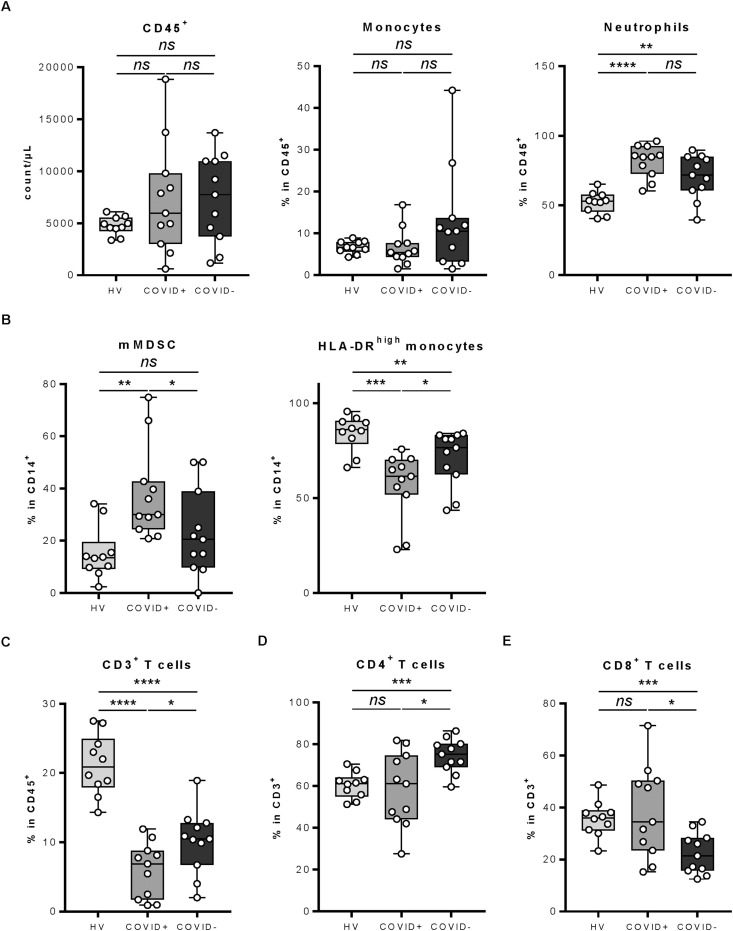
We first examined the number and proportion of immune cells in peripheral blood [9]. Total leucocyte number did not vary between healthy donors, COVID-19–positive and non-COVID cancer patients (Fig. 3 A). When looking at the proportion of myeloid cells, we observed no difference in the proportion of monocytes, but a marked increase in the proportion of neutrophils in cancer patients, with a higher proportion in COVID-19–positive cancer patients (Fig. 3A). No particular variation was observed for the frequency of CD15+ CD16low eosinophils (Fig. SD1). For monocyte subsets, we observed a decrease in the absolute number of CD14low CD16+ monocytes in COVID-19–positive patients compared with both HVs and non–COVID-19 cancer patients. CD14high CD16+ monocytes accumulated in all cancer patients, but more marked extent in COVID-19–negative cancer patients (Fig. SD2). Regarding maturation of monocytes, we observed that HLA-DRlow (Human Leukocyte Antigen – DR isotype) monocytes, which harbour a monocytic MDSC (Myeloid-Derived Suppressor Cells) phenotype, were increased in COVID-19–positive cancer patients, while HLA-DRhigh-activated monocytes were decreased (Fig. 3B).
Fig. 3.
Leucocyte population analysis between COVID-19–positive and –negative patients and healthy volunteers. Whole blood of healthy volunteers or COVID-19–positive and –negative patients was stained with anti-CD16, anti-CD56, anti-CD25, anti–HLA-DR, anti-CD14, anti-CD4, anti-CD8, anti-CD3, anti-CD15, anti-CD45, anti-CD127, anti-CCR7 and anti-CD45RA antibodies and analysed by flow cytometry. A: The count of CD45+ leucocytes (left panel) and the frequency of CD14+ monocytes (middle panel) and CD15+ CD16+ neutrophils (right panel) among CD45+ cells is depicted. B: The frequency of mMDSC (monocytic Myeloid-Derived Suppressor Cells) (HLA-DRlow (Human Leukocyte Antigen – DR isotype) CD14+) (left panel) and HLA-DRhigh (Human Leukocyte Antigen – DR isotype) monocytes (right panel) among CD14+ monocytes is depicted. C: The frequency of CD3+ T cells among CD45+ cells is depicted. D–E: The frequency of CD4+ T cells (D) and CD8+ T cells (E) among CD3+ T cells is depicted. The data presented constitute the analyses performed on 11 COVID-19–positive patients, 11 COVID-19–negative patients and 10 healthy volunteers. Statistical difference is determined by a Mann–Whitney test. ns = no significant, ∗ = p< 0.05, ∗∗ = p< 0.01, ∗∗∗ = p< 0.001 and ∗∗∗∗ = p<0.0001.
When looking at lymphoid cells, we did not observe any difference in the proportion of NK subsets, γδT cells or NKT cells between COVID-19–positive and non-COVID cancer patients (Fig. SD3). However, the frequency of these three cell types was lower in cancer patients than that in HVs (Fig. SD3). We observed a significant decrease in the proportion of CD3 T cells and a respective increase in the B cell proportion in cancer patients compared with HVs (Fig. 3C and not shown). We noted a modification of the CD4/CD8 ratio between COVID-19–positive and –negative patients, with an increase in the CD8 proportion and a decrease in the CD4 proportion in COVID-19–positive patients (Fig. 3D and E).
Together these data underline the induction of CD3 lymphopenia, with an inversion of the CD4/CD8 ratio, a change in monocyte activation, accumulation of mMDSC-like cells and a decrease in activated monocytes in COVID-19–positive cancer patients.
3.3. Analysis of T-cell phenotypic and secretion parameters
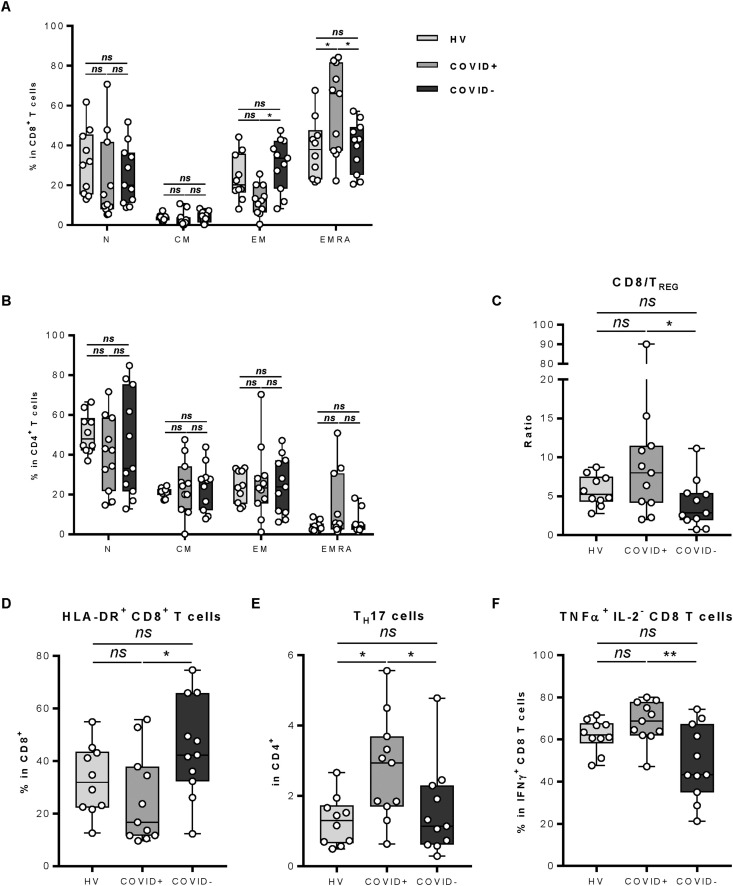
Looking at CD4 and CD8 subsets, we observed a decrease in effector memory cells in CD8 T cells and an increase in effector memory RA+ (EMRA+) cells, suggesting an accumulation of terminally differentiated cells in COVID-19–positive patients (Fig. 4 A). For the CD4 subset, we did not observe any difference for each subtype in COVID-19–positive patients with exception of a decrease of naïve CD4 T cells (Fig. 4B). The Treg proportion in CD4 T cells did not change in COVID-19–positive patients, but as for the overall increase in CD8 T cells, we observed a decrease in the Treg/CD8 ratio (Fig. 4C). Interestingly, we observed in the basal peripheral blood a marked decrease in HLA-DR expression in CD8 T cells in COVID-19–positive patients (Fig. 4D), which is marker of T-cell activation [10,11]. Concerning secretory function of T cells, we observed an increase in IL-17A and Th17 cells in COVID-19–positive patients (Fig. 4E) and an accumulation of cytotoxic (IFNγ+) CD8 T cells producing TNFα (Fig. 4F). The study of CD8 T-cells secretion capacities showed no other differences between COVID-19–positive and –negative patients (Fig. SD4).
Fig. 4.
Leucocyte populations and lymphocyte function analysis between COVID-19–positive and –negative patients and healthy volunteers. A–D: Whole blood of healthy volunteers or COVID-19–positive and –negative patients was stained with anti-CD16, anti-CD56, anti-CD25, anti–HLA-DR, anti-CD14, anti-CD4, anti-CD8, anti-CD3, anti-CD15, anti-CD45, anti-CD127, anti-CCR7 and anti-CD45RA antibodies and analysed by flow cytometry. A: The frequency of naïve (N: CD45RA+ CCR7+), central memory (CM: CD45RA− CCR7+), effector memory (EM: CD45RA− CCR7−) and effector memory RA+ (EMRA: CD45RA+ CCR7−) among CD8+ T cells is depicted. B: The frequency of naïve (N: CD45RA+ CCR7+), central memory (CM: CD45RA− CCR7+), effector memory (EM: CD45RA− CCR7−) and effector memory RA+ (EMRA: CD45RA+ CCR7−) among CD4+ T cells is depicted. C: The ratio of CD8 to regulatory T cells (TREG) is depicted. D: The frequency of HLA-DR (Human Leukocyte Antigen – DR isotype)–positive CD8+ T cells among CD8+ T cells is depicted. E–F: Whole blood of healthy volunteers or COVID-19–positive and –negative patients was activated and then stained with anti-IFNγ, anti-CD25, anti-CD4, anti-IL-4, anti-Foxp3, anti-TNFα, anti-IL-17A, anti-CD8 and anti-IL-2 antibodies and analysed by flow cytometry. E: The frequency of IL-17A–positive CD4+ T cells (Th17 cells) among CD4+ T cells is depicted. F: The frequency of TNFα–positive CD8+ T cells among IFNγ+ CD8+ T cells is depicted. The data presented constitute the analyses performed on 11 COVID-19–positive patients, 11 COVID-19–negative patients and 10 healthy volunteers. Statistical difference is determined by a Mann–Whitney test. ns = no significant, ∗ = p< 0.05 and ∗∗ = p< 0.01.
Together these data underline the induction of a T-dependent inflammatory response with an accumulation of Th17, TNFα-producing CD8 T cells and a concomitant decrease in CD8 HLA-DR–positive cells, suggesting a reduction in effector CD8 T cells in COVID-19–positive cancer patients.
During follow-up, the blood immune modifications persisted for 2 weeks. Interestingly, death from COVID-19 was associated with higher neutrophil and mMDSC levels and also with a lower number of inflammatory monocytes (IMs), CD3 T cells, CD8 HLA-DR–positive cells and EMRA+ CD8 T cells (Fig. SD5).
4. Discussion
To the best of our knowledge, this is the first preliminary study to describe the immunological characteristics of cancer patients with SARS-CoV-2 infection. A growing body of data is emerging regarding the clinical and epidemiological features of patients with COVID-19 [9]. In addition, there have been some reports on immune response during SARS-CoV-2 infection [[12], [13], [14]]. The results obtained in this study could be strengthened by a larger number of patients and by the addition of analyses on COVID-19–positive non-cancer patients.
With regard to the myeloid compartment, we observed an accumulation of HLA-DRlowCD14+ cells, similar to mMDSC cells, as well as concomitant decrease in HLA-DRhigh CD14+ cells. Previous studies in non-cancer, symptomatic COVID-19 patients have shown a similar accumulation of HLA-DRlow CD14+ IMs[[15], [16], [17]]. The expansion and activation of these cells frequently depends on the cytokines IL-1 and IL-6 [18,19]. This accumulation could be linked to an inflammatory signature found during COVID-19 [20]. Significantly elevated systemic levels of the pro-inflammatory cytokine IL-6 have been reported in several COVID-19 patient cohorts and shown to correlate with disease severity [21] and the presence of inflammatory cytokines in serum. As in non-cancer patients, SARS-CoV-2 infection appears to affect CD14 myeloid cell differentiation.
T cells play a fundamental role in SARS-CoV-2 infection. A major marker associated with this disease is lymphopenia, with drastically reduced numbers of both CD4 and CD8 T cells in moderate and severe COVID-19 cases [9,[22], [23], [24], [25]]. Previous reports have shown predominant CD8 T-cell depletion, which seems to correlate with COVID-19–associated disease severity and mortality [9,22,24,[26], [27], [28], [29]]. In cancer patients, we also observed major CD3 T-cell depletion. COVID-19 accentuates cancer-related CD3 lymphopenia, but CD4 lymphopenia is more pronounced than CD8 depletion.
Regarding the T-cell phenotype, many studies have underlined the increased presence of activated T cells during COVID-19 [19,[30], [31], [32], [33], [34], [35]]. Functional analysis showed impaired function of both CD4 and CD8 T cells, with reduced frequencies of polyfunctional T cells [9,25,35]. Our data showed marked CD4 lymphopenia, with a trend towards a decreased naïve CD4 T-cell fraction, which may suggest an overall reduction in CD4 T-cell help, with absence of precursors. From a functional point of view, we observed a strong accumulation of IL-17A-producing Th17 cells. These are inflammatory cells that are involved in neoangiogenesis and accumulation of myeloid cells at infection or tumour sites [36], as frequently observed during SARS-Cov2 infection [37]. While IL-6 production is observed during SARS-Cov2 infection [21] and because this cytokine is involved in Th17 differentiation, the accumulation of Th17 is logical and suggests IL-6–dependent inflammation in these patients.
Regarding CD8 T cells, we observed an accumulation of EMRA cells, which in contrast suggests an accumulation of terminally activated T cells and a marked CD8 immune response. CD8 T cells remained functional with accumulation of IFNγ+ TNFα+ cells. Surprisingly, we observed a substantial reduction in HLA-DR expression on CD8 T cells. HLA-DR is recognised as a marker of T-cell activation [10,11] and has been shown to be increased in cytotoxic T lymphocytes in autoimmune diseases [38] and in patients with HIV infection [39]. Thus, CD8 immune response is ambivalent, with on the one hand, a decrease in the T-cell count and activated HLA-DR CD8 T cells, and, on the other hand, a concomitant decrease in the Treg/CD8 ratio and an accumulation of polyfunction CD8 T cells, suggesting a marked CD8-dependent inflammatory response that might be deleterious.
5. Conclusions
This manuscript underlines the particular immune response in cancer patients with COVID-19. As in other reports, we observed marked T-cell lymphopenia and a decrease in HLA-DR+ CD8 T cells. However, we observed some particularities, with an inversion of the CD4/CD8 ratio, and an induction of an inflammatory response, with accumulation of MDSC, Th17 and TNFα+ inflammatory CD8 T cells. This information could be important in broadening our understanding of the complications that occurs in cancer patients infected with SARS-CoV-2 virus.
Financial support
None.
Author contributions
M.T., J.D.F. and F.G. contributed to literature search, data collection, data analysis, data interpretation and writing of the manuscript. M.T., J.D.F., E.L. and F.G. contributed to the study design. M.T., J.D.F., M.B. and L.H. made contributions to collection of the data and the data analysis. M.T., J.D.F., E.L. and F.G. participated in drafting the article and revising it critically for important intellectual content. All authors reviewed the manuscript and gave final approval of the submitted version.
Conflict of interest statement
F. Ghiringhelli reports receiving honoraria for oral communications from Lilly, Sanofi, Bristol-Myers Squibb, Astra Zeneca and Amgen; receiving funding for clinical trials from Astra Zeneca; receiving travel grants from Roche France, Amgen and Servier; and being an advisory board member for Merck Serano, Amgen, Roche France and Sanofi.
The other authors have no potential conflict of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2020.08.013.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1: Leucocyte population analysis between COVID-19 positive and negative patient and healthy volunteers. Whole blood of healthy volunteers or COVID-19 positive and negative patients was stained with anti-CD16, anti-CD56, anti-CD25, anti-HLA-DR, anti-CD14, anti-CD4, anti-CD8, anti-CD3, anti-CD15, anti-CD45, anti-CD127, anti-CCR7 and anti-CD45RA antibodies and analyzed by flow cytometry. The frequency of CD15+ CD16low eosinophils among CD45+ cells is depicted. The data presented constitute the analyses performed on 11 COVID-19 positive patients, 11 COVID-19 negative patients and 10 healthy volunteers. Statistical difference is determined by a Mann-Whitney test, ns = no significant. Supplementary figure 2: Leucocyte population analysis between COVID-19 positive and negative patient and healthy volunteers. Whole blood of healthy volunteers or COVID-19 positive and negative patients was stained with anti-CD16, anti-CD56, anti-CD25, anti-HLA-DR, anti-CD14, anti-CD4, anti-CD8, anti-CD3, anti-CD15, anti-CD45, anti-CD127, anti-CCR7 and anti-CD45RA antibodies and analyzed by flow cytometry. A-B: The count of CD16+ CD14low monocytes (A) and CD16+ CD14+ monocytes is depicted. The data presented constitute the analyses performed on 11 COVID-19 positive patients, 11 COVID-19 negative patients and 10 healthy volunteers. Statistical difference is determined by a Mann-Whitney test. ns = no significant, ∗ = p< 0.05 , ∗∗ = p< 0.01, ∗∗∗ = p< 0.001 and ∗∗∗∗ = p<0.0001.Supplementary figure 3: Leucocyte population analysis between COVID-19 positive and negative patient and healthy volunteers. Whole blood of healthy volunteers or COVID-19 positive and negative patients was stained with anti-CD16, anti-CD56, anti-CD25, anti-HLA-DR, anti-CD14, anti-CD4, anti-CD8, anti-CD3, anti-CD15, anti-CD45, anti-CD127, anti-CCR7 and anti-CD45RA antibodies and analyzed by flow cytometry. A-C: The count of CD56+ NK cells (A), γδ T cells (B) and CD56+ CD3+ NKT cells is depicted. The data presented constitute the analyses performed on 11 COVID-19 positive patients, 11 COVID-19 negative patients and 10 healthy volunteers. Statistical difference is determined by a Mann-Whitney test. ns = no significant, ∗ = p< 0.05 , ∗∗ = p< 0.01, ∗∗∗ = p< 0.001.Supplementary figure 4: Lymphocyte function analysis between COVID-19 positive and negative patient and healthy volunteers. Whole blood of healthy volunteers or COVID-19 positive and negative patients was activated and then stained with anti-IFNγ, anti-CD25, anti-CD4, anti-IL-4, anti-Foxp3, anti-TNFα, anti-IL-17A, anti-CD8 and anti-IL-2 antibodies and analyzed by flow cytometry. The frequency of TNFα- IL-2-, TNFα- IL-2+, TNFα+ IL-2- and TNFα+ IL-2+ among IFNγ+ CD8+ T cells is depicted. The data presented constitute the analyses performed on 11 COVID-19 positive patients, 11 COVID-19 negative patients and 10 healthy volunteers. Statistical difference is determined by a Mann-Whitney test. ns = no significant and ∗∗∗ = p< 0.001. Supplementary figure 5: Blood immune modification during follow up between COVID-19 positive and negative patient. Whole blood of COVID-19 positive was stained with anti-CD16, anti-CD56, anti-CD25, anti-HLA-DR, anti-CD14, anti-CD4, anti-CD8, anti-CD3, anti-CD15, anti-CD45, anti-CD127, anti-CCR7 and anti-CD45RA antibodies and analyzed by flow cytometry. Patients were monitored for blood levels during their hospitalization and were separated into 2 groups: patients with a severe COVID19 (dead group) versus a mild COVID19 (alive group). For each group, the frequency of neutrophils among CD45+ cells, mMDSC and HLA-DRhigh monocytes among CD14+ cells is depicted. As regards T cell analysis, the frequency of CD3+ T cells among CD45+ cells, the frequency of HLA-DR positive CD8 T cells and EMRA CD8 T cells is also depicted. Supplementary figure 6: Gating strategy for the “Leucocyte population identification and numeration” panel. Supplementary figure 7: Gating strategy for the “Lymphocyte function analysis” panel.
Baseline demographics for each patient individually
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu M., Gao Y., Shi S., Chen Y., Yang K., Tian J. Drinking no-links to the severity of COVID-19: a systematic review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.05.042. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.296. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyashita H., Mikami T., Chopra N., Yamada T., Chernyavsky S., Rizk D., et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York city. Ann Oncol Off J Eur Soc Med Oncol. 2020 doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The OpenSAFELY Collaborative. Williamson E., Walker A.J., Bhaskaran K.J., Bacon S., Bates C., et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. Epidemiology. 2020 doi: 10.1101/2020.05.06.20092999. [DOI] [Google Scholar]
- 9.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baecher-Allan C., Wolf E., Hafler D.A. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 11.Viallard J.-F., Blanco P., André M., Etienne G., Liferman F., Neau D., et al. CD8+HLA-DR+ T lymphocytes are increased in common variable immunodeficiency patients with impaired memory B-cell differentiation. Clin Immunol. 2006;119:51–58. doi: 10.1016/j.clim.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet Lond Engl. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet Lond Engl. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. S1931312820302365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y., et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. MedRxiv. 2020 doi: 10.1101/2020.03.24.20042655. 2020.03.24.20042655. [DOI] [Google Scholar]
- 17.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., qi Y., et al. Aberrant pathogenic GM-CSF + T cells and inflammatory CD14 + CD16 + monocytes in severe pulmonary syndrome patients of a new coronavirus. Immunology. 2020 doi: 10.1101/2020.02.12.945576. [DOI] [Google Scholar]
- 18.Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:1–18. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo C., Li B., Ma H., Wang X., Cai P., Yu Q., et al. Tocilizumab treatment in severe COVID-19 patients attenuates the inflammatory storm incited by monocyte centric immune interactions revealed by single-cell analysis. 2020. [DOI]
- 20.Ong E.Z., Chan Y.F.Z., Leong W.Y., Lee N.M.Y., Kalimuddin S., Haja Mohideen S.M., et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nie S., Zhao X., Zhao K., Zhang Z., Zhang Z., Zhang Z. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study. 2020. [DOI]
- 24.Zeng Q., Li Y., Huang G., Wu W., Dong S., Xu Y. Mortality of COVID-19 is associated with cellular immune function compared to immune function in Chinese han population. Infectious Diseases (except HIV/AIDS) 2020 doi: 10.1101/2020.03.08.20031229. [DOI] [Google Scholar]
- 25.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020:11. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5 doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., qi Y., et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020 doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., et al. Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors. 2020. [DOI] [PubMed]
- 31.Dong C., Ni L., Ye F., Chen M.-L., Feng Y., Deng Y.-Q., et al. Characterization of anti-viral immunity in recovered individuals infected by SARS-CoV-2. 2020. [DOI]
- 32.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;1–3 doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 33.Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0819-2. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X., Dai T., Zhou X., Qian H., Guo R., Lei L., et al. Analysis of adaptive immune cell populations and phenotypes in the patients infected by SARS-CoV-2. MedRxiv. 2020 doi: 10.1101/2020.03.23.20040675. 2020.03.23.20040675. [DOI] [Google Scholar]
- 35.Zheng H.-Y., Zhang M., Yang C.-X., Zhang N., Wang X.-C., Yang X.-P., et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin F., Apetoh L., Ghiringhelli F. Controversies on the role of Th17 in cancer: a TGF-β-dependent immunosuppressive activity? Trends Mol Med. 2012;18:742–749. doi: 10.1016/j.molmed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015432. 0:null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viallard J.F., Bloch-Michel C., Neau-Cransac M., Taupin J.L., Garrigue S., Miossec V., et al. HLA-DR expression on lymphocyte subsets as a marker of disease activity in patients with systemic lupus erythematosus. Clin Exp Immunol. 2001;125:485–491. doi: 10.1046/j.1365-2249.2001.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sáez-Cirión A., Lacabaratz C., Lambotte O., Versmisse P., Urrutia A., Boufassa F., et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Leucocyte population analysis between COVID-19 positive and negative patient and healthy volunteers. Whole blood of healthy volunteers or COVID-19 positive and negative patients was stained with anti-CD16, anti-CD56, anti-CD25, anti-HLA-DR, anti-CD14, anti-CD4, anti-CD8, anti-CD3, anti-CD15, anti-CD45, anti-CD127, anti-CCR7 and anti-CD45RA antibodies and analyzed by flow cytometry. The frequency of CD15+ CD16low eosinophils among CD45+ cells is depicted. The data presented constitute the analyses performed on 11 COVID-19 positive patients, 11 COVID-19 negative patients and 10 healthy volunteers. Statistical difference is determined by a Mann-Whitney test, ns = no significant. Supplementary figure 2: Leucocyte population analysis between COVID-19 positive and negative patient and healthy volunteers. Whole blood of healthy volunteers or COVID-19 positive and negative patients was stained with anti-CD16, anti-CD56, anti-CD25, anti-HLA-DR, anti-CD14, anti-CD4, anti-CD8, anti-CD3, anti-CD15, anti-CD45, anti-CD127, anti-CCR7 and anti-CD45RA antibodies and analyzed by flow cytometry. A-B: The count of CD16+ CD14low monocytes (A) and CD16+ CD14+ monocytes is depicted. The data presented constitute the analyses performed on 11 COVID-19 positive patients, 11 COVID-19 negative patients and 10 healthy volunteers. Statistical difference is determined by a Mann-Whitney test. ns = no significant, ∗ = p< 0.05 , ∗∗ = p< 0.01, ∗∗∗ = p< 0.001 and ∗∗∗∗ = p<0.0001.Supplementary figure 3: Leucocyte population analysis between COVID-19 positive and negative patient and healthy volunteers. Whole blood of healthy volunteers or COVID-19 positive and negative patients was stained with anti-CD16, anti-CD56, anti-CD25, anti-HLA-DR, anti-CD14, anti-CD4, anti-CD8, anti-CD3, anti-CD15, anti-CD45, anti-CD127, anti-CCR7 and anti-CD45RA antibodies and analyzed by flow cytometry. A-C: The count of CD56+ NK cells (A), γδ T cells (B) and CD56+ CD3+ NKT cells is depicted. The data presented constitute the analyses performed on 11 COVID-19 positive patients, 11 COVID-19 negative patients and 10 healthy volunteers. Statistical difference is determined by a Mann-Whitney test. ns = no significant, ∗ = p< 0.05 , ∗∗ = p< 0.01, ∗∗∗ = p< 0.001.Supplementary figure 4: Lymphocyte function analysis between COVID-19 positive and negative patient and healthy volunteers. Whole blood of healthy volunteers or COVID-19 positive and negative patients was activated and then stained with anti-IFNγ, anti-CD25, anti-CD4, anti-IL-4, anti-Foxp3, anti-TNFα, anti-IL-17A, anti-CD8 and anti-IL-2 antibodies and analyzed by flow cytometry. The frequency of TNFα- IL-2-, TNFα- IL-2+, TNFα+ IL-2- and TNFα+ IL-2+ among IFNγ+ CD8+ T cells is depicted. The data presented constitute the analyses performed on 11 COVID-19 positive patients, 11 COVID-19 negative patients and 10 healthy volunteers. Statistical difference is determined by a Mann-Whitney test. ns = no significant and ∗∗∗ = p< 0.001. Supplementary figure 5: Blood immune modification during follow up between COVID-19 positive and negative patient. Whole blood of COVID-19 positive was stained with anti-CD16, anti-CD56, anti-CD25, anti-HLA-DR, anti-CD14, anti-CD4, anti-CD8, anti-CD3, anti-CD15, anti-CD45, anti-CD127, anti-CCR7 and anti-CD45RA antibodies and analyzed by flow cytometry. Patients were monitored for blood levels during their hospitalization and were separated into 2 groups: patients with a severe COVID19 (dead group) versus a mild COVID19 (alive group). For each group, the frequency of neutrophils among CD45+ cells, mMDSC and HLA-DRhigh monocytes among CD14+ cells is depicted. As regards T cell analysis, the frequency of CD3+ T cells among CD45+ cells, the frequency of HLA-DR positive CD8 T cells and EMRA CD8 T cells is also depicted. Supplementary figure 6: Gating strategy for the “Leucocyte population identification and numeration” panel. Supplementary figure 7: Gating strategy for the “Lymphocyte function analysis” panel.
Baseline demographics for each patient individually