Abstract
Background and Aims
Most patients with perianal Crohn’s fistula receive medical treatment with anti-tumour necrosis factor [TNF], but the results of anti-TNF treatment have not been directly compared with chronic seton drainage or surgical closure. The aim of this study was to assess if chronic seton drainage for patients with perianal Crohn’s disease fistulas would result in less re-interventions, compared with anti-TNF and compared with surgical closure.
Methods
This randomised trial was performed in 19 European centres. Patients with high perianal Crohn’s fistulas with a single internal opening were randomly assigned to: i] chronic seton drainage for 1 year; ii] anti-TNF therapy for 1 year; and iii] surgical closure after 2 months under a short course anti-TNF. The primary outcome was the cumulative number of patients with fistula-related re-intervention[s] at 1.5 years. Patients declining randomisation due to a specific treatment preference were included in a parallel prospective PISA registry cohort.
Results
Between September 14, 2013 and November 20, 2017, 44 of the 126 planned patients were randomised. The study was stopped by the data safety monitoring board because of futility. Seton treatment was associated with the highest re-intervention rate [10/15, versus 6/15 anti-TNF and 3/14 surgical closure patients, p = 0.02]. No substantial differences in perianal disease activity and quality of life between the three treatment groups were observed. Interestingly, in the PISA prospective registry, inferiority of chronic seton treatment was not observed for any outcome measure.
Conclusions
The results imply that chronic seton treatment should not be recommended as the sole treatment for perianal Crohn’s fistulas.
Keywords: Crohn’s disease, perianal fistula, anti-TNF
1. Introduction
The lifetime risk of fistula development in patients with Crohn’s disease [CD] ranges from 14% to 38%.1 Perianal CD fistulas cause pain, purulent discharge, and sphincter and perineal tissue destruction, resulting in a significant impairment of quality of life [QoL].2 Also, the impact on health care resources is considerable, due to multiple surgical interventions and biologic drugs.3 In daily clinical practice, no consensus has been reached on the optimal treatment of high perianal fistulas with a single internal opening.4 Currently, the three standard treatment options are: i] surgical approach by chronic seton drain drainage; ii] medical approach by anti-tumour necrosis factor alpha antibodies [anti-TNFα]; and iii] surgical closure with or without anti-TNF induction treatment. The choice of treatment is at the discretion of the patient, after shared decision making with the treating physician, preferably after discussion within a multidisciplinary team.
Since two randomised controlled trials [RCTs] reported increased fistula closure rates, reduced fistula discharge, and improved quality of life [QoL] following anti-TNF compared with placebo, most patients receive anti-TNF.5,6 Nonetheless, the long-term effect of anti-TNF is not as favourable, due to high recurrence rates and serious side effects. Systematic reviews suggested similar fistula closure rates between these three treatment options [43–50%].7–9 However, the surgical treatment options are generally less popular due to concerns regarding wound healing problems in CD.4–6,10 The advantages of seton drainage include patency preservation of the fistula tract, preventing side branching of the tract and recurrent abscess formation. Subsequently, the reported re-intervention rates seemed substantially lower with seton drainage [10–20%] as compared with anti-TNF and surgical closure [30–50%].7–9 However, rates varied widely, and no definite conclusion could be drawn. Previous studies were flawed by a high risk of bias, had short follow-up, and none of the studies directly compared seton drainage with anti-TNF treatment and/or surgical closure.
Therefore, we conducted an international, multicentre, prospective randomised controlled trial to identify the optimal treatment of Crohn’s high perianal fistulas. It was hypothesised that chronic seton drainage for perianal fistulas in CD would be the most effective treatment approach, as it would reduce re-interventions in the short term when compared with anti-TNF and surgical closure following anti-TNF, and overall long-term closure rates would be comparable between the three groups.
2. Materials and Methods
2.1. Study design
The PISA trial is an international, prospective multicentre, pragmatic, randomised, controlled, open-label, parallel group, superiority trial. The trial compared chronic seton drainage with anti-TNF and with surgical closure after anti-TNF induction. The study was conducted at 19 teaching hospitals and tertiary care centres in The Netherlands, Belgium, Spain, and Italy [seven centres were tertiary referral centres, five of which were in the Netherlands].
The study was performed in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The trial received central approval from the medical ethics committee at the Amsterdam UMC, location AMC, and from the corresponding committees in all participating centres. A data and safety monitoring board [DSMB] monitored the trial at predefined time points. Additionally, the study was monitored by the clinical research unit of the Amsterdam UMC, location AMC in accordance with the moderate risk classification of the Dutch federation of Academic Centres [NFU]. The study protocol has been published previously.11 This trial is registered at the Dutch Trial Registry [NTR4137].
2.2. Participants
Adult patients with a newly diagnosed or recurrent draining high tract [intersphincteric, trans-sphincteric or suprasphincteric] Crohn’s perianal fistula located in the upper two-thirds of the external sphincter were screened for eligibility. Main exclusion criteria were: multiple internal fistula openings. based on magnetic resonance imaging [MRI] or inspection under anaesthesia [the number of external fistulas was not taken into account]; proctitis [defined as any active mucosal inflammation or ulcer >5 mm in the rectum]; anorectal stenosis [defined as the impossibility of introducing a proctoscope]; a rectovaginal fistula; a seton in situ for more than 3 months; anti-TNF treatment in the preceding 3 months; patients not eligible for anti-TNF treatment [e.g., due to previous anti-TNF treatment without any effect on perianal fistula[s]; previously demonstrated allergy to anti-TNF medication; immunocompromised statuss]; and presence of a stoma. All participants provided written informed consent.
2.3. Randomisation and masking
Patients were allocated [1:1:1] to chronic seton drainage, long-term anti-TNF, or surgical closure after anti-TNF induction. Random block randomisation with block sizes of six participants was performed by a central web-based system [ALEA Clinical B.V., The Netherlands] and was not stratified. Patients and study staff masking was not possible because of the differing nature of the interventions [medical versus surgical]. Treatment preference, if explored at consultation before randomisation, was registered. In case this was reason to decline participation in the trial, the patients were asked for consent to be prospectively included in the PISA registration study, to maintain external validity. These patients met the same inclusion criteria and were treated according to the same protocol as the patients included in the PISA RCT.
2.4. Interventions
The procedures have been published previously [Supplementary Figure 1, available as Supplementary data at ECCO-JCC online].11 Before randomization, all patients underwent seton insertion [vessel loop] under general anaesthesia in a day care setting and received a 2-week antibiotic course. Furthermore, 6-mercaptopurine [6MP] was added. Patients were followed for 1.5 years.
For patients allocated to chronic seton drainage, the seton was scheduled to be removed after 1 year.
For patients allocated to anti-TNF, the choice of infliximab or adalimumab was left to the discretion of the treating gastroenterologist. Anti-TNF treatment was continued for at least 1 year. Any dose adaptation was allowed. The seton was removed 6 weeks after start of anti-TNF treatment, as it has been demonstrated that seton removal before 2 months is associated with higher closure rates.12 However, ultimately the decision of seton removal is at the discretion of the treating physician.
For patients allocated to surgical closure after anti-TNF induction, surgical closure was either performed by advancement flap or ligation of the intersphincteric tract [LIFT] procedure. The choice of treatment was left to the discretion of the treating surgeon. Surgical closure was performed in a day care setting and was combined with seton removal. Surgical closure was planned after completion of the anti-TNF induction, generally within 8–12 weeks after starting anti-TNF. Anti-TNF was stopped after 4 months. The procedure was performed by a specialised colorectal surgeon. When the participating centres lacked expertise, the patient was referred to the Amsterdam UMC, location AMC.
2.5. Outcomes
The primary outcome was the proportion of patients with fistula-related re-intervention[s], defined as surgical re-interventions and/or [re]start of anti-TNF therapy due to suspicion of recurrent abscess or new fistula tract[s] within 1 year. This was assessed by the trial physician and derived from operation and medical reports. A planned seton change without a suspicion of an abscess, e.g., due to a knotless seton, or [re]start of anti-TNF for general CD symptoms, were not considered as a re-intervention. Secondary outcomes included: i] the proportion of patients with clinically relevant severe Perianal Crohn’s Disease Activity Index [PCDAI > 7, as this is associated with the need of therapy13], evaluated by a physician at the outpatient clinic at Months 0, 6, 12, and 18; ii] the proportion of patients with a closed fistula, defined as a fibrotic tract on MRI14 after 1.5 years; iii] results of [disease-specific] quality of life [QoL] questionnaires (Inflammatory Bowel Disease Questionnaire [IBDQ] and EuroQol Visual Analogue Scale [EQ-VAS]); and iv] cost-effectiveness [including the EQ-5D-3L, antibiotic courses, number of sick leave or in-hospital days according to the health and labour questionnaire] assessed by questionnaires sent by email [LimeSurvey 2.6.7, Hamburg, Germany] or, if the patient preferred, by regular mail at Months 0, 3, 6, 9, 12, 15, and 18.
Patients were seen at the outpatient clinic at Months 6, 12, and 18 after inclusion. Patients were contacted by telephone every 3 months to verify adverse events, re-interventions, and any changes in medical therapy. Serious adverse events included those resulting in death or those that were life-threatening, requiring or prolonging admission to hospital, or resulting in persistent or substantial disability or incapacity. The local investigator and trial coordinator collected the data in an electronic database [Oracle Clinical 4.6.2, Redwood Shores, USA].
2.6. Statistical analyses
All analyses, including the analyses of the registry data, were based on the intention-to-treat principle. To detect a clinically relevant reduction of 30% of re-interventions [50% anti-TNF and surgical closure versus 20% seton drainage] with a power of at least 80% at a two-sided α level of 0.05 considering a 5% drop out rate, it was necessary to include 42 patients in each group [total target sample size of 126 patients]. The 30% decrease in re-interventions was based on systematic reviews.7–9 Chi square or Fisher’s exact test was used as appropriate, to analyse differences between the proportion of patients with fistula-related re-intervention[s] and patients with severe perianal disease activity [PCDAI > 7] among the three treatment groups. The change in IBDQ and EQ-VAS over time in the three study arms was investigated using linear mixed models with repeated measures analysis of variance adjusted for baseline value. QoL data are presented as model-based estimated means and corresponding confidence intervald [CI]. A two-sided p-value of less than 0.05 was considered significant. All statistical analyses were performed with SPSS software, version 24.0 [IBM Corp., Armonk, New York, USA].
2.7. Early termination of the trial
After an accrual of 33% of the total sample size, the [serious] adverse events per treatment group were reported to the DSMB as stipulated in the protocol. Most events entailed re-interventions [Supplementary Document 1, available as Supplementary data at ECCO-JCC online]. The proportion of patients with a re-intervention was highest in the chronic seton group. At the discretion of the DSMB, it was decided to perform an interim analysis. Conditional powers under the null trend [treatments are equally efficient] and the alternative trend [chronic seton is superior] were calculated to assess futility of continuing the trial. For both trends, the likelihood of showing superiority of the chronic seton arm at the completion of the trial was less than 1%. In case of continuation of the trial with the remaining two arms [anti-TNF versus surgical closure after anti-TNF], the conditional power to observe a 30% difference [20% versus 50% re-interventions] between these arms was <1% and 9% under the null and alternative hypothesis, respectively. The DSMB recommended termination of the trial due to futility [Supplementary Document 1]. The PISA steering committee decided to follow the advice and the METC accepted this decision on notification. A meeting was organised to discuss the crucial aspects of small numbers.15 As the chance of type 1 errors increased, the following decisions were made: to only statistically test the primary outcome at the original α level of 0·0; to complete the dataset by awaiting a minimal follow-up of 6 months; to report all outcome events till the end of study; and to evaluate the data of the registry patients. Because not all patients had completed the study, Kaplan-Meier analyses with log-rank testing to assess data for categorical outcomes were used. As described in the protocol, the study also intended to report fistula closure rates and a cost-effectiveness analysis. However, as closure of perianal fistula was only measured with MRI at 1.5 years, it was decided to await these data. Since chronic seton treatment was considered to be clinically too unfavourable, the cost-effectiveness analysis was considered no longer opportune. The funders shared that view.
3. Results
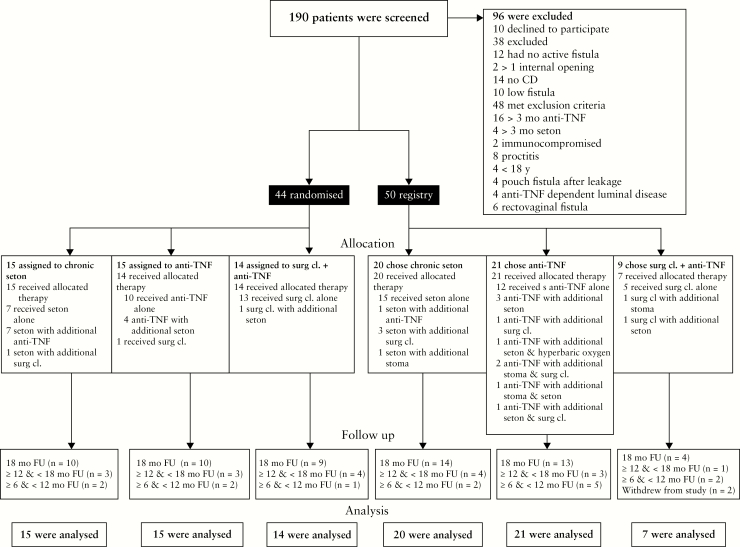
Between September 14, 2013 and November 20, 2017 [termination of the trial], 190 patients were screened for eligibility, of whom 96 were excluded; 44 patients were randomised and 50 patients were included in the PISA registry. Patients in the randomised trial were assigned to chronic seton drainage [n = 15], anti-TNF treatment [n = 15], or surgical closure after anti-TNF induction [n = 14]. In the PISA registry, 20 patients chose chronic seton drainage, 21 anti-TNF treatment, and nine surgical closure after anti-TNF induction. Two patients in the registry, both in the surgical closure group, withdrew from the study within 1 month and were excluded from outcome analyses [Figure 1]. The remaining 92 patients had a follow-up of at least 6 months, of whom 60 patients completed the 1.5-year follow-up.
Figure 1.
Trial profile according to the CONSORT diagram. Surg cl, surgical closure; mo, months; FU, follow-up.
Patient baseline characteristics of the RCT and the registry are shown in Tables 1 and 2. The mean age of the randomised patients, as well as of the registry patients, was 38 years (standard deviation [SD] 14 and 12, respectively). The baseline characteristics between the three treatment groups in the RCT, as well as in the registry, did not differ. In all groups, there were no differences in adherence to the protocol. At least 80% started with antibiotics and more than 80% were still on thiopurine at the end of follow-up. Also, the baseline characteristics between the patients in the RCT and the registry were comparable [Supplementary Table 1, available as Supplementary data at ECCO-JCC online].
Table 1.
Baseline characteristics of randomised patients.
| Seton [n = 15] | Anti-TNF [n = 15] | Surgical closure [n = 14] | |
|---|---|---|---|
| Age mean years, [SD] | 35 [13] | 43 [15] | 36 [15] |
| Female | 11 [73%] | 8 [53%] | 8 [57%] |
| Smoking | 5 [36%] | 5 [33%] | 2 [14%] |
| Luminal disease activitya | 0 [0%] | 2 [14%] | 0 [0%] |
| Prior anti-TNF usage | 1 [10%] | 4 [29%] | 6 [46%] |
| Disease years perianal fistula, median [IQR] | 1 [1–4] | 2 [1–8] | 1 [1–5] |
| Number of previous fistula interventions, median [range] | 1 [0–4] | 1 [0–3] | 2 [0–3] |
| Severe perianal disease activity [PCDAI >7]b | 9 [64%] | 7 [54%] | 11 [79%] |
| IBDQ [max 224 points], mean [SD]c | 151 [46] | 148 [35] | 146 [44] |
| EQ-VAS, mean [SD]d | 61 [21] | 59 [23] | 60 [20] |
| Number external openings, median [range] | 1 [0–2] | 1 [0–3] | 1 [0–2] |
| MRI imaging | |||
| Number external fistula tracts >1 | 12 [80%] | 8 [5%] | 5 [36%] |
| Rectal wall involvement | 2 [15%] | 4 [29%] | 0 [0%] |
TNF, tumour necrosis factor; SD, standard deviation; IQR, interquartile range; PCDAI, Perianal Crohn’s Disease Activity Index; IBDQ, Inflammatory Bowel Disease Questionnaire; EQ-VAS, EuroQol Visual Analogue Scale; MRI, magnetic resonance imaging.
aLuminal disease activity requiring anti-TNF. Assessed by colonoscopy within 3 months prior to randomisation.
bPCDAI assessed five items: i] fistula production, ii] pain, iii] limitation of sexual activities, iv] type of perianal disease, and v] severity of induration. Every category includes a scale ranging from 0 to 4 points, higher scores representing higher disease activity. The total score can range from 0 to 20 points.
cIBDQ score consists of 32 questions, each with a 1–7 scale. The total score can range from 32 to 224 points, with higher scores representing higher quality of life [Qo]L.
dThe EQ-VAS is a generic, standardised measure of health-related quality of life over the preceding week, consisting of the EQ-VAS descriptive system and the EQ visual analogue scale [EQ-VAS]. The EQ-VAS is a vertical scale grading the overall health status, ranging from 0 [worst imaginable health state] to 100 [best imaginable health state].
Table 2.
Baseline characteristics of registry patients [none of the parameters were significantly different].
| Seton group [n = 20] | Anti-TNF group [n = 21] | Surgical closure group [n = 9] | |
|---|---|---|---|
| Age, mean [SD] | 42 [13] | 36 [9] | 31 [9] |
| Female | 13 [68%] | 9 [45%] | 4 [44%] |
| Smoking | 5 [25%] | 4 [22%] | 6 [67%] |
| Luminal disease activity | 3 [19%] | 2 [13%] | 1 [17%] |
| Prior anti-TNF usage | 8 [42%] | 7 [41%] | 5 [71%] |
| Disease years perianal fistula, median [IQR] | 1 [0–9] | 2 [0–5] | 2 [1–6] |
| Number of previous fistula interventions, median [range] | 1 [0–9] | 0 [0–5] | 2 [0–4] |
| Severe perianal disease activity [PCDAI > 7] | 13 [81%] | 12 [67%] | 4 [57%] |
| IBDQ [maximum 224 points], mean [SD] | 140 [45] | 143 [28] | 142 [45] |
| EQ-VAS, mean [SD] | 54 [24] | 54 [23] | 59 [23] |
| Number external opening, median [range] | 1 [0–2] | 1 [0–2] | 1 [0–2] |
| MRI imaging | |||
| Number external fistula tracts >1 | 9 [45%] | 14 [67%] | 5 [56%] |
| Rectal wall involvement | 2 [11%] | 2 [13%] | 0 [0%] |
TNF, tumour necrosis factor; SD, standard deviation; IQR, interquartile range; PCDAI, Perianal Crohn’s Disease Activity Index; IBDQ, Inflammatory Bowel Disease Questionnaire; EQ-VAS, EuroQol Visual Analogue Scale; MRI, magnetic resonance imaging.
The proportion of patients with a fistula-related re-intervention[s] among the randomised patients was significantly associated with chronic seton drainage: 10 patients [74%] versus six patients [42%] in the anti-TNF group and three patients [23%] in the surgical closure after anti-TNF group, p = 0.02. In the registry patients, the proportion of patients with a re-intervention was similar between the groups, with eight patients [42%] in the chronic seton group versus nine patients [48%] in the anti-TNF group and two patients [44%] in the surgical closure after anti-TNF group, p = 0.78 [Table 3].
Table 3.
Re-interventions in RCT and registry patients till end of study, assessed using Kaplan-Meier analyses.
| Re-interventions | Seton drainage n [%] | Anti-TNF n [%] | Surgical closure n [%] |
|---|---|---|---|
| RCT* Registry | 10 [74%] 8 [42%] | 6 [42%] 9 [48%] | 3 [23%] 2 [44%] |
Re-interventions till end of study were significantly higher in the seton group of the randomised patients [p log-rank = 0.02]
RCT, randomised controlled trial; TNF, tumour necrosis factor.
Re-interventions occurred earliest in the chronic seton group: for the randomised patients after a median of 4 months (interquartile range [IQR] 1–9) versus 6 months [3–8] in the anti-TNF group, and 11 months [IQR 10–11] in the surgical closure after anti-TNF group. For the registry patients, re-interventions occurred after a median of 2 months [IQR 1–11] in the chronic seton group versus 3 months [IQR 1–11] in the anti-TNF group and 13 months [IQR 8–13] in the surgical closure group. Re-interventions per group per time point are shown in Supplementary Figures 2 and 3, available as Supplementary data at ECCO-JCC online.
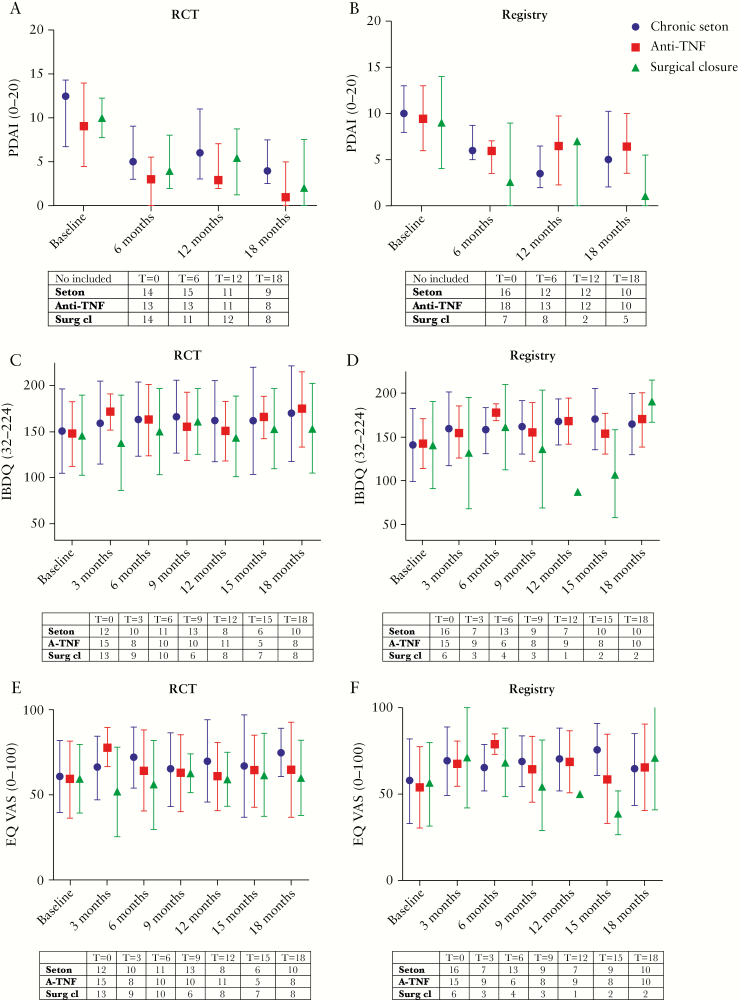
Baseline PCDAI was comparable for the three treatment groups in both the RCT and the registry [Tables 1 and 2]. The PCDAI improved in all groups [Figure 2a and b]. In the RCT, the number of patients per group with severe perianal disease activity [score >7] till end of study included five patients [40%] in the chronic seton group, two patients [19%] in the anti-TNF group, and three patients [31%] in the surgical closure after anti-TNF group. In the registry, severe perianal disease activity till end of study was: five patients [40%] in the chronic seton group, five patients [44%] in the anti-TNF group, and one patient [20%] in the surgical closure after anti-TNF group. For one patient in the RCT and nine patients in the registry, the PCDAI was not assessed during follow-up.
Baseline disease-specific QoL and general QoL were both comparable between the three treatment groups in both the RCT and the registry [Tables 1 and 2]. The QoL is shown in Supplementary Table 2, available as Supplementary data at ECCO-JCC online, and in Figure 2 c–f. In the RCT, the disease-specific QoL till end of study was higher in the anti-TNF group compared with the other two groups, whereas the general QoL was lower in the surgical closure after anti-TNF group. The registry showed no considerable differences for disease-specific and/or general QoL. The disease-specific QoL [IBDQ] and general QoL [EQ-VAS] could be assessed for 39 [89%] patients in the RCT and in 34 [71%] patients in the registry.
4. Discussion
This study is the first prospective randomised controlled trial comparing surgical treatment options with anti-TNF for Crohn’s disease high perianal fistulas. After the first interim analysis, the trial was terminated based on futility. Refuting the original hypothesis, the trial showed an inferior outcome of chronic seton treatment with respect to re-interventions in the randomised patients. None of the secondary outcomes in the RCT group demonstrated results favouring chronic seton drainage. Continuation of the study with the remaining two treatment arms would also be futile, as the re-intervention rates in these arms were lower than expected. The outcomes of this study should be interpreted with caution, since both the number of included patients and the number of events [re-interventions] were considerably smaller than the minimum required sample size for sufficient power. Therefore, it is uncertain as to what extent over- or underestimation of treatment effects may have occurred. Consequently, not the exact reported numbers and rates of the treatment effects, but rather the relative differences between the treatments arms have potential value for drawing conclusions.16 In addition, the discrepancies found between the RCT and registry results make it hard to draw firm conclusions.
The unexpected differences in re-intervention rates per treatment group in the randomised patients can be explained by various factors. The original hypothesis was based on retrospective studies with different inclusion criteria with a rather short duration of follow-up, especially for seton treatment.9 As a result, these studies might have been prone to bias, leading to under-reporting of re-interventions after seton treatment. In contrast, the number of re-interventions in the anti-TNF group and surgical closure after anti-TNF group were lower than previously described. In the anti-TNF group, all patients were treated with seton drainage before the start of anti-TNF, in order to prevent recurrent abscess formation. In previous studies this was not done on a consistent basis, which could explain the low re-intervention rate in our study.9 Furthermore, during the PISA trial, most surgical closures were LIFT procedures. Previous study results are probably outdated, as they generally describe the treatment effect of an advancement flap and reported outcomes without concomitant anti-TNF.7,8 It is hypothesised that a LIFT procedure combined with anti-TNF may account for the superior results observed in this study.
In our RCT, the disease-specific QoL was highest in the anti-TNF group. This can be expected, as anti-TNF may also have a favourable effect on the overall disease burden in CD.5 The general QoL was lower in the surgical closure group. Since the surgical intervention is only applied after some months, awaiting a complete follow-up will probably improve these results.
As the results of the PISA RCT were different from those expected and the baseline characteristics of the PISA registry patients were not different from those of randomised patients, it seemed justified to compare these results. In the registry data, chronic seton drainage was not associated with significantly more re-interventions. This was a somewhat striking finding, especially as severe perianal disease activity between the randomised and registry chronic seton treatment group was comparable at each point in time. Seton is known to be an uncomfortable treatment. Patients who consciously chose seton treatment in the registry, might have preferred to avoid surgery or the side effects of biologicals. In contrast, patients randomised to chronic seton treatment might be more disappointed about the discomfort, especially as it takes considerable time for seton stability to be achieved. This is further emphasised by the fact that most of the re-interventions occurred within 6 months in the seton group. Discomfort discussed at the outpatient clinic could lead to inspection under anaesthesia in daily clinical practice. These events count as a re-intervention, even in the absence of an abscess. It is argued that a seton procedure was tolerated more by patients who chose chronic seton drainage willingly as opposed to patients who were randomly allocated to it. Consequently, the primary endpoint re-intervention [which was thought to be an objective endpoint] is likely influenced by patient preference. This could explain the different results between the RCT and preference groups.
This was the first RCT comparing the three different treatment options head to head. Initially, the conclusion based on the PISA RCT was very clear; instead of showing superiority, chronic seton drainage was significantly associated with inferior results. Upon PISA counselling, strong patient preference was noted and was followed by a low inclusion ratio. Therefore, we also initiated the PISA registry parallel to the RCT. In accordance with the RCT, the PISA registry results did not suggest superiority of chronic seton treatment. However, it did not confirm inferiority of chronic seton treatment. Hence, if a patient chooses chronic seton treatment, it might still be a valid alternative. Interestingly, the registry data also revealed that relatively few patients chose surgery. It touches upon a more extensive problem that patients may not be well informed about the surgical treatment options. A fundamental factor driving this situation is probably that the majority of Crohn’s fistula patients have a long medical history with a gastroenterologist who might be less aware of the surgical treatment options and outcomes to be able to support thorough shared decision making.
Apart from interesting clinical data [albeit small numbers], we learned that a classical RCT might not be the optimal design for trials which compare treatments with substantially different characteristics [medical versus surgical].17 This type of study design, with a high internal validity due to homogeneity [including unknown confounders] between the study groups and the possibility of blinding, was originally designed to compare medical versus placebo therapy.18 However, when performing an RCT which compares treatments of substantially different natures, patient treatment preferences can be expected. In such cases, only presenting the RCT data will inevitably result in a less representative study group, which would not be in accordance with the transparency statement. We are aware that this might introduce a bias in the registry data, but withholding this information could result in an unbalanced and possibly unjustified conclusion. This study provided valuable lessons learned when designing future studies.
A limitation of the study is lack of patient involvement in trial design, particularly relating to design of the intervention to be included: the major pitfall of this study is that we did not consider patient preferences in the original design. A key lesson learned is that trial participants are not passive recipients of interventions. As described above, results of the RCT are likely to be influenced by patient preference. The influence of this occurrence can be mitigated by applying a more pragmatic design, such as a patient preference design or alternatively a cohort-embedded RCT [also known as TWICS].19,20 These designs incorporate patient preference instead of excluding patients with a distinct treatment preference, resulting in a higher external validity. These design have their own limitations. However, modern research should try to find a fine balance between the focus on limiting bias for study results [mainly concerning internal validity] and at the same time drawing externally valid conclusions that also take into account the applicability of study results. In conclusion, chronic seton treatment as the sole treatment is not the superior treatment for patients with perianal Crohn’s fistulas.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
Figure 2.
PCDAI, IBDQ, EQ-VAS over time [from baseline to 18 months] in RCT and registry patients. Blue represents the chronic seton group, red the anti-TNF group, and green the surgical closure after anti-TNF group. A lower PCDAI characterises less perianal disease activity. Higher IBDQ and EQ-VAS scores indicate a better quality of life [QoL]. The change in IBDQ and EQ-VAS over time of the three study arms was investigated using linear mixed-models with repeated measures analysis of variance adjusted for baseline value. QoL data are presented as model-based estimated means and corresponding confidence intervals [CIs]. The arrows represent a re-intervention of a treatment of the other treatment group [seton placement, start anti-TNF therapy of surgical closure]. Stripes without any specification are re-interventions that are the same as the original treatment. TNF, tumour necrosis factor; PCDAI, Perianal Crohn’s Disease Activity Index; IBDQ, Inflammatory Bowel Disease Questionnaire; EQ-VAS, EuroQol Visual Analogue Scale.
Anonymised patient level data can be made available on reasonable request after approval from the trial management committee and after signing a data access agreement. Proposals should be directed to the corresponding author. Consent was not obtained for data sharing, but the presented data are anonymised and the risk of identification is low.
Funding
The work was supported by The Netherlands Organization for Health Research and Development [ZonMw, grant number 837002002] and the Crohn and Colitis Foundation [grant number 210270]. The funders of the study had no role in the study design, data collection, analysis, interpretation, or writing of the report.
Conflict of Interest
GRD’H has: served as adviser for Abbvie, Ablynx, Allergan, Amakem, Amgen, AM Pharma, Arena Pharmaceuticals, AstraZeneca, Avaxia, Biogen, Bristol Meiers Squibb, Boerhinger Ingelheim, Celgene/Receptos, Celltrion, Cosmo, Covidien/Medtronics, Echo Pharmaceuticals, Eli Lilly, Engene, Ferring, DrFALK Pharma, Galapagos, Genentech/Roche, Gilead, Glaxo Smith Kline, Gossamerbio, Hospira/Pfizer, Immunic, Johnson and Johnson, Lycera, Medimetrics, Millenium/Takeda, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Nextbiotics, Novonordisk, Otsuka, Pfizer/Hospira, Photopill, Prometheus laboratories/Nestle, Progenity, Protagonist, Robarts Clinical Trials, Salix, Samsung Bioepis, Sandoz, Seres/Nestle, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant, and Vifor; received speaker fees from Abbvie, Biogen, Ferring, Johnson and Johnson, Merck Sharp Dome, Mundipharma, Norgine, Pfizer, Samsung Bioepis, Shire, Millenium/Takeda, Tillotts and Vifor. CYP has served as adviser for Abbvie, Takeda, and Pliant, declares a grant from Takeda, and received speaker’s fees from Abbvie, Tillotts, and Takeda. KBG has served as speaker and/or adviser for Amgen, AbbVie, Biogen, Boehringer Ingelheim, Ferring, Hospira, MSD, Pfizer, Samsung Bioepis, Sandoz, Takeda, and Tigenix. AS has served as speaker and/or adviser for Takeda. SD has served as a speaker, consultant, and advisory board member for Abbvie, Ferring, Hospira, Johnson & Johnson, Merck, Millennium Takeda, Mundipharma, Pfizer, Tigenix, UCB Pharma, and Vifor. The authors have no competing interests.
Authors Contributions
EJdG, MGD, WAB, and CJB designed the trial. All authors except the statistician recruited and treated patients. KAW, EJdG, MES collected the data. KAW and MGD analysed the data. KAW, GRD’H, CYP, KG, MGD, WAB, and CJB interpreted the data. KAW, MGD, WAB, and CJB drafted the manuscript. EJdG, MES, GRD’H, CYP, KBG, MFG, SAvT, JMJ, AP, KFB, DDZ, AS, SD, JvdB, and MWM critically revised the manuscript for important intellectual content.
Acknowledgments
We would like to thank E. J. M. Nieveen van Dijkum, M. W. T. Tanck, and J. J. G. H. M. Bergman for their participation in the Data Safety Monitoring Board, P. J. Tanis for his participation as independent expert, all staff at the participating centres of the PISA trial for their efforts, the Dutch Initiative on Crohn’s and Colitis, F. A. B. M. Wasmann for linguistics editing, and especially the patients for participating in the trial.
This study was presented at: the European Crohn and Colitis Organisation twice, March 7 and March 8, 2019, Copenhagen, Denmark; the Dutch Digestive Disease days, March 20, 2019, Veldhoven, The Netherlands; the Dutch Surgical days, May 16, 2019, Veldhoven, The Netherlands; and the European Society of Coloproctology, September 27, 2019, Vienna, Austria.
References
- 1.Hellers G, Bergstrand O, Ewerth S, Holmström B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut 1980;21:525–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viganò C, Losco A, Caprioli F, Basilisco G. Incidence and clinical outcomes of intersphincteric abscesses diagnosed by anal ultrasonography in patients with Crohn’s disease. Inflamm Bowel Dis 2011;17:2102–8. [DOI] [PubMed] [Google Scholar]
- 3.Hakkaart- van Roijen L, Tan SS. Handleiding voor kostenonderzoek: methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. College voor zorgverzekeringen 2010. zorginstituutnederland.nl. [Google Scholar]
- 4.de Groof EJ, Cabral VN, Buskens CJ, et al. . Systematic review of evidence and consensus on perianal fistula: an analysis of national and international guidelines. Colorectal Dis 2016;18:O119–34. [DOI] [PubMed] [Google Scholar]
- 5.Present DH, Rutgeerts P, Targan S, et al. . Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999;340:1398–405. [DOI] [PubMed] [Google Scholar]
- 6.Lichtiger S, Binion DG, Wolf DC, et al. . The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn’s disease who failed prior infliximab therapy. Aliment Pharmacol Ther 2010;32:1228–39. [DOI] [PubMed] [Google Scholar]
- 7.Stellingwerf ME, van Praag EM, Bemelman WA, Buskens CJ. P426 Meta-analysis of endorectal advancement flap vs. ligation of the intersphincteric fistula tract for Crohn’s and cryptoglandular high perianal fistulas. J Crohns Colitis 2018;12[Suppl_1]: S320. [Google Scholar]
- 8.Soltani A, Kaiser AM. Endorectal advancement flap for cryptoglandular or Crohn’s fistula-in-ano. Dis Colon Rectum 2010;53:486–95. [DOI] [PubMed] [Google Scholar]
- 9.de Groof EJ, Sahami S, Lucas C, Ponsioen CY, Bemelman WA, Buskens CJ. Treatment of perianal fistula in Crohn’s disease: a systematic review and meta-analysis comparing seton drainage and anti-tumour necrosis factor treatment. Colorectal Dis 2016;18:667–75. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka S, Matsuo K, Sasaki T, et al. . Clinical advantages of combined seton placement and infliximab maintenance therapy for perianal fistulizing Crohn’s disease: when and how were the seton drains removed? Hepatogastroenterology 2010;57:3–7. [PubMed] [Google Scholar]
- 11.de Groof EJ, Buskens CJ, Ponsioen CY, et al. . Multimodal treatment of perianal fistulas in Crohn’s disease: seton versus anti-TNF versus advancement plasty [PISA]: study protocol for a randomized controlled trial. Trials 2015;16:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaertner WB, Decanini A, Mellgren A, et al. . Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis Colon Rectum 2007;50:1754–60. [DOI] [PubMed] [Google Scholar]
- 13.Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. . Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol 2016;14:348–54.e17. [DOI] [PubMed] [Google Scholar]
- 14.Van Assche G, Vanbeckevoort D, Bielen D, et al. . Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn’s disease. Am J Gastroenterol 2003;98:332–9. [DOI] [PubMed] [Google Scholar]
- 15.Lachin JM. A review of methods for futility stopping based on conditional power. Stat Med 2005;24:2747–64. [DOI] [PubMed] [Google Scholar]
- 16.Viele K, McGlothlin A, Broglio K. Interpretation of clinical trials that stopped early. JAMA 2016;315:1646–7. [DOI] [PubMed] [Google Scholar]
- 17.Preference Collaborative Review Group. Patients’ preferences within randomised trials: systematic review and patient level meta-analysis. BMJ 2008;337:a1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bothwell LE, Greene JA, Podolsky SH, Jones DS. Assessing the gold standard–lessons from the history of RCTs. N Engl J Med 2016;374:2175–81. [DOI] [PubMed] [Google Scholar]
- 19.Wasmann KA, Wijsmann P, van Dieren S, Bemelman W, Buskens C.. Partially randomised patient preference trials as an alternative design to randomised controlled trials: systematic review and meta-analyses. BMJ Open 2019;9:e031151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed HU, Berge V, Bottomley D, et al. ; Prostate Cancer RCT Consensus Group . Can we deliver randomized trials of focal therapy in prostate cancer? Nat Rev Clin Oncol 2014;11:482–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.