Abstract
Background
COVID-19 pandemic has affected around 20million patients worldwide and 2.0 million cases from India. The lockdown was employed to delay the pandemic. However, it had an unintentional impact on acute cardiovascular care, especially acute myocardial infarction (AMI). Observational studies have shown a decrease in hospital admissions for AMI in several developed countries during the pandemic period. We aimed to evaluate the impact of COVID-19 on the AMI admissions patterns across India.
Methods
In this multicentric, retrospective, cross-sectional study, we included all AMI cases admitted to participating hospitals during the study period 15th March to 15th June 2020 and compared them using a historical control of all cases of AMI admitted during the corresponding period in the year 2019. Major objective of the study is to analyze the changes inthe number of hospital admissions for AMI in hospitals across India. In addition, we intend to evaluate the impact of COVID-19 on the weekly AMI admission rates, and other performance measures like rates of thrombolysis/primary percutaneous interventions (PCI), window period, door to balloon time, and door to needle time. Other objectives include evaluation of changes in the major complications and mortality rates of AMI and its predictors during COVID-19 pandemic.
Conclusions
This CSI-AMI study will provide scientific evidence about the impact of COVID-19 on AMI care in India. Based on this study, we may be able to suggest appropriate changes to the existing MI guidelines and to educate the public regarding emergency care for AMI during COVID-19 pandemic.
Keywords: Acute coronary syndrome, Acute myocardial infarction, Corona virus, COVID-19, Outcome
Abbreviations: COVID 19, corona virus disease of 2019; AMI, acute myocardial infarction; ACS, Acute coronary syndrome; STEMI, ST elevation myocardial infarction; NSTEMI, non-STEMI; CSI, cardiology society of India; ICMR, Indian council of medical research; GCP, good clinical practice
1. Introduction
India is among the worst affected Asian nations and overall has the third-highest number of COVID-19 confirmed cases in the world with more than 2.1 million cases.1 COVID-19 has overwhelmed healthcare systems across the world and in India also. To address the pandemic, nationwide policy was implemented, and public level lockdown was declared to prevent the progression of the pandemic. Hospitals and healthcare facilities shifted the focus fully onto the COVID-19. Most of the hospitals postponed the care for non-emergent conditions. Though this strategy helped in slowing down the growth of the pandemic in India, it had affected the management of emergency conditions and especially acute cardiovascular conditions. Lock-down approach has an unintentional effect on the management of ACS (acute coronary syndrome) and in particular STEMI (ST-elevation myocardial infarction), and high-risk NSTEMI (non ST-elevation myocardial infarction) where time is the most important determinant of the outcome.
Observational studies from Northern Italy showed a significant decline in the number of ACS cases presenting to hospitals. The incidence rate ratio decreased by 30% as compared to the previous year and the decrease was in seen in all forms of ACS admissions including STEMI, NSTEMI and unstable angina.2 In another study from Italy carried out by the Italian Society of Cardiology in 54 hospitals taking care of acute myocardial infarction (AMI) patients, it was found that AMI admissions decreased 48.4% through out a one-week period during the COVID era compared to equivalent week in 2019.3 STEMI case fatality rate during the pandemic increased substantially compared with 2019. Similarly, a study from Austria showed the number of ACS cases within the pandemic period (during the early and late phase of a pandemic) decreased by 42%.4 A single-center study from Hong Kong showed a decrease in the number of primary PCI as well as an increase in the time to first medical contact and time to revascularization.5 Similar data from Spain also showed a decrease in cardiac interventions by 40%.6 A recent large analysis from 9 high volume centers across the United States of America also suggested a 38% reduction in cardiac catheterization laboratory activations for STEMI during the pandemic period.7 A recent study from England using the ‘secondary uses service admitted patient care database’showed that the admission rates for ACS paralleled the COVID-19 pandemic with low rates of admissions in the peak phases of pandemic and an increase in admission rates with the decline in pandemic in the country.8
The apparent decrease in rates of AMI admissions in hospitals during the COVID-19 pandemic lockdown period could be due to true decreases in ACS rates or could be due to the unavailability of transportation access and fear of COVID-19 in hospitals. Significant decrease in air pollution and less job stress were implicated for the decrease in ACS admissions, but recent observations of the pattern of ACS trends paralleling the pandemic trend suggest otherwise. There were reports from Italy showing a statistically significant increase in out of hospital cardiac arrests.9
Management of ACS, especially STEMI offers significant challenges in low-middle income countries (LMIC) like India.10,11 The challenges get further amplified during the COVID-19 pandemic.12 For instance, Indian CREATE registry13 has shown that most of the ACS patients reach the hospital by public or private transport. With the extended phases of lockdown, most of the public transport has ceased and private vehicles were off the road for varying periods across different states of India. This can have a significant impact on ACS admission rates especially given the wide variations in health care systems across different states in India. ACS has a very high mortality in India possibly related to lesser number of patients receiving timely interventions.13,14 Both the number of patients receiving timely interventions and the timing of interventions during an AMI are likely to worsen due to the pandemic. Primary PCI is the standard of care for STEMI and fibrinolysis may be considered as an option during these pandemic times for STEMI, if the index of suspicion of COVID infection is high as suggested by recent guidelines.12,15 The decline in the admission rates of STEMI cases due to the prevailing situation may have long term effects like increased future heart failure cases due to ischemic cardiomyopathy and increased sudden cardiac deaths due to VT.
The status of hospital admissions rates for ACS and the management strategy in India during the pandemic period is largely unknown. A study of ACS admission rates in India is essential given the impact of delayed or denied acute care during an episode of ACS on long term outcomes in terms of cardiovascular morbidity and mortality. The study is expected to provide some insights into future directions for cardiovascular care in the later phase/recurrences of this pandemic. This manuscript provides the study rationale, design, organization, and process analysis.
1.1. The rationale for the study
The status of AMI admissions in India during the COVID-19 pandemic period is largely unknown. Studies from Italy, Austria, Hongkong, England and United states have shown a significant decrease in AMI admissions, an increase in the window period, and an increase in out of hospital cardiac arrests during the pandemic period. We believe the pandemic has significantly impacted the AMI care in India at multiple levels. The motivating premise for this study is to analyze this impact and to issue appropriate guidelines to improve acute cardiovascular care during this pandemic.
1.2. Aims and objectives of the study
-
1.
To find out the change in the number of AMI admissions in the various hospitals in India during the period from the 15th of March to the 15th of June 2020 (COVID-19 period) as compared to the corresponding period in 2019.
-
2.
To find out weekly AMI admissions during the same period in different parts of India and to assess the impact of various phases of lockdown and its relaxations in AMI admissions.
-
3.
To find out the mortality rate of AMI patients admitted to the various hospitals in India during the COVID-19 era as compared to the corresponding period in 2019.
-
4.
To find out the type of AMI (STEMI vs NSTEMI), rate of coronary angiography, rate of PCI, thrombolysis or no revascularization, time windows for STEMI, and rates of major complications of AMI in India during COVID-19 era compared to the corresponding 2019 period.
2. Study design and methods
This is a Nationwide, multicenter, retrospective, cross-sectional study of all AMI admitted during the study period from 15th of March to 15th of June in the year 2020 using a historical control of all cases of AMI admitted during the corresponding period in the year 2019 designed by the cardiological Society of India (CSI).
2.1. Trial organization and process flow
CSI is the largest organization of cardiologists in India focused on improving cardiovascular care and research. It has 26 chapters with organized working committees spread all over India. We approached all the 26 state chapters for participation in the study and 22 chapters agreed to participate. The steering committee of the study requested the chapter coordinators to identify cardiac care facilities that were managing patients with AMI, which also have retrievable and reliable medical records of all patients during the study period. We contacted individual centers located in states that do not have a chapter of CSI. Finally, we included a total of 257 hospitals across India who were willing to participate in the study. The hospitals selected included both public and private hospitals in various urban and rural settings so that the impact of the pandemic can be studied across various realms of urban, rural, private, and public hospitals. The major locations of the participating hospitals are shown in Fig. 1.
Fig. 1.
Indian map showing all the participating centers across India.
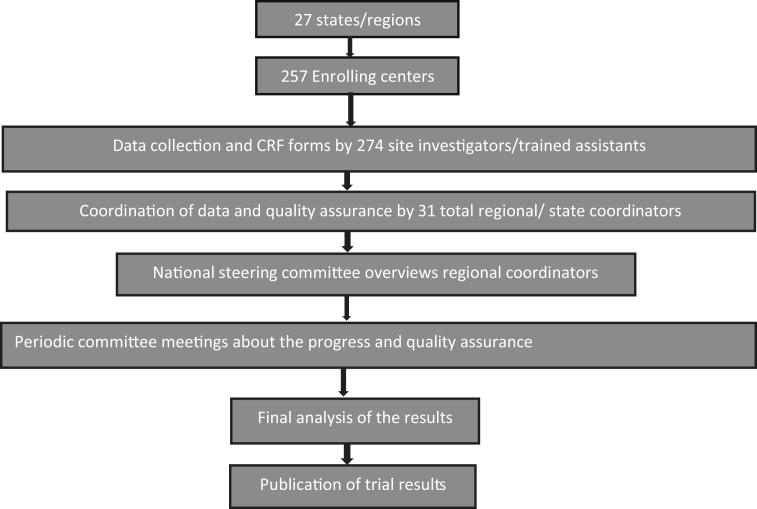
The trial organization is divided into a national steering committee, an advisory committee, State coordinators, and site investigators designated for each hospital (Fig. 2). The national steering committee comprised of Convener, CSI Registry Data Council, and five eminent cardiologists experienced in research related to acute cardiac care in India along with 4 members of CSI Registry Data Council. This steering committee coordinates the data collection process by interacting with state coordinators with periodic meetings. Overall study was monitored by a separate advisory committee comprising of past presidents of CSI and eminent researchers from India.
Fig. 2.
CSI-AMI- COVID-19 study organization and process flow.
The site investigators had a responsibility to collect all consecutive case records. The patient data is anonymized in the case report forms (CRF). The data once completed will be uploaded to a website. The state/regional coordinators will supervise the filling of the case record forms and periodically check for errors and missing information. Once the data collection process is completed, the state coordinators will verify and forward it to the steering committee for analysis and publication. National steering committee and advisors will randomly verify 5% CRFs with source documents to confirm accuracy.
We are including a total of 257 hospitals, with 274 site investigators, 31 state/regional coordinators from across 27 states covering almost all regions of India.
2.2. Inclusion criteria
All adult patients (age >18years) presenting to the hospital with a diagnosis of AMI.
AMI (Acute Myocardial Infarction): includes STEMI and NSTEMI.
STEMI: defined as per ACC/AHA guidelines16
-
•
New ST-segment elevation at the J point in 2 contiguous leads with the cut-off point as greater than 0.1 mV in all leads other than V2 or V3
-
•
In leads V2–V3 the cut-off point is greater than 0.2 mV in men older than 40 years old and greater than 0.25 in men younger than 40 years old, or greater than 0.15 mV in women.
-
•
Modified Sgarbossa criteria are used for diagnosing STEMI in pre-existing LBBB.
For STEMI to be included, the presentation should be within 14 days of onset of symptoms (should be typical of myocardial ischemia). ECG must show evolutionary changes of STEMI. If treated elsewhere within 14 days, the state coordinators must satisfy that the patient is not included in the participants list of another hospital.
NSTEMI: defined as new-onset chest pain consistent with ACS and troponin elevation but without ECG changes consistent with STEMI. There should be evidence of myocardial injury shown by elevation of troponins to diagnose NSTEMI. Whether this is acute or not is to be decided ideally by serial Troponins showing rise and/or fall. However, since this may not be always possible, we will rely on clinician’s impression to decide whether this is acute or not acute based on the clinical presentation. In addition, there should be one other criteria like typical symptoms, typical ECG changes, new regional wall motion abnormalities or other manifestations as outlined in the fourth universal definition of AMI. If NSTEMI treated elsewhere, either fully or partially, and referred for further evaluation to the participating centre, the patient will be included if Troponins are still elevated irrespective of duration, provided the patient is not included in the study by the other hospital.
2.3. Exclusion criteria
The exclusion criteria included STEMI outside the 14-day window period, NSTEMI having troponin negative at the time of hospitalization, cases already enrolled in to the study by another centre, and cases where the required details are not available in the medical records.
2.4. Data collection
Data about all the patients admitted with the diagnosis of AMI during the 3 months from 15th of March to 15th of June, 2020 (this period is selected, as nationwide preparedness was in full effect, and hospitals had their dedicated protocols for managing AMI, pandemic period). Similarly, data about all the patients admitted with the diagnosis of AMI during the three months from 15thof March to 15thof June 2019 (pre-pandemic period) will be collected retrospectively from all the centers. This data of the pandemic periodwill be compared and analyzed with the pre-pandemic period.
Multiple online meetings of the steering committee and state coordinators were held. During these meetings, clarity of definition of MI, definition of NSTEMI, duration of MI to be considered, need for troponin value were discussed and significant changes made in the proforma as suggested by the group. The individual patient data will be anonymized. Each patient is given a unique case id. The participating centers cannot access other centers data. The e-CRF is provided as supplement. The study is being conducted as per ICMR collaborative study guidelines and GCP guidelines.17,18
2.4.1. Study period
Pandemic phase: March 15, 2020, to June 15, 2020.
Pre pandemic phase (control period for comparison): March 15, 2019, to June 15, 2019.
The study organization and process flow are shown in Fig. 2.
3. Statistical considerations
All statistical analysis will be performed using SPSS STATA 14 software. A p value of <0.05 will be considered as significant. Quantitative variables will be expressed as mean and standard deviation. Qualitative variables will be expressed as frequencies and percentages. Comparison of these categorical variables among the groups will be carried out by using chi-square or Fischer exact test. CSI Registry Data Council will be maintaining the data.
3.1. Study timelines
Identify state coordinators: before 21st June 2020.
Forming national steering committee: before 21st June 2020.
Identifying the participating hospitals and site investigators: before 30th June 2020.
Ethics committee clearance and study registration: before 15th July 2020.
Collection of data and submission of case record forms: before 15th August 2020.
Data analysis and publication: before 15th September.
3.2. Work in progress
The necessary state coordinators and site investigators were identified. The national steering and advisory committeesare formed. The study also got ethical approval and registration. The study is designed to collect retrospective data from the respective hospital records. Since this is a retrospective study and not disclosing any confidential data related to the patient, informed consent has been waived off by the Ethics committee of CSI Kerala Chapter. Individual participating centers are required to either get an ethical approval from respective Institutional ethics committee or get a no objection certificate from administration and use the ethical approval obtained by the Ethics committee of CSI Kerala Chapter. Participating centers have started entering data in to eCRF platform after obtaining appropriate regulatory approvals. Entry in to the eCRF is the primary mode of data collection, which is monitored by the state co-ordinations and national principal investigator along with the steering committee. Mistakes, if any were identified and the site investigators were required to do appropriate correction.
4. Summary
This study designed by the largest organization of cardiologists in India to address the COVID-19 pandemic impact on AMI care in India. If the pandemic is significantly affecting the AMI care, appropriate guidelines will be issued to the scientific society. Based on this study, we will be able to provide suitable recommendations to the government regarding the need to inform the public by print and visual media regarding emergency cardiac care for acute MI.
Declaration of competing interest
The authors are solely responsible for the design of the study, the conduct of the study, drafting and editing of the paper, and its final contents.
Acknowledgments
Our sincere thanks tothe Cardiological Society of India (CSI) especially the headquarters and executive members for supporting the study. We also acknowledge the support of the presidents, secretaries and executive members of the various participating chapters, and the site investigators for making the study a reality.
The study is funded by the headquarters and the participating chapters of CSI.
Footnotes
What is already Known?
What this study adds?
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.COVID-19 situation update worldwide, as of 9 May 2020. European Centre for Disease Prevention and Control. 2020 https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases [Google Scholar]
- 2.De Filippo O., D’Ascenzo F., Angelini F. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Rosa Salvatore, Spaccarotella Carmen, Basso Cristina. Reduction of hospitalizations for myocardial infarction in Italy in the COVID – 19 era. Eur Heart J. 2020:1–6. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzler B., Siostrzonek P., Binder R.K., Bauer A., Reinstadler S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam C.-C.F., Cheung K.-S., Lam S. Impact of Coronavirus disease 2019 (COVID-19) outbreak on ST-segment–elevation myocardial infarction care in Hong Kong, China. Circ. Cardiovascular Quality and Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Leor O., Cid-Álvarez B., Ojeda S. Impact of the COVID-19 pandemic on interventional cardiology activity in Spain. RECICE. 2020:4060. [Google Scholar]
- 7.Garcia S., Albaghdadi M.S., Meraj P.M. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mafham M.M., Spata E., Goldacre R. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldi E., Sechi G.M., Mare C. COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J. 2020;41:3045–3054. doi: 10.1093/eurheartj/ehaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guha S., Sethi R., Ray S. Cardiological Society of India: position statement for the management of ST elevation myocardial infarction in India. Indian Heart J. 2017 Apr;69 doi: 10.1016/j.ihj.2017.03.006. S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra S., Ramakrishnan S., Babu A.S. Management algorithms for acute ST elevation myocardial infarction in less industrialized world. Indian Heart J. 2017;69(Suppl 1):S98–S103. doi: 10.1016/j.ihj.2017.03.005. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerkar P.G., Naik N., Alexander T. Cardiological society of India: document on acute MI care during COVID-19. Indian Heart J. 2020;72:70–74. doi: 10.1016/j.ihj.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xavier D., Pais P., Devereaux P. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 14.Mohanan P.P., Mathew R., Harikrishnan S. Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: results from the Kerala ACS Registry. Eur Heart J. 2013;34:121–129. doi: 10.1093/eurheartj/ehs219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FGP Welt, Shah P.B., Aronow H.D. Catheterization laboratory Considerations during the Coronavirus (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2372–2375. doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Gara P.T., Kushner F.G., Ascheim D.D. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2013;61:78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Guidelines | Indian Council of Medical Research | Government of India n.d.https://main.icmr.nic.in/content/guidelines.
- 18.ClinicalResearch Regulation for India | ClinRegsn.D.https://clinregs.niaid.nih.gov/country/india.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.