Abstract
It is increasingly clear that mechanotransduction pathways play important roles in regulating fundamental cellular functions. Of the basic mechanical functions, the determination of cellular morphology is critical. Cells typically use many mechanosensitive steps and different cell states to achieve a polarized shape through repeated testing of the microenvironment. Indeed, morphology is determined by the microenvironment through periodic activation of motility, mechanotesting, and mechanoresponse functions by hormones, internal clocks, and receptor tyrosine kinases. Patterned substrates and controlled environments with defined rigidities limit the range of cell behavior and influence cell state decisions and are thus very useful for studying these steps. The recently defined rigidity sensing process provides a good example of how cells repeatedly test their microenvironment and is also linked to cancer. In general, aberrant extracellular matrix mechanosensing is associated with numerous conditions, including cardiovascular disease, aging, and fibrosis, that correlate with changes in tissue morphology and matrix composition. Hence, detailed descriptions of the steps involved in sensing and responding to the microenvironment are needed to better understand both the mechanisms of tissue homeostasis and the pathomechanisms of human disease.
Keywords: mechanotransduction, cell morphology, cell fate, integrin adhesions, cytoskeleton
1. INTRODUCTION
1.1. Cell Form and Function
For proper function of most mammalian organisms, the cells should initially create the proper form during development and then maintain their proper morphology through normal events, despite the fact that the cell components are dynamic and the system is subjected to a variety of external stresses. Once an organ is formed during development, the morphology is primarily maintained by the extracellular matrix (ECM) that also guides neighboring cells. This idea is strongly supported by the finding that growing stem cells in decellularized matrices of organs will enable regeneration of the organs to a significant extent (1, 2).
The important parameters of the microenvironment that shape cells and ultimately create tissue morphology are rigidity, architecture, and composition (both of the ECM and of neighboring cells) (see the sidebar titled Mechanosensitive Cell Responses). It remains unknown how during development of the organs individual cells maintain the proper tension and morphology over time, as the cytoskeletal proteins are constantly being remodeled and matrices are quite malleable. A way to start to understand the dynamic interplay between cells and their environment during development of tissue morphology is to continuously follow cells as they establish their morphology in different defined environments through changes in actin dynamics and myosin contractility in accordance with their initial state (3–5). From the sequence of biomechanical and biochemical events that occur, a more complete understanding of the important processes in tissue morphogenesis may emerge. In this review, we consider the early steps that cells undergo in binding to matrix-coated surfaces of different rigidities and morphologies. In general, active cell processes test the environment and respond by activating cytoskeletal motility processes that progressively lead the cell to a proper state for its environment. On longer timescales, it is difficult to trace the steps that the cells use or the states that they experience as they transition to new behaviors in the developmental or regenerative context (6–8). To encourage further definition of the many steps in determining the final morphology, we consider a few examples of the initial steps and then the current fragmentary evidence of how those steps may be involved in later cell behaviors, including in disease states.
1.2. Morphology During Development, Homeostasis, and Repair
To introduce the general problem of morphology determination in cells of tissues, we discuss the important steps in cell mechanical processes that underlie the transition of a cell from one shape to another. First, it is useful to introduce the concept of standard cellular and subcellular motile functions that are the tools used by cells to change their shape, such as the extension of lamellipodia, apical contractions in epithelia, and rigidity sensing contractions. These motile functions are activated by a variety of environmental and cell-derived signals (e.g., hormones, tugs on cells, and cellular clocks) (see Figure 1), and they will naturally enable the cell to test mechanical aspects of the environment. Because the cycle of activation, movement, testing, and response commonly takes only a few minutes, many such cycles will typically occur in cells after hours, much less days. As a result of multiple activation, motility, testing, and response events, cells will assume a quasi-equilibrium morphology, tension, and area, which are in turn linked to cell functional states. This includes the most fundamental states of cells, such as proliferation, death, migration, and differentiation (9, 10).
Figure 1.

This diagram shows the basic cycles that underlie the shaping of cells and tissues. In most cases, intrinsic signals in cells activate motility processes, involving small G proteins, kinases, and actomyosin that change cell morphology or tug on neighboring cells to enable tests of the mechanical environment. The outcomes of those tests will cause cellular responses that can alter but often maintain the cell morphology. Although there is considerable variability in the duration of such cycles, the typical times are approximately 10 min for the complete cycle, meaning that many cycles will occur during a normal experimental period.
Much of our knowledge about the effects of cell morphologies on functional states comes from studies involving controlled adhesive environments to regulate cell size and shape. These studies date back 40 years (11) and began with the notion that anchorage-dependent cells behave differently on different matrices that either do or do not allow their proliferation. More controlled studies using defined adhesive islands showed a direct correlation between cell area and proliferation (12, 13) and an inverse correlation between cell area and apoptosis (12). The same approach was also used in numerous studies to analyze the effects of cell shape and size on stem cell differentiation; those showed various outcomes depending on the type of cells, patterns, and growth media that were used (13–15). Even more subtle effects can be observed in the functions of specific cell types; for example, micropatterning of surfaces to force macrophages into round versus elongated shapes can drive macrophage cells toward the M1 versus M2 phenotypes, respectively (16). Cell orientation was also shown to affect the orientation of the cell division axis (17) and cell–cell junction positioning (18). More recently, it was shown that gene expression profiles of cells grown on triangular matrices versus circular matrices of the same area are dramatically different after only 4 h (19). The adhesion area and force on adhesions can explain many of the differences because the level of force and the adhesion area are critical factors in determining the signals from the adhesions (20). Indeed, inhibition of actomyosin contractility can abrogate the effects of the micropatterned cell shapes on their function (15, 16). Such morphological factors have also been implemented as part of models developed to predict traction forces, largely based upon stress fibers (21). At a more mechanistic level, there is evidence that highly pointed or elongated portions of cells with a high membrane area to volume ratio can produce dramatic differences in cAMP signaling. Because adenylyl cyclase that forms cAMP is a membrane enzyme, and the cAMP phosphodiesterase is a cytoplasmic enzyme, a high membrane to cytoplasm ratio in such regions will result in high concentrations of cAMP (22). More recent studies have focused on the theoretical basis of matrix shape–dependent effects on signaling pathways, as several pathways, including PIP2 and PIP3 synthesis and degradation, have both membrane and cytoplasmic components (23). These (and numerous other) observations indicate that the matrix pattern is a major factor in determining the development of cell state, which raises the question of how matrix morphology is translated into a cellular functional state.
1.3. Cell-Matrix Mechanotransduction and Morphology
One of the most important sources of environmental signals is the rigidity of the ECM, which can affect major cellular behaviors, including migration (24), proliferation (25), differentiation (26), gene expression (27), apoptotis (28), and epithelial-to-mesenchymal transition (EMT) (29). With normal cells, these processes are part of the maintenance and repair in tissues; however, pathological changes of ECM stiffness or, conversely, improper cellular responses to matrix stiffness, can lead to malignancies (30–32). As such, of the many mechanical factors that cells will respond to while establishing their proper role in a tissue, the property of matrix rigidity has received the most attention.
When interacting with a two-dimensional matrix of particular rigidity, a cell will form a defined morphology as the result of many decisions that are made as repeated testing and response events are activated by the cell. Over the last several years, it has emerged that rigidity sensing often involves the periodic formation and breakdown of a standard sensory module that is normally built at the edges of cells for approximately one minute to determine whether or not the matrix is rigid and then to develop the appropriate signals (32). The sensing process itself is mediated by transmembrane integrin molecules that bind the ECM at their extracellular side, and the actin cytoskeleton, via adapter proteins, at their intracellular side (see sidebar titled Integrin-Matrix Adhesions). Mechanosensing requires the application of force through the actomyosin contractions of the integrin adhesions on the newly attached matrix (4). In response to the applied forces and to resistance of the matrix, the adhesions alter their size, strength, and dynamics (33–37). This results in larger adhesions on rigid matrices and more force-dependent signaling (see more below). The formation of strong adhesions will enable the activation of additional lamellipodial extension and retraction (38) and the next round of mechanosensing of matrix rigidity.
Thus, a repeated pattern of cell edge activity, adhesion formation, and matrix testing occurs over time and space that eventually will determine the final cell morphology when it reaches steady state. Patterns of adhesions can be controlled by the shape and density of matrix sites, and modeling of the forces from the actin flow at the cell edge can explain some of the patterns that arise (39, 40). Nevertheless, there is a major gap between our understanding of the elementary sensing processes and the signals that they produce in terms of the molecules regulating actin and myosin dynamics as well as nuclear expression profiles. Further, most assays of cell morphology changes with mutations or drugs involve measurements of cell shape and size only after hours of spreading, wherein countless cycles of pulling and signal generation have occurred during that time. In reviewing this situation, we first describe the relatively simple process of spreading on fibronectin and then move to more complex systems, such as cell stretching or cell motility, where the number of molecular steps between the mechanosensors and the morphology changes is even greater. In all cases, virtually continuous observation of the forces and shape changes as well as the critical molecular players is needed to gain an understanding of how morphology is determined at a quasi-equilibrium time period of days.
2. CELL SPREADING ON FIBRONECTIN
2.1. From Initial Attachment to Mechanosensing
As an example of a reasonably well-understood shape change, we consider the simple morphological change from a cell in suspension to a full spread cell on a fibronectin-coated surface. The process is highly reproducible in many different cell lines, which makes it relatively easy to study. However, new aspects of the process continue to be found that highlight important steps in mechanosensing and belie a very complex process (Figure 2). Each one of these steps that is part of the mechanotransduction process involves the actin cytoskeleton, and many involve myosin contractility, but they share little else in terms of common themes or common transduction pathways. Further, as a cell goes from suspension to a polarized, spread state, it transitions through over four different states where distinct motility behaviors occur (41, 42).
Figure 2.
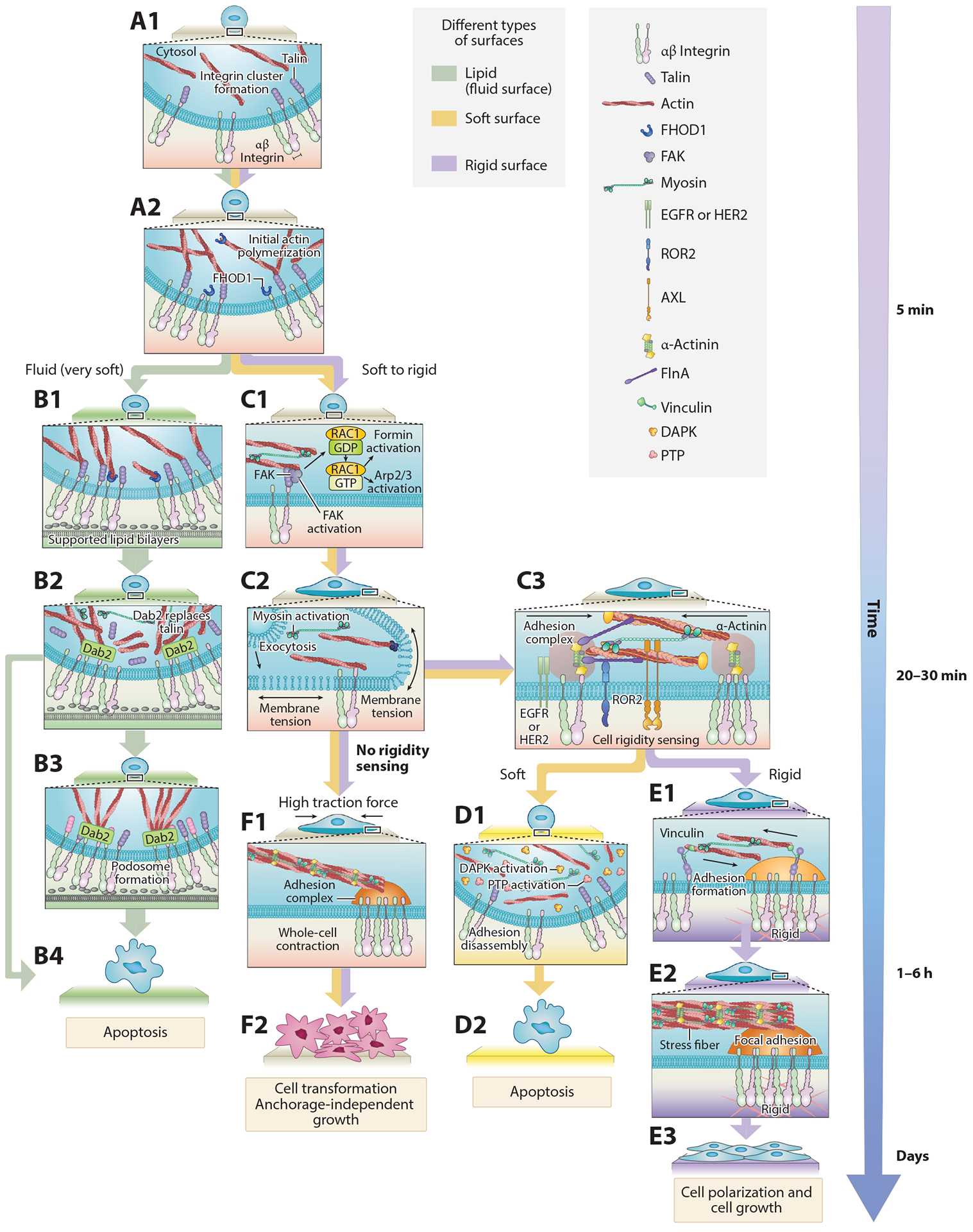
Mechanosensing occurs through a series of steps that involve adhesion formation, probing, and response. The cycle begins when (A1–A2) the cell contacts RGD ligand and forms integrin clusters that activate actin polymerization (4, 44). (B1–B4) Step-by-step mechanosensing of fibroblast cells on supported lipid bilayers (45, 114). (C1–C3) When the cell edge encounters new matrix, it forms nascent integrin adhesions and begins to contract the matrix through contractile units in order to test its rigidity (32, 49, 54). (D1–D2) Newly formed nascent adhesions disassemble when the matrix is soft. (E1–E3) If the matrix is stiff enough and the forces reach a threshold of approximately 25 pN, adhesion is reinforced via recruitment of additional proteins from the cytoplasm. (F1–F2) Transformed cells lose the rigidity sensing process and generate high traction forces on matrix. Abbreviations: AXL, anexelekto; Dab2, disabled homolog 2; DAPK, death-associated protein kinase; EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase; FHOD1, formin homology (FH)1/FH2 domain-coating protein 1; FlnA, filamin A; HER2, human epidermal growth factor receptor 2; PTP, protein tyrosine phosphatase; RGD, arginylglycylaspartic acid; ROR2, receptor tyrosine kinase-like orphan receptor 2.
As a cell in suspension initially interacts with the fibronectin-coated surface, nascent integrin clusters are formed in a myosin-independent but talin-dependent manner (43) (Figure 2, A1). Then, actin begins to polymerize from these clusters through a pathway involving integrin activation of Src and recruitment and activation of the formin FHOD1 (44, 44a) (Figure 2, A2). If myosin pulls on the actin filaments anchored to the integrin clusters and develops a significant force, then RAC-dependent actin polymerization occurs [possibly through a guanine-nucleotide-exchange factor (GEF) that is mechanically activated]. However, if inadequate force is produced [for example, in cases where ligands for the integrins are in fluid lipid bilayers and therefore cannot resist the force (44)], then spreading will not be activated, and cells will develop podosomes (45) before transitioning to apoptosis (Figure 2, B1–B4). In some cells on stiff matrices, actin polymerization is extensive, the cell spreads isotropically until it runs out of excess membrane, and tension in the membrane spikes briefly (46) (Figure 2, C1–C2). This leads to rapid activation of myosin contraction and membrane exocytosis, causing the cells to transition to a slower spreading phase (46, 47). In this phase, the spreading is based on actin polymerization at the cell edge, which pushes the cell edge outward while myosin pulls actin filaments toward the cell center, resulting in radial actin flow (48) and radial traction forces (49). This is part of the repeated protrusion-retraction cycles, which lead to an approximately 40% increase in plasma membrane area [notably, these cycles are also observed during cell migration (50)].
During the protrusion-retraction cycles, as the cell edge extends to new matrix areas, nascent adhesions of ~100 nm in diameter with ~50 integrin molecules are formed (51). Then, contractile actomyosin units of approximately 2 μm in length pull locally on those adhesions for about 1 min (32, 52). These contractions correlate with rigidity sensing, and altering them changes cells’ responses to matrix rigidity (32, 49, 52–54) (Figure 2, C3). Over at least a tenfold range in rigidity, the rate of pillar contraction is constant, with steps of myosin of approximately 2.5 nm occurring every 330 ms until the maximum matrix displacement is reached, and then a similar rate of relaxation of elastic matrix contacts is observed (32, 49). During the contraction process, force is developed on the matrix through the actomyosin links to the integrins, and if the force is great enough, the cell decides to reinforce the links between the cytoskeleton and the integrins. Reinforcement occurs when the contractions pause for 1–2 s at a force of ~25 pN. This pause correlates with a dramatic increase in the rate of accumulation of α-actinin at the adhesions, followed significantly later by other proteins such as vinculin and paxillin (32). Once the cell determines that the surface is rigid, it will typically reinforce adhesions and enter the cell cycle, i.e., a growth state (Figure 2, E1–E3). This is accompanied by gradual growth and cross-linking of actin stress fibers that are attached to the adhesions (55). Thus, with a rigid substrate, a time- and force-dependent, stepwise process occurs, starting from the integrin clusters, which leads to increased adhesion maturation and stabilization and increased organization of the actin cytoskeleton. However, if the matrix is too soft to support the formation of such force, then the adhesions will disassemble (32), and the cell will gradually transition into an apoptotic pathway, possibly through DAPK1 activation (56) (Figure 2, D1–D2).
For proper rigidity sensing, several additional biochemical steps were found. At a very early stage, calpain cleavage of talin is needed for proper adhesion development and for cell growth through the production of talin rod fragments (57). Also, Src family kinase activation of epidermal growth factor receptor [EGFR, or human epidermal growth factor receptor (HER2)] activity is needed to continue spreading and polarization as well as for rigidity sensing (53). Following adhesion maturation through α-actinin recruitment (32, 58), a transition from integrin αvβ3 to α5β1 occurs (59). Then contractions in adhesions, as well as force on adhesions coming from the flow of actin, are needed for continued adhesion growth (60). Rigidity-dependent strengthening of adhesions could occur through stick-slip of talin, where there is stretching and recruitment of vinculin and release upon talin relaxation (61). In development, the stretch-relaxation could prepare vinculin for interaction with mitogen-activated protein kinase (MAPK1) (62). When myosin force drops, adhesions dissipate, FHL2 moves to the nucleus to activate p21 synthesis (63), and cells eventually activate apoptotic pathways.
This is most certainly an incomplete picture of the early events and major steps in actin polymerization, as anchoring of actin and myosin activation is poorly understood. What is clear is that many different steps are needed to complete this simple morphological change, and many occur in a parallel but linked fashion. It is important to be able to put the steps into the context of larger motility processes, because it is only through an understanding of the sequence of molecular events in spreading that the process and related motility processes can be understood.
2.2. Adhesion Maturation and Actin flow: The Role of the Clutch in Force Development
After approximately 30 min on a surface, fibroblasts will develop mature adhesions that produce traction forces on the matrix. Blocking myosin activity will cause the disassembly of these adhesions, a feature that has been used to define a set of force-dependent components of the integrin adhesome (64–66). Traction forces are linked to the general flow of actin from the periphery to the nucleus that can produce drag forces on early nascent adhesions needed for adhesion maturation. If strong adhesions are established (on stiff matrices), the actin flow operates on them in a stick-slip manner in that the actin will pull on proteins like talin in the adhesions and release them periodically, resulting in stretch-relaxation cycles (67, 68) (Figure 3). In a stick-slip situation, the strength of the actin-integrin-matrix linkage will increase to match high pulling forces, perhaps as a result of the increased binding to stretched molecules (69), whereas a clutch model predicts that the rapid rise in force with rigid matrices will cause a rapid break of the linkage (70) (see sidebar titled Clutch, Drag, or Stick-Slip Models to Explain Matrix Tension). Many studies have shown that cells will normally increase the strength of links with the energy to give higher forces on rigid surfaces; e.g., one possible mechanism is the binding of vinculin to stretched talin (7). However, depletion of talin will cause cells to develop lower forces on stiff substrates as predicted by the clutch model (71). Thus, talin is an important component in the adhesions and affects the relative traction forces that are generated on the matrix, which in turn are dictated by the matrix rigidity: The more rigid the matrix, the stronger the force.
Figure 3.
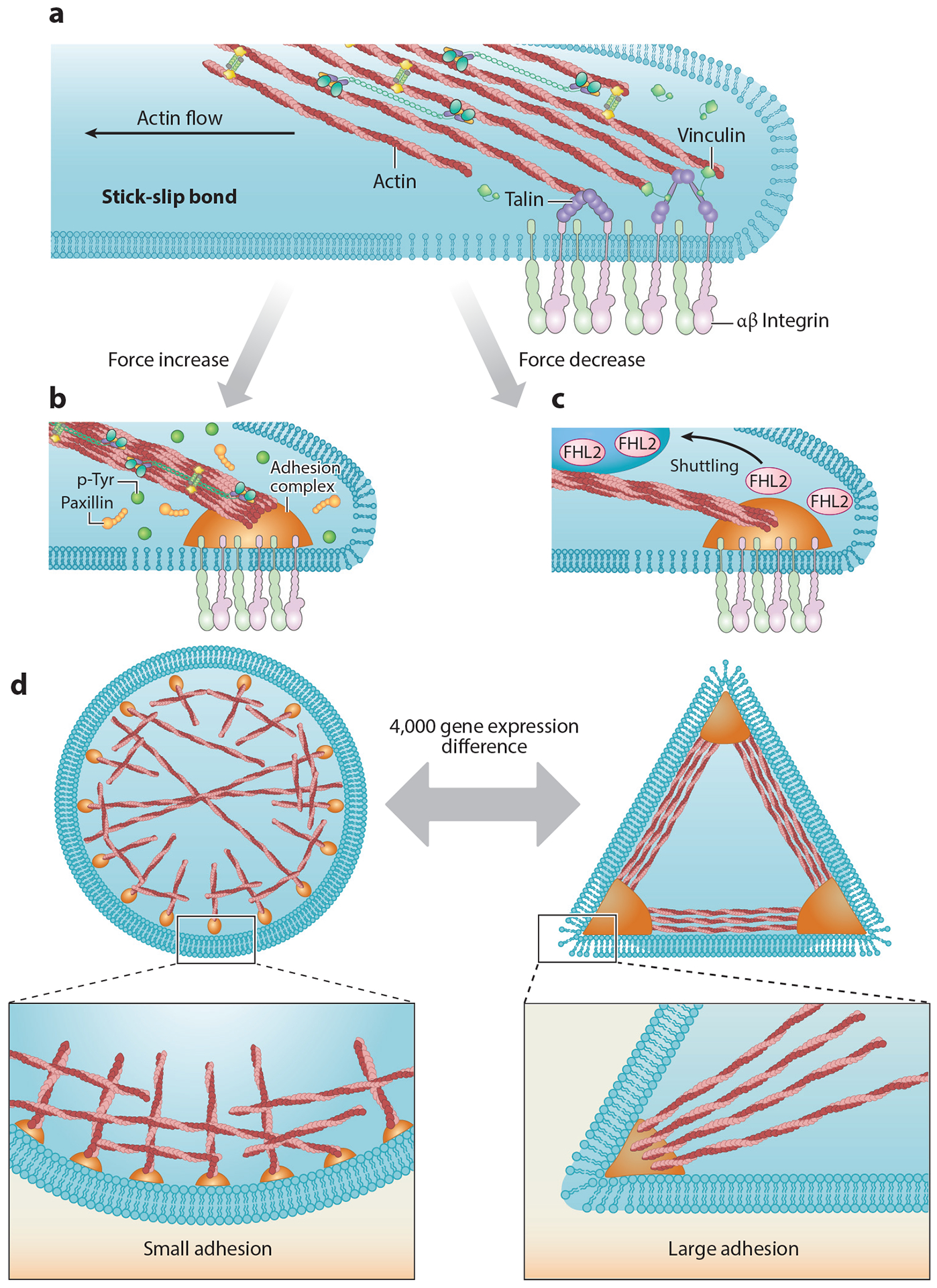
(a) Established adhesions experience drag forces from actin flowing to the cell center, resulting in a stick-slip behavior at the actin-adhesion interface (68). At later time points, when the adhesions mature, actin drag forces lead to repeated talin stretch-relaxation events that involve the binding of several vinculin molecules to a single talin (7). (b) Increasing forces cause increases in local tyrosine phosphorylation levels and reinforce adhesion formation. (c) Decreasing force leads to FHL2 shuttling to the nucleus (63). (d) Within 4 h of plating, cells on circular patterns show different expression levels of more than 4,000 genes compared with cells on triangle patterns (19). Abbreviations: FHL2, four and a half LIM domains protein 2; p-Tyr, phosphotyrosine.
A major role for the adhesions is to provide the proper mechanical signals from the matrix (including rigidity, density, and geometry) for the cell to properly respond. In the case of differentiation, for example, although stem cells test the matrix with local contractions initially, it is the longer-term integration of the rigidity that is important for the decision into which lineage to differentiate (72) and for the modification of lamin A expression level (73). In the adhesions, mechanosensitive proteins need to respond to the mechanical signals to transmit them downstream in the form of biochemical cues. Indeed, the flow of actin past proteins like talin causes it to stretch and bind vinculin (perhaps causing vinculin modification) until it relaxes and releases vinculin (74). Similar processes could occur for p130Cas, filamin, and many other actin-binding proteins in adhesions. A notable example is vinculin, which is known to be under force in adhesions (75), but it does not have a clear role in supporting traction forces (76). A careful analysis of traction forces with different vinculin isoforms concluded that “vinculin is not required for transmission of adhesive or traction forces but is necessary for myosin contractility-dependent adhesion strength and traction force and for the coupling of cell area and traction force” (76, p. 9788). Nevertheless, its interaction with MAPK1 is linked to the differentiation of stem cells to a cardiac lineage (62) that has excited modeling of the steps from vinculin to the final cell behavior (77). However, over the days needed for stem cell differentiation, we do not know when this interaction is important and under what conditions it contributes to the final outcome. It should, however, be possible to follow the steps in the stem cell differentiation process over time to better understand where the critical interactions occur (see sidebar titled Methods to Study Mechanosensing) and how they may fit into other patterns of cell motility and adhesion dynamics. In other words, a complete analysis of the steps involved is important.
In the tissue context, rigidity is normally maintained for long periods of time during the life of the organism (much longer than typical timescales of cellular processes). A way to maintain the proper mechanical properties of the tissue is to periodically test them with repeated cycles, as discussed above, and to become locked into a differentiated state that will produce the proper tension (Figure 2). Further, forces from matrices or neighboring cells can stimulate cell growth or activity (see sidebar titled Cell Stretching and External Force Effects). Activation of the mechanosensing processes can be mediated by hormones, cell stretching, or internal circadian clocks that activate the testing of the environment and adjustment of mechanical properties. The hormones, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and others can cause an increase in cytoskeletal activity that will test the cellular environment. Recent studies show that EGF activates rigidity sensing of cells on rigid surfaces but not cells on soft surfaces (53), which indicates that the effect of the hormone on the cell will depend on the environment of the cell. In the case of the circadian clock genes, there are roles in neoplastic transformation that belie important functions for the clocks in regulating motility and mechanosensing (78).
2.3. Different Adhesions for Different Tasks
The steps involved in defining cellular morphologies are also manifested at the subcellular and molecular levels. It has long been recognized that different steps in formation, growth, and disassembly of adhesions involve alteration in not only their size and strength but also their composition. Recent proteomics studies have compared integrin adhesions under different force regimes. The consensus composition of adhesions appears to center around four axes comprising ILK–PINCH–kindlin, FAK–paxillin, talin–vinculin, and α-actinin–zyxin–VASP (79). The latter axis appears to be a major step in adhesion maturation, as force-induced recruitment of LIM domain proteins (including zyxin and FHL2, among others) is often observed in the transition of focal complexes into mature adhesions. Upon inhibition of force, LIM domain proteins are typically the earliest ones to exit the adhesions, having relatively weak binding affinities and high turnover rates (34). Importantly, LIM domain proteins can also be shuttled to the nucleus, where they play major roles in transcription regulation, the most notable of these being zyxin (80), FHL1, and FHL2 (63). Thus, on relatively soft matrices, which do not promote the formation of strong adhesions and forces, LIM domain proteins are excluded from the adhesions, and this appears to act as a biomechanical switch (81).
3. THE REGULATORY ROLE OF RECEPTOR TYROSINE KINASES
The receptor tyrosine kinases (RTKs) typically are activated by hormones or other ligands and then phosphorylate components to activate Ras and other signaling pathways (82). A knockdown screen of all RTKs in the human genome showed that many RTKs are needed for proper cellular responses to the stimulus of surfaces of different rigidities (35). Depletion of some RTKs decreased rigidity sensing and adhesion formation, whereas depletion of others caused increased adhesion formation. Two RTKs, AXL and ROR2, which caused increased adhesion and stress fiber formation on soft surfaces after knockdown, were tested for changes in rigidity-sensing contractility. Knockdown of AXL caused an increase in the contraction displacements from 120 nm to 180 nm, whereas ROR2 knockdown caused a twofold increase in the duration of the contractions. However, no role was found for ligands in both cases (54). Thus, changes of RTK activity can have important effects on regulating tension at the adhesions, as well as on adhesion maturation and growth. For example, AXL and its ligand Gas6 are both overexpressed in several cancers, and addition of Gas6 decreases rigidity-sensing contraction length and creates lower forces on rigid matrices (54). At the same time, it also activates the AXL kinase that further activates MAPK kinases and growth pathways (83, 84). Although there is overall inhibition of the cell cycle by Gas6, which seems to contradict the effects of growth pathway activation, there is also increased resistance to apoptosis in response to taxol chemotherapy (85). Despite the seemingly contradictory roles of the RTKs in different cell contexts, it is clear that they can act in motility processes in the absence of ligands.
On the basis of genetic studies of Drosophila, many RTKs are involved in defining tissue morphology and tissue function (8). Recent findings indicate that RTKs may have an active role in the absence of their ligand to control the parameters of mechanical sensing and activation. Many roles of the RTKs appear to involve their hormone-independent roles, including their function as mechanoregulators of contractility (54) or activators of sensory functions downstream of Src family kinases or other kinases (53). In the context of contractility, the two EGF receptor family members, EGFR and HER2, regulate the density of local actomyosin contractions on rigid surfaces but not on soft surfaces (53). It will be important in the future to understand the roles of the RTKs in developing tissue morphology, in maintenance of morphology, and in response to ligands. Furthermore, the functions of the RTKs may change with the cellular type and the local environment of the cells.
4. MEMBRANE TENSION AND CELL POLARIZATION
As adhesions mature, the cells will polarize and migrate in some cases. The basis for polarization has been linked to the polarized growth of actin filaments at the leading edge of the cell that depends on a gradient of membrane tension (86). In recent studies, the membrane tension appears to act on phospholipase D2 and mTORC2 to limit the area of actin polymerization to the leading edge (87). With depletion of those proteins, the cells are less polarized and their extensions are less polar. Moreover, an increase in myosin light chain activity and presumably contractility will also result in less polarization (88). For fibroblasts there is also a major role for vinculin in polarization (74).
5. STEPS IN CADHERIN JUNCTION FORMATION
Similar to ECM adhesions, the cell–cell cadherin junctions appear to form through a series of steps that involve cluster formation (89, 90), early adhesion formation (91), consolidation through force-dependent actin interactions (92), and myosin-dependent movements (93, 94). We have also found similar contractile activity with submicrometer cadherin-coated pillars (Yang, Nguyen, Mege, Ladoux, and Sheetz, unpublished results) that appears connected with sensing the rigidity of cadherin contacts (95). The intercellular forces on adhesions correlate with the growth of epithelial cells (96). Thus, we suggest that a similar list of steps is involved in the formation of cadherin-mediated adhesions with a similar complexity that will include many different proteins for the many specific functions involved in epithelial shape determination through cadherin-dependent adhesions (97).
6. ABERRANT MECHANOTRANSDUCTION
Although we are only beginning to understand how malformations of tissues occur, it is clear that many diseases manifest through alterations in mechanosensing. The contractile machinery responsible for mechanosensing is composed of multiple components (Figure 2), with some having important regulatory roles. A particularly important aspect of aberrant mechanosensing is in the case of cancer that typically involves transformation or growth on soft agar. Recent studies provide preliminary evidence that transformed cells lack rigidity-sensing complexes (32, 52). The inability to sense rigidity might explain why cancer cells can grow on soft surfaces (Figure 4) (32, 98). A common theme emerging from mechanosensing studies of transformed cancer cells is that they generate abnormally high traction forces irrespective of rigidity and that they do not form proper mechanosensing contractile units (99). Potentially, these high forces are hyperactivating mechanosensitive proteins in the adhesions, which then leads to abnormal activation of growth pathways. The exact pathways that are involved are not clear, nor are the long-term kinetics of this process. Moreover, it is highly likely that there are still numerous unidentified adhesion-related proteins that are involved in transformation, given that close to 80% of the genes of the adhesome (100) are prognostic markers in cancer (Figure 5).
Figure 4.
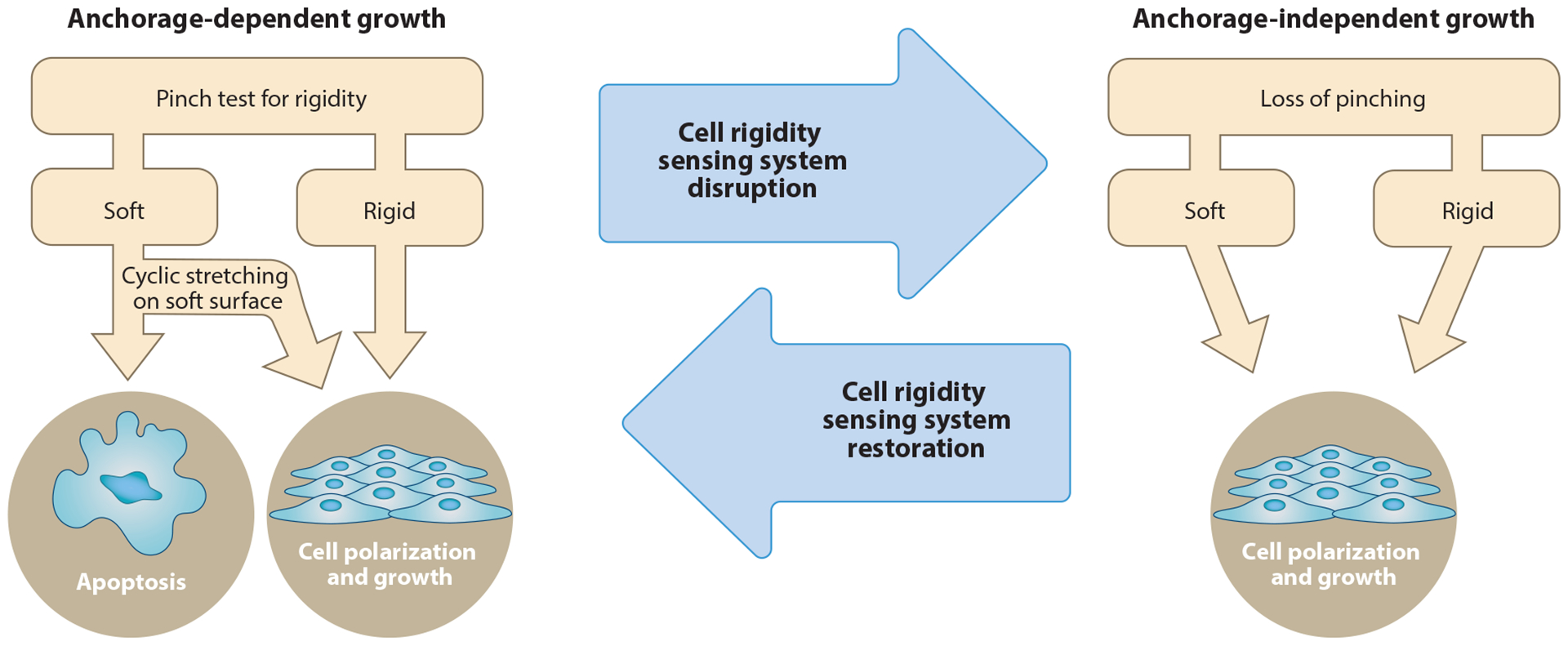
Rigidity-sensing contractile units are needed for inhibition of growth on soft surfaces. When nontransformed cells properly form contractile units, they will detect when the matrix is stiff or soft and respond appropriately by growing or undergoing apoptosis, respectively. Depletion of many proteins in the contractile units causes transformation of the cells (32, 52, 99), which do not form contractile units and therefore cannot properly sense rigidity.
Figure 5.
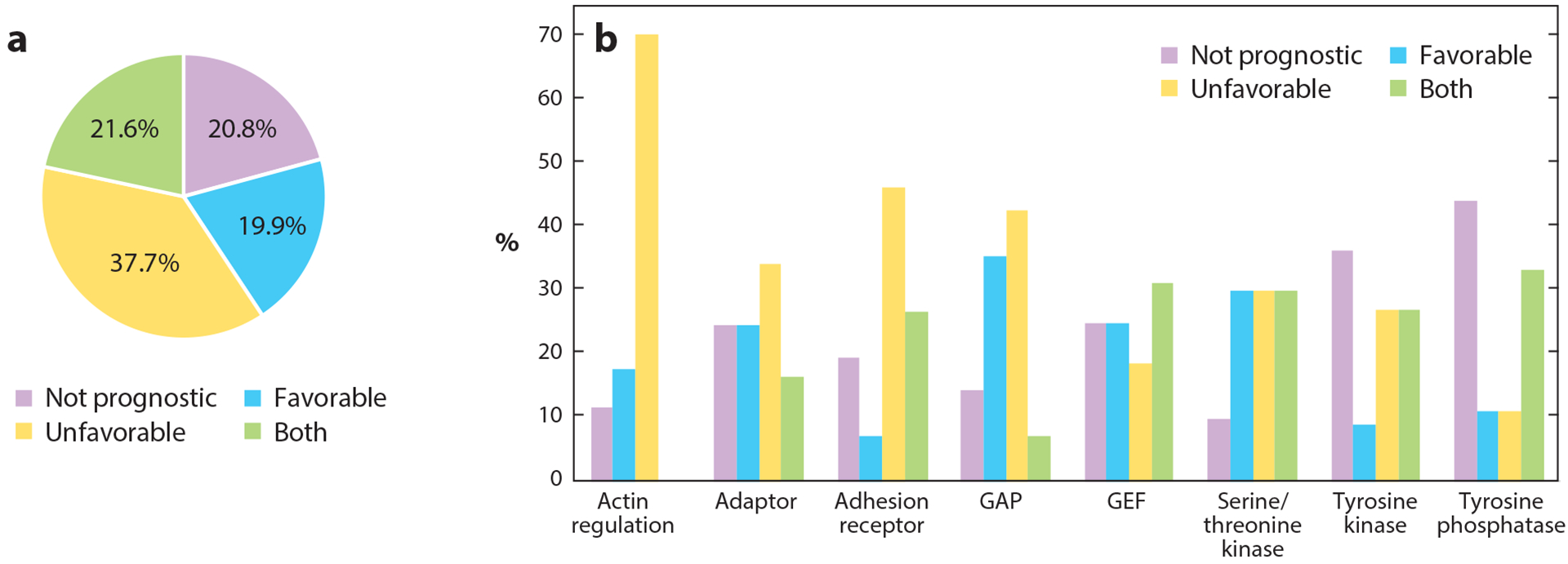
Involvement of adhesome components in cancer. Analysis is based on the Pathology Atlas, which is part of the Human Protein Atlas (https://www.proteinatlas.org). (a) Out of the 231 genes of the adhesome, 183 (~80%) are prognostic markers in cancer. Of those, 87 genes are unfavorable in specific cancer types (i.e., high mRNA expression correlates with shorter survival time), 46 genes are favorable in specific cancer types (high mRNA expression correlates with longer survival times), and 50 genes are favorable in some cancers and unfavorable in others. The remaining 48 genes are not prognostic, but 17 of those are still considered cancer related, i.e., often mutated in cancer. (b) Major categories of adhesome proteins involved in cancer and their division based on prognosis. Abbreviations: GAP, GTPase-activating proteins/GTPase-accelerating proteins; GEF, guanine nucleotide exchange factors.
Another important mechanobiological aspect of cancer is that tumors are typically stiffer than the tissues in which they grow, due to increased deposition and cross-linking of ECM proteins in the tumor stroma (101, 102). This property was shown to promote cancer progression (103). Because there is a direct correlation between matrix stiffness and the level of cellular forces through matrix adhesions (24, 49, 104), a stiff tumor environment will support the formation of high intracellular forces and will promote an increase in cell proliferation (105, 106). In addition, increased matrix stiffness and high forces can stimulate the EMT (28, 29), allow avoidance of anoikis (32), and induce cell migration (107), all of which are critical steps in the progression of metastasis. Thus, both extrinsic and intrinsic factors can contribute to aberrant activation of mechanosensory pathways in cancer.
In addition to cancer, aberrant mechanical responses are linked to tissue fibrosis and scarring. The process of wound healing is significantly affected by mechanical signals. For example, external mechanical stress leads to hypertrophic scarring due to infiltration of inflammatory cells and decreased apoptosis of local cells in the wound (108). Such scars can eventually lead to tissue fibrosis due to an irregular balance between collagen production and degradation. It is thought that aberrant mechanical responses of ECM-producing fibroblasts in the wound region are a major determinant of tissue fibrosis. Indeed, several mechanotransduction pathways in fibroblasts were shown to be involved in fibrosis, most notably the FAK-ERK pathway (109, 110). Fibrosis is also involved in vascular stiffening in atherosclerosis. In this case, a major role for matrix metalloproteinases (MMPs) was observed, particularly MMP-2 and MMP-9 (111). Further, early stages of atherosclerosis are linked to regions of low blood flow, where deposition of fibronectin and fibrinogen lead to monocyte recruitment (112). There is increasing evidence that physical activity does help to prolong life and enable a better quality of life. This is particularly relevant for atherosclerosis, and indeed, in a diabetic mouse model, physical activity led to a significant decrease in sclerotic lesions, decreased MMPs concentration, and less macrophage infiltration in the lesions (113). This suggests that at least some of the effects of physical activity could be transmitted through mechanical signals from the ECM. As we better understand the relationship between specific types of mechanosensing and various maladies, we can hope to better treat those maladies through mechanical therapy in combination with existing therapies. As many of the body repair systems involve mechanical movements to close wounds or repair ligaments, it is logical that a better understanding of the mechanosensing and motility steps involved will lead to better treatments.
7. CONCLUSIONS AND PERSPECTIVES
Mechanosensing and mechanotransduction are basic elements of shaping a tissue by cells. These are not continuous processes but are periodically activated either through normal cycles within the cells (circadian rhythms) or through acute activation by hormones, physical activity, or stresses. Naturally, long-term changes in tissue morphology are the result of alterations in the set points for the physical parameters brought about by disease processes, stresses, or natural aging. Nonetheless, there is normally a standard series of steps that cells follow in testing and responding to many aspects of their mechanical environment. Thus, the final cellular morphology is the result of multiple rounds of sensing matrix rigidity, shape, and curvature as well as environmental activation by mechanical stimulation or hormones. By understanding the molecular steps in the standard mechanosensing processes and, in particular, how cells integrate the different signals to yield a final response (regulation of cell shape, migration, growth, feedback on the ECM, etc.), we can possibly alter the malformations caused by disease processes and better aid recovery processes.
MECHANOSENSITIVE CELL RESPONSES.
ECM mechanical cues affect most fundamental cellular processes. These effects are transmitted over varying timescales and involve activation or inhibition of signaling pathways, as well as control over gene expression. Many of these effects involve changes in cell morphology, which are closely linked to cellular functions. For instance, differentiation of mesenchymal stem cells into an osteogenic lineage involves a high cellular aspect ratio and high cellular contractility (115), both of which are induced by stiff matrices (35). In contrast, lower polarization and decreased forces (both induced by low rigidity) favor an adipogenic outcome (115). Regulation of cell morphology is largely dependent upon Rho GTPases, which activate actomyosin contractility through Rho-associated protein kinase and myosin light chain kinase, and are recruited to the cell edge and activated there downstream of the FAK/Src pathway. Cell contractility and shape are also part of cellular decisions to grow or die. Differentiated cells that are plated on very soft matrices typically do not proliferate and eventually activate apoptosis. The mechanism that activates these pathways is not clear, but it seems to involve death-associated protein kinase (DAPK), which is known to phosphorylate myosin and tropomyosin, both major components of the mechanosensing machinery.
INTEGRIN-MATRIX ADHESIONS.
ECM mechanosensing relies upon integrin-based adhesions, which are dynamic multimolecular assemblies with transmembrane integrins at their core (116). Integrins are present at the cell surface as heterodimers of alpha and beta chains. There are 18 alpha isoforms and 8 beta isoforms in mammals that assemble into 24 known different dimers. The formation of adhesions begins when integrins are activated, either through an outside-in mechanism (binding the ECM at the integrin extracellular domain) or inside-out mechanism (integrin activation through binding of proteins such as talin or kindlin to their cytoplasmic tails). This triggers the recruitment of cytoplasmic proteins to the integrin tail to form the adhesion plaque, which consists of both signaling molecules such as FAK and Src as well as adapter proteins that mediate the connection between integrins and actin. This latter connection is critical for cellular responses to mechanical cues, as mechanosensing involves cytoskeletal-based forces that are transmitted to the matrix through the adhesions. More than 200 different proteins are associated with integrin adhesions, with each molecule having on average 8–10 different potential binding partners (100). This complexity is manifested in the kinetics and regulation of responses of adhesions to different biochemical and mechanical cues (117, 118).
CLUTCH, DRAG, OR STICK-SLIP MODELS TO EXPLAIN MATRIX TENSION.
An important aspect of cell-matrix adhesions is that rapid actin flow inward (<50–60 nm/s) (48) produces force on matrix adhesions that increases with substrate rigidity. The simple clutch model proposes that actin filaments transiently bind to adhesion proteins, producing force on matrices. When modeled (70), stiffer matrices caused rapid breaking of bonds, resulting in lower forces on stiff matrices compared to soft matrices, contrary to experiments. In recent studies (71), depletion of talin produced the predicted clutch behavior, but normal behavior required an increased number of bonds with rigidity. In the drag model, multiple, transient weak interactions between actin filaments and adhesion proteins create force on the adhesions (119). This explains formation of immune synapses and other large focal clusters but does not explain higher traction forces on rigid matrices. The stick-slip model proposes that transient stretching of talin by actin causes vinculin binding (67), which adds actin-binding sites. Talin stretching and vinculin and actin binding could scale with rigidity because stretching should increase on rigid matrices. A critical assumption is that the rate of force rise on stretchable proteins correlates with rigidity (120, 121). Currently, actin-matrix linkages and reinforcement are not understood; however, tyrosine phosphatases and kinases are implicated (122).
METHODS TO STUDY MECHANOSENSING.
Several tools were developed over the years to measure mechanosensing-related forces. The first demonstration of cellular traction forces on matrices was the wrinkling of elastic silicone surfaces by adherent cells (123). Later, in order to quantify the forces more precisely, this technique was modified into the widely used traction force microscopy method, which utilizes tracking the movements of fluorescent beads embedded in elastic gels (124) whose rigidities could be modulated by changing the cross-linking properties of its different components (125). Later, the flexible pillar array system was developed to track forces (126), in which the effective rigidity of the pillars is modulated by changing their height or width. In this method, live-cell brightfield or fluorescent imaging tracks themovements of the pillars as cells aremoving on top of them (33, 49); this system has allowed improved resolution of cellular forces (32). At the molecular level, atomic force microscopy has played a crucial role in elucidating the submolecular mechanisms of mechanosensitive protein unfolding, in particular talin (127) and titin (128). More recently, fluorescence resonance energy transfer paired within force-bearing proteins enabled measurement of the approximate forces on those proteins (75).
CELL STRETCHING AND EXTERNAL FORCE EFFECTS.
In addition to the cell testing the matrix, the matrix can also stimulate the cell by pulling or pushing on it. Cells within tissues are stretched periodically as part of normal blood flow and breathing, and changes in physical activity alter the lengths and frequencies of this stretch with consequences for cell expression patterns (129). Furthermore, periodic stretching of soft pillar arrays showed that forces from soft surfaces can stimulate growth (130). There has been considerable interest in characterizing cell responses to external mechanical perturbations (reviewed in 3, 131). Stretching experiments have shown that endothelial cells and fibroblasts will orient relative to uniaxial stretches dependent upon the strain and the frequency (132–135). This has been explained theoretically by the dependence on the dynamics of deformation of the matrix through the adhesions, which then affect the cytoskeletal organization (136). The important parameters are the time constants for the actin filament turnover and adhesion dynamics. In addition, actin filaments are broken easily by bending forces but can support much higher axial forces. Thus, once again, the interplay between mechanical forces and actomyosin dynamics strongly affects the shape of the cell.
SUMMARY POINTS.
The mechanical properties of the cellular environment take part in regulating fundamental cellular functions. The best understood property is the rigidity of the extracellular matrix.
Cells sense and respond to their environment through a standard series of steps that lead over time to the final morphology and behavior, which are further tested periodically at longer times.
Whereas mechanosensing is a short-term event, its effects are long term, indicating that mechanochemical steps form a progressive series that develops cell morphology over time and space.
Both testing and response involve cross talk between the adhesion and cytoskeletal machineries and biochemical pathways.
Aberrant mechanosensing of ECM rigidity is linked to various diseases, including cancer. This is (partially) attributed to the improper activation of biochemical pathways that control growth and migration.
Glossary
- Lamellipodia
broad, flat extensions of the plasma membrane commonly used by fibroblasts to reach new areas of surrounding surfaces
- cAMP signaling
heterotrimeric G proteins activated by G protein–coupled receptors (GPCRs) that activate adenylyl cyclase to produce the second messenger, cAMP
- Podosome
cylindrical bundles of actin filaments formed by Arp2/3 activation and actin polymerization to exert a downward force on the membrane
- SRC family kinases
nonreceptor tyrosine kinases that commonly associate with matrix adhesions and affect adhesion function both mechanically and biochemically
- Drag forces
forces generated by dragging actin filament networks from the lamellipodium edge toward the nucleus through actin-binding proteins in adhesions
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Taylor DA, Sampaio LC, Ferdous Z, Gobin AS, Taite LJ. 2018. Decellularized matrices in regenerative medicine. Acta Biomater. 74:74–89 [DOI] [PubMed] [Google Scholar]
- 2.Yu Y, Alkhawaji A, Ding Y, Mei J. 2016. Decellularized scaffolds in regenerative medicine. Oncotarget 7:58671–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorland YL, Huveneers S. 2017. Cell–cell junctional mechanotransduction in endothelial remodeling. Cell. Mol. Life Sci 74:279–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iskratsch T, Wolfenson H, Sheetz MP. 2014. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol 15:825–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roca-Cusachs P, Conte V, Trepat X. 2017. Quantifying forces in cell biology. Nat. Cell Biol 19:742–51 [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Vicente-Manzanares M, Potvin-Trottier L, Wiseman PW, Horwitz AR. 2012. The integrinligand interaction regulates adhesion and migration through a molecular clutch. PLOS ONE 7:e40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu X, Margadant F, Yao M, Sheetz M. 2017. Molecular stretching modulates mechanosensing pathways. Protein Sci. 26:1337–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sopko R, Perrimon N. 2013. Receptor tyrosine kinases in Drosophila development. Cold Spring Harb. Perspect. Biol 5 10.1101/cshperspect.a009050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vining KH, Mooney DJ. 2017. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol 18:728–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnans C, Chou J, Werb Z. 2014. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol 15:786–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folkman J, Moscona A. 1978. Role of cell shape in growth control. Nature 273:345–49 [DOI] [PubMed] [Google Scholar]
- 12.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. 1997. Geometric control of cell life and death. Science 276:1425–28 [DOI] [PubMed] [Google Scholar]
- 13.Watt FM, Jordan PW, O’Neill CH. 1988. Cell shape controls terminal differentiation of human epidermal keratinocytes. PNAS 85:5576–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tay CY, Yu H, Pal M, Leong WS, Tan NS, et al. 2010. Micropatterned matrix directs differentiation of human mesenchymal stem cells towards myocardial lineage. Exp. Cell Res 316:1159–68 [DOI] [PubMed] [Google Scholar]
- 15.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6:483–95 [DOI] [PubMed] [Google Scholar]
- 16.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. 2013. Modulation of macrophage phenotype by cell shape. PNAS 110:17253–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Théry M, Racine V, Pépin A, Piel M, Chen Y, et al. 2005. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol 7:947–53 [DOI] [PubMed] [Google Scholar]
- 18.Tseng Q, Duchemin-Pelletier E, Deshiere A, Balland M, Guillou H, et al. 2012. Spatial organization of the extracellular matrix regulates cell–cell junction positioning. PNAS 109:1506–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain N, Iyer KV, Kumar A, Shivashankar GV. 2013. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. PNAS 110:11349–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Abdeen AA, Tang X, Saif TA, Kilian KA. 2015. Geometric guidance of integrin mediated traction stress during stem cell differentiation. Biomaterials 69:174–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roux C, Duperray A, Laurent VM, Michel R, Peschetola V, et al. 2016. Prediction of traction forces of motile cells. Interface Focus 6:20160042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neves SR, Tsokas P, Sarkar A, Grace EA, Rangamani P, et al. 2008. Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell 133:666–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangamani P, Lipshtat A, Azeloglu EU, Calizo RC, Hu M, et al. 2013. Decoding information in cell shape. Cell 154:1356–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo CM, Wang HB, Dembo M, Wang YL. 2000. Cell movement is guided by the rigidity of the substrate. Biophys. J 79:144–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sessions AO, Engler AJ. 2016. Mechanical regulation of cardiac aging in model systems. Circ. Res 118:1553–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126:677–89 [DOI] [PubMed] [Google Scholar]
- 27.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, et al. 2011. Role of YAP/TAZ in mechanotransduction. Nature 474:179–83 [DOI] [PubMed] [Google Scholar]
- 28.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. 2012. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol. Biol. Cell 23:781–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, et al. 2015. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol 17:678–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, et al. 2014. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med 20:360–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michor F, Weaver VM. 2014. Understanding tissue context influences on intratumour heterogeneity. Nat. Cell Biol 16:301–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfenson H, Meacci G, Liu S, Stachowiak MR, Iskratsch T, et al. 2016. Tropomyosin controls sarcomere-like contractions for rigidity sensing and suppressing growth on soft matrices. Nat. Cell Biol 18:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trichet L, Le Digabel J, Hawkins RJ, Vedula SR, Gupta M, et al. 2012. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. PNAS 109:6933–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfenson H, Bershadsky A, Henis YI, Geiger B. 2011. Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J. Cell Sci 124:1425–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prager-Khoutorsky M, Lichtenstein A, Krishnan R, Rajendran K, Mayo A, et al. 2011. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol 13:1457–65 [DOI] [PubMed] [Google Scholar]
- 36.Hamadi A, Bouali M, Dontenwill M, Stoeckel H, Takeda K, Ronde P. 2005. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J. Cell Sci 118:4415–25 [DOI] [PubMed] [Google Scholar]
- 37.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. 2007. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol 176:573–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, et al. 2007. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell 128:561–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shemesh T, Bershadsky AD, Kozlov MM. 2012. Physical model for self-organization of actin cytoskeleton and adhesion complexes at the cell front. Biophys. J 102:1746–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tee YH, Shemesh T, Thiagarajan V, Hariadi RF, Anderson KL, et al. 2015. Cellular chirality arising from the self-organization of the actin cytoskeleton. Nat. Cell Biol 17:445–57 [DOI] [PubMed] [Google Scholar]
- 41.Dobereiner HG, Dubin-Thaler B, Giannone G, Xenias HS, Sheetz MP. 2004. Dynamic phase transitions in cell spreading. Phys. Rev. Lett 93:108105. [DOI] [PubMed] [Google Scholar]
- 42.Wolfenson H, Iskratsch T, Sheetz MP. 2014. Early events in cell spreading as a model for quantitative analysis of biomechanical events. Biophys. J 107:2508–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. 2008. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol 10:1039–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu CH, Law JB, Suryana M, Low HY, Sheetz MP. 2011. Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. PNAS 108:20585–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Iskratsch T, Yu CH, Mathur A, Liu S, Stevenin V, et al. 2013. FHOD1 is needed for directed forces and adhesion maturation during cell spreading and migration. Dev. Cell 27:545–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu CH, Rafiq NB, Krishnasamy A, Hartman KL, Jones GE, et al. 2013. Integrin-matrix clusters form podosome-like adhesions in the absence of traction forces. Cell Rep 5:1456–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gauthier NC, Fardin MA, Roca-Cusachs P, Sheetz MP. 2011. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. PNAS 108:14467–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pontes B, Monzo P, Gole L, Le Roux AL, Kosmalska AJ, et al. 2017. Membrane tension controls adhesion positioning at the leading edge of cells. J. Cell Biol 10.1083/jcb.201611117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. 2004. Periodic lamellipodial contractions correlate with rearward actin waves. Cell 116:431–43 [DOI] [PubMed] [Google Scholar]
- 49.Ghassemi S, Meacci G, Liu S, Gondarenko AA, Mathur A, et al. 2012. Cells test substrate rigidity by local contractions on submicrometer pillars. PNAS 109:5328–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doyle AD, Kutys ML, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. 2012. Micro-environmental control of cell migration–myosin IIA is required for efficient migration in fibrillar environments through control of cell adhesion dynamics. J. Cell Sci 125:2244–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Changede R, Xu X, Margadant F, Sheetz MP. 2015. Nascent integrin adhesions form on all matrix rigidities after integrin activation. Dev. Cell 35:614–21 [DOI] [PubMed] [Google Scholar]
- 52.Meacci G, Wolfenson H, Liu S, Stachowiak MR, Iskratsch T, et al. 2016. α-Actinin links extracellular matrix rigidity-sensing contractile units with periodic cell-edge retractions. Mol. Biol. Cell 27:3471–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saxena M, Liu S, Yang B, Hajal C, Changede R, et al. 2017. EGFR and HER2 activate rigidity sensing only on rigid matrices. Nat. Mater 16:775–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang B, Lieu ZZ, Wolfenson H, Hameed FM, Bershadsky AD, Sheetz MP. 2016. Mechanosensing controlled directly by tyrosine kinases. Nano Lett. 16:5951–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naumanen P, Lappalainen P, Hotulainen P. 2008. Mechanisms of actin stress fibre assembly. J. Microsc 231:446–54 [DOI] [PubMed] [Google Scholar]
- 56.Ivanovska J, Mahadevan V, Schneider-Stock R. 2014. DAPK and cytoskeleton-associated functions. Apoptosis 19:329–38 [DOI] [PubMed] [Google Scholar]
- 57.Saxena M, Changede R, Hone J, Wolfenson H, Sheetz MP. 2017. Force-induced calpain cleavage of talin is critical for growth, adhesion development, and rigidity sensing. Nano Lett. 17:7242–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roca-Cusachs P, del Rio A, Puklin-Faucher E, Gauthier NC, Biais N, Sheetz MP. 2013. Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation. PNAS 110:E1361–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zamir E, Katz M, Posen Y, Erez N, Yamada KM, et al. 2000. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol 2:191–96 [DOI] [PubMed] [Google Scholar]
- 60.Oakes PW, Beckham Y, Stricker J, Gardel ML. 2012. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. Cell Biol 196:363–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. 2012. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151:1513–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holle AW, Tang X, Vijayraghavan D, Vincent LG, Fuhrmann A, et al. 2013. In situ mechanotransduction via vinculin regulates stem cell differentiation. Stem Cells 31:2467–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakazawa N, Sathe AR, Shivashankar GV, Sheetz MP. 2016. Matrix mechanics controls FHL2 movement to the nucleus to activate p21 expression. PNAS 113:E6813–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schiller HB, Friedel CC, Boulegue C, Fassler R. 2011. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 12:259–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuo JC, Han X, Hsiao CT, Yates JR 3rd, Waterman CM. 2011. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol 13:383–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Byron A, Humphries JD, Craig SE, Knight D, Humphries MJ. 2012. Proteomic analysis of α4β1 integrin adhesion complexes reveals α-subunit-dependent protein recruitment. Proteomics 12:2107–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu X, Jing C, Xu X, Nakazawa N, Cornish VW, et al. 2016. Cooperative vinculin binding to talin mapped by time resolved super resolution microscopy. Nano Lett. 16:4062–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Margadant F, Chew LL, Hu X, Yu H, Bate N, et al. 2011. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLOS Biol. 9:e1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sawada Y, Sheetz MP. 2002. Force transduction by Triton cytoskeletons. J. Cell Biol 156:609–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan CE, Odde DJ. 2008. Traction dynamics of filopodia on compliant substrates. Science 322:1687–91 [DOI] [PubMed] [Google Scholar]
- 71.Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Pérez-González C, et al. 2016. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol 18:540–48 [DOI] [PubMed] [Google Scholar]
- 72.Discher DE, Mooney DJ, Zandstra PW. 2009. Growth factors, matrices, and forces combine and control stem cells. Science 324:1673–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, et al. 2013. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341:1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thievessen I, Fakhri N, Steinwachs J, Kraus V, McIsaac RS, et al. 2015. Vinculin is required for cell polarization, migration, and extracellular matrix remodeling in 3D collagen. FASEB J. 29:4555–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, et al. 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466:263–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dumbauld DW, Lee TT, Singh A, Scrimgeour J, Gersbach CA, et al. 2013. How vinculin regulates force transmission. PNAS 110:9788–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garakani K, Shams H, Mofrad MRK. 2017. Mechanosensitive conformation of vinculin regulates its binding to MAPK1. Biophys. J 112:1885–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katamune C, Koyanagi S, Shiromizu S, Matsunaga N, Shimba S, et al. 2016. Different roles of negative and positive components of the circadian clock in oncogene-induced neoplastic transformation. J. Biol. Chem 291:10541–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horton ER, Byron A, Askari JA, Ng DH, Millon-Fremillon A, et al. 2015. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol 17:1577–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nix DA, Beckerle MC. 1997. Nuclear–cytoplasmic shuttling of the focal contact protein, zyxin: a potential mechanism for communication between sites of cell adhesion and the nucleus. J. Cell Biol 138:1139–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X, Wei X, Yuan Y, Sun Q, Zhan J, et al. 2018. Src-mediated phosphorylation converts FHL1 from tumor suppressor to tumor promoter. J. Cell Biol 217:1335–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schneider IC, Hays CK, Waterman CM. 2009. Epidermal growth factor-induced contraction regulates paxillin phosphorylation to temporally separate traction generation from de-adhesion. Mol. Biol. Cell 20:3155–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gustafsson A, Fritz HKM, Dahlbäck B. 2017. Gas6-Axl signaling in presence of Sunitinib is enhanced, diversified and sustained in renal tumor cells, resulting in tumor-progressive advantages. Exp. Cell Res 355:47–56 [DOI] [PubMed] [Google Scholar]
- 84.Zweemer AJM, French CB, Mesfin J, Gordonov S, Meyer AS, Lauffenburger DA. 2017. Apoptotic bodies elicit Gas6-mediated migration of AXL-expressing tumor cells. Mol. Cancer Res 15:1656–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee E, Decker AM, Cackowski FC, Kana LA, Yumoto K, et al. 2016. Growth arrest-specific 6 (GAS6) promotes prostate cancer survival by G1 arrest/S phase delay and inhibition of apoptosis during chemotherapy in bone marrow. J. Cell Biochem 117:2815–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diz-Muñoz A, Fletcher DA, Weiner OD. 2013. Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 23:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diz-Muñoz A, Thurley K, Chintamen S, Altschuler SJ, Wu LF, et al. 2016. Membrane tension acts through PLD2 and mTORC2 to limit actin network assembly during neutrophil migration. PLOS Biol. 14:e1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lou SS, Diz-Muñoz A, Weiner OD, Fletcher DA, Theriot JA. 2015. Myosin light chain kinase regulates cell polarization independently of membrane tension or Rho kinase. J. Cell Biol 209:275–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu Y, Kanchanawong P, Zaidel-Bar R. 2015. Actin-delimited adhesion-independent clustering of E-cadherin forms the nanoscale building blocks of adherens junctions. Dev. Cell 32:139–54 [DOI] [PubMed] [Google Scholar]
- 90.Strale PO, Duchesne L, Peyret G, Montel L, Nguyen T, et al. 2015. The formation of ordered nanoclusters controls cadherin anchoring to actin and cell-cell contact fluidity. J. Cell Biol 210:333–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nejsum LN, Nelson WJ. 2009. Epithelial cell surface polarity: the early steps. Front. Biosci 14:1088–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, et al. 2014. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 346:1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu SK, Budnar S, Yap AS, Gomez GA. 2014. Pulsatile contractility of actomyosin networks organizes the cellular cortex at lateral cadherin junctions. Eur. J. Cell Biol 93:396–404 [DOI] [PubMed] [Google Scholar]
- 94.Acharya BR, Wu SK, Lieu ZZ, Parton RG, Grill SW, et al. 2017. Mammalian diaphanous 1 mediates a pathway for E-cadherin to stabilize epithelial barriers through junctional contractility. Cell Rep. 18:2854–67 [DOI] [PubMed] [Google Scholar]
- 95.Collins C, Denisin AK, Pruitt BL, Nelson WJ. 2017. Changes in E-cadherin rigidity sensing regulate cell adhesion. PNAS 114:E5835–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benham-Pyle BW, Pruitt BL, Nelson WJ. 2015. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science 348:1024–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zaidel-Bar R 2013. Cadherin adhesome at a glance. J. Cell Sci 126:373–78 [DOI] [PubMed] [Google Scholar]
- 98.Wang HB, Dembo M, Wang YL. 2000. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol 279:C1345–50 [DOI] [PubMed] [Google Scholar]
- 99.Yang B, Wolfenson H, Nakazawa N, Liu S, Hu J, Sheetz M. 2018. Stopping transformed growth with cytoskeletal proteins: turning a devil into an angel. bioRxiv 221176 10.1101/221176 [DOI] [Google Scholar]
- 100.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. 2007. Functional atlas of the integrin adhesome. Nat. Cell Biol 9:858–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Plodinec M, Loparic M, Monnier CA, Obermann EC, Zanetti-Dallenbach R, et al. 2012. The nanomechanical signature of breast cancer. Nat. Nanotechnol 7:757–65 [DOI] [PubMed] [Google Scholar]
- 102.Acerbi I, Cassereau L, Dean I, Shi Q, Au A, et al. 2015. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol 7:1120–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, et al. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell 8:241–54 [DOI] [PubMed] [Google Scholar]
- 104.Ghibaudo M, Saez A, Trichet L, Xayaphoummine A, Browaeys J, et al. 2008. Traction forces and rigidity sensing regulate cell functions. Soft Matter 4:1836 [Google Scholar]
- 105.Mih JD, Marinkovic A, Liu F, Sharif AS, Tschumperlin DJ. 2012. Matrix stiffness reverses the effect of actomyosin tension on cell proliferation. J. Cell Sci 125:5974–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kong HJ, Polte TR, Alsberg E, Mooney DJ. 2005. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. PNAS 102:4300–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saez A, Ghibaudo M, Buguin A, Silberzan P, Ladoux B. 2007. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. PNAS 104:8281–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, et al. 2007. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 21:3250–61 [DOI] [PubMed] [Google Scholar]
- 109.Wong VW, Levi K, Akaishi S, Schultz G, Dauskardt RH. 2012. Scar zones: region-specific differences in skin tension may determine incisional scar formation. Plast. Reconstr. Surg 129:1272–76 [DOI] [PubMed] [Google Scholar]
- 110.Lagares D, Busnadiego O, Garcia-Fernandez RA, Kapoor M, Liu S, et al. 2012. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 64:1653–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Giannandrea M, Parks WC. 2014. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model Mech 7:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. 2005. The subendothelial extracellular matrix modulates NF-κB activation by flow: a potential role in atherosclerosis. J. Cell Biol 169:191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kadoglou NP, Moustardas P, Kapelouzou A, Katsimpoulas M, Giagini A, et al. 2013. The anti-inflammatory effects of exercise training promote atherosclerotic plaque stabilization in apolipoprotein E knockout mice with diabetic atherosclerosis. Eur. J. Histochem 57:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu CH, Rafiq NB, Cao F, Zhou Y, Krishnasamy A, et al. 2015. Integrin-β3 clusters recruit clathrin-mediated endocytic machinery in the absence of traction force. Nat. Commun 6:8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kilian KA, Bugarija B, Lahn BT, Mrksich M. 2010. Geometric cues for directing the differentiation of mesenchymal stem cells. PNAS 107:4872–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolfenson H, Lavelin I, Geiger B. 2013. Dynamic regulation of the structure and functions of integrin adhesions. Dev. Cell 24:447–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lavelin I, Wolfenson H, Patla I, Henis YI, Medalia O, et al. 2013. Differential effect of actomyosin relaxation on the dynamic properties of focal adhesion proteins. PLOS ONE 8:e73549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zaidel-Bar R, Geiger B. 2010. The switchable integrin adhesome. J. Cell Sci 123:1385–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. 2008. T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys. J 94:3286–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choquet D, Felsenfeld DP, Sheetz MP. 1997. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88:39–48 [DOI] [PubMed] [Google Scholar]
- 121.Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. 2006. Rigidity sensing at the leading edge through αvβ3 integrins and RPTPα. Biophys. J 90:1804–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giannone G, Sheetz MP. 2006. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 16:213–23 [DOI] [PubMed] [Google Scholar]
- 123.Harris AK, Wild P, Stopak D. 1980. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science 208:177–79 [DOI] [PubMed] [Google Scholar]
- 124.Oliver T, Dembo M, Jacobson K. 1995. Traction forces in locomoting cells. Cell Motil. Cytoskelet 31:225–40 [DOI] [PubMed] [Google Scholar]
- 125.Pelham RJ Jr., Wang Y 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility. PNAS 94:13661–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. 2003. Cells lying on a bed of microneedles: an approach to isolate mechanical force. PNAS 100:1484–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. 2009. Stretching single talin rod molecules activates vinculin binding. Science 323:638–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rief M, Gautel M, Oesterhelt F, Fernandez JM, Gaub HE. 1997. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science 276:1109–12 [DOI] [PubMed] [Google Scholar]
- 129.Chen LJ, Wei SY, Chiu JJ. 2013. Mechanical regulation of epigenetics in vascular biology and patho-biology. J. Cell. Mol. Med 17:437–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cui Y, Hameed FM, Yang B, Lee K, Pan CQ, et al. 2015. Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun 6:6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. 2016. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig 126:821–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Greiner AM, Chen H, Spatz JP, Kemkemer R. 2013. Cyclic tensile strain controls cell shape and directs actin stress fiber formation and focal adhesion alignment in spreading cells. PLOS ONE 8:e77328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heil P, Spatz JP. 2010. Lateral shear forces applied to cells with single elastic micropillars to influence focal adhesion dynamics. J. Phys. Condens. Matter 22:194108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goldyn AM, Kaiser P, Spatz JP, Ballestrem C, Kemkemer R. 2010. The kinetics of force-induced cell reorganization depend on microtubules and actin. Cytoskeleton 67:241–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jungbauer S, Gao H, Spatz JP, Kemkemer R. 2008. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys. J 95:3470–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Livne A, Geiger B. 2016. The inner workings of stress fibers—from contractile machinery to focal adhesions and back. J. Cell Sci 129:1293–304 [DOI] [PubMed] [Google Scholar]


