Abstract
Background and Aims
Vedolizumab was shown to be safe and effective for the treatment of Crohn’s disease [CD] and ulcerative colitis [UC] in the GEMINI Long-Term Safety [LTS] study. The vedolizumab Extended Access Program [XAP] provides patients with continued treatment. This XAP pharmacokinetics [PK] sub-study investigated vedolizumab efficacy, safety, and PK.
Methods
Vedolizumab dosing frequency was reduced from every 4 weeks [Q4W] to every 8 weeks [Q8W] at XAP enrolment, and patients were followed for 56 weeks. Outcomes included: efficacy, loss of clinical benefit, and re-escalation to Q4W dosing; and vedolizumab PK, immunogenicity, and adverse events.
Results
Among 167 enrolled patients [CD = 88, UC = 79], 80 [91%] with CD and 73 [92%] with UC completed 56 weeks; 76 [86%] and 71 [90%] with CD and UC, respectively, remained on Q8W dosing for 56 weeks. Clinical remission, corticosteroid-free clinical remission, and C-reactive protein levels were stable among patients remaining on Q8W through Week 56. Four patients with CD and two with UC resumed Q4W dosing [three with CD regained clinical response]. Patients with CD who completed Week 56 on Q8W dosing had median trough vedolizumab concentrations of 43.6 µg/mL at enrolment and 10.4 µg/mL at Week 56; concentrations were 42.4 µg/mL and 13.3 µg/mL, respectively, in patients with UC. Treatment-related adverse events were infrequent; no new or serious adverse events related to vedolizumab were reported.
Conclusions
In the XAP-PK sub-study, adherence to Q8W dosing was high, with no loss of efficacy; very few patients required re-escalation to Q4W. There were no new safety signals.
Keywords: Clinical trials, Vedolizumab, Crohn’s disease, ulcerative colitis
1. Introduction
Crohn’s disease [CD] and ulcerative colitis [UC] are chronic inflammatory diseases of the gastrointestinal tract. Clinical manifestations of both diseases include diarrhoea [typically bloody in patients with UC], as well as abdominal pain, faecal urgency, and incontinence. With more extensive disease, systemic characteristics such as fever, weight loss, malaise, and fatigue often occur.1,2
Vedolizumab is a humanised monoclonal antibody that reduces lymphocyte migration to the gut by targeting α 4β 7 integrin on lymphocytes, preventing docking with mucosal addressin cell adhesion molecule-1 [MAdCAM-1] on gastrointestinal mucosal endothelial cells.3–5 In the pivotal phase 3 GEMINI 1 [UC] and GEMINI 2 studies [CD], vedolizumab 300 mg intravenously [IV] was effective and well tolerated in patients at both 8-week [Q8W] and 4-week [Q4W] dosing.6,7 In addition, the recent VERSIFY trial [CD] demonstrated the ability of vedolizumab Q8W dosing to induce and sustain endoscopic improvements, along with good safety/tolerability.8 Vedolizumab was approved in 2014 in the USA and Europe, and has since been approved for the treatment of patients with moderately to severely active UC or CD in more than 60 countries.9,10
Patients who participated in GEMINI 1 [UC] or GEMINI 2 [CD], and completed the study through Week 52 on either placebo, vedolizumab Q4W, or vedolizumab Q8W, and patients who withdrew from GEMINI 1 or GEMINI 2 early, due to sustained non-response, could enrol in the GEMINI long-term safety [LTS] study. Patients who had participated in a previous phase 2 safety study and received up to 78 weeks of vedolizumab, and patients from GEMINI 3 [induction-only study in patients with CD], were also eligible to continue receiving treatment in the GEMINI LTS. Additionally, a cohort of vedolizumab-naïve patients were enrolled in the study. All patients enrolled in GEMINI LTS received vedolizumab 300 mg IV Q4W. After GEMINI LTS, an Extended Access Program [XAP] with Q8W dosing was initiated to provide patients who experienced clinical benefit in GEMINI LTS or VERSIFY continued access to vedolizumab and to monitor safety. Patients received vedolizumab with Q8W dosing in both VERSIFY and the XAP. Patients received vedolizumab Q4W in GEMINI LTS and then at a reduced dosing frequency of Q8W upon enrolment into the XAP study. To date, there are limited data from patients whose vedolizumab dosing frequency was reduced from Q4W to Q8W. The XAP pharmacokinetics [PK] sub-study was initiated to assess clinical efficacy, PK, and safety for patients whose vedolizumab dosing frequency was reduced from Q4W to Q8W.
2. Methods
2.1. Study design
The Extended Access Program of Vedolizumab IV in Ulcerative Colitis and Crohn’s Disease [vedolizumab XAP, NCT02743806] is a phase 3b/4, prospective, open-label, multinational, interventional study established to monitor safety of patients who received vedolizumab, and to provide continued vedolizumab access to eligible patients. Patients eligible for the XAP included those who received vedolizumab in the two qualifying studies GEMINI LTS or VERSIFY, experienced continued clinical benefit, and did not have access to commercially available vedolizumab. Results from GEMINI LTS and VERSIFY have been previously reported.8,11,12
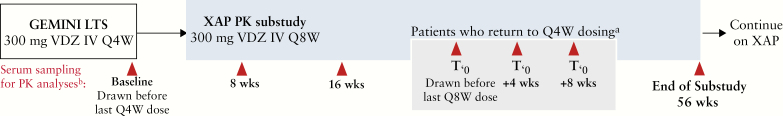
The XAP-PK sub-study was available to patients from GEMINI LTS who enrolled in the XAP and were receiving vedolizumab Q4W. Patients from VERSIFY were not eligible for the XAP-PK sub-study because they received vedolizumab Q8W during the study. The study protocol was approved by an institutional review board or ethics committee at each study site, and all patients provided written informed consent. Enrolment occurred at the last visit at which patients received Q4W vedolizumab in GEMINI LTS. Patients then reduced vedolizumab dosing frequency from 300 mg IV Q4W to 300 mg IV Q8W, and were followed for up to 56 weeks [Figure 1]. Re-escalation of Q4W dosing due to loss of response was allowed based on the treating physician’s clinical judgement and with approval of the study medical monitor. Patients could leave the sub-study if vedolizumab became commercially available to the patients. In this case, patients were considered as having completed the sub-study early.
Figure 1.
XAP-PK sub-study schema. Patients from GEMINI LTS were eligible to enrol in the XAP-PK sub-study. Baseline assessments were obtained at the last study visit in GEMINI LTS and then at Weeks 8, 16, and 56. If patients required re-escalation to Q4W dosing, additional serum samples were obtained before the last Q4W dose and 4 and 8 weeks after returning to Q4W dosing. aPatients could be re-escalated to Q4W dosing based on the physician’s judgment and with medical monitor approval. bSerum titres of anti-vedolizumab antibodies and neutralising antibodies were determined using validated assays. IV, intravenous; LTS, long-term safety; PK, pharmacokinetics; Q4W, every 4 weeks; Q8W, every 8 weeks; XAP, Extended Access Program.
Outcomes included efficacy as measured by Harvey-Bradshaw Index [HBI] score [for patients with CD] or partial Mayo score [for patients with UC], loss of clinical benefit and re-escalation to Q4W dosing, vedolizumab PK, and adverse events [AEs]. The full analysis set included all enrolled patients.
2.2. XAP-PK sub-study endpoints
Clinical remission was assessed at baseline [defined as the last Q4W dosing visit in GEMINI LTS] and Weeks 8, 16, and 56, and was defined for patients with CD as an HBI score ≤4, and for patients with UC as a partial Mayo score ≤2 with no sub-score >1. Corticosteroid [CS]-free clinical remission was defined as clinical remission and no ongoing concomitant CS use at the time of remission assessment, for both CD and UC patients. For patients who resumed Q4W dosing, clinical response to dosing re-escalation was defined as a ≥3-point decrease in HBI score from the time of dosing re-escalation [CD], or a ≥2-point decrease in partial Mayo score and a ≥25% decrease from score at time of dosing re-escalation, with accompanying ≥1-point decrease in rectal bleeding sub-score [RBS] from the time of dosing re-escalation or an absolute RBS of ≤1 point [UC].
Blood samples for PK analyses of anti-vedolizumab antibodies [AVA] and neutralising AVA were obtained at baseline and pre-dose at Weeks 8, 16, and 56. Vedolizumab trough concentration [Ctrough] was measured using a validated enzyme-linked immunosorbent assay [ELISA]. ELISA-based AVA and neutralising AVA assessment methods have been previously described.13 C-reactive protein [CRP] concentrations were measured as a biomarker of clinical remission.
2.3. Statistical analyses
The sample size for the XAP-PK sub-study was not based on statistical assumptions; the number of patients enrolled resulted from the patients who consented and qualified for the XAP-PK sub-study from GEMINI LTS. All response-type endpoints were summarised using point estimates and 95% confidence intervals [CI] for the proportion of patients achieving response. Partial Mayo score, HBI score, CRP, and pre-dose vedolizumab Ctrough assessments were summarised descriptively.
For patients who discontinued before Week 56, data obtained at the last sub-study visit were imputed as the end of sub-study [EOSS] observation for efficacy analyses. Patients who gained access to commercial vedolizumab before Week 56 were considered to have completed the sub-study as of their last study visit.
3. Results
3.1. Sub-study population
A total of 167 patients from GEMINI LTS—88 of 1349 [7%] patients with CD and 79 of 894 [9%] with UC—were enrolled in the XAP-PK sub-study. In the total population, the mean time since disease diagnosis was 13.9 ± 6.8 years and the mean time since vedolizumab treatment initiation in an earlier study was 6.4 ± 1.3 years [Table 1]. The majority of patients were anti-tumour necrosis factor [TNF]–naïve at the start of vedolizumab therapy [68.9%] and had no concomitant CS or immunomodulator use [68.3%] at enrolment in the XAP-PK sub-study. At the time of XAP enrolment, 73 patients [83.0%] with CD and 74 patients [93.7%] with UC were in clinical remission. Rates of CS-free clinical remission were 77.3% and 88.6%, respectively.
Table 1.
Patient demographics and disease characteristics at the time of XAP-PK sub-study enrolment.
| CD [n = 88] | UC [n = 79] | Total [n = 167] | |
|---|---|---|---|
| Age, mean [SD], years | 41.5 [11.5] | 48.1 [11.8] | 44.6 [12.1] |
| Sex, male, n [%] | 48 [54.5] | 45 [57.0] | 93 [55.7] |
| Time since diagnosis, mean [SD], years | 13.8 [6.3] | 13.9 [7.3] | 13.9 [6.8] |
| Time since start of vedolizumab therapy in previous studies, mean [SD], years | 6.2 [1.0] | 6.6 [1.5] | 6.4 [1.3] |
| Previous anti-TNF exposure, n [%] | |||
| Naïve | 56 [63.6] | 59 [74.7] | 115 [68.9] |
| Experienced | 32 [36.4] | 20 [25.3] | 52 [31.1] |
| Concomitant medication use at enrolment, n [%] | |||
| CS only | 6 [6.8] | 5 [6.3] | 11 [6.6] |
| IMM only | 23 [26.1] | 17 [21.5] | 40 [24.0] |
| CS + IMM | 1 [1.1] | 1 [1.3] | 2 [1.2] |
| No concomitant CS/IMM | 58 [65.9] | 56 [70.9] | 114 [68.3] |
| Clinical remission at enrolment, n [%] | 73 [83.0] | 74 [93.7] | 147 [88.0] |
| CS-free clinical remission at enrolment, n [%] | 68 [77.3] | 70 [88.6] | 138 [82.6] |
| Baseline CRP, n [%] | |||
| Normal [≤5.0 mg/L] | 57 [64.8] | 69 [87.3] | 126 [75.4] |
| High [>5.0 mg/L] | 27 [30.7] | 6 [7.6] | 33 [19.8] |
| Missing | 4 [4.5] | 4 [5.1] | 8 [4.8] |
| Baseline HBI score, mean [SD] | 2.1 [3.2] | — | — |
| Baseline partial Mayo score, mean [SD] | — | 0.5 [1.0] | — |
CD, Crohn’s disease; CRP, C-reactive protein; CS, corticosteroid; HBI, Harvey-Bradshaw Index; IMM, immunomodulator; PK, pharmacokinetics; SD, standard deviation; TNF, tumour necrosis factor; UC, ulcerative colitis; XAP, Extended Access Program.
3.2. Patient disposition and treatment persistence
Among patients with CD, 80 of 88 [90.9%] completed the sub-study to Week 56; one patient completed the sub-study early due to availability of commercial vedolizumab, four withdrew voluntarily, two patients discontinued due to loss of benefit, and one discontinued due to pregnancy. Among patients with UC: 73 of 79 [92.4%] completed the sub-study to Week 56; two patients completed the sub-study early due to availability of commercial vedolizumab; three voluntarily withdrew; and one patient was lost to follow-up [Table 2].
Table 2.
Patient disposition.
| CD [n = 88] | UC [n = 79] | Total [n = 167] | |
|---|---|---|---|
| Completed XAP-PK sub-study to Week 56, n [%] | 80 [90.9] | 73 [92.4] | 153 [91.6] |
| Remained on Q8W dosing to Week 56, n [%] | 76 [86.4] | 71 [89.9] | 147 [88.0] |
| Changed dosing from Q8W to Q4W, n [%] | 4 [4.5]a | 2 [2.5] | 6 [3.6] |
| Completed main study before Week 56b | 1 [1.1] | 2 [2.5] | 3 [1.8] |
| Premature discontinuation, n [%] | 7 [8.0] | 4 [5.1] | 11 [6.6] |
| Voluntary withdrawal | 4 [4.5] | 3 [3.8] | 7 [4.2] |
| No longer adequate benefit | 2 [2.3] | 0 | 2 [1.2] |
| Lost to follow-up | 0 | 1 [1.3] | 1 [0.6] |
| Pregnancy | 1 [1.1] | 0 | 1 [0.6] |
CD, Crohn’s disease; PK, pharmacokinetic; Q4W, every 4 weeks; Q8W, every 8 weeks; UC, ulcerative colitis; XAP, Extended Access Program.
aOne additional patient re-escalated to Q4W dosing but prematurely discontinued the study due to loss of treatment benefit.
bPatients discontinued the XAP study and continued treatment with commercially available vedolizumab.
Through Week 56, 76 [86.4%] and 71 [89.9%] patients with CD and UC, respectively, remained on Q8W dosing. Four patients with CD and two patients with UC re-escalated to Q4W dosing during the sub-study; one additional patient with CD re-escalated to Q4W but discontinued the study prematurely [Table 2; and Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. The median time to loss of clinical benefit and dosing re-escalation was 107 days [range, 57–367 days] for patients with CD and 198 days [170–225 days] for patients with UC.
3.3. Efficacy
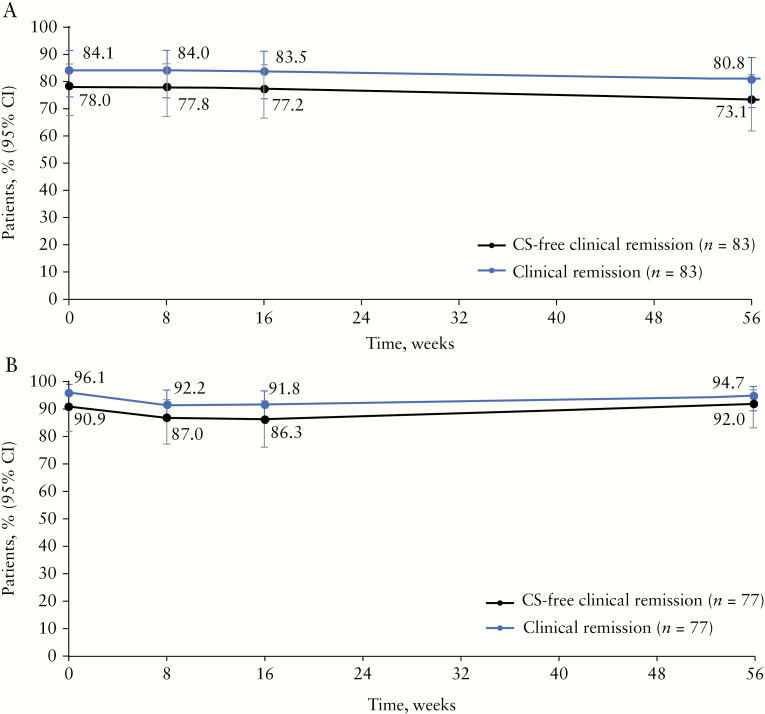
Of the 83 patients with CD who remained on Q8W dosing [including patients who completed Week 56 or discontinued early with EOSS values carried forward], 69 [84.1%] and 63 [80.8%] were in clinical remission at baseline and at Week 56, respectively. CS-free clinical remission was observed in 64 [78%] and 57 [73.1%] of patients with CD at baseline and Week 56, respectively [Figure 2A]. Among the 77 patients with UC who remained on Q8W dosing [including patients who completed Week 56 or discontinued early with EOSS values carried forward], 74 [96.1%] and 71 [94.7%] were in clinical remission at baseline and Week 56, respectively, with 70 [90.9%] in CS-free remission at baseline and 69 [92.0%] in CS-free clinical remission at Week 56 [Figure 2B]. Of patients who remained on Q8W dosing and were in CS-free clinical remission at baseline, 52 of 59 [88%] patients with CD and 68 of 69 [99%] with UC were in CS-free clinical remission at Week 56. Patients with CD or UC who were anti-TNF–naïve had numerically higher rates of clinical remission at Week 56 than patients with previous anti-TNF treatment [Supplementary Figure 2A and B, available as Supplementary data at ECCO-JCC online]. Loss of clinical remission after reduction of dosing frequency by patients in clinical remission at the start of the study was observed for 13 of 73 patients with CD and 6 of 74 patients with UC.
Figure 2.
Clinical remission and corticosteroid-free clinical remission in patients with CD [A] and UC [B] who completed the sub-study on Q8W dosing. Remission, defined as an HBI score ≤4 for patients with CD, or a partial Mayo score ≤2 with no sub-score >1 for patients with UC, and CS use was assessed at baseline [Week 0] as well as Weeks 8, 16, and 56. Week 56 includes patients who dropped out before Week 56 with EOSS values mapped to Week 56. EOSS, end of sub-study; CD, Crohn’s disease; CI, confidence interval; CS, corticosteroid; HBI, Harvey-Bradshaw Index; Q8W, every 8 weeks; UC, ulcerative colitis.
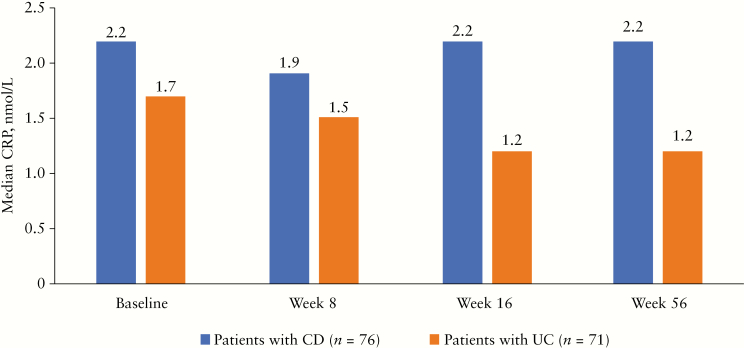
Among patients who remained on Q8W dosing and completed Week 56 of the sub-study, median CRP levels were stable over time, supporting findings of continued clinical remission [Figure 3]. Median CRP levels were 2.2 mg/L [range, 0.30–72.9 mg/L] at baseline and 2.2 mg/L [range, 0.20–34.0 mg/L] at Week 56 for patients with CD. Median CRP levels for patients with UC were 1.7 mg/L [range, 0.20–11.3 mg/L] at baseline and 1.2 mg/L [range, 0.20–255.3 mg/L] at Week 56.
Figure 3.
CRP levels [observed median] in patients with CD and UC who completed Week 56 of the sub-study on Q8W dosing. Serum samples were obtained before vedolizumab dosing at enrolment in the sub-study [baseline] and at Weeks 8, 16, and 56. CD, Crohn’s disease; CRP, C-reactive protein; Q8W, every 8 weeks; UC, ulcerative colitis.
Four patients with CD required dosing re-escalation to Q4W and remained in the sub-study; a fifth patient re-escalated to Q4W but discontinued. Three of the four patients were in CS-free clinical remission at baseline and two of these three patients regained clinical response after returning to Q4W dosing. The two patients who regained clinical response after returning to Q4W dosing were also in CS-free remission at Week 56. One patient who was not in CS-free remission at baseline also regained clinical response after returning to Q4W dosing. The remaining patient who did not regain clinical response was not in CS-free clinical remission at baseline. The median time to dosing re-escalation was 107 days [range, 57–367 days].
Two patients with UC required re-escalation of dosing frequency to Q4W. At the time of return to Q4W dosing, both patients had a partial Mayo score of 6.0. Neither patient was in CS-free clinical remission at baseline and neither patient regained clinical response, nor were they in clinical remission at any time point in the sub-study. Their times to dosing re-escalation were 170 and 225 days, respectively.
3.4. Pharmacokinetics and immunogenicity
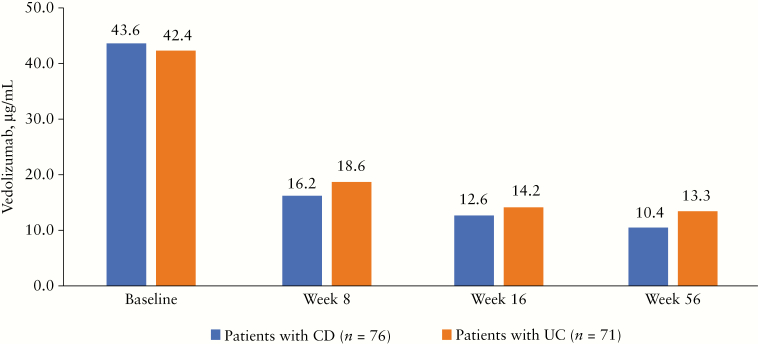
In patients with CD who remained on Q8W dosing for 56 weeks, the median vedolizumab Ctrough levels decreased from 43.6 µg/mL [range, 0–108.0 µg/mL] at baseline to 16.2 µg/mL [range, 0–128.0 µg/mL] at Week 8 and 10.4 µg/mL [range, 0–45.2 µg/mL] at Week 56. Likewise, in patients with UC who remained on Q8W dosing for 56 weeks, median vedolizumab Ctrough levels were 42.4 µg/mL [range, 14.1–102.0 µg/mL], 18.6 µg/mL [range, 2.8–120.0 µg/mL], and 13.3 µg/mL [range, 0–44.7 µg/mL] at baseline, Week 8, and Week 56, respectively [Figure 4].
Figure 4.
Serum vedolizumab Ctrough levels [observed median] in patients with CD and UC who completed Week 56 of the sub-study on Q8W dosing. Serum samples were obtained before vedolizumab dosing at enrolment in the sub-study [baseline] and at Weeks 8, 16, and 56. CD, Crohn’s disease; Ctrough, trough concentration; Q8W, every 8 weeks; UC, ulcerative colitis.
Patients with CD who were in clinical remission at baseline [n = 60] had a median vedolizumab Ctrough value of 41.4 µg/mL [range, 0.0–108.0 µg/mL] versus 49.8 µg/mL [range, 37.3–89.6 µg/mL] in patients who were not in clinical remission [n = 9]. At Week 56, the median vedolizumab Ctrough values were 10.3 µg/mL [range, 0.0–45.2 µg/mL] in patients with CD on Q8W dosing in clinical remission [n = 59] and 11.2 µg/mL [range, 0.0–20.0 µg/mL] in patients on Q8W dosing who were not in clinical remission [n = 13]. Likewise, the median baseline vedolizumab Ctrough value for patients with UC who were in clinical remission [n = 66] was 44.8 µg/mL [range, 14.1–102.0 µg/mL] versus 42.9 µg/mL [range, 20.2–53.5 µg/mL] for those not in clinical remission [n = 4]. At Week 56, the median vedolizumab Ctrough value of patients with UC on Q8W dosing who were in clinical remission [n = 57] was 13.4 µg/mL [range, 0.0–44.7 µg/mL] versus 9.8 µg/mL [range, 2.7–17.6 µg/mL] for those not in clinical remission [n = 4]. Finally, there were no clinically meaningful differences in vedolizumab Ctrough values between anti-TNF–naïve and anti-TNF–experienced CD or UC patients at any time point [Supplementary Figure 3A and B, available as Supplementary data at ECCO-JCC online].
Median baseline vedolizumab Ctrough values were similar between patients who required dosing re-escalation and regained response (n = 2; 44.9 µg/mL [range, 42.2–47.6 µg/mL]), patients who required dosing re-escalation and did not regain clinical response (n = 3; 47.5 µg/mL [range, 25.4–53.5 µg/mL]), and those who remained on Q8W dosing through Week 56 (n = 121; 42.6 µg/mL [range, 0.0–108.0 µg/mL]). At Week 56, median vedolizumab Ctrough values in patients who regained response after dosing re-escalation [n = 2] were numerically higher than in those who did not regain response [n = 3] but were lower than baseline values (33.0 µg/mL [range, 20.1–45.9 µg/mL] versus 22.8 µg/mL [range, 12.0–54.7 µg/mL]).
One patient with CD was positive for AVAs at baseline and remained positive through Week 56. Although the patient’s AVAs were neutralising, the patient nevertheless remained on Q8W dosing throughout the sub-study. No patient with UC had a positive AVA finding.
3.5. Safety/tolerability
Adverse events related to vedolizumab in the XAP-PK sub-study were rare: two patients with CD [pharyngitis and worsening of CD] and one patient with UC [proctitis] [Table 3]. Five patients [three with CD and two with UC] experienced serious AEs including worsening of CD [two patients], worsening of UC [one patient], intestinal stenosis [one patient], and large intestine polyp [one patient]. No serious AEs were related to vedolizumab.
Table 3.
Summary of adverse events.
| CD [n = 88] | UC [n = 79] | Total [n = 167] | ||||
|---|---|---|---|---|---|---|
| Events, n | Patients, n [%] | Events, n | Patients, n [%] | Events, n | Patients, n [%] | |
| Any AEs | 55 | 32 [36.4] | 49 | 25 [31.6] | 104 | 57 [34.1] |
| Related to treatmenta | 2 | 2 [2.3] | 1 | 1 [1.3] | 3 | 3 [1.8] |
| Not related to treatment | 53 | 30 [34.1] | 48 | 24 [30.4] | 101 | 54 [32.3] |
| Mild | 25 | 15 [17.0] | 31 | 14 [17.7] | 56 | 29 [17.4] |
| Moderate | 28 | 15 [17.0] | 16 | 10 [12.7] | 44 | 25 [15.0] |
| Severe | 2 | 2 [2.3] | 2 | 1 [1.3] | 4 | 3 [1.8] |
| Serious AEs | 3 | 3 [3.4] | 2 | 2 [2.5] | 5 | 5 [3.0] |
| Related to treatment | 0 | 0 | 0 | 0 | 0 | 0 |
| Not related to treatment | 3 | 3 [3.4] | 2 | 2 [2.5] | 5 | 5 [3.0] |
| Leading to treatment discontinuation | 0 | 0 | 1 | 1 [1.3] | 1 | 1 [0.6] |
| Deaths | – | 0 | – | 0 | – | 0 |
AE, adverse event; CD, Crohn’s disease; UC, ulcerative colitis.
a Treatment-related AEs included pharyngitis and worsening of CD [one patient each, CD] and proctitis [one patient, UC].
4. Discussion
Both CD and UC are chronic inflammatory diseases of the gastrointestinal tract, and long-term maintenance therapy is an important part of disease management. There is a need to establish safe and efficacious long-term treatment options. Previously, the GEMINI 1 and GEMINI 2 trials randomised patients to placebo, vedolizumab Q4W, or vedolizumab Q8W for up to 52 weeks of maintenance therapy. These trials showed that vedolizumab was safe and efficacious at both Q8W and Q4W dosing frequencies.6,7 Patients from GEMINI 1 and GEMINI 2, a previous phase 2 safety study, GEMINI 3, and vedolizumab-naïve patients, were enrolled in GEMINI LTS on Q4W dosing to continue monitoring the safety of long-term vedolizumab treatment. The vedolizumab XAP was initiated to provide continued access for patients who benefited from vedolizumab in these trials but could not yet access the drug commercially. During the vedolizumab development programme there were no data from patients who underwent dosing reduction from Q4W to Q8W. In the XAP, vedolizumab dosing frequency was Q8W, which provided a unique and valuable opportunity to specifically investigate long-term efficacy and safety, as well as vedolizumab PK, as patients transitioned from Q4W dosing in GEMINI LTS to Q8W in the XAP. Results from this study may provide needed clinical support for more dose adjustments in patients receiving vedolizumab in the future.
In the XAP-PK sub-study cohort, rates of both clinical remission and CS-free clinical remission were high at the time of enrolment and remained generally stable over 56 weeks for both UC and CD patients. This pattern was parallelled by stable median CRP levels over time. For both UC and CD, patients who were anti-TNF–naïve had numerically higher rates of clinical remission than patients who had previously been treated with an anti-TNF therapy. As expected, as patients reduced dosing frequency from Q4W to Q8W, there was a corresponding decrease in serum vedolizumab trough levels, which remained at a steady state for the duration of the sub-study. Vedolizumab trough concentrations in the XAP study are consistent with published reports in real-world patient populations which reported that Q4W dosing consistently results in significantly higher vedolizumab trough concentrations than Q8W dosing.14–16 In particular, Schulze et al. and Al-Bawardy et al. report vedolizumab concentrations with Q4W dosing [28.50 µg/mL and 32.0 µg/mL, respectively] which are similar to the concentrations we report at enrolment in the XAP [43.6 µg/mL]. Similarly, trough concentrations with Q8W dosing [12.80 µg/mL and 15.0 µg/mL] were also similar to trough concentrations with Q8W dosing in the XAP [16.2 µg/mL].14,15 Reducing dosing frequency did not result in increased immunogenicity. Only one patient had persistently positive AVAs [which were neutralising], whereas all other patients were AVA-negative. Importantly, there were no new safety signals with de-escalation or re-escalation of vedolizumab treatment.
Patient compliance in the XAP sub-study was high and few patients discontinued prematurely. In addition, adherence to Q8W dosing was very high. Only 5.6% of patients with CD and 2.5% of patients with UC required dosing re-escalation to Q4W within the 56 weeks of the PK sub-study. Of those, approximately half of the patients regained clinical response. Dosing re-escalation did not appear to be associated with immunogenicity. This finding may be due in part to the use of an ELISA-based assay which may have underestimated the number of patients positive for AVAs. Due to the small number of patients who returned to Q4W dosing, no additional conclusions could be drawn regarding patient characteristics that predicted the loss of response to Q8W dosing.
It should be noted that this sub-study was conducted in a selected sub-population of patients with more than 6 years on vedolizumab treatment and overall well-controlled disease. In addition, patients in the sub-study had variable previous exposure to vedolizumab because they may have entered GEMINI LTS after completing GEMINI 1 or 2, after leaving GEMINI 1 or 2 due to loss of response or the need for rescue medication, after induction therapy in GEMINI 3, or as vedolizumab de novo patients. As such, Q4W dosing in GEMINI LTS may not be reflective of Q4W dosing in real-world practice; findings from this sub-study cannot be used to infer how the broader patient population may respond to reductions in dosing frequency. In addition, endoscopies were not performed in the XAP and faecal calprotectin levels were not measured; hence, a limitation of the analysis is the lack of insight into disease activity [ie, stability of mucosal healing]. Given these limitations, clinical monitoring may be required for patients who transition to reduced vedolizumab dosing frequency. Although the number of patients in the XAP-PK sub-study is relatively small, the outcomes support further studies on the appropriate time point for reducing vedolizumab dosing frequency to better understand the clinical and economic impact of reduced-frequency vedolizumab dosing. In conclusion, in a well-controlled cohort of CD and UC patients, high treatment persistence [>85%] was observed on long-term maintenance therapy following reduction of dosing frequency to 300 mg vedolizumab IV Q8W. Both clinical remission and CS-free clinical remission rates were maintained over 56 weeks in UC and CD patients whose dosing frequency was reduced from Q4W to Q8W. Only a minority of patients required re-escalation to Q4W dosing frequency within 56 weeks of the PK sub-study. Taken together, these data support reduction of vedolizumab dosing frequency as a safe and clinically relevant long-term treatment strategy in well-controlled patients with CD or UC.
Takeda makes patient-level, de-identified datasets and associated documents available after applicable marketing approvals and commercial availability have been received, an opportunity for the primary publication of the research has been allowed, and other criteria have been met as set forth in Takeda’s Data Sharing Policy; see www.TakedaClinicalTrials.com/Approach for details. To obtain access, researchers must submit a legitimate academic research proposal for adjudication by an independent review panel, who will review the scientific merit of the research and the requester’s qualifications and conflict of interest that can result in potential bias. Once approved, qualified researchers who sign a data sharing agreement are provided access to these data in a secure research environment.
Funding
This work was sponsored by Takeda.
Conflict of Interest
SVe: financial support for research: AbbVie, Takeda, Pfizer, Johnson & Johnson; lecture fee[s]: Merck Sharp & Dohme Corp., AbbVie, Takeda, Ferring, Centocor, Hospira, Pfizer, Johnson & Johnson, Genentech/Roche, Tillotts; consultancy: Merck Sharp & Dohme Corp., AbbVie, Takeda, Ferring, Centocor, Hospira, Pfizer, Johnson & Johnson, Genentech/Roche, Celgene, Mundipharma, Celltrion, SecondGenome, Prometheus, Gilead, Galapagos, ProDigest, Abivax, GSK, Tillotts. ML: financial support for research and educational activities: Takeda, Janssen, Pfizer; advisory board member: Janssen, Takeda, Egis. FM: lecture fees: AbbVie, Ferring, Hospira, Johnson and Johnson, Merck, MSD, Takeda, Pfizer Inc., UCB Pharma, Vifor, Biogen, Celgene, Celltrion, Sandoz, Falk, Laboratórios Vitória. SA: employee of Takeda; holds Takeda stock or stock options. DLr: employee of Takeda; holds Takeda stock or stock options. MR: employee of Takeda at the time that this research was conducted; has granted patents and pending patent applications relating to the clinical pharmacology of vedolizumab. JR: employee of Takeda; holds Takeda stock or stock options. SD: lecture fees: AbbVie, Ferring, Hospira, Johnson and Johnson, Merck, MSD, Takeda, Mundipharma, Pfizer Inc., Tigenix, UCB Pharma, Vifor, Biogen, Celgene, Allergan, Celltrion, Sandoz, Boehringer Ingelheim; consultancy: AbbVie, Ferring, Hospira, Johnson and Johnson, Merck, MSD, Takeda, Mundipharma, Pfizer Inc., Tigenix, UCB Pharma, Vifor, Biogen, Celgene, Allergan, Celltrion, Sandoz, Boehringer Ingelheim.
Supplementary Material
Acknowledgments
Medical writing support was provided by Liz Gooch, PhD, of ProEd Communications, Inc., and was funded by Takeda.
Author Contributions
All authors contributed to the development of this manuscript. SV, MR, and SD were involved in the study concept and design and MR acquired the data. All authors contributed to the analysis and interpretation of the data, had full access to all study data, and had final responsibility for the decision to submit for publication.
References
- 1.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 2.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 3.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 4.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol 1994;153:517–28. [PubMed] [Google Scholar]
- 5.Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther 2009;330:864–75. [DOI] [PubMed] [Google Scholar]
- 6.Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 7.Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group . Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 8.Danese S, Sandborn WJ, Colombel JF, et al. Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn’s disease. Gastroenterology 2019;157:1007–18.e7. [DOI] [PubMed] [Google Scholar]
- 9.Entyvio [Vedolizumab] [Package Insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.; 2018. [Google Scholar]
- 10.Vedolizumab [Summary of Product Characteristics]. Taastrup, Denmark: Takeda Pharma A/S; 2019. [Google Scholar]
- 11.Loftus EV Jr, Colombel JF, Feagan BG, et al. Long-term Efficacy of Vedolizumab for Ulcerative Colitis. J Crohns Colitis 2017;11:400–11. [DOI] [PubMed] [Google Scholar]
- 12.Vermeire S, Loftus EV Jr, Colombel JF, et al. Long-term efficacy of vedolizumab for Crohn’s disease. J Crohns Colitis 2017;11:412–24. [DOI] [PubMed] [Google Scholar]
- 13.Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther 2015;42:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Bawardy B, Ramos GP, Willrich MAV, et al. Vedolizumab drug level correlation with clinical remission, biomarker normalization, and mucosal healing in inflammatory bowel disease. Inflamm Bowel Dis 2019;25:580–6. [DOI] [PubMed] [Google Scholar]
- 15.Schulze H, Esters P, Hartmann F, et al. A prospective cohort study to assess the relevance of vedolizumab drug level monitoring in IBD patients. Scand J Gastroenterol 2018;53:670–6. [DOI] [PubMed] [Google Scholar]
- 16.Ungaro RC, Yarur A, Jossen J, et al. Higher trough vedolizumab concentrations during maintenance therapy are associated with corticosteroid-free remission in inflammatory bowel disease. J Crohns Colitis 2019;13:963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.