Abstract
The coronavirus disease 2019 (COVID-19) pandemic has greatly affected demand for imaging services, with marked reductions in demand for elective imaging and image-guided interventional procedures. To guide radiology planning and recovery from this unprecedented impact, three recovery models were developed to predict imaging volume over the course of the COVID-19 pandemic: (1) a long-term volume model with three scenarios based on prior disease outbreaks and other historical analogues, to aid in long-term planning when the pandemic was just beginning; (2) a short-term volume model based on the supply-demand approach, leveraging increasingly available COVID-19 data points to predict examination volume on a week-to-week basis; and (3) a next-wave model to estimate the impact from future COVID-19 surges. The authors present these models as techniques that can be used at any stage in an unpredictable pandemic timeline.
Key Words: COVID-19, supply and demand, recovery planning, predictive models
Introduction
The coronavirus disease 2019 (COVID-19) pandemic continues to have tremendous impact on the global economy and health care systems, and its effects on imaging volume have been substantial [1, 2, 3, 4]. Similar to other health care facilities, our radiology department experienced a drastic reduction in imaging volume, ranging from 48% to 93% by imaging modality [5]. In the context of this unprecedented drop, radiology departments need robust estimates on how soon volumes and operations may recover to effectively plan for the immediate and long-term financial stability of their institution.
The most recent analogue to the COVID-19 pandemic was the severe acute respiratory syndrome (SARS) epidemic of 2002 to 2004. Health systems were overburdened [6,7], and the fear of SARS spreading globally led to financial losses in health care and other industries [8,9]. Overall, despite widespread media coverage of the epidemic, the deleterious effect on world economies was relatively short lived because of efficient and swift containment [10]. More recently, Middle East respiratory syndrome and H1N1 influenza wreaked havoc with health care utilization, with profound financial impacts, in various regions, but not to the same degree as SARS [11, 12, 13, 14]. Beyond health care, major natural disasters (such as hurricanes Katrina and Harvey) have produced similar far-reaching economic effects [15, 16, 17, 18, 19].
Most recently, the great recession of 2007 to 2009 decreased health care utilization because of economic hardship and a drop in patients’ willingness to accept copayments [20,21], especially those with high-deductible health plans [22]. Economic downturns have been shown to variably affect many aspects of health care in many countries [23, 24, 25, 26].
However, none of the aforementioned examples have compared with the devastating economic and health care effects wrought by the COVID-19 pandemic. In addition, recovery predictions in health care have not been investigated rigorously, possibly because there has been no recent disaster on the scale of COVID-19. Facing the unprecedented nature and length of the COVID-19 outbreak, many radiology departments have sought clear guidance to better prepare for the future of the pandemic. To address this need within our department, we developed three novel models that could predict imaging volume over the course of the COVID-19 pandemic:
-
1.
A long-term volume model, estimating major scenarios of radiology volume recovery;
-
2.
a short-term volume model, predicting examination volume on a week-to-week basis throughout the course of the pandemic; and
-
3.
a next-wave model, forecasting the impact of future (subsequent) COVID-19 surges on imaging volume.
The main aim of this work was to guide radiology operations through the immediate and long-term recovery using these three complementary models. Furthermore, these models explicitly account for the potential interaction among baseline volumes, patient sentiment, and state and national policy.
Modeling the Pandemic Recovery
Our radiology department is centered within a large urban academic hospital (main hospital campus) and its affiliated imaging centers. Our institution is a 1,011-bed quaternary care urban academic medical center in Boston and yearly sees approximately 50,000 inpatients, 110,000 emergency department patients, and 1.5 million outpatients, culminating in about 750,000 imaging studies, which include diagnostic imaging and interventional radiologic procedures. These data were used as a baseline for the three models presented here.
Long-Term Volume: Modeling Imaging Recovery on the Basis of Historical Analogues
Our first recovery model was developed during the initial wave of COVID-19 in the United States (April 2020), as our department observed a severe drop in imaging volume. At the time, little was known about how a potential recovery would proceed, and reopening as a concept was not actively discussed. Thus, we relied on historical analogues such as the SARS epidemic of 2002 to 2004. This research [27] revealed that
-
•
forecasting imaging volume rebound must consider both the expected duration of the pandemic and the shape of the recovery curves;
-
•
even during the same pandemic outbreak, each health care facility may have its own recovery pattern; and
-
•
once health effects are minimized, because of a marked reduction in new cases and reduced fear of contracting the disease, the economic effects will have a profound impact on the subsequent recovery.
Using historical precedents, we identified three possible scenarios to model the course of imaging volume recovery: swift, gradual, and muted (Table 1 ).
Table 1.
Three scenarios for long-term imaging volume recovery
| Scenario | Description | Timeline | Historical Examples |
|---|---|---|---|
| Swift | Restrictions are lifted quickly. Pent-up demand built over the course of several months. Positive media coverage and public outreach of medical centers would portray imaging centers as safe, which could accelerate recovery. | Volumes return to 80%-90% of prepandemic within 1-2 months. | First phase (March to April) of the 2003 SARS epidemic in Toronto [27] 2003 SARS epidemic in Taiwan [32,33] |
| Gradual | Even if restrictions are lifted quickly, the rate of recovery is dampened. This scenario is fundamentally built upon a general fear of contracting the virus at health care facilities and economic effects that relate to loss of income, insurance, and the consequent reduction in health care utilization. | Volumes take 2-3 months to return to 80%-90% of prepandemic levels. | 2003 SARS epidemic in Taipei: designated infection hospital that was shut down for 1 month before reopening [34,35] Hotel demand after the SARS epidemic in Toronto (first and second phases) [36] 2007-2009 great recession [37] COVID-19 economic recovery in China [38] |
| Muted | Demand remains persistently low. Reoccurrences of infection require repeat physical distancing measures and interruption of elective imaging and image-guided interventional services. In the United States, sporadic outbreaks of COVID-19 will continue to occur, and although containable, these will contribute to a fear of health care facilities, resulting in a muted recovery response. Again, the economic effects resulting from COVID-19 could contribute to a muted recovery as certain segments of the population decline to undergo imaging examinations. | Gradual recovery, but only to 50%-80% of prepandemic volumes before plateau. Full demand does not recover until a vaccine/treatment/cure is available. | Second phase (May-June) of the 2003 SARS epidemic in Toronto [27] Ongoing waves of COVID-19 |
Note: The description, timeline, and historical examples upon which each scenario is based are shown. COVID-19 = coronavirus disease 2019; SARS = severe acute respiratory syndrome.
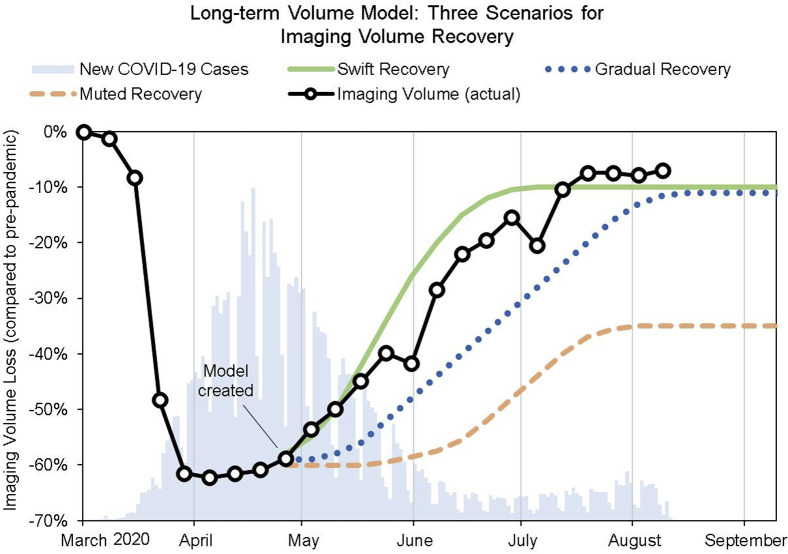
In a swift recovery scenario, imaging volume would experience a quick return to normal as pent-up demand, growing desensitization to the virus, adoption of safety protocols, and refined hospital safety protocols contribute to a 1- to 2-month recovery to 80% to 90% of normal levels; the pandemic is at the cusp of complete containment. A gradual recovery would result in a slow return to normal, about 2 to 3 months for 80% to 90% recovery, because of lingering fear of the virus, recessionary effects on health care utilization, and adjusting to the new capacity constraints needed for sanitation and safety. A muted recovery would be similar to a gradual recovery, except that volume would plateau at 50% to 80% of normal levels. This could be caused by lost demand, fear of contracting the virus at major hospitals, or subsequent surges of the virus. Each scenario was implemented for planning purposes as shown in Figure 1 .
Fig 1.
Long-term volume model with swift, gradual, and muted recovery scenarios. The shape and length of the recovery were determined by study of historical analogues. Abrupt drops in imaging volume correspond to weeks with Memorial Day and Independence Day holidays. These scenarios offered key insight in the early stages of the pandemic: (1) the overall length of recovery, which the swift model predicted well, and (2) the flattening of demand as volume reached prepandemic levels. COVID-19 = coronavirus disease 2019.
To draw these curves, we used the information in Table 1 as a guide. We took the higher end of the range for the duration: swift recovery would take 2 months, while gradual and muted recovery would take 3 months. For swift and gradual recovery, we forecasted a return to 90% of prepandemic volume to account for the increased time (and subsequent decreased capacity) for sanitation and hygiene practices; for example, just 3 to 4 min of additional cleaning time added to a 30-min examination would decrease volume (holding hours constant) by about 10%. For muted recovery, we forecasted a return to 65% on the basis of the experience of a hospital in Taiwan immediately after the SARS epidemic [28].
These three scenarios created a framework of competing assumptions on the basis of historical analogues. They also determine the scale of impact on the radiology department for the purpose of long-term planning. All recovery scenarios provided valuable insight for projecting the long-term impact of the COVID-19 pandemic. The only feasible way to improve these scenarios is to develop more precise recovery-predictive models, as more pandemic data become available.
Short-Term Volume: Predicting Recovery With Supply and Demand
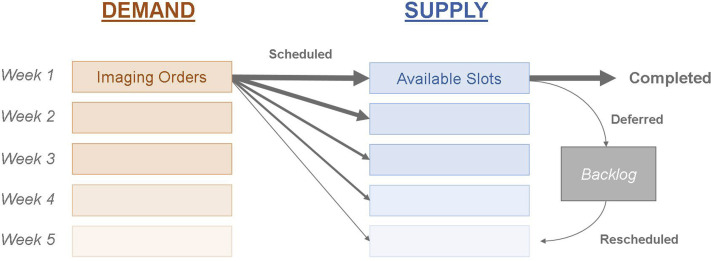
As the COVID-19 recovery timeline progressed, we observed steady, linear growth in imaging volume, largely corresponding to the swift recovery scenario described earlier. However, to plan for weekly operational improvements, a new and more accurate week-to-week model was needed on the basis of supply and demand in the context of radiology (Fig. 2 ). Supply is the total amount of imaging studies that can be conducted by a department; this value is determined by staffing, equipment resources, scheduling, scanner protocols, and other patient-processing components. Demand is approximated by the number of open imaging orders, driven primarily by referring physicians.
Fig 2.
Supply-and-demand model for imaging volume. Imaging orders (demand) are scheduled for available slots in the future (supply); the thickness of the arrows corresponds to the relative number of examinations scheduled for each slot, with most scheduled for the same week. Most of these scheduled examinations are completed. Some are deferred, put into the backlog, and rescheduled. Several factors affected supply and demand during the pandemic: supply was decreased because of government restrictions and enhanced precautions, while demand was reduced because of patients’ cancelling examinations, deferring care, and otherwise not wanting to be in a hospital during a pandemic, while doctors ordered fewer examinations for many reasons.
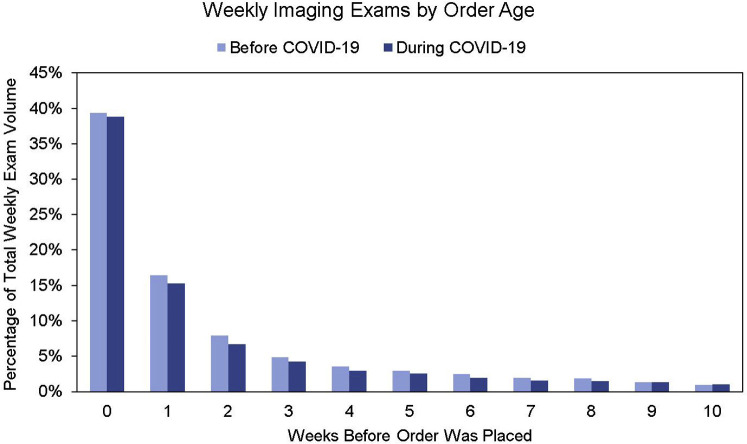
When an outpatient examination is ordered, it is scheduled for an open slot in the future. This could be during the same day or up to 1 year in the future. However, analyzing orders on a weekly basis and correcting for holiday weeks, the proportion of examinations scheduled for the same week, 1 week out, 2 weeks out, and so on, appears consistent (Fig. 3 ). Knowing this, it is possible to predict examination volume using ordering volume.
Fig 3.
Percentage of radiology orders converted to performed examinations after a number of weeks. The number of orders scheduled for the same week, 1 week out, and so on, stayed very constant after the coronavirus disease 2019 (COVID-19) period.
Volume planning, and the supply-demand approach in particular, assumes that the ordering volume will have an impact on the future volume of imaging examinations. Radiology departments have little control over their inpatient and emergency patient volumes, which are typically ordered and scheduled on the same day. Therefore, the ability to plan and intervene derives from outpatient examinations; thus these examinations were chosen for our model.
To examine how ordering volume drives outpatient examinations, we considered the total number of orders for a given week and estimated the number of these orders that would eventually be fulfilled (rather than cancelled or deferred) during the following weeks. Then, we found the typical proportion of orders that were executed for the same week, 1 week out, 2 weeks out, and so on (Fig. 3). As a result, we found that the recovery demand volume D N at week N can be accurately predicted from the previous 10 weeks with the following linear regression model:
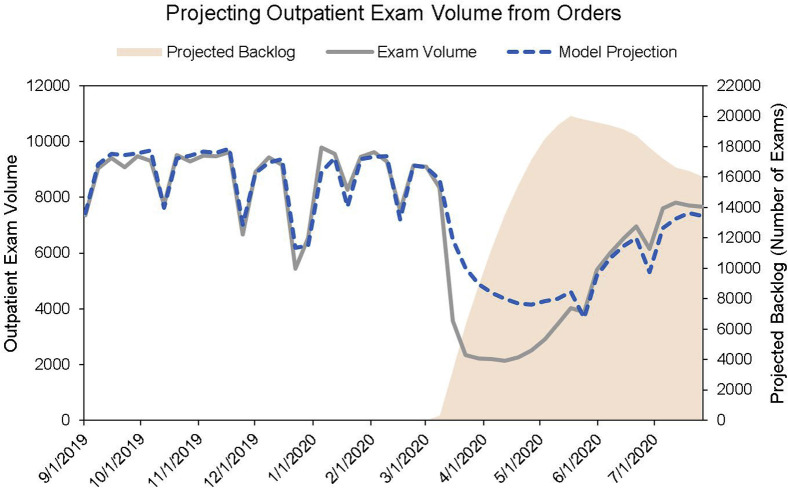
where D N is the demand (examination volume) in week N, P i is the proportion of orders scheduled for i weeks in the future, O N − i is the number of orders made in week N − i, and is a factor accounting for orders older than 10 weeks. was chosen by first running the model without and then comparing against actual historical data to find the error of predicted versus actual examination volume. In our case, can take on two values: one for holiday and another for nonholiday weeks. With this formula, we were able to calculate examination volume from ordering volume (Fig. 4 ).
Fig 4.
Projecting outpatient imaging volume from orders. The model effectively shows how many examinations should have been done considering the drop in orders. The gap between the model output and the actual examination volume accounts for the examinations added to the backlog (shown in light orange), which in our case are only the examinations that were scheduled and then cancelled, awaiting rescheduling. After the surge, model output is lower than actual examination volume; this is explained by technologists’ working through backlogged examinations. Abrupt drops in imaging volume correspond to weeks with holidays.
For the supply component S N of our model, we decided to use a linear trend of the three most recent nonholiday week supplies (the examinations conducted). We consistently updated our model with the current department operational protocols to ensure that our assumptions remained accurate.
The mismatch between supply and demand (S N − D N) and the underestimation of examination volume compared with the actual examination volume can be explained because of the backlog shown in Figure 4. Using our estimate of the backlog—in our case, examinations that were scheduled but then deferred and queued for rescheduling—we were able to conclude in the short term whether the department would “run out” of backlogged examinations to perform in lieu of lagging orders.
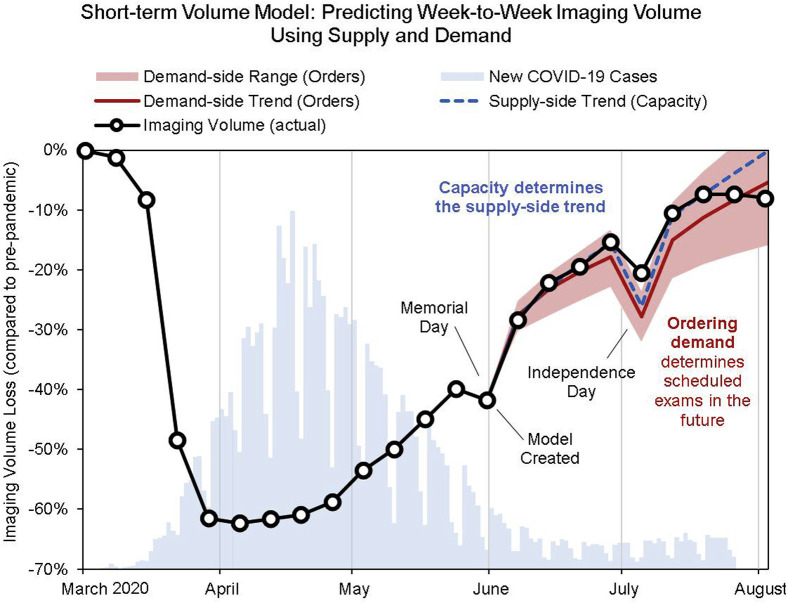
The resulting model provided a very accurate prediction for the next-week volume for our radiology department. We used this model to predict the demand-side examination volume trend and range (corresponding to ±50% of the previous weeks’ growth rate) as well as the supply-side trend (Fig. 5 ). Using this model to predict 8 weeks out, all predicted imaging volumes were within 5% of the actual volumes (aside from holiday-week volumes, which were 7% off). Ideally, the supply trend should exceed the demand trend to ensure timely access but fall within the demand range to limit excess capacity.
Fig 5.
Short-term volume model, showing projection of examination volume 9 weeks out after Memorial Day using supply and demand. The red line is the demand-side trend, which is dependent on the current ordering trend. The shaded red area showcases the range of ordering-based examination volume corresponding to ±50% growth rate compared with the trend before Memorial Day. The dotted blue line is the supply-side trend, which is an extrapolation of the current examination volume trend without considering ordering volume. This is intended to show how many examinations can be done independent of ordering demand. Additional supply constraints may be considered to reflect other capacity limitations that may account for the discrepancy seen in late August. COVID-19 = coronavirus disease 2019.
As recovery approaches 100%, additional supply constraints may be considered to reflect other capacity limitations, such as those imposed by patient social distancing and hygiene requirements.
The Next Wave: Modeling the Impact of Subsequent Waves
The experience of many previous pandemics [29,30] foretold the possibility of subsequent waves of COVID-19, and interest notably picked up after relaxations in lockdown policies were seriously discussed [31]. At our institution, the first wave of COVID-19 had disrupted 10% to 15% of our yearly imaging volume. Thus, forecasting the impact of a subsequent wave on imaging volume was vital to planning for the operational and financial health of the radiology department.
Unlike the first wave of COVID-19, clear policy guidance on pandemic recovery was available, and many countries had attempted different recovery strategies while trying to contain the outbreak. By looking at the interplay between the policy and the amount of radiology volume that was recovered, we discovered a strategy by which to predict the impact of a subsequent wave on imaging volume. Our aim was to forecast the impact on yearly imaging volume; we did not aim to predict when exactly a subsequent wave of COVID-19 would occur, only its shape, length, and depth.
When COVID-19 first surfaced, fear of contracting the disease and government restrictions catalyzed a severe reduction in imaging volume. Therefore, we viewed the imaging volume completed despite an unprecedented pandemic as the most critical volume, consisting of essential examinations that could not be delayed. In our case, this consisted of approximately 38% of cases; for 3 weeks at the height of the surge, only these examinations were performed.
Our estimates relied on a key element of government recovery plans: their phased nature. Many plans had separate phases in which certain businesses and activities were allowed to resume. Each phase was separated by at least 2 weeks, which is the incubation period of the virus; this allowed each of the phases to be studied to determine whether containment was on track.
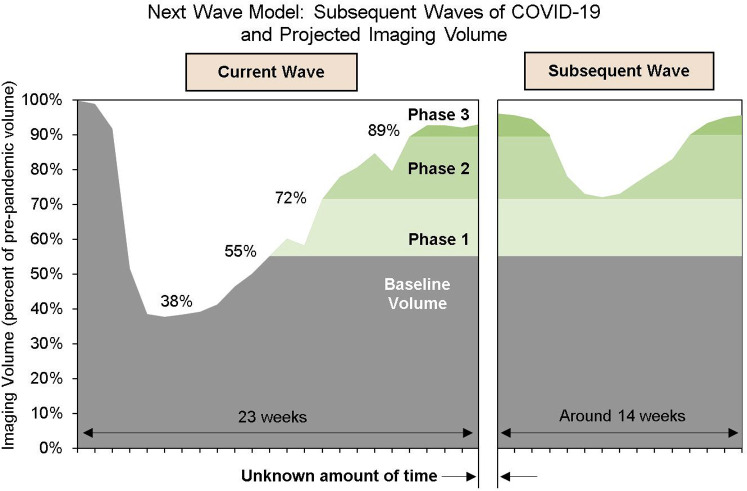
Radiology volume increased by a certain amount during each recovery phase. This allowed us to estimate what volume of imaging examinations would return in similar phase transitions in the future. We expected that if daily new COVID-19 cases were to increase again, policymakers would return back to the most lenient phase that allowed historical containment of the virus. Thus, we estimated that even if a similarly sized wave of COVID-19 were to strike again, the increased adherence to precautions coupled with the knowledge that a certain level of activities are possible to maintain while still containing viral spread would result in a lower decrement in imaging volume than during the initial wave (Fig. 6 ).
Fig 6.
Next-wave model: predicting the shape, depth, and length of a subsequent wave of coronavirus disease 2019 (COVID-19) using the interplay between current radiology recovery and a phased reopening policy. By looking at how much volume is regained during each phase without resurgence of the virus, we can assume that policy would be rolled back to the most lenient but safe phase, having the same effect on imaging volume as during the corresponding phase of the first wave. With this model, in our case, we estimated that a second wave of COVID-19 would result in an overall yearly drop in imaging volume of about 1% to 3%, compared with the 10% to 15% total yearly drop that we predicted from the first wave.
As a result, we aimed to describe the shape, depth, and length of an imaging volume decline on the basis of this technique. If a second wave of COVID-19 were to occur, we assumed that
-
•
the subsequent impact on imaging volume would resemble that of the first wave: a steep drop-off and a slow recovery;
-
•
a roll-back to an earlier phase would at worst cause a drop back to imaging volume that was observed during that phase;
-
•
the initial drop in volume would take the same amount of time as during the surge (although it would drop by far less);
-
•
recovery in imaging volume would proceed at this same rate as the first wave;
-
•
innovations in radiology operations (implemented at our sites) would allow a return to 100% of imaging volume despite the increased needs of social distancing and sanitization;
-
•
restrictions would be as effective as they were during the first wave and that they would require a similar amount of time.
With these assumptions, in our case, we estimated that a second wave of COVID-19 would result in an overall yearly drop in imaging volume of about 1% to 3%, compared with the 10% to 15% total yearly drop that we predicted from the first wave. This estimate can vary greatly for different radiology departments, but we assume that our methodology, described earlier, would remain accurate.
Limitations
Our work has several limitations inherited from the uncertainty of the pandemic. First, historical analogues may not entirely represent future events. Our early-stage long-term volume model was based in part on the SARS epidemic of 2002 to 2004, but the critical difference between the current COVID-19 pandemic and the SARS epidemic was their lengths: waves of SARS only lasted about 1 to 2 months, much shorter than COVID-19 waves. With SARS, imaging examination deferral for 1 to 2 months was possible. Thus, it may have been reasonable to assume that recovery would begin about 1 to 2 months after the COVID-19 pandemic began.
Second, the long-term volume model is good at predicting the overall shape and length of the imaging volume recovery, but the exact start of that recovery is hard to determine. Therefore, the model needs to be adjusted to the start of the recovery. Also, the imaging volume reduction was consequent to government restrictions but also patient behavior and preferences, which were not surveyed for our model.
For predicting subsequent waves, our model relies on having a phased recovery approach and assumes the government would roll back to an earlier phase that allowed containment progress. This does not take into account response to a rollback, for example, how well it would be followed compared with the first wave. The importance of policy cannot be understated when estimating ordering volume. Predicting the most likely policymaker response was necessary to have an effective estimate for ordering volume as well as subsequent wave modeling.
Despite these limitations, we believe that the methods described in this work will equip radiology departments with a reasonably robust, structured approach to recovery planning.
Conclusions
In addition to the specific model results described here, our approach produced the following conclusions.
In an unprecedented pandemic, both short- and long-term radiology recovery models are required to plan for recovery interventions and goals; these models can be developed using historical analysis combined with a supply-and-demand approach. The models we offer enable radiology departments to start with major recovery scenarios and refine them as more information becomes known, backing up assumptions with data-driven methods. These three methods will be valuable for use, not just for the current COVID-19 pandemic but in any sudden and unprecedented situations in which clear insight is needed for critical planning, adjustment, and decision making.
Take-Home Points
-
▪
Scenario models based on historical analogues are valuable to predict overall long-term impact, accurately predicting swifter recovery in inpatient and emergency volume and more muted outpatient radiology recovery, which plateaued at 80% of pre-COVID volume at a single institution.
-
▪
Radiology volume can be accurately predicted from examination ordering trends.
-
▪
Supply-demand creates a realistic framework for health care recovery models.
-
▪
Modeling subsequent waves of COVID-19 provides valuable insight into what the pandemic holds for radiology operations in the future.
Footnotes
Dr Brink has received personal fees from Accumen, outside the submitted work. All other authors state that they have no conflict of interest related to the material discussed in this article. Mr Guitron and Drs Pianykh, Succi, Lang, and Brink are employees.
References
- 1.Cavallo J.J., Forman H.P. The economic impact of the COVID-19 pandemic on radiology practices. Radiology. 2020;296:E141–E144. doi: 10.1148/radiol.2020201495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naidich J.J., Boltyenkov A., Wang J.J., Chusid J., Hughes D., Sanelli P.C. Impact of the coronavirus disease 2019 (COVID-19) pandemic on imaging case volumes. J Am Coll Radiol. 2020;17:865–872. doi: 10.1016/j.jacr.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin H, Abdelhalim A, Chau S, Shah S, Desai B, Gholamrezanezhad A. Responding to coronavirus disease 2019: LA County hospital experience. Emerg Radiol. In press. [DOI] [PMC free article] [PubMed]
- 4.Phillips C.D., Shatzkes D.R., Moonis G., Hsu K.A., Doshi A., Filippi C.G. From the eye of the storm: multi-institutional practical perspectives on neuroradiology from the COVID-19 outbreak in New York City. AJNR Am J Neuroradiol. 2020;41:960–965. doi: 10.3174/ajnr.A6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang M, Yeung T, Mendoza DP, et al. Imaging volume trends and recovery during the COVID-19 pandemic: a comparative analysis between a large urban academic hospital and its affiliated imaging centers. Acad Radiol. In press. [DOI] [PMC free article] [PubMed]
- 6.Chen W.-K., Cheng Y.-C., Chung Y.-T., Lin C.-C. The impact of the SARS outbreak on an urban emergency department in Taiwan. Med Care. 2005;43:168–172. doi: 10.1097/00005650-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Munir M.M., Martions R.S., Mian A.I. Emergency department admissions during COVID-19: implications from the 2002-2004 SARS epidemic. Western J Emerg Med. 2020;21:744–745. doi: 10.5811/westjem.2020.5.48203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beutels P., Jia N., Zhou Q.-Y., Smith R., Cao W.-C., de Vlas S.J. The economic impact of SARS in Beijing, China. Trop Med Intern Health. 2009;14(suppl 1):85–91. doi: 10.1111/j.1365-3156.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 9.Brahmbhatt M., Dutta A. On SARS type economic effects during infectious disease outbreaks. Policy Research Working Paper 4466. The World Bank. http://documents1.worldbank.org/curated/en/101511468028867410/pdf/wps4466.pdf Available at:
- 10.Keogh-Brown M.R., Smith R.D. The economic impact of SARS: how does the reality match the predictions? Health Policy. 2008;88:110–120. doi: 10.1016/j.healthpol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galante M., Garin O., Sicuri E. Health services utilization, work absenteeism and costs of pandemic influenza A (H1N1) 2009 in Spain: a multicenter-longitudinal study. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0031696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joo H., Maskery B.A., Berro A.D., Rotz L.D., Lee Y.-K., Brown C.M. Economic impact of the 2015 MERS outbreak on the Republic of Korea’s tourism-related industries. Health Security. 2019;17:100–108. doi: 10.1089/hs.2018.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung H., Park M., Hong K., Hyun E. The impact of an epidemic outbreak on consumer expenditures: an empirical assessment for MERS Korea. Sustainability. 2016;8:454. [Google Scholar]
- 14.Jeffery D.D., Cohen M., Brooks A., Linton A., Gromadzki R., Hunter C. Impact of the 2009 influenza (H1N1) pandemic on the United States military health care system. Military Med. 2013;178:653–658. doi: 10.7205/MILMED-D-12-00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baade R.A., Baumann R., Matheson V. Estimating the economic impact of natural and social disasters, with an application to Hurricane Katrina. Urban Studies. 2007;44:2061–2076. [Google Scholar]
- 16.Bayard K., Decker R.A., Gilbert C. Natural disasters and the measurement of industrial production: Hurricane Harvey, a case study. FEDS Notes. https://www.federalreserve.gov/econres/notes/feds-notes/natural-disasters-and-the-measurement-of-industrial-production-hurricane-harvey-a-case-study-20171011.htm Available at:
- 17.Bodenreider C., Wright L., Barr O., Xu K., Wilson S. Assessment of social, economic, and geographic vulnerability pre- and post-Hurricane Harvey in Houston, Texas. Environ Justice. 2019;12:182–193. [Google Scholar]
- 18.Vigdor J. The economic aftermath of Hurricane Katrina. J Econ Perspect. 2008;22:135–154. [Google Scholar]
- 19.Pelling M., Özerdem A., Barakat S. The macro-economic impact of disasters. Prog Dev Studies. 2002;2:283–305. [Google Scholar]
- 20.Lusardi A., Schneider D.J., Tufano P. National Bureau of Economic Research; Cambridge, Massachusetts: 2010. The economic crisis and medical care usage. [Google Scholar]
- 21.Sen B., Blackburn J., Morrisey M.A. Did copayment changes reduce health service utilization among CHIP enrollees? Evidence from Alabama. Health Serv Res. 2012;47:1603–1620. doi: 10.1111/j.1475-6773.2012.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fendrick A.M., Buxbaum J.D., Tang Y. Association between switching to a high-deductible health plan and discontinuation of type 2 diabetes treatment. JAMA Network Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonanzas F. The impact of the economic downturn on healthcare in Spain: consequences and alternatives. Expert Rev Pharmacoecon Outcomes Res. 2013;13:433–439. doi: 10.1586/14737167.2013.815418. [DOI] [PubMed] [Google Scholar]
- 24.De Belvis A.G., Ferrè F., Specchia M.L., Valerio L., Fattore G., Ricciardi W. The financial crisis in Italy: implications for the healthcare sector. Health Policy. 2012;106:10–16. doi: 10.1016/j.healthpol.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Perelman J., Felix S., Santana R. The great recession in Portugal: impact on hospital care use. Health Policy. 2015;119:307–315. doi: 10.1016/j.healthpol.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Waters H., Saadah F., Pradhan M. The impact of the 1997-98 East Asian economic crisis on health and health care in Indonesia. Health Policy Planning. 2003;18:172–181. doi: 10.1093/heapol/czg022. [DOI] [PubMed] [Google Scholar]
- 27.Low D.E. Learning from SARS: preparing for the next disease outbreak—workshop summary. The National Academies Press; Washington, District of Columbia: 2004. SARS: lessons from Toronto; pp. 63–70. [Google Scholar]
- 28.Chu D., Chen R.-C., Ku C.-Y., Chou P. The impact of SARS on hospital performance. BMC Health Serv Res. 2008;8:228. doi: 10.1186/1472-6963-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ofner-Agostini M., Wallington T., Henry B. Investigation of the second wave (phase 2) of severe acute respiratory syndrome (SARS) in Toronto, Canada. What happened? Can Commun Dis Rep. 2008;34:1–11. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention The 1918 influenza pandemic occurred in three waves and was the most severe pandemic in history. May 11, 2018. https://www.cdc.gov/flu/pandemic-resources/1918-commemoration/three-waves.htm Available at:
- 31.Gallagher J. Coronavirus: what is a second wave and is one coming? BBC. June 24, 2020. https://www.bbc.com/news/health-53113785 Available at:
- 32.McDonald L.C., Simor A.E., Su I.-J. SARS in healthcare facilities, Toronto and Taiwan. Emerg Infect Dis. 2004;10:777–781. doi: 10.3201/eid1005.030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K.-T., Twu S.-J., Chang H.-L. SARS in Taiwan: an overview and lessons learned. Int J Infect Dis. 2005;9:77–85. doi: 10.1016/j.ijid.2004.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization Regional Office for the Western Pacific . World Health Organization; Geneva, Switzerland: 2006. SARS: how a global epidemic was stopped. [Google Scholar]
- 35.Wu J., Ho M., Huang T., Chen K., Hsu K., Su I. CDC-Taiwan; Taipei: 2003. Epidemiological investigation of the SARS outbreak in the Taipei Municipal Hoping Hospital. Memoir of severe acute respiratory syndrome control in Taiwan; pp. 45–48. [Google Scholar]
- 36.Johnson Tew P., Lu Z., Tolomiczenko G., Gellatly J. SARS: lessons in strategic planning for hoteliers and destination marketers. Int J Contemp Hospitality Manag. 2008;20:332–346. [Google Scholar]
- 37.Dranove D., Garthwaite C., Ody C. The economic downturn and its lingering effects reduced Medicare spending growth by $4 billion in 2009-12. Health Aff (Millwood) https://www.healthaffairs.org/doi/10.1377/hlthaff.2015.0100 Available at: [DOI] [PubMed]
- 38.Chen H., Qian W., Wen Q. The impact of the COVID-19 pandemic on consumption: learning from high frequency transaction data. April 6, 2020. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3568574 Available at: