The California Institute of Regenerative Medicine (CIRM) and the International Alliance for Biological Standardization (IABS) conducted the 4th Cell Therapy Conference Manufacturing and Testing of Pluripotent Stem Cells. The conference was held on June 5–6, 2018 in Los Angeles, and covered pre-clinical and clinical pluripotent stem cell product development. Several development programs based on pluripotent stem cells were described. The meeting addressed unresolved issues related to the manufacture and testing of pluripotent stem cell-based therapies.
The overall goal for this conference was to provide participants with a strategic roadmap, so that the newly developed therapies are well positioned to achieve the current regulatory standards, are designed to present minimized risk to developers and patients and be made available to patients around the world. Proceedings of the Conference were recently published [1].
Here we present a strategic road map to filing a Biologics License Application (BLA) that the CIRM and IABS colleagues along with pluripotent stem cell manufacturing experts composed as an outcome of the conference. The BLA road map provides current and future pluripotent stem cell-based therapy developers with strategic manufacturing options for navigating this exciting regenerative medicine therapeutic area and delivering products to the market for patients with unmet medical needs.
Three paths are outlined below for the Chemistry, Manufacturing, Control (CMC) sections of a cell therapy development plan road map. The three paths capture developers’ potential strategies and thus vary, based on fundamental evaluation and availability of resources, risk tolerance, and product development timelines in an expedited process review environment for innovative products for patients with unmet medical needs.
A BLA must contain the CMC plan for the drug substance (un-formulated active substance) and the drug product (the finished dosage form of the product). This write-up focuses on CMC details for the drug product.
The CMC BLA section for the drug product should include descriptions of the:
Development of the quality management system (this will include the standards adopted at different stages of manufacture and establishment of risk assessment and change control procedures)
Development of the product, process and control strategy
Consistent and validated manufacturing process
Analytical tools for process & product characterization (identity, purity, physical state)
Analytical tools for product QC, in process testing, in process monitoring
Potency assay or assays
Analytical validation packages
Product stability packages
Process validation packages
Licensable facility description and validation summary
Raw material specification and controls
Validated container and closure system
Storage and shipment modalities
Cell bank history & characterization data
Genetic modifications and vectors, including the traceability of any added genetic material (if relevant)
Said another way, health authorities are looking for a CMC package which shows that the developer understands what is important about the product to support its safety and efficacy, and has controls in place which will ensure consistent performance throughout the lifecycle of the product. Health Authorities are also looking for evidence that the commercial product comes from a facility which complies with cGMP and is able to satisfy the commercial demand for the product. These are the CMC requirements for product licensure that support results from nonclinical and clinical studies.
In addition, it is vital to maintain a strong element of scientific rigor and review throughout all stages of development. This approach ensures that product development and risk assessment meet current best and sound scientific practice.
CMC Development Pathway
The initial key regulatory step for the development of a given drug product leading to a BLA is the submission of an Investigational New Drug Application (IND) to initiate human clinical studies to demonstrate product safety and efficacy. The content and review of the CMC information for human Somatic Cell Therapy are summarized in a CBER/FDA guidance document [2]. Upon opening of the IND, both clinical and CMC development of the product could proceed to support the eventual BLA and licensure of the product.
It is important to note that the CMC requirements for a BLA approval to permit commercial product distribution are substantially higher than those for an IND. Most of the items of the list above, which are required for the BLA, are absent from an IND, or only discussed in more simplified terms. For example, there is little discussion of a Quality Risk Management (QRM) plan or a development package in a typical IND. Process, facility, and analytical validation and the potency assay are not required for the IND, and product stability data are encouraged but not mandated. Process and product controls are rudimentary at the IND stage, compared to the robust controls expected in a BLA. Simply put, a BLA should contain a complete CMC story with supporting data of how the product is made, tested, controlled, stored, shipped to the end user, and prepared for delivery to the patient; by contrast, an IND contains a likely outline of how this information might be collected in future to develop the story, with simply initial data regarding the first 1–2 batches of product.
Three different development pathways, Cases A, B, or C can be chosen, depending on a given firm’s experience level with biologics product development, risk tolerance, and resources availability. The fundamental assumptions for each pathway are detailed in Table 1.
Table 1.
Assumptions about three development pathways.
| Case A | I. Some firms will be experienced in development of licensed biological products, II. Some experienced firms will be willing and able to invest in the proposed cell therapy to ensure rapid development and licensure of the product. This can lead to compressed timelines but it may also place investment at risk even prior to achieving clinical proof-of-concept. This is especially relevant for regenerative medicine advanced therapies (RMAT) and Breakthrough-eligible products, which could be licensed with modest sized pivotal clinical trials similar to Phase 2 studies, if substantial clinical benefit is demonstrated for high unmet medical needs in Phase 1/2 studies. III. If the firm has internal manufacturing capability, no investment is needed for a commercial plant. If not, then capacity must be found at a CMO or built. |
| Case B | I. Although experienced with the development of biological products, some firms may not be willing or able to invest at risk to ensure rapid development and licensure. Investment beyond that to enable Phase 1 and 2 studies will not occur, except for critical items, until clear achievement of clinical proof-of-concept. II. If the firm has internal manufacturing capability, no investment is needed for a commercial plant. If not, then capacity must be found at a CMO or built. |
| Case C | I. Some firms and other non-commercial organizations will not invest beyond the minimum level necessary to initiate Phase 1/2 studies until clinical proof of concept is achieved II. In many of these cases, the strategy is to sell the product or the organization to a pharmaceutical company that will then invest in late development. |
All three paths look similar in early development to enable first in human trials. But once Phase 1 supplies are made, the paths begin to diverge from each other and investigators are encouraged to understand product licensure requirements by this Phase 1 stage. Currently, an organization developing a cell therapy will typically follow one of the three paths outlined above. While all three paths can lead to successful licensure of a biological product, they each have different timelines and resource requirements. It is important to understand which path has been chosen so that expectations are consistent with the choice made. It is also possible to start on one path and switch to another path, as long as expectations and resources are consistent with the new chosen plan. Another important assumption is that all changes made to product, process, analytical methods, and site of manufacture development lead to products which are demonstrated to be comparable in terms of safety and efficacy.
In case A, CMC development work starts and continues on a commercializable manufacturing process and analytical tools to support late development studies. This CMC work continues in parallel with first in human studies, even though it may take some years to reach proof-of-concept from early clinical studies. If the product is eligible for RMAT or Breakthrough designation after Phase 1 studies, this is the only path which enables a pivotal Phase 2 study with a licensable process and analytical methods especially for rare diseases. This pathway typically takes 3–5 years from the start of first-in-human studies to BLA filing, even with adequate and timely investment in process and analytical development, and if needed, investment in a commercial manufacturing facility. The therapeutic indication dictates the number of patients that would be an acceptable representation for size of the safety data base for product licensure. Thus, rare and genetic diseases often include significantly smaller patient trials as compared to more prevalent disease indications involving cardiovascular, metabolic, or neurological targets. To reduce the risk of failure in this approach, attention must be paid to CMC design and development to ensure that even though CMC development parallels first-in-human studies, it is poised for a commercial phase development.
In case B, CMC optimization work on the process and analytical methods mostly stops after Phase 1 supplies are prepared, tested, and released. Since most investigational products fail to reach the marketplace, this approach presents the least risk and could be the most efficient use of limited resources. However, delay to market is inevitable since no work to create a commercializable process and analytical tools begins until clinical proof-of-concept is reached. Exceptions may be made when critical items are recognized as essential for product success which are relatively inexpensive to fund (e.g., improved cell line to meet commercial expectations, development of a functional/potency bioassay method, development of improved formulations to support product shelf life, stability and transport). These are typically long-lead time efforts which do not require many resources, but are critical to the success of the product and take several years to complete. These additional investments do not materially change the size of investment at risk, but can substantially reduce the delay in reaching the marketplace after clinical proof of concept is reached.
In case C, no additional effort occurs beyond that required to prepare Phase 1 supplies. This approach is efficient in terms of use of limited resources, but inevitably results in major delays to market if clinical proof-of-concept is demonstrated while necessary product development work is to be completed later. This approach is common among start-up firms with limited capital and projects done in academic settings with limited commercial development experience and understanding of types of studies required to support an approvable BLA. This approach is not consistent with a product which is RMAT or Breakthrough eligible, because the CMC part of the BLA will not be ready to support product commercialization if/when the clinical results are encouraging and positive.
All three cases can be further complicated when a delivery device is needed to directly apply the active ingredient to a sequestered target site in the body (instead of a simple parenteral injection at a superficial site). Devices have a development life of their own separate from the active ingredient, and in many jurisdictions, health authorities have a separate set of regulatory requirements for devices. In some jurisdictions like the USA, the device and the active ingredient will be regulated as a combination product with its own special rules and regulations. Use of delivery devices typically adds time, complexity, and investment cost to the development effort, especially when custom devices, not already on the market, are required. The developer will need to obtain clear advice from the regulator on how such devices impact the manufacturing process and the regulatory approval process; failure to include this in early assessments could have serious time and cost implications at a later stage. Some of these delays can be circumvented by re-purposing a previously approved device.
The above development concepts are illustrated in Table 2 below. Early development is defined as those activities conducted prior to obtaining clinical proof of concept. Late development is defined as those activities conducted after obtaining proof-of-concept. Proof-of-concept is the demonstration of substantial and desirable biological activity in humans, which if duplicated in an adequately designed pivotal study, is likely to be considered evidence of efficacy. Proof-of-concept may be observed at the conclusion of clinical phase 1/2 or 2, or at an intermediate look in some cases.
Table 2.
Resources investment patterns in late development/commercial activity of biologics product development.
| Case | Mfg. Processa | QC assaysb | Bioassay | Licensable Mfg. Plantc |
|---|---|---|---|---|
| A | Early | Early | Early & Late | Early & Late (if needed) |
| B | Critical Elements Only Early; All Late | Critical Elements Only Early; All Late | Critical Elements Only Early; All Late | Late (if needed) |
| C | Minimal early; Late criticald | Minimal Early; Late | Minimal Early; Late | Late |
Need to describe the key elements of a manufacturing process: raw materials, flow charts, process controls.
Need to spell out intent of QC assays: assays for purity, potency, identity, physical state.
Need to articulate what it takes to license a Mfg. plant: flow diagram, product, personnel, equipment waste & airflow, HVAC & computer system.
Focus on selection of appropriate raw materials, vectors and cell source to avoid critical problems at a later stage.
Fig. 1 illustrates a description of what the FDA expects firms to follow, courtesy of Dr. Steven Oh (OTAT, CBER, USFDA), that would be consistent with the path chosen for a given product development. All tests that determine a drug product safety are to be developed early and continued through-out development. That category includes product characterization and analytical development studies, while qualification and validation studies could follow, and a potency assay matching mechanism of action may be started early but not fully developed until later in the product development process.
Fig. 1.
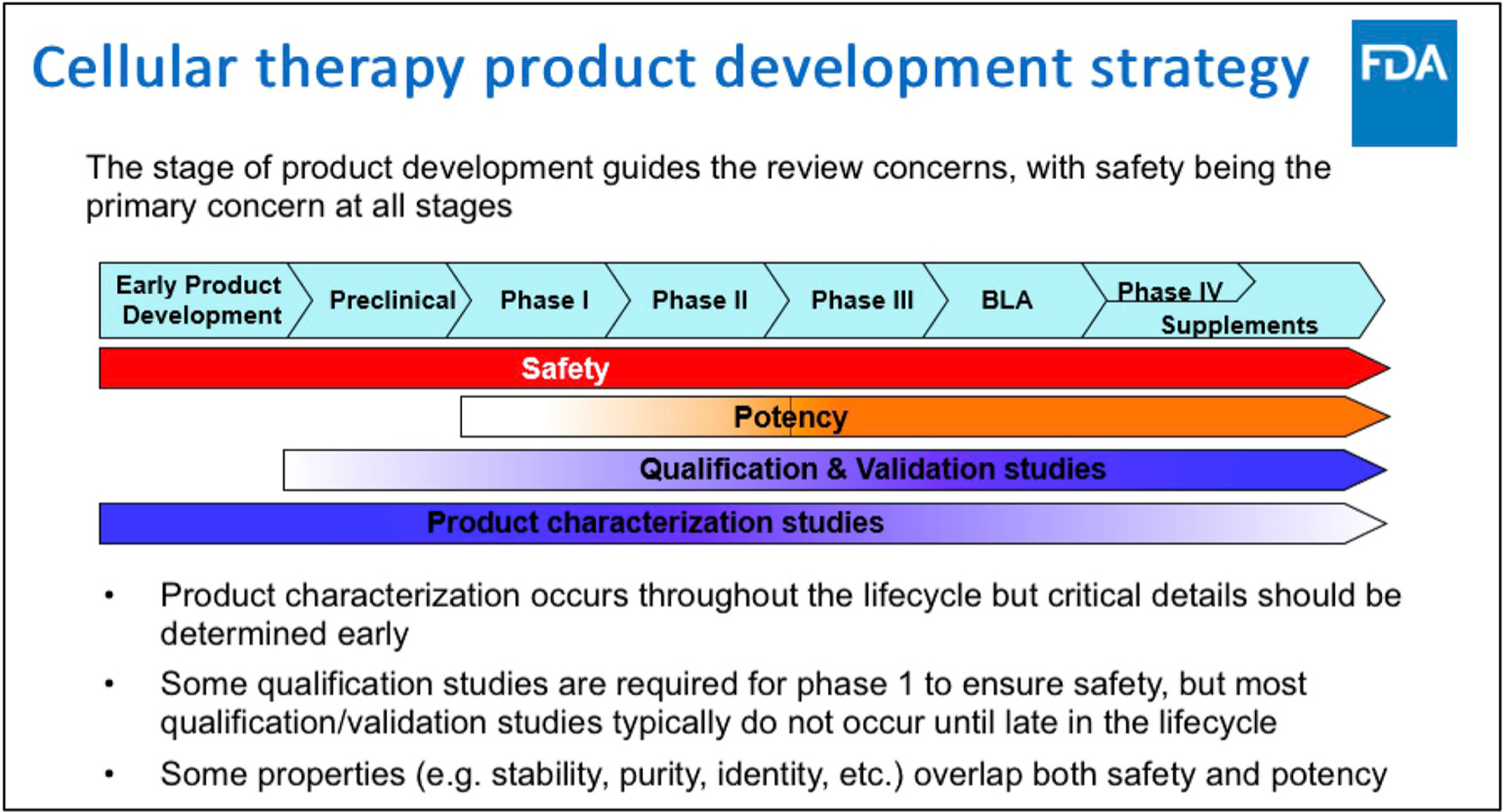
Clinical product Development. Courtesy of Dr. Steven Oh, Deputy Director, Division of cellular and gene therapies, OTAT, CBER, US FDA [3].
The conference also highlighted expedited development of a given regenerative medicine product currently means understanding licensure requirements early i.e. by phase 1. Hence, a summary of key activities under the most aggressive development path such as scenario A is included for all firms (see below).
| Scenario A. | ||
|---|---|---|
| Understand & Prioritize | Optimize Implementation | Finalize Testing & Enhance Surveillance |
| Starting material Source | Supply Chain | Global distribution |
| Proper Cell Characterization & Analytics | Impurities | Satellite centers for product testing |
| Scalability | Aseptic processing | Potency Assay |
| Raw materials controls | Final container | |
| Process consistency: yield & failure mode | Potency assay | |
| Definition of critical quality attributes relate to MOA | Sterility testing | |
| Potency Assay | Equipment Enhancement | |
| Cell preservation | ||
| Facility needs & cost | ||
| Stability, Transport & tracking | ||
| Release Assays | ||
| Tools and protocols for patient delivery (non-GMP) |
The current Health Authority experience with expedited pathways development, i.e. RMAT, breakthrough designation, for cell and gene therapy identified CMC issues as a key variable that appear to hinder the acceleration of the cell therapy products. The following are important topics that appear common: the need for clear definition of analytical methodologies and specifications, comparability assessment protocols as key in determining changes in the drug product without undermining quality and safety, potency assays related to stability and mechanism of action, raw materials sourcing and supply chain. Thus, earlier definition and control of these variables are needed to enable the product development acceleration as applicable to Case A above.
In conclusion, all paths for the development of a regenerative medicine cell therapy product include key quality standards that comply with cGMP, define product quality attributes as related to safety and efficacy as well as product specifications that defined limits for safety. The key is to have a firm knowledge of all the requirements and how best to manage them in relationship to resources availability, risk tolerance, and the desired timelines. Early and regular communication with the Health Authorities is highly recommended.
Acknowledgement
The authors thank the California Institute for Regenerative Medicine (CIRM) and the International Alliance for Biological Standardization (IABS) for the close cooperation in organizing the international conference and the resultant publications. In addition, sincere appreciation is extended to All the conference participants, as well as the National Institute of Health (NIH), the Food and Drug Administration (FDA), and the National Institute of Health Sciences, Japan.
Contributor Information
Abla A. Creasey, California Institute for Regenerative Medicine (CIRM), Oakland, CA, USA.
Glyn Stacey, International Alliance for Biological Standardization (IABS), Geneva, Switzerland.
Kapil Bharti, National Eye Institute, National Institute of Health, Bethesda, MD, USA.
Yoji Sato, National Institute of Health Sciences, Kawasaki, Japan.
Anthony Lubiniecki, International Alliance for Biological Standardization (IABS), Geneva, Switzerland.
References
- [1].Abbot S, Agbanyo F, Ahlfors J, Baghbaderami BA, Bartido S, et al. Report of the international conference on manufacturing and testing of pluripotent stem cell. Biologicals 2018;56:67–83. 2018 10.1016/j.biologicals.2018.08.004. [DOI] [PubMed] [Google Scholar]
- [2].Guidance for FDA Reviewers and Sponsors. Content and review of Chemistry, manufacturing, and control (CMC) information for human gene therapy investigational new drug applications (INDs). April 2008. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM078694.pdf.
- [3].Steven Oh. Cellular product manufacturing and comparability considerations IABS CIRM 4th cell therapy conference: manufacturing and testing of pluripotent stem cells, June 5, 2018, vol 56 Los Angeles: California Biologicals; 2018. p. 67–83 10.1016/j.biologicals.2018.08.004. [DOI] [Google Scholar]


