Abstract
Background
The coronavirus disease 2019 (COVID-19) has reportedly affected almost 23 million people, with more than 800 thousand deaths globally. There have been a few reports on the ocular manifestations of COVID-19 patients in China but no reports in Korea. The present study aimed to examine ocular manifestations of COVID-19 patients in Korea.
Methods
COVID-19 patients admitted from March 2020 to April 2020 at Keimyung University Dongsan Hospital and Keimyung University Daegu Dongsan Hospital were reviewed retrospectively for ocular manifestations. During the period of hospitalization, ocular symptoms as well as blood test results were noted and analyzed. Patients were then divided into the first-episode and relapsed group and ocular symptoms were analyzed in the groups.
Results
A total of 103 patients were included in this study. Among them, 71patients were in the first-episode group and 32 patients in the relapsed group. No significant differences were determined in terms of positivity of ocular symptoms between the first-episode group (12 patients, 16.9%) and the relapsed group (10 patients, 31.3%, P > 0.05). Symptoms of positive upper respiratory infection and lower creatine phosphokinase were determined to be related to positive ocular symptoms. Conjunctival congestion was noted in seven patients. In the subgroup analysis, the conjunctival congestion-positive patients exhibited higher positivity of upper respiratory infection symptoms (100%) as compared with those in the negative group (40%, P = 0.017).
Conclusion
Positive upper respiratory infection symptoms and lower creatine phosphokinase were determined to be related to ocular symptoms in COVID-19 patients. Among these patients, positive upper respiratory infection symptoms were associated with conjunctival congestion.
Keywords: Coronavirus, COVID-19, Conjunctival Congestion, Ocular Manifestations
Graphical Abstract
INTRODUCTION
The novel coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China, in December 2019, and it has been declared as a public health emergency of interest by the World Health Organization in January 2020. To date, COVID-19 has reportedly infected almost 23 million people, with more than 800 thousand deaths globally. However, ocular manifestations were not reported in the initial clinical reports.1,2,3 In China, Guan et al.4 have reported that 9 of the 1099 cases have conjunctival congestion; furthermore, Chen et al.5 demonstrated various ocular symptoms in COVID-19 patients such as conjunctival congestion, dry eyes, blurred vision, and foreign-body sensation. Wu et al.6 also reported conjunctivitis as an ocular finding in COVID-19 patients, demonstrating a positive correlation with higher white blood cell and neutrophil counts and higher levels of procalcitonin, C-reactive protein, and lactate dehydrogenase in Hubei, China. Similar to Hubei province, Daegu has also recorded a high number of COVID-19 cases in Korea. However, to the best of our knowledge, no study on the ocular manifestations of COVID-19 patients has been reported in the country.
Here, we evaluated the characteristics of ocular manifestations of patients with COVID-19 in Daegu.
METHODS
Study design and subjects
This study was designed as a retrospective, comparative case series conducted in two hospitals (Keimyung University Dongsan Hospital and Keimyung University Daegu Dongsan Hospital). The study adhered to the tenets of the Declaration of Helsinki. We retrospectively reviewed the electronic medical records of COVID-19 patients who were admitted from March to April 2020. Patients who were transferred from other hospitals were excluded. The patients were divided into two groups: the first-episode group and relapsed group. Differences in terms of laboratory results and the prevalence of ocular symptoms were examined by groups. Among the ocular symptom-positive patients, we also performed a subgroup analysis of conjunctival congestion-positive and -negative patients.
Collection of clinical data
Demographic characteristics, including age, gender, total duration of hospitalization, presence of hypertension, diabetes, cerebrovascular attack, and hyperlipidemia, were assessed. We also confirmed any positive upper respiratory infection (URI) symptoms (at least one of the following: runny nose, coughing, sore throat, and fever ≥ 38°C) and pneumonia at the time of COVID-19 diagnosis. Data on ocular symptoms (ocular discomfort, ocular pain, conjunctival congestion, visual disturbance, epiphora, and itching sensation) were obtained each day through an ophthalmologist via telephone and recorded in the electronic medical record. For patients with conjunctival congestion, a nurse took a photograph of the eye and forwarded it to the ophthalmologist for confirmation. Blood test results on the day of admission, including complete blood cell count, procalcitonin, C-reactive protein, creatine phosphokinase, lactate phosphokinase, lactate dehydrogenase, sodium, potassium, chloride, calcium, inorganic phosphorus, glucose, blood urea nitrogen, creatinine, total protein, albumin, total bilirubin, alkaline phosphatase, aspartate phosphatase, and alanine transaminase, were also included in this study.
Statistical analysis
Statistical analysis was performed using SPSS version 12.0 (IBM, Armonk, NY, USA). Between-group differences in age, total duration of hospitalization, and blood test results were compared using an independent t-test or Mann–Whitney U test. Categorical variables such as gender, positive URI symptoms or pneumonia,and the presence of systemic diseases such as diabetes, hypertension, and hyperlipidemia were compared using a chi-square test or Fisher exact test. P values of less than 0.05 were considered statistically significant.
Ethics statement
The protocol of the current study was reviewed and approved by the Institutional Review Board of Keimyung University Dongsan Hospital (approval No.2020-06-109). Because of the retrospective and noninvasive study design, the requirement for informed consent was waived by the board.
RESULTS
Demographics and clinical laboratory results of the first-episode and relapsed groups
A total of 103 patients were included in this study. Among them, 71 patients were in the first-episode group and 32 patients in the relapsed group. Demographics and laboratory results have been summarized in Table 1. The mean patient age was 49 ± 18 years in the first-episode group and 44 ± 16 years in the relapsed group, a difference that was deemed not statistically significant (P = 0.18). In total, 15 patients (21.1%) in the first-episode group and 8 patients (25.0%) in the relapsed group were male. The total hospitalization period was found to be significantly longer in the first-episode group than in the relapsed group (34 ± 18 vs. 13 ± 7 days, P < 0.01). The presence of pneumonia was significantly different between the two groups (first-episode group versus relapsed group: 49.3% vs. 21.9%, P < 0.01). There was also a significant difference in positive URI symptoms (first-episode group versus relapsed group: 46.5% vs. 15.6%, P < 0.01). There was no difference in systemic diseases such as hypertension, diabetes mellitus, and hyperlipidemia between the two groups (P > 0.05). Blood test results indicated significantly higher values of procalcitonin, neutrophil count, monocyte count, C-reactive protein, lactate dehydrogenase, alkaline phosphatase, aspartate transaminase, and alanine transaminase in the first-episode group compared to the relapsed group (P < 0.05). Other test results showed no differences between the two groups (P > 0.05) (Table 1).
Table 1. Demographics and laboratory results of patients with coronavirus disease 2019.
| Parameters | First-episode group (n = 71) | Relapsed group (n = 32) | P value |
|---|---|---|---|
| Age, yr | 49 ± 18 | 44 ± 16 | 0.18 |
| Total hospitalization period, day | 34 ± 18 | 13 ± 7 | < 0.01a |
| Sex, male | 15 (21.1) | 8 (25.0) | 0.66 |
| Pneumonia | 35 (49.3) | 7 (21.9) | 0.01a |
| URI symptoms | 33 (46.5) | 5 (15.6) | < 0.01a |
| Diabetes | 6 (8.5) | 3 (9.4) | 1.00 |
| Hypertension | 12 (16.9) | 4 (12.5) | 0.77 |
| Hyperlipidemia | 6 (8.5) | 0 (0.0) | 0.17 |
| Procalcitonin, ng/mL | 0.04 ± 0.03 | 0.03 ± 0.01 | < 0.01a |
| White blood cell count, /μL | 5,478 ± 1,631 | 4,853 ± 1,306 | 0.06 |
| Red blood cell count, 106/μL | 4.28 ± 4.88 | 4.29 ± 5.04 | 0.93 |
| Hemoglobin, g/dL | 12.67 ± 1.44 | 12.87 ± 1.41 | 0.51 |
| Hematocrit, % | 38.28 ± 3.76 | 38.93 ± 3.69 | 0.41 |
| Platelet count, 103/μL | 260.01 ± 96.02 | 227.59 ± 66.72 | 0.09 |
| Neutrophil count, /μL | 3,133 ± 1,137 | 2,621 ± 1,034 | 0.03a |
| Monocyte count, /μL | 478 ± 161 | 387 ± 144 | < 0.01a |
| Lymphocyte count, /μL | 1,744 ± 803 | 1,695 ± 489 | 0.70 |
| Eosinophil count, /μL | 101 ± 99 | 125 ± 93 | 0.24 |
| Basophil count, /μL | 23 ± 17 | 24 ± 14 | 0.64 |
| C-reactive protein, mg/dL | 1.3 ± 2.5 | 0.2 ± 0.4 | < 0.01a |
| Creatine phosphokinase, U/L | 98 ± 86 | 61 ± 21 | < 0.01a |
| Lactate dehydrogenase, U/L | 457 ± 135 | 353 ± 82 | < 0.01a |
| Sodium, mmol/L | 141 ± 2 | 141 ± 1 | 0.08 |
| Potassium, mmol/L | 4.2 ± 0.5 | 4.3 ± 0.4 | 0.25 |
| Chloride, mmol/L | 101 ± 3 | 102 ± 2 | 0.11 |
| Calcium, total, mg/dL | 9.1 ± 0.5 | 9.2 ± 0.4 | 0.08 |
| Inorganic phosphorus, mg/dL | 3.1 ± 0.6 | 3.2 ± 0.5 | 0.87 |
| Glucose, mg/dL | 119 ± 80 | 100 ± 28 | 0.09 |
| Blood urea nitrogen, mg/dL | 13 ± 4 | 13 ± 3 | 0.59 |
| Creatinine, mg/dL | 0.72 ± 0.19 | 0.69 ± 0.14 | 0.49 |
| Protein, total, g/dL | 7.0 ± 0.4 | 7.1 ± 0.5 | 0.13 |
| Albumin, g/dL | 4.2 ± 0.4 | 4.3 ± 0.3 | 0.28 |
| Bilirubin, total, mg/dL | 0.51 ± 0.55 | 0.45 ± 0.21 | 0.58 |
| Alkaline phosphatase, U/L | 81 ± 48 | 63 ± 16 | < 0.01a |
| Aspartate transaminase, U/L | 28 ± 20 | 19 ± 7 | < 0.01a |
| Alanine transaminase, U/L | 28 ± 22 | 20 ± 14 | 0.02a |
Data are presented as mean ± standard deviation or number (%).
aStatistically significant by independent two-sample t-test or Pearson χ2 test.
Ocular manifestations of COVID-19 patients in the first-episode and relapsed groups
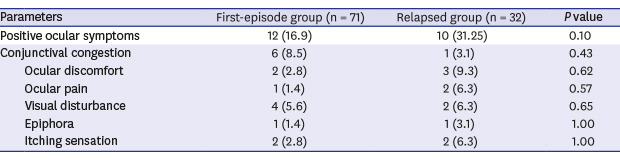
Table 2 compares ocular manifestations between the two groups. There were no significant differences in the positivity of ocular symptoms between the first-episode group (12 patients, 16.9%) and relapsed group (10 patients, 31.3%, P = 0.18). Conjunctival congestion was visible in six patients (8.5%) in the first-episode group and one patient (3.1%) in the relapsed group. Complaints of ocular discomfort were noted in two patients (2.8%) in the first-episode group and three patients (9.3%) in the relapsed group. Ocular pain was noted in one patient (1.4%) in the first-episode group and in two patients (6.3%) in the relapsed group. Visual disturbance was reported in four patients (5.6%) in the first-episode group and two patients (6.3%) in the relapsed group. Epiphora was shown in one patient (1.4%) in the first-episode group and one (3.1%) in the relapsed group. Two patients (2.8%) in the first-episode group and two (6.3%) in the relapsed group reported itching sensation. All of the above ocular symptoms were not statistically different between the two groups (P > 0.05). Seventeen patients were identified to have only one ocular symptom, while five patients exhibited two ocular symptoms. No patients were determined to have more than three ocular symptoms. Among the seven patients with conjunctival congestion, one patient had ocular discomfort, two patients had epiphora, and one patient had itching sensation simultaneously.
Table 2. Ocular manifestations of patients with coronavirus disease 2019.
| Parameters | First-episode group (n = 71) | Relapsed group (n = 32) | P value | |
|---|---|---|---|---|
| Positive ocular symptoms | 12 (16.9) | 10 (31.25) | 0.10 | |
| Conjunctival congestion | 6 (8.5) | 1 (3.1) | 0.43 | |
| Ocular discomfort | 2 (2.8) | 3 (9.3) | 0.62 | |
| Ocular pain | 1 (1.4) | 2 (6.3) | 0.57 | |
| Visual disturbance | 4 (5.6) | 2 (6.3) | 0.65 | |
| Epiphora | 1 (1.4) | 1 (3.1) | 1.00 | |
| Itching sensation | 2 (2.8) | 2 (6.3) | 1.00 | |
Data are presented as number (%).
Analysis of the factors associated with the positivity of ocular symptoms
Table 3 summarizes the demographics and laboratory results of the ocular symptom-positive group and -negative group. The occurrence of positive URI symptoms was significantly greater in the ocular symptom-positive group (13 patients, 59.1%) than in the ocular symptom-negative group (25 patients, 30.9%, P = 0.02). Creatine phosphokinase was also observed to be significantly lower in the ocular symptom-positive group (61 ± 33 vs. 93 ± 80 U/L, P = 0.02). Other factors such as age; total duration of hospitalization; gender; presence of pneumonia, diabetes, or hypertension; and other blood test results showed no significant difference between the ocular symptom-positive and -negative groups (P > 0.05).
Table 3. Demographics and laboratory results of ocular symptom-positive and -negative groups with coronavirus disease 2019.
| Parameters | Ocular symptom-positive group (n = 22) | Ocular symptom-negative group (n = 81) | P value |
|---|---|---|---|
| Age, yr | 53 ± 12 | 46 ± 18 | 0.20 |
| Total hospitalization period, day | 30 ± 16 | 27 ± 19 | 0.50 |
| Sex, male | 3 (13.6) | 20 (24.7) | 0.39 |
| Pneumonia | 10 (45.5) | 32 (39.5) | 0.62 |
| URI symptoms | 13 (59.1) | 25 (30.9) | 0.02* |
| Diabetes | 2 (9.1) | 7 (8.6) | 1.00 |
| Hypertension | 4 (18.2) | 12 (14.8) | 0.74 |
| Hyperlipidemia | 1 (4.5) | 5 (6.2) | 1.00 |
| Procalcitonin, ng/mL | 0.03 ± 0.02 | 0.03 ± 0.01 | 0.11 |
| White blood cell count, /μL | 5,208 ± 1,372 | 5,305 ± 1,612 | 0.79 |
| Red blood cell count, 106/μL | 4.31 ± 0.40 | 4.28 ± 0.51 | 0.67 |
| Hemoglobin, g/dL | 12.63 ± 1.08 | 12.76 ± 1.51 | 0.79 |
| Hematocrit, % | 38.48 ± 2.79 | 38.48 ± 3.96 | 0.99 |
| Platelet count, 103/μL | 229.00 ± 71.28 | 255.63 ± 92.71 | 0.18 |
| Neutrophil count, /μL | 2,904 ± 961 | 2,993 ± 1,172 | 0.66 |
| Monocyte count, /μL | 434 ± 189 | 454 ± 153 | 0.18 |
| Lymphocyte count, /μL | 1,718 ± 596 | 1,732 ± 751 | 0.74 |
| Eosinophil count, /μL | 129 ± 102 | 103 ± 95 | 0.16 |
| Basophil count, /μL | 22 ± 12 | 23 ± 17 | 0.79 |
| C-reactive protein, mg/dL | 0.57 ± 1.00 | 1.04 ± 2.38 | 0.77 |
| Creatine phosphokinase, U/L | 61 ± 33 | 93 ± 80 | 0.02a |
| Lactate dehydrogenase, U/L | 387 ± 93 | 435 ± 137 | 0.14 |
| Sodium, mmol/L | 142 ± 2 | 141 ± 2 | 0.05 |
| Potassium, mmol/L | 4.3 ± 0.3 | 4.2 ± 0.4 | 0.16 |
| Chloride, mmol/L | 102 ± 2 | 101 ± 3 | 0.15 |
| Calcium, total, mg/dL | 9.2 ± 0.4 | 9.1 ± 0.5 | 0.29 |
| Inorganic phosphorus, mg/dL | 3.1 ± 0.5 | 3.2 ± 0.6 | 0.82 |
| Glucose, mg/dL | 101 ± 22 | 116 ± 76 | 0.57 |
| Blood urea nitrogen, mg/dL | 13 ± 3 | 13 ± 4 | 0.32 |
| Creatinine, mg/dL | 0.67 ± 0.14 | 0.72 ± 0.18 | 0.27 |
| Protein, total, g/dL | 7.0 ± 0.4 | 7.0 ± 0.4 | 0.80 |
| Albumin, g/dL | 4.3 ± 0.3 | 4.3 ± 0.4 | 0.86 |
| Bilirubin, total, mg/dL | 0.40 ± 0.18 | 0.51 ± 0.52 | 0.32 |
| Alkaline phosphatase, U/L | 70 ± 20 | 77 ± 45 | 0.86 |
| Aspartate transaminase, U/L | 20 ± 7 | 26 ± 19 | 0.05 |
| Alanine transaminase, U/L | 21 ± 9 | 27 ± 19 | 0.58 |
Data are presented as mean ± standard deviation or number (%).
aStatistically significant by Mann–Whitney U test, Pearson χ2 test.
Subgroup analysis based on the presence of conjunctival congestion
Table 4 presents the demographics and laboratory results of the conjunctival congestion-positive and -negative groups. A higher rate of positive URI symptoms was observed in the conjunctival congestion-positive group (7 patients, 100%) than in the conjunctival congestion-negative group (6 patients, 40%, P = 0.02). Other factors such as age; total duration of hospitalization; symptom onset from diagnosis; gender; presence of pneumonia, diabetes, or hypertension; and other blood test results showed no significant difference between the conjunctival congestion-positive and -negative groups (P > 0.05).
Table 4. Demographics and laboratory results between conjunctival congestion-positive and -negative patients with ocular symptom-positive coronavirus disease 2019.
| Parameters | Conjunctival congestion-positive (n = 7) | Conjunctival congestion-negative (n = 15) | P value |
|---|---|---|---|
| Age, yr | 57 ± 6 | 51 ± 14 | 0.41 |
| Total hospitalization period, day | 38 ± 10 | 26 ± 18 | 0.11 |
| Time to symptom onset from diagnosis, day | 9 ± 15 | 4 ± 3 | 0.63 |
| Sex, male | 1 (14.3) | 2 (13.3) | 1.00 |
| Pneumonia | 5 (71.4) | 5 (33.3) | 0.17 |
| URI symptoms | 7 (100.0) | 6 (40.0) | 0.02a |
| Diabetes | 1 (14.3) | 1 (6.7) | 1.00 |
| Hypertension | 0 (0.0) | 4 (26.7) | 0.26 |
| Hyperlipidemia | 1 (14.3) | 0 (0.0) | 0.32 |
| Procalcitonin, ng/mL | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.07 |
| White blood cell count, /μL | 4,929 ± 1,137 | 4,987 ± 1,487 | 0.68 |
| Red blood cell count, 106/μL | 4.32 ± 0.42 | 4.30 ± 0.41 | 0.84 |
| Hemoglobin, g/dL | 12.69 ± 0.97 | 12.61 ± 1.16 | 0.95 |
| Hematocrit, % | 38.53 ± 2.56 | 38.45 ± 2.98 | 0.95 |
| Platelet count, 103/μL | 213.58 ± 41.06 | 236.20 ± 82.00 | 0.95 |
| Neutrophil count, /μL | 2,675 ± 780 | 3,011 ± 1,042 | 0.63 |
| Monocyte count, /μL | 428 ± 136 | 437 ± 213 | 0.95 |
| Lymphocyte count, /μL | 1,725 ± 751 | 1,715 ± 540 | 0.84 |
| Eosinophil count, /μL | 82 ± 56 | 152 ± 113 | 0.14 |
| Basophil count, /μL | 19 ± 11 | 24 ± 13 | 0.33 |
| C-reactive protein, mg/dL | 1.23 ± 1.54 | 0.26 ± 0.38 | 0.08 |
| Creatine phosphokinase, U/L | 51 ± 17 | 66 ± 38 | 0.27 |
| Lactate dehydrogenase, U/L | 439 ± 115 | 363 ± 73 | 0.16 |
| Sodium, mmol/L | 142 ± 2 | 141 ± 2 | 0.45 |
| Potassium, mmol/L | 4.3 ± 0.2 | 4.3 ± 0.4 | 0.95 |
| Chloride, mmol/L | 102 ± 3 | 102 ± 2 | 0.30 |
| Calcium, total, mg/dL | 9.0 ± 0.4 | 9.3 ± 0.4 | 0.24 |
| Inorganic phosphorus, mg/dL | 3.0 ± 0.4 | 3.1 ± 0.5 | 0.33 |
| Glucose, mg/dL | 97 ± 16 | 103 ± 25 | 0.84 |
| Blood urea nitrogen, mg/dL | 14 ± 3 | 13 ± 2 | 0.19 |
| Creatinine, mg/dL | 0.66 ± 0.15 | 0.68 ± 0.13 | 0.94 |
| Protein, total, g/dL | 6.9 ± 0.4 | 7.1 ± 0.4 | 0.21 |
| Albumin, g/dL | 4.1 ± 0.2 | 4.3 ± 0.3 | 0.12 |
| Bilirubin, total, mg/dL | 0.39 ± 0.23 | 0.41 ± 0.15 | 0.37 |
| Alkaline phosphatase, U/L | 78 ± 27 | 66 ± 15 | 0.33 |
| Aspartate transaminase, U/L | 21 ± 4 | 19 ± 9 | 0.16 |
| Alanine transaminase, U/L | 22 ± 10 | 20 ± 9 | 0.54 |
Data are presented as mean ± standard deviation or number (%).
aStatistically significant by Fisher exact test.
DISCUSSION
There are already a few studies reporting on the ocular symptoms or signs of COVID-19; however, most of these studies have been performed in China.5,6 The present study retrospectively analyzed the ocular manifestations of COVID-19 patients in Korea. The comparison between the first-episode group and relapsed group showed longer total hospitalization period, higher incidence of pneumonia, higher incidence of URI symptoms, and higher levels of procalcitonin, neutrophil count, C-reactive protein, creatinine phosphokinase, lactate dehydrogenase, alkaline phosphatase, aspartate transaminase, and alanine transaminase. It has already been determined that elevated levels of procalcitonin, aspartate transaminase, and lactate dehydrogenase are related to the severity of COVID-19.7,8 Several studies have reported that a positive COVID-19 test result in discharged patients is due to the prolonged viral shedding that is close to the limit of detection.9,10 Based on these studies, the relapsed group in the present research might have resulted from the low viral shedding that is barely detected and the blood test results that show low disease severity.
The commonly reported ocular manifestations of COVID-19 are itching, red eye, tearing, foreign body sensation, and chemosis.11 These symptoms and signs were also included in the present study. One previous study reported that 31.6% of COVID-19 patients showed that ocular abnormalities and higher procalcitonin level, white blood cell count, neutrophil count, C-reactive protein level, and lactate dehydrogenase level have a correlation to ocular symptoms.6 In our study, 31.25% of the relapsed group and 16.9% of the first-episode group were determined to have ocular symptoms. The factors associated with the ocular symptoms were positive URI symptoms and creatine phosphokinase. Possible explanations for these discrepancies include the fact that the relationship between ocular symptoms and blood test results is not yet fully understood and that the mental status of COVID-19 patients might have affected their subjective reports of ocular symptoms. Kitazawa et al.12 reported that anxiety and depression were associated with the subjective symptoms of dry eye disease. There have been no studies regarding depression and anxiety in relapsed COVID-19 patients; however, it is estimated that patients who have been hospitalized and recovered from COVID-19 will manifest persistent psychiatric disorders such as anxiety and depression.13,14 Relapsed COVID-19 patients might be more vulnerable to these psychiatric disorders as they are being requarantined, resulting in a higher prevalence of subjective ocular symptoms. Further studies are required to confirm the correlation between the subjective ocular symptoms and psychiatric health status in patients with COVID-19.
Subgroup analysis revealed that the conjunctival congestion-positive group had a significantly greater incidence of positive URI symptoms. It was determined that patients with viral conjunctivitis typically have had recent contact with a sick person or a recent history of URI.15 We did not perform a slit-lamp examination in this study; therefore, other causes of conjunctival congestion could not be ruled out. However, considering that no patient experienced ocular pain, it is more likely that viral conjunctivitis was the cause of conjunctival congestion. Colavita et al.16 reported a case of bilateral conjunctivitis with URI symptoms in a female patient with COVID-19 in Italy. One previous study demonstrated one positive COVID-19 conjunctival swab among three conjunctival hyperemia patients.6 Conjunctival swab was not performed in this study; thus, further research performing conjunctival swab in a large number of conjunctival congestion-positive COVID-19 patients is needed.
The present study has some limitations. First, the sample size is small, and no healthy population was included in this study as a control. Second, a comprehensive ophthalmologic evaluation was not performed because COVID-19 patients were isolated. Regardless, these results can be valuable to ophthalmologists worldwide in an effort to evaluate ocular manifestations in COVID-19.
In conclusion, positive URI symptoms and decreased creatine phosphokinase levels were determined to be associated with ocular symptoms in COVID-19 patients. Among these patients, positive URI symptoms are associated with conjunctival congestion.
Footnotes
Funding: This work was supported by the Research Program of Medicity Daegu Council funded by Daegu Metropolitan City (fund code: COVID19_DM12).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee YH.
- Data curation: Lee YH.
- Formal analysis: Lee YH.
- Methodology: Lee YH, Kim YC.
- Writing - original draft: Lee YH.
- Writing - review & editing: Lee YH. Shin JP, Kim YC.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Deng C, Chen X, Zhang X, Chen B, Yu H, et al. Ocular manifestations and clinical characteristics of 534 cases of COVID-19 in China: a cross-sectional study. MedRxiv. 2020 doi: 10.1101/2020.03.12.20034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24(6):3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 8.Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020;56(2):106051. doi: 10.1016/j.ijantimicag.2020.106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan J, Kou S, Liang Y, Zeng J, Pan Y, Liu L. PCR assays turned positive in 25 discharged COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang X, Zhao S, He D, Yang L, Wang MH, Li Y, et al. Positive RT-PCR tests among discharged COVID-19 patients in Shenzhen, China. Infect Control Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Duan C, Zeng Y, Tong Y, Nie Y, Yang Y, et al. Ocular Findings and Proportion with Conjunctival SARS-COV-2 in COVID-19 Patients. Ophthalmology. 2020;127(7):982–983. doi: 10.1016/j.ophtha.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitazawa M, Sakamoto C, Yoshimura M, Kawashima M, Inoue S, Mimura M, et al. The relationship of dry eye disease with depression and anxiety: a naturalistic observational study. Transl Vis Sci Technol. 2018;7(6):35. doi: 10.1167/tvst.7.6.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817–818. doi: 10.1001/jamainternmed.2020.1562. [DOI] [PubMed] [Google Scholar]
- 14.Holmes EA, O'Connor RC, Perry VH, Tracey I, Wessely S, Arseneault L, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7(6):547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solano D, Virgile J, Czyz CN. Viral Conjunctivitis. Treasure Island (FL): Stat Pearls Publishing; 2020. [Google Scholar]
- 16.Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med. 2020;173(3):242–243. doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]


