Abstract
Colorectal cancer (CRC) is one of the most widely recognized and deadly malignancies worldwide. Although death rates have declined over the previous decade, mainly because of enhanced screening or potential treatment alternatives, CRC remains the third leading cause of cancer-related mortality globally, with an estimated incidence of over 1 million new cases and approximately 600 000 deaths estimated yearly. Therefore, many scientific efforts are put into the development of new diagnostic biomarkers for CRC. MicroRNAs (miRNAs), one of the epigenetics categories, have demonstrated significant roles in carcinogenesis and progression through regulating epithelial-mesenchymal transition (EMT), oncogenic signaling pathways, and metastasis. Dysregulation of miRNAs expression has been reported in many cancers, including CRC. The expression profile of miRNAs is reproducibly altered in CRC, and their expression patterns are associated with diagnosis, prognosis, and therapeutic outcomes in CRC. Recently, many studies were conducted on the dysregulation of miRNAs as a diagnostic and prognostic biomarker in CRC. Among them, some miRNAs, which include miR-21, miR-34 family, miR-155, miR-224, and miR-378, have been more studied in CRC with more prominent roles in diagnosis, prognosis, and therapy. In the present review, we summarized the latest information regarding the dysregulated miRNAs in CRC and the advantages of using miRNAs as a biomarker for CRC diagnosis, treatment, and their function in different signaling pathways involved in CRC progression. Moreover, we described the translation of miRNA research to potential therapeutic applications in the management of CRC in clinical settings.
Keywords: microRNA, Biomarker, Colorectal cancer, Diagnosis and prognosis, Signaling pathways
1. Introduction
Colorectal cancer (CRC) is the third most frequently diagnosed cancer worldwide, with an annual incidence of 1.4 million new cases and 694 000 deaths [1]. About 15% of CRCs are diagnosed in metastatic stages (stage IV), with an average survival rate of 2.5 years. In the last decade, CRC incidence rates increased by 22%, and CRC death rates increased by 13% among adults aged less than 50 years in the USA [2]. However, the precise aetiologic factors of these onset cases have yet to be elucidated. According to recent studies, CRC develops from precancerous lesions; thus, early diagnosis can reduce incidence and mortality. Also, finding a potential diagnostic and prognostic biomarkers will help us assess tumor initiation, progression, and response to treatment [3,4].
MicroRNAs (miRNAs) are a family of endogenous, small nonprotein coding RNA molecules that conduct their suppressive functions by direct binding to the 3′‐untranslated regions (3′-UTR) of target mRNAs [5]. Approximately two-thirds of the protein-coding genes are known to be regulated by miRNAs [5]. MiRNAs participate in various biological functions such as apoptosis, cell development, and differentiation [6,7]. Because of their central role in tumorigenesis regulation, miRNAs have attracted a great deal of interest as potential therapeutic targets or disease biomarkers [8,9]. Dysregulated expression of miRNAs in human tumors is shown in several studies. In oncology, miRNAs are classified as oncogenes or tumor suppressors, depending on the function of their target genes [6]. Dysregulation of miRNA expression is related to the promotion of tumor mass growth, metastasis, increased malignancy of tumor cells. Moreover, the association between miRNAs expression and the risk of recurrence and response to the therapeutic regimen has been uncovered.
Therefore, miRNA profiling may be a novel tool for the diagnosis and prognosis of many types of tumors, including CRC. In this review, we summarized the differential expressions of miRNAs and their functions in CRC, and miRNA roles in CRC diagnosis, prognosis, and treatment.
2. Molecular pathogenesis of CRC
The suppressor pathway or pathway of chromosomal instability (CIN) was first proposed as the colorectal carcinogenesis [10]. The accumulation of mutations leads to oncogene activation such as Kirsten rat sarcoma (KRAS) and inactivation of TS genes such as Deleted in Colorectal Cancer (DCC), Total Protein-53 (TP-53), SMAD family member 4, Mothers against decapentaplegic homolog 4 (SMAD4), and Adenomatous polyposis coli (APC) [11]. Mutations in the genes MSH2, MSH3, MSH6, Exo1, PMS1, PSM2, MLH1, and MLH3 responsible for DNA repair during replication are associated with the second mechanism of colorectal carcinogenesis. Accumulation of errors in repetitive DNA fragments causes mutations in target genes [12]. The last pathway of aberrant hypermethylation was identified as a mechanism of gene function silencing in epigenetics [13]. Examples of these genes are the calcium voltage-gated channel subunit a1G (CACNA1G), the protein-coding gene, suppressor of cytokine signaling-1 (SOCS1), Runt-related transcription factor-3 (RUNX3), the induction of neuronal differentiation by the overexpression of NEUROG-1, and finally the insulin-like growth factor 2 (IGF2) [14].
3. A brief overview of microRNA
MiRNA represents the most studied non-coding RNAs, responsible for negative modulating of up to 60% of protein-coding gene expression [15]. Shortly, the biogenesis of miRNA starts in the nucleus, with the transcription of a long hairpin transcript (pri-miRNA) of hundreds or thousands of nucleotides. Further, by an enzymatic process coordinated by RNA polymerase III Drosha and DiGeorge syndrome critical region 8 (DGCR8), pri-miRNA is reduced to a smaller transcript of about 70 nucleotides, called pre-miRNA. After it is exported in the cytoplasm by nuclear receptor exportin, pre-miRNA is firstly reduced by Dicer complex to a mature miRNA duplex of about 22 nucleotides lengths and then to a single-stranded mature miRNA. Further, mature miRNA-loaded AGO2 and RNA-induced silencing complex (RISC) will function as a guide to target specific mRNA transcripts by sequence complementarity, usually in the 3′-UTR, leading to translational repression or mRNA degradation.
4. Aberrant miRNAs expression in CRC initiation and progression
4.1. miRNA expression on CRC proliferation
Aberrant expression of miRNAs and their roles in various biological processes have been observed to be associated with colorectal carcinogenesis. There is overwhelming evidence supporting a mechanistic role of miRNAs in various processes inside the cell, such as metastasis, cell proliferation, and apoptosis, which are considered a hallmark of CRC. During the last decade, scientific studies have been investigated the functional role of aberrant miRNAs expression profile in CRC proliferation.
MiR-143 with a tumor suppressive activity was identified in proliferation, and its expression is substantially reduced in CRC. Also, down-regulation of miR-143 can frequently precede APC gene mutations; thus, down-regulation of this miRNA is essential for the proliferation of CRC. Up-regulation of miR-21 targeted many genes, such as PDCD4, PTEN, transforming growth factor-beta receptor II (TGF-βR2), and cell division cycle 25A (CDC25A), that are involved in controlling CRC cell proliferation. Moreover, a recent study indicated that overexpression of miR-21 elevated cell proliferation and inhibited apoptosis against the treatment of chemotherapeutic agent Fluorouracil (5-FU) in an HT29 CRC cell [16,17]. Additionally, the silencing of miR-21 suppressed cell proliferation and restored the sensitivity of chemotherapy in HT-29 CRC cells.
MiR-155 by binding to the 3′-UTR of protein tyrosine phosphatase, receptor type J (PTPRJ) mRNA suppresses the expression of PTPRJ through miR-155/PTPRJ/AKT axis could affect the proliferation of CRC cells. MiR-143 and miR-145 can regulate cell growth and proliferation in vitro by targeting different oncogenic protein-coding genes [18,19]. MiR-143 by directly repressing the translation of KRAS [20] and DNMT3A [21] functions to suppress cell growth and proliferation [19]. MiR-148 induces cell proliferation and cell cycle progression in CRC by suppressing p55PIK. Moreover, the overexpression of miR-451 in CRC leads to decreased cell proliferation by targeting the oncogene macrophage migration factor (MIF) [22]. MiR-31 has been shown to increase CRC proliferation and tumorigenesis by directly binding to the 3′-UTR of RAS p21 GTPase activating protein 1 (RASA1) transcripts. Additionally, a recent study has shown that miR-29b suppress proliferation and induce apoptosis in CRC cells and mediate the inhibition of epithelial-mesenchymal transition (EMT) [23]. An overview of the studies investigating the role of miRNAs associated with CRC development is depicted in Table 1.
Table 1.
miRNAs involved in CRC development.
| miRNAs | Targets | Functions | References |
|---|---|---|---|
| miR-21 | PDCD4 | Invasion and metastasis promotion | [45] |
| miR-194 | MAP4K4, AKT2 | Proliferation, apoptosis, invasion, migration, cell cycle | [72,73] |
| miR-497 | VEGFA | Inhibition of invasion and metastasis | [74] |
| miR-148b | CCK2R | Induction of cell proliferation | [75] |
| miR-409-3p | GAB1 | Inhibition of tumor progression and metastasis | [76] |
| miR-92a | KLF4 | Promotion of cell growth and migration | [77] |
| miR-100 | RAP1B | Cell proliferation, invasion, apoptosis | [78] |
| miR-34a | E2F1, SIRT1, FMNL2, E2F5, SNHG7 | Proliferation, invasiveness, metastasis, apoptosis,chemo-resistance | [[79], [80], [81]] |
| miR-638 | SOX2 | Cell invasion, migration, EMT | [82] |
| miR-320 | FOXO4 and PDCD4 | Inhibition of cell proliferation | [83] |
| miR-126 | PI3K, VCAM-1, CXCR4, VEGFA,IRS1, RhoA | Proliferation, invasion, migration, cell cycle, angiogenesis, hematopoiesis | [84,85] |
| Let-7c | KRAS, MMP11 and PBX3 | Metastasis induction | [86] |
| miR-503 | calcium-sensing receptor | Induction of proliferation migration and invasion | [87] |
| miR-206 | NOTCH3 | Cell proliferation, migration, apoptosis, cell cycle arrest | [88] |
| miR-375 | Bcl-2 | Inhibition of tumor progression | [89] |
| miR-18a | K-Ras | Cell proliferation, anchorage-independent growth | [90] |
| miR-133a | FSCN1,LASP1 | Cell proliferation, invasion, migration, tumor growth, intrahepatic and pulmonary metastasis, phosphorylation of ERK/MEK | [91,92] |
| miR-1246 | CCNG2 | Induction of cell growth and metastasis | [93] |
| MiR-330 | CDC42 | Proliferation | [94] |
| miR-320a | β-catenin,Rac1 | Cell proliferation, migration, invasion, cell cycle arrest | [95] |
| miR-181b | RASSF1A | Proliferation and enhance cell survival | [96] |
| miR-124 | STAT3 | Cell proliferation, apoptosis, tumor growth, differentiation, prognosis | [97] |
| miR-144 | GSPT1 | Inhibition of proliferation and migration | [98] |
| miR-145 | Fascin-1 | Cell proliferation, invasion, tumor growth, pulmonary metastasis | [99] |
| miR-218 | BMI-1 | Cell proliferation, apoptosis, cell cycle arrest | [100] |
| miR-99b-5p | mTOR | Inhibition of metastasis formation | [101] |
| miR-429 | Onecut2 | Cell migration, invasion, EMT | [102] |
| miR-139 | IGF-IR, NOTCH1 |
Cell proliferation, migration, invasion, apoptosis, tumor growth, cell cycle arrest | [103] |
4.2. miRNAs affect the invasion in CRC
During CRC development, neoplastic cells may acquire the ability to invade or spread to distant organs through complex processes, including directional activation of proteolytic enzymes, EMT and translocation of cancer cells. During EMT, cancer cells undergo several processes that modify their phenotype, leading to cell motility, the acquisition of stemness properties, inhibition of apoptosis, and immunosuppression.
One of the critical miRNAs involved in the regulation of EMT-MET plasticity in CRC is miR-200. The overexpression of this miRNA in CRC cells contributed to MET through increased E-cadherin and reduced vimentin expression. miR-34a is another miRNA involve in EMT through snail1 as a target gene, which leads to induce EMT in CRC. In another relevant study, the expression levels of miR-155 promoting CRC cell invasion by regulating claudin-1 expression can act as a mediator of EMT. Moreover, miR-301 involves the regulation of invasion by targeting the downstream gene TGF-βR2 or NF-κB/STAT3 to promote tumorigenesis. MiR-29 family (miR-29a, miR-29b, and miR-29c) by regulating EMT are involved in the tumor progression. Specifically, the down-regulation of miR-29c has a vital role in CRC cell invasion via suppressing EMT in vitro. Also, miR-29b suppresses EMT and plays a vital role in cell invasion via negatively regulating the MAPK/ERK and PI3K/AKT pathways. Moreover, miR-126 within the 7th intron of epidermal growth factor-like domain 7 (EGFL7) could contribute to the progression of invasion and cell survival in CRC via inactivation of the oncogene signaling pathway.
4.3. miRNAs involve in metastasis
Metastasis is the final step during CRC progression. Recent studies implicate the components of miRNA-regulating networks in EMT with traits associated with metastasis formation in CRC. According to the recent evidence, miR-34a suppresses metastasis in CRC through EMT-regulating network in SNAIL/ZNF81 and IL6R/STAT3. MiR-20-5p and mir-224 can induce EMT and metastasis of CRC cells by negative regulation of SMAD4 as a mediator of the TGF-β pathway. Also, miR-34a inhibited CRC cell metastasis through the down-regulation of formin-like 2 (FMNL2) and E2F transcription factor 5 (E2F5) expressions. Additionally, miR-200c has a critical role in the regulation of EMT and metastatic behavior in CRC via the negative regulation of the target genes such as ETS proto-oncogene 1, transcription factor (ETS1), fms related tyrosine kinase 1 (FLT1) and zinc finger E-box binding homeobox 1 (ZEB1), which, in turn, regulates the EMT markers (E-cadherin and vimentin). Moreover, miR-224 can induce CRC tumor growth and metastasis by targeting SMAD4 [24]. Additionally, decrease the expression of miR-335, miR-132, and miR-192 can induce CRC metastasis via increasing expression of the ZEB2 target gene. Moreover, miR-126 inhibited the expression of vascular cell adhesion molecule-1 (VCAM-1), which led to metastasis in CRC. Another example is given by miR-200, whose increasing serum levels are significantly associated with CRC progression and metastasis.
5. miRNA involved in signaling pathways related to CRC
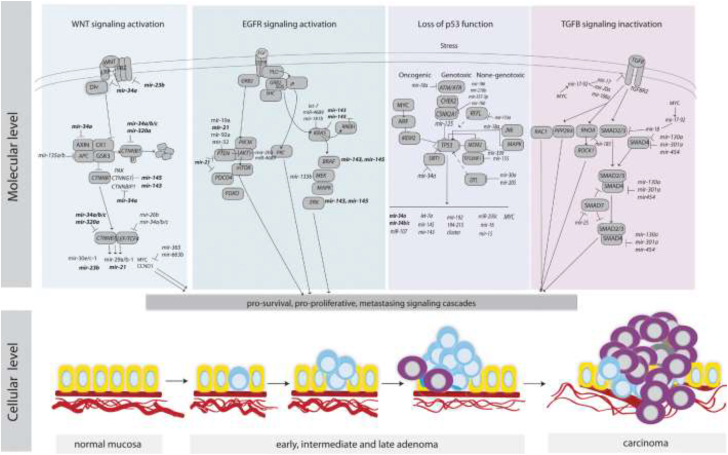
One of the significant causes of CRC is the activation of driven genes in the oncogenic signaling pathways, such as TGF-β, Wnt, inflammatory signaling pathways, and Ras (Fig. 1). Also, these signaling pathways are regulated by miRNAs.
Fig. 1.
An overview of key signaling pathways in CRC and the regulation of their components by miRNAs.
Recent studies indicated that miR-135a/b could regulate the Wnt signaling pathway by targeting APC the critical elements of the Wnt pathway, which leads to the repression of APC expression and induces of Wnt signaling pathway [25]. Also, the miR-34 family (miR-34a/b/c) can directly target Wnt ligands that interact with β-catenin [26], which leads to Wnt signaling repression [26].
Recent studies have reported that aberrant activation of the oncogenic EGFR pathway may occur due to TS-miRNAs loss of function. MiR-143 and miR-145 are the two most important TS in the EGFR pathway that decreases proliferation and migration by targeting KRAS and BRAF [27]. Most recent studies indicated that the crosstalk between the TGF-β signaling pathway and some miRNAs [28,29]. Several studies have shown that miR-20a, promotes CRC progression by facilitating CRC cell line migration, invasion and upregulating the expression of EMT markers, and further enhances the ability of TGF-β to drive cancer cell migration, invasion, and metastasis [30,31]. Similarly, miR-106 a/b also increases EMT and metastasis by targeting TGF-β receptor TGFBR2. MiR-20-5p and miR-224 by negative regulation of SMAD4 induce EMT, invasion, and metastasis of CRC cells [32,33].
One of the critical drivers of CRC is inflammatory signaling pathways, and miR-21 as a most recognized oncogene appears to be a key modulator of several pro-oncogenic and immunomodulatory factors, such as NF-κB, and MyD88, an adapter of Toll-like receptors (TLRs) needed for NF-κB activation by TLR ligands [[34], [35], [36]]. Similarly, miR-221 and miR-222 can activate NF-κB and STAT3 by indirectly modulating their protein stability through miR-221/222-mediated positive feedback loops to elevate the expression of STAT3 and RelA [37]. Therefore, the miR-21 family acts as a key modulator in inflammatory signaling pathways in which these miRNAs maintain a positive loop with the modulation factors PDCD4, NF-κB, and STAT3. More data about miRNAs involved in the signaling pathways in CRC developing and progression are presented in Table 2.
Table 2.
The target genes of the dysregulated miRNAs and key signaling pathways contributing to CRC development.
| miRNAs | Targets | Signaling Pathways | Oncogenic roles | Reference |
|---|---|---|---|---|
| miR-10b | PIK3CA, TGF-β, SM α-actin; TWIST-1, E-cadherin; KLF4; HOXD10, RhoC | PI3K/Akt/mTOR pathway, TGF-s signaling pathway | Tumorigenesis and metastasis; proliferation; vascular invasion, tumor differentiation and metastasis | [104,105] |
| miR-638 | SOX2, TSPAN1 | Suppressing of TP53 function | EMT, invasion, migration, proliferation | [82,106] |
| miR-574-5p | Qki 6/7 | Wnt/β-catenin pathway | Proliferation, tumorigenesis differentiation, angiogenesis | [107] |
| miR-21 | TGFBR2, CTNNB1, PIK3CA, ZFHX3, BRAF, SFRP1, ITGb4, PDCD4,PTEN, TIAM1, TIPM3, SPRY2, RECK, and Sec23A | Activation of Wnt/s-catenin pathway; TGF-s signaling pathway | Tumor progression, proliferation, EMT, metastasis; invasion, inhibition of apoptosis induction of stemness | [[108], [109], [110], [111]] |
| miR-217 | MAPK1, KRAS, Raf-1 | EGFR signaling pathway | Tumor growth, apoptosis | [112] |
| miR-137 | CTNNB1, WNT3a | Wnt/β-catenin pathway | Cell cycle progression | [113] |
| miR-19a | KRAS, VEGFA | EGFR signaling pathway | Proliferation, angiogenesis | [114] |
| miR-96 | RECK, TP53INP1, FOXO1, and FOXO3a | Activation of Wnt signaling pathway | Cell cycle progression, decreased apoptosis | [113] |
| miR-504 | TP53 | Suppressing of TP53 function | CRC progression | [115] |
| miR-106a | PTEN, PI3K, and AKT; TGFBR2 | PTEN/PI3K/AKT signaling pathway; TGF-s signaling pathway | Tumorigenesis, inhibition of cell apoptosis and autophagy; increased migration, invasion and metastasis | [116] |
| miR-182 | ST6GALNAC2, PI3K/AKT | EGFRsignaling pathway | Proliferation, invasion | [117] |
| miR-222 | PTEN | PTEN signaling | Radio resistance, metastatic activity | [118] |
| miR-384 | KRAS, CDC42 | EGFR signaling pathway | Invasion, migratiuon, metastasis | [119] |
| miR-150-5p | TP53 | Deficiency of TP53 function | Promotion of proliferation, cell cycle progress, invasion/migration, reduction of cell apoptosis | [120] |
| miR-146a | NUMB | Wnt/β-catenin pathway | Progression, stemness | [121] |
| miR-26b | PTEN, WNT5A | Induction of EGFR signaling pathway | EMT, proliferation, and metastasis | [122] |
| miR-181a | SRC kinase signaling inhibitor 1 (SRCIN1); WIF-1, E-cadherin, β-catenin, and vimentin | Promotion of SRC/VEGF signaling pathway; wnt/β-catenin signaling | Angiogenesis; cell motility, invasion, tumor growth and liver metastasis | [123,124] |
| miR-125a-3p | FUT5-FUT6 | EGFR signaling pathway | Proliferation, migration, invasion, angiogenesis | [125] |
| miR-135b | TGFBR2 | Inactivation of TGF-β signaling pathway | Progression, inhibiting of apoptosis | [126] |
| miR-92a | PTEN, SMAD2, SMAD4, and TGFBR2; KLF4; matrix metalloproteinase 2 and E-cadherin | PI3K/Akt pathway | Proliferation, EMT, invasion, venous invasion, and metastases, cell growth and migration. | [127,128] |
| miR-494 | APC | wnt/β-catenin signaling | Proliferation, tumorigenesis | [129] |
| miR-224 | SMAD4 | Inactivation of TGF-β signaling pathway | Invasion, metastasis | [33] |
| miR-185 | MYC, CCND1 | Wnt/β-catenin pathway | Proliferation, progression | [130] |
6. miRNA could act as diagnosis and prognosis biomarker in CRC
MiRNAs with high stability in many types of biological samples have become an outstanding candidate for discovering new cancer biomarkers [38,39]. In recent years, several reports have demonstrated that miRNAs can be a potential biomarker for the diagnosis and prognosis of CRC [40,41]. For example, a panel of six miRNAs, including miR-21, let-7g, miR-31, miR-92a, miR-181b, and miR-203 are reliable biomarkers in CRC diagnosis with over 80% specificity and sensitivity [42]. Another study conducted by Hibner et al. [43] shows the panel of 7 miRNAs (let-7a, miR-1229, miR-1246, miR-150, miR-21, miR-223, and miR-23a) act as a potential biomarker for CRC diagnosis and prognosis with high sensitivity and specificity [44]. One of the most identified oncogenic miRNAs, which are highly expressed in CRC is miR-21, which has been linked to carcinogenic processes [[45], [46], [47]]. These findings highlight the importance of miR-21 as a molecular biomarker [48,49]. In the study that the overexpression of miR-21 was found in serum samples from CRC patients [50], the authors proposed a three-miRNAs panel (miR-21, miR-19a-3p, and miR-425-5p) for diagnosis of CRC with the high sensitivity and specificity in CRC serum samples (0.875 and 0.744, respectively) with an area under the ROC curve of 0.88 [51]. Additionally, another study indicated that miR-21 as an early diagnostic biomarker for CRC with a sensitivity and specificity of 0.77 and 0.84, respectively [52], with an AUC of 0.81. An interesting study was conducted by Pan et al. [52], in which the expression level of 30 miRNAs in plasma samples was analyzed using qRT-PCR. These authors showed that analysis of plasma expression levels of five miRNAs, such as miR-15b, miR-17, miR-21, miR-26b, and miR-145, together with carcinoembryonic antigen (CEA), can improve the diagnostic accuracy of CRC (AUC = 0.85 in the training cohort, AUC = 0.818 in the validation cohort).
The increased expression levels of miR-155 in CRC tissues compared to normal samples showed by Zhang et al. [53] after analyzing clinical samples of patients with CRC. Moreover, based on the recent study conducted by Lv et al. [54], they discovered that there is no change in serum miR-155 expression level between controls and stage Ⅰ CRC patients after measuring the serum specimens of CRC patients compared to healthy controls. However, the up-regulation of miR-155 in stages Ⅱ-Ⅳ patients was found. Thus, miR-155 cannot be used as an early diagnostic biomarker in serum [54]. Moreover, a recent study has shown that overexpression of miR-155 in CRC patients shows poor overall survival (OS) and disease-free survival (DFS), this study proposed that miR-155 has independent prognostic values for OS and DFS in CRC patients [55]. Thus, miR-155 might serve as a new tumor biomarker in the clinicopathological diagnosis and prognostic assessment in CRC.
Moreover, another example of miRNA in plasma with the highest predictive capability in CRC is miR-378 [56]. According to the association between the miR-378 decrease and increased tumor volume, metastasis, and short OS of CRC patients and the tumor suppressor role of this miRNA, all of the above suggested that miR-378 could serve as a biomarker to predict the outcome of CRC [57]. The list of some crucial miRNAs as diagnostic and prognostic biomarkers in CRC is summarized in Table 3, Table 4.
Table 3.
Dysregulation of miRNAs as diagnostic and prognostic biomarkers of CRC.
| miRNAs | Sources of miRNA | Expression | Sensitivity | Specificity | Biomarker | References |
|---|---|---|---|---|---|---|
| miR-19a | Serum | up-regulation | 66.7% | 63.9% | Prognostic | [131] |
| miR-144 | Serum | up-regulation | 74% | 87% | Diagnostic | [132] |
| miR-106a | plasma | up-regulation | 62.3% | 68.2% | Diagnostic | [133] |
| miR-21 | Serum | up-regulation | 82.8% | 90.6% | Diagnostic and Prognostic | [47] |
| miR-601 | Plasma | down-regulation | 69.2% | 72.4% | Diagnostic | [134] |
| miR-18a | Serum | up-regulation | 61% | 69% | Diagnostic | [135] |
| miR-92 | Plasma | up-regulation | 89% | 70% | Diagnostic | [136] |
| miR-183 | Plasma | up-regulation | 73.7% | 88.5% | Diagnostic and Prognostic | [137] |
| miR-145 | Serum | down-regulation | – | – | Diagnostic and Prognostic | [138] |
| miR-23a-3p, miR-27a-3p, miR-142-5p, miR-376c-3p | Serum | up-regulation | 89% | 81% | Diagnostic | [139] |
| miR-92a | Plasma | up-regulation | 71.6% | 73.3% | Diagnostic | [136] |
| miR-451 | Plasma | up-regulation | 88.2% | 100% | Diagnostic | [140] |
| miR-139-3p, miR-431 | Plasma | up-regulation | 91% | 57% | Diagnostic and Prognostic | [141] |
| miR-96 | Plasma | up-regulation | 65.4% | 73.3% | Prognostic | [142] |
| miR-7, miR-93 | Plasma | down-regulation | 91% | 88% | Diagnostic | [143] |
| miR-17-3p | Plasma | up-regulation | 64% | 70% | Diagnostic and Prognostic | [138] |
| miR-760 | plasma | down-regulation | 80% | 72.4% | Diagnostic | [143] |
| miR-375 | Plasma | down-regulation | 76.92% | 64.63% | Diagnostic and Prognostic | [144] |
| miR-422a | Serum | down-regulation | – | – | Prognostic | [145] |
| miR-1290 | Serum | up-regulation | 70.01% | 91.2% | Diagnostic | [146] |
| miR-221 | plasma | up-regulation | 86% | 41% | Diagnostic | [135] |
Table 4.
Identifying prognostic values of miRNA for CRC via univariate and multivariate analysis.
| miRNAs | Sources of miRNA | Expression | HR (95% CI), P value |
Outcome | References | |
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| miR-17-3p | Serum | up-regulation | 2.72 (1.58–4.69), P < 0.0001 | 2.24 (1.28–3.92), P = 0.035 | DFS | [138] |
| miR-96 | Plasma | up-regulation | P = 0.002 | 2.27 (1.15–4.51), P = 0.019 | OS | [142] |
| miR-23a-3p, miR-376c-3p | Serum | up-regulation | – | 2.30 (1.44–3.66), P < 0.0004 | OS | [139] |
| miR-106a | Serum | up-regulation | 2.81 (1.64–4.80), P < 0.0001 | 3.02 (1.36–6.73), P = 0.007 | DFS | [138] |
| miR-141 | Plasma | up-regulation | 3.61(1.96–6.65) | 2.40 (1.18–4.86) | OS | [147] |
| miR-1290 | Serum | up-regulation | 3.43 (1.83–6.67) | 4.51 (1.23–23.69), P = 0.0096 | OS | [146] |
7. miRNA can act as a therapeutic target in the CRC
One of the significant troubles for CRC treatment is the acquired chemotherapy resistance. As the miRNAs are involved in cancer progression, they can be considered therapeutic targets [58]. Different tools may be used for the miRNA inhibition, such as the miRNA sponges, antisense oligonucleotides, or molecule inhibitors. Downregulation of miR-211 sponge was indicated against the TUSC7 in the CRC tissues compared to the normal ones. Furthermore, The survival rate of high-expression miR-211 in patients is superior to those with low expression [59]. Moreover, one of the most critical issues in miRNAs therapeutics is the use of miRNA as a replacement therapy via inhibition of miRNA function through anti-miRs and miRNA mimics [60].
MiRNAs can be silenced by anti-miRs, antagomiRs, locked nucleic acids (LNAs), or miRNA sponges. A recent study showed that LNA-anti-miR-21 inhibited cell growth and invasiveness in LS174T CRC cells, suggesting the therapeutic potential of LNA-anti-miR-21 in CRC [61]. Similar studies showed the action by the anti-miRs against the miR-20a [62], miR-21 [63], miR-95 [64], miR-675 [65], and miR-31 [66] in the CRC cell lines. One of the most exciting strategies in cell culture of CRC by targeting overexpressed oncogenic miRNAs is that miRNAs bind to the RISC complexes, leading to blocking the interaction of miRNAs with their endogenous mRNA targets. Specific inhibition of miR-20a, miR-21, miR-95, and miR-675 has been achieved in human CRC cell lines to inhibit cell proliferation and induce apoptosis [62,65,67]. Also, miRNAs can act as predictive biomarkers for therapeutic response in CRC due to the high tissue specificity and stability.
One of the main components of therapy for CRC treatment and its proven effect on survival in CRC patients is 5-FU [68]. The low expression levels of miR-34 were observed in 5-FU-resistant CRC DLD-1 cells, and this miRNA was investigated as a recurrence biomarker due to sensitizing cells to 5-FU treatment inhibits cell growth [69]. Additionally, miR-21 by down-regulation of MutS homolog 2 (MSH2) confer resistance to 5-FU chemotherapy; conversely, the up-regulation of this miRNA suppresses apoptosis induced by 5-FU and G2/M arrest [63].
Downregulation of miR-34a was shown to mediate resistance to 5-FU in the CRC cell line, reversing the resistance by downregulating Sirt1 and E2F3 via ectopic expression miR-34a [69]. More importantly, treatment with miR-21 and miR-30d antagonists were sensitized hypoxic and resistant CRC cells to 5-FU [69]. Moreover, according to the study conducted on the expression profiles of miRNAs in the plasma from 24 CRC patients before and after four cycles of 5-FU/oxaliplatin treatment. The significant upregulation of (miR-106a, miR-484, and miR-130b) in non-responders before treatment was observed. According to the recent experiments, overexpression of miR-153 increased CRC resistance to oxaliplatin both in vitro and in vivo [70].The upregulation of miR-409-3p inhibited cell autophagic activity and enhanced the sensitivity to oxaliplatin, abrogated by the restoration of beclin-1, suggesting that miR-409-3p sensitized CRC to oxaliplatin by inhibiting beclin-1-mediated autophagy [71]. Several studies have focused on miRNAs regulatory roles in the induction of chemo-resistance and their involvement in treatment success (Table 5).
Table 5.
miRNAs and their expression pattern in response to drugs in CRC.
| miRNAs | Treatment regimen | Expression | Ref. |
|---|---|---|---|
| miR-21 | 5-FU | High | [46] |
| miR-214 | 5-Fu | Low | [148] |
| miR-143 | Oxaliplatin | Low | [149] |
| miR-126 | oxaliplatin | Low | [150] |
| miR-10b | 5-Fu | High | [151] |
| miR-519c | 5-FU | Low | [152] |
| miR-129 | 5-Fu | High | [153] |
| miR-625-3p | oxaliplatin | High | [154] |
| miR-148a | 5-FU | Low | [155] |
| miR-106a, miR-130b, miR-484 | 5-FU, oxaliplatin | High | [156] |
| miR-143 | Oxaliplatin | Low | [157] |
| miR-215, miR-190b, miR-29b-2 | 5-FU | High | [158] |
| miR-625-3p | Oxaliplatin | High | [154] |
| miR-320e | 5-FU, oxaliplatin | High | [159] |
| miR-150 | 5-FU | Low | [68] |
| miR-143 | 5-Fu | Low | [160] |
| miR-203 | Oxaliplatin | High | [161] |
| miR-494 | 5-Fu | Low | [68] |
| miR-1914 | oxaliplatin | Low | [162] |
| miR-34a | 5-Fu | Low | [69] |
In sum, there are a limited number of studies have been conducted in miRNA-based therapy; thus, there may be a long way for the first miRNA-based therapy for CRC in the future.
8. Discussion
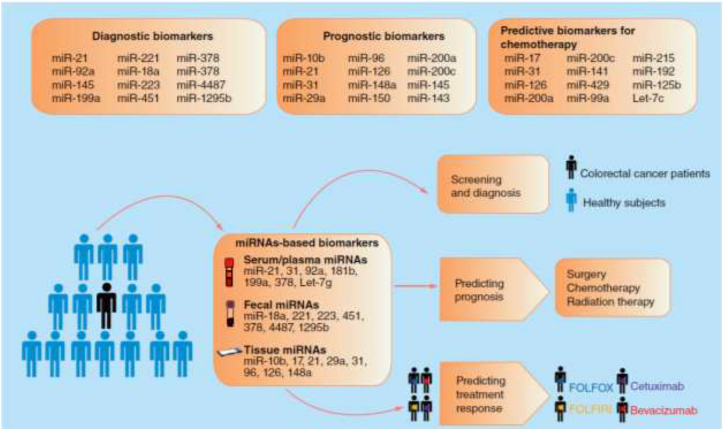
Driving and modulation of the progression in CRC may be occurred by dysregulation of miRNAs. By understanding the regulatory roles of miRNAs in CRC initiation and progression, we can find new insight into finding novel diagnostic and prognostic tools for CRC screening and personalized therapy. The clinical perspective of miRNAs and the importance as a diagnostic and prognostic biomarkers for CRC are summarized in Fig. 2.
Fig. 2.
The overview of the clinical application of miRNAs in colorectal cancer.
Recent studies indicated that due to the insufficient specificity and sensitivity using the expression profiles of a single miRNA as a diagnostic or prognostic biomarker of CRC, it is not most effective. It may be helpful to use the miRNAs signature together and conventional biomarkers, such as CEA, to increase the sensitivity and specificity. Many researchers are currently investigating miRNA panels as CRC biomarkers, which appears to be a more promising strategy than single miRNA tests. The development of panels containing many miRNA biomarkers seems essential and may enable more accurate diagnoses and prognoses of CRC in the future. Moreover, there are many challenges in the development use of miRNAs-based therapeutics for CRC. The potential use of miRNAs in the clinical management of CRC patients is summarized in Fig. 3. One of the main challenges in the therapeutic of CRC is the identification of miRNAs that affect CRC and determine how to achieve effective delivery without causing undesirable side effects. For example, one of the adverse effects of replacement therapy is the initial clinical experience with miR-34; thus, the correction of miRNA dysregulation is a promising therapeutic approach for CRC treatment.
Fig. 3.
The potential usage of miRNAs in the clinical management of the colorectal cancer patients.
In conclusion, despite the numerous studies of miRNAs in CRC have been conducted, but the roles and functions of many individual miRNAs in CRC remain poorly understood. Thus, the integrated analysis of multiple miRNA targets for a given miRNA, and the integrated bioinformatic analysis of mRNAs, proteins, copy number variants, and mutations, are strongly needed.
Declaration of competing interest
The author declares that they have no conflict of interest.
References
- 1.Torre L., Siegel R., Jemal A. vol. 2. American Cancer Society; Atlanta: 2015. (Global Cancer Facts & Figures). [Google Scholar]
- 2.Srmkf S.A. Colorectal cancer statistics. CA A Cancer J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. 2017. [DOI] [PubMed] [Google Scholar]
- 3.Chung D.C. Genetic testing and early onset colon cancer. Gastroenterology. 2018;154(4):788–789. doi: 10.1053/j.gastro.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Yiu A.J., Yiu C.Y. Biomarkers in colorectal cancer. Anticancer Res. 2016;36(3):1093–1102. [PubMed] [Google Scholar]
- 5.Friedman R.C., Farh K.K.-H., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melo S.A., Esteller M. Dysregulation of microRNAs in cancer: playing with fire. FEBS Lett. 2011;585(13):2087–2099. doi: 10.1016/j.febslet.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L-y, Liu M., Li X., Tang H. miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3) J. Biol. Chem. 2013;288(6):4035–4047. doi: 10.1074/jbc.M112.410506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asadi M., Shanehbandi D., Zafari V., Khaze V., Somi M.H., Hashemzadeh S. Transcript level of MicroRNA processing elements in gastric cancer. J. Gastrointest. Canc. 2019;50(4):855–859. doi: 10.1007/s12029-018-0154-8. [DOI] [PubMed] [Google Scholar]
- 9.Masuda T., Hayashi N., Kuroda Y., Ito S., Eguchi H., Mimori K. MicroRNAs as biomarkers in colorectal cancer. Cancers. 2017;9(9):124. doi: 10.3390/cancers9090124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 11.Worthley D.L., Leggett B.A. Colorectal cancer: molecular features and clinical opportunities. Clin. Biochem. Rev. 2010;31(2):31. [PMC free article] [PubMed] [Google Scholar]
- 12.Loeb L.A. A mutator phenotype in cancer. Canc. Res. 2001;61(8):3230–3239. [PubMed] [Google Scholar]
- 13.Baylin S.B., Ohm J.E. Epigenetic gene silencing in cancer–a mechanism for early oncogenic pathway addiction? Nat. Rev. Canc. 2006;6(2):107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 14.Weisenberger D.J., Siegmund K.D., Campan M., Young J., Long T.I., Faasse M.A. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 2006;38(7):787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 15.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 16.Volinia S., Calin G.A., Liu C.-G., Ambs S., Cimmino A., Petrocca F. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selcuklu S.D., Donoghue M.T., Spillane C. Portland Press Ltd.; 2009. miR-21 as a Key Regulator of Oncogenic Processes. [DOI] [PubMed] [Google Scholar]
- 18.Michael M.Z., O'Connor S.M., van Holst Pellekaan N.G., Young G.P., James R.J. Reduced accumulation of specific MicroRNAs in colorectal Neoplasia11Note: susan M. O'Connor and nicholas G. van Holst pellekaan contributed equally to this work. Mol. Canc. Res. 2003;1(12):882–891. [PubMed] [Google Scholar]
- 19.Akao Y., Nakagawa Y., Naoe T. MicroRNA-143 and-145 in colon cancer. DNA Cell Biol. 2007;26(5):311–320. doi: 10.1089/dna.2006.0550. [DOI] [PubMed] [Google Scholar]
- 20.Chen X., Guo X., Zhang H., Xiang Y., Chen J., Yin Y. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28(10):1385–1392. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- 21.Ng E., Tsang W., Ng S., Jin H., Yu J., Li J. MicroRNA-143 targets DNA methyltransferases 3A in colorectal cancer. Br. J. Canc. 2009;101(4):699–706. doi: 10.1038/sj.bjc.6605195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X., Zhang Z., Sun L., Chai N., Tang S., Jin J. MicroRNA-499-5p promotes cellular invasion and tumor metastasis in colorectal cancer by targeting FOXO4 and PDCD4. Carcinogenesis. 2011;32(12):1798–1805. doi: 10.1093/carcin/bgr213. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B., Yang Y., Shi X., Liao W., Chen M., Cheng A.S.-L. Proton pump inhibitor pantoprazole abrogates adriamycin-resistant gastric cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin signaling and epithelial–mesenchymal transition. Canc. Lett. 2015;356(2):704–712. doi: 10.1016/j.canlet.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Liao W.-T., Li T.-T., Wang Z.-G., Wang S.-Y., He M.-R., Ye Y.-P. microRNA-224 promotes cell proliferation and tumor growth in human colorectal cancer by repressing PHLPP1 and PHLPP2. Clin. Canc. Res. 2013;19(17):4662–4672. doi: 10.1158/1078-0432.CCR-13-0244. [DOI] [PubMed] [Google Scholar]
- 25.Nagel R., le Sage C., Diosdado B., van der Waal M., Vrielink J.A.O., Bolijn A. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Canc. Res. 2008;68(14):5795–5802. doi: 10.1158/0008-5472.CAN-08-0951. [DOI] [PubMed] [Google Scholar]
- 26.Kim N.H., Kim H.S., Kim N.-G., Lee I., Choi H.-S., Li X.-Y. p53 and microRNA-34 are suppressors of canonical Wnt signaling. Sci. Signal. 2011;4(197):ra71. doi: 10.1126/scisignal.2001744. ra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wee P., Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers. 2017;9(5):52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dews M., Fox J.L., Hultine S., Sundaram P., Wang W., Liu Y.Y. The myc–mir-17~ 92 axis blunts TGFβ signaling and production of multiple TGFβ-dependent antiangiogenic factors. Canc. Res. 2010;70(20):8233–8246. doi: 10.1158/0008-5472.CAN-10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mestdagh P., Boström A.-K., Impens F., Fredlund E., Van Peer G., De Antonellis P. The miR-17-92 microRNA cluster regulates multiple components of the TGF-β pathway in neuroblastoma. Mol. Cell. 2010;40(5):762–773. doi: 10.1016/j.molcel.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokolova V., Fiorino A., Zoni E., Crippa E., Reid J.F., Gariboldi M. The effects of miR‐20a on p21: two mechanisms blocking growth arrest in TGF‐β‐responsive colon carcinoma. J. Cell. Physiol. 2015;230(12):3105–3114. doi: 10.1002/jcp.25051. [DOI] [PubMed] [Google Scholar]
- 31.Chang Y., Liu C., Yang J., Liu G., Feng F., Tang J. MiR-20a triggers metastasis of gallbladder carcinoma. J. Hepatol. 2013;59(3):518–527. doi: 10.1016/j.jhep.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Cheng D., Zhao S., Tang H., Zhang D., Sun H., Yu F. MicroRNA-20a-5p promotes colorectal cancer invasion and metastasis by downregulating Smad4. Oncotarget. 2016;7(29) doi: 10.18632/oncotarget.9900. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Ling H., Pickard K., Ivan C., Isella C., Ikuo M., Mitter R. The clinical and biological significance of MIR-224 expression in colorectal cancer metastasis. Gut. 2016;65(6):977–989. doi: 10.1136/gutjnl-2015-309372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasry A., Zinger A., Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016;17(3):230. doi: 10.1038/ni.3384. [DOI] [PubMed] [Google Scholar]
- 35.Oshima H., Oshima M. The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J. Gastroenterol. 2012;47(2):97–106. doi: 10.1007/s00535-011-0523-6. [DOI] [PubMed] [Google Scholar]
- 36.Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11(1):115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 37.Liu S., Sun X., Wang M., Hou Y., Zhan Y., Jiang Y. A microRNA 221–and 222–mediated feedback loop maintains constitutive activation of NFκB and STAT3 in colorectal Cancer cells. Gastroenterology. 2014;147(4):847–859. doi: 10.1053/j.gastro.2014.06.006. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber J.A., Baxter D.H., Zhang S., Huang D.Y., How Huang K., Jen Lee M. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010;56(11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lan H., Lu H., Wang X., Jin H. MicroRNAs as potential biomarkers in cancer: opportunities and challenges. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Z., Huang D., Ni S., Peng Z., Sheng W., Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int. J. Canc. 2010;127(1):118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 41.Ng E.K., Chong W.W., Jin H., Lam E.K., Shin V.Y., Yu J. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58(10):1375–1381. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 42.Wang J., Huang S-k, Zhao M., Yang M., Zhong J-l, Gu Y-y. Identification of a circulating microRNA signature for colorectal cancer detection. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0087451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hibner G., Kimsa-Furdzik M., Francuz T. Relevance of microRNAs as potential diagnostic and prognostic markers in colorectal cancer. Int. J. Mol. Sci. 2018;19(10):2944. doi: 10.3390/ijms19102944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogata-Kawata H., Izumiya M., Kurioka D., Honma Y., Yamada Y., Furuta K. Circulating exosomal microRNAs as biomarkers of colon cancer. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oue N., Anami K., Schetter A.J., Moehler M., Okayama H., Khan M.A. High miR‐21 expression from FFPE tissues is associated with poor survival and response to adjuvant chemotherapy in colon cancer. Int. J. Canc. 2014;134(8):1926–1934. doi: 10.1002/ijc.28522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schetter A.J., Leung S.Y., Sohn J.J., Zanetti K.A., Bowman E.D., Yanaihara N. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. Jama. 2008;299(4):425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toiyama Y., Takahashi M., Hur K., Nagasaka T., Tanaka K., Inoue Y. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J. Natl. Cancer Inst. 2013;105(12):849–859. doi: 10.1093/jnci/djt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mei M., Ren Y., Zhou X., Yuan X-b, Han L., Wang G-x. Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol. Canc. Res. Treat. 2010;9(1):77–86. doi: 10.1177/153303461000900109. [DOI] [PubMed] [Google Scholar]
- 49.Peng Q., Zhang X., Min M., Zou L., Shen P., Zhu Y. The clinical role of microRNA-21 as a promising biomarker in the diagnosis and prognosis of colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(27) doi: 10.18632/oncotarget.16488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu M., Huang Z., Zhu D., Zhou X., Shan X., Qi L-w. A panel of microRNA signature in serum for colorectal cancer diagnosis. Oncotarget. 2017;8(10) doi: 10.18632/oncotarget.15059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang B., Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J. Canc. Res. Clin. Oncol. 2012;138(10):1659–1666. doi: 10.1007/s00432-012-1244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H., Li P., Ju H., Pesta M., Kulda V., Jin W. Diagnostic and prognostic value of microRNA-21 in colorectal cancer: an original study and individual participant data meta-analysis. Canc. Epidemiol. Prevention Biomarkers. 2014;23(12):2783–2792. doi: 10.1158/1055-9965.EPI-14-0598. [DOI] [PubMed] [Google Scholar]
- 53.Zhang G.-J., Xiao H.-X., Tian H.-P., Liu Z.-L., Xia S.-S., Zhou T. Upregulation of microRNA-155 promotes the migration and invasion of colorectal cancer cells through the regulation of claudin-1 expression. Int. J. Mol. Med. 2013;31(6):1375–1380. doi: 10.3892/ijmm.2013.1348. [DOI] [PubMed] [Google Scholar]
- 54.Lv Z-c, Fan Y-s, Chen H-b, Zhao D-w. Investigation of microRNA-155 as a serum diagnostic and prognostic biomarker for colorectal cancer. Tumor Biol. 2015;36(3):1619–1625. doi: 10.1007/s13277-014-2760-9. [DOI] [PubMed] [Google Scholar]
- 55.Shibuya H., Iinuma H., Shimada R., Horiuchi A., Watanabe T. Clinicopathological and prognostic value of microRNA-21 and microRNA-155 in colorectal cancer. Oncology. 2010;79(3–4):313–320. doi: 10.1159/000323283. [DOI] [PubMed] [Google Scholar]
- 56.Zanutto S., Pizzamiglio S., Ghilotti M., Bertan C., Ravagnani F., Perrone F. Circulating miR-378 in plasma: a reliable, haemolysis-independent biomarker for colorectal cancer. Br. J. Canc. 2014;110(4):1001–1007. doi: 10.1038/bjc.2013.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandenberghe R. Classification of the primary progressive aphasias: principles and review of progress since 2011. Alzheimer's Res. Ther. 2016;8(1):1–9. doi: 10.1186/s13195-016-0185-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yi R., Li Y., Wang F.-L., Miao G., Qi R.-M., Zhao Y.-Y. MicroRNAs as diagnostic and prognostic biomarkers in colorectal cancer. World J. Gastrointest. Oncol. 2016;8(4):330. doi: 10.4251/wjgo.v8.i4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J., Zhao J., Zhang R. The novel long noncoding RNA TUSC7 inhibits proliferation by sponging MiR-211 in colorectal cancer. Cell. Physiol. Biochem. 2017;41(2):635–644. doi: 10.1159/000457938. [DOI] [PubMed] [Google Scholar]
- 60.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16(3):203. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 61.Nedaeinia R., Sharifi M., Avan A., Kazemi M., Rafiee L., Ghayour-Mobarhan M. Locked nucleic acid anti-miR-21 inhibits cell growth and invasive behaviors of a colorectal adenocarcinoma cell line: LNA-anti-miR as a novel approach. Canc. Gene Ther. 2016;23(8):246–253. doi: 10.1038/cgt.2016.25. [DOI] [PubMed] [Google Scholar]
- 62.Chai H., Liu M., Tian R., Li X., Tang H. miR-20a targets BNIP2 and contributes chemotherapeutic resistance in colorectal adenocarcinoma SW480 and SW620 cell lines. Acta Biochim. Biophys. Sin. 2011;43(3):217–225. doi: 10.1093/abbs/gmq125. [DOI] [PubMed] [Google Scholar]
- 63.Valeri N., Gasparini P., Braconi C., Paone A., Lovat F., Fabbri M. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2) Proc. Natl. Acad. Sci. Unit. States Am. 2010;107(49):21098–21103. doi: 10.1073/pnas.1015541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang Z., Huang S., Wang Q., Liang L., Ni S., Wang L. MicroRNA-95 promotes cell proliferation and targets sorting Nexin 1 in human colorectal carcinoma. Canc. Res. 2011;71(7):2582–2589. doi: 10.1158/0008-5472.CAN-10-3032. [DOI] [PubMed] [Google Scholar]
- 65.Tsang W.P., Ng E.K., Ng S.S., Jin H., Yu J., Sung J.J. Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis. 2010;31(3):350–358. doi: 10.1093/carcin/bgp181. [DOI] [PubMed] [Google Scholar]
- 66.Wang C.-J., Stratmann J., Zhou Z.-G., Sun X.-F. Suppression of microRNA-31 increases sensitivity to 5-FU at an early stage, and affects cell migration and invasion in HCT-116 colon cancer cells. BMC Canc. 2010;10(1):1–11. doi: 10.1186/1471-2407-10-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wheler J.J., Janku F., Naing A., Li Y., Stephen B., Zinner R. Cancer therapy directed by comprehensive genomic profiling: a single center study. Canc. Res. 2016;76(13):3690–3701. doi: 10.1158/0008-5472.CAN-15-3043. [DOI] [PubMed] [Google Scholar]
- 68.Chai J., Dong W., Xie C., Wang L., Han D.L., Wang S. Micro RNA‐494 sensitizes colon cancer cells to fluorouracil through regulation of DPYD. IUBMB Life. 2015;67(3):191–201. doi: 10.1002/iub.1361. [DOI] [PubMed] [Google Scholar]
- 69.Akao Y., Noguchi S., Iio A., Kojima K., Takagi T., Naoe T. Dysregulation of microRNA-34a expression causes drug-resistance to 5-FU in human colon cancer DLD-1 cells. Canc. Lett. 2011;300(2):197–204. doi: 10.1016/j.canlet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L., Pickard K., Jenei V., Bullock M.D., Bruce A., Mitter R. miR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Canc. Res. 2013;73(21):6435–6447. doi: 10.1158/0008-5472.CAN-12-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan S., Shi H., Ba M., Lin S., Tang H., Zeng X. miR-409-3p sensitizes colon cancer cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int. J. Mol. Med. 2016;37(4):1030–1038. doi: 10.3892/ijmm.2016.2492. [DOI] [PubMed] [Google Scholar]
- 72.Wang B., Shen Z-l, Gao Z-d, Zhao G., Wang C-y, Yang Y. MiR-194, commonly repressed in colorectal cancer, suppresses tumor growth by regulating the MAP4K4/c-Jun/MDM2 signaling pathway. Cell Cycle. 2015;14(7):1046–1058. doi: 10.1080/15384101.2015.1007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao H.-J., Ren L.-L., Wang Z.-H., Sun T.-T., Yu Y.-N., Wang Y.-C. MiR-194 deregulation contributes to colorectal carcinogenesis via targeting AKT2 pathway. Theranostics. 2014;4(12):1193. doi: 10.7150/thno.8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu Y., Yu H., Shi X., Xu K., Tang Q., Liang B. microRNA‐497 inhibits invasion and metastasis of colorectal cancer cells by targeting vascular endothelial growth factor‐A. Cell Prolif. 2016;49(1):69–78. doi: 10.1111/cpr.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song Y., Xu Y., Wang Z., Chen Y., Yue Z., Gao P. MicroRNA‐148b suppresses cell growth by targeting cholecystokinin‐2 receptor in colorectal cancer. Int. J. Canc. 2012;131(5):1042–1051. doi: 10.1002/ijc.26485. [DOI] [PubMed] [Google Scholar]
- 76.Bai R., Weng C., Dong H., Li S., Chen G., Xu Z. Micro RNA‐409‐3p suppresses colorectal cancer invasion and metastasis partly by targeting GAB1 expression. Int. J. Canc. 2015;137(10):2310–2322. doi: 10.1002/ijc.29607. [DOI] [PubMed] [Google Scholar]
- 77.Lv H., Zhang Z., Wang Y., Li C., Gong W., Wang X. MicroRNA-92a promotes colorectal cancer cell growth and migration by inhibiting KLF4. Oncol. Res. 2016;23(6):283–290. doi: 10.3727/096504016X14562725373833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng H., Luo J., Hao H., Hu J., Xie S.-K., Ren D. MicroRNA-100 regulates SW620 colorectal cancer cell proliferation and invasion by targeting RAP1B. Oncol. Rep. 2014;31(5):2055–2062. doi: 10.3892/or.2014.3075. [DOI] [PubMed] [Google Scholar]
- 79.Jiang H., Ge F., Hu B., Wu L., Yang H., Wang H. rs35301225 polymorphism in miR-34a promotes development of human colon cancer by deregulation of 3′ UTR in E2F1 in Chinese population. Canc. Cell Int. 2017;17(1):1–7. doi: 10.1186/s12935-017-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamakuchi M., Ferlito M., Lowenstein C.J. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105(36):13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu G., Sun Y., An S., Xin S., Ren X., Zhang D. MicroRNA-34a targets FMNL2 and E2F5 and suppresses the progression of colorectal cancer. Exp. Mol. Pathol. 2015;99(1):173–179. doi: 10.1016/j.yexmp.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 82.Ma K., Pan X., Fan P., He Y., Gu J., Wang W. Loss of miR-638 in vitro promotes cell invasion and a mesenchymal-like transition by influencing SOX2 expression in colorectal carcinoma cells. Mol. Canc. 2014;13(1):118. doi: 10.1186/1476-4598-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao H., Dong T., Zhou H., Wang L., Huang A., Feng B. miR-320a suppresses colorectal cancer progression by targeting Rac1. Carcinogenesis. 2014;35(4):886–895. doi: 10.1093/carcin/bgt378. [DOI] [PubMed] [Google Scholar]
- 84.Li Z., Li N., Wu M., Li X., Luo Z., Wang X. Expression of miR-126 suppresses migration and invasion of colon cancer cells by targeting CXCR4. Mol. Cell. Biochem. 2013;381(1–2):233–242. doi: 10.1007/s11010-013-1707-6. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y., Wang X., Xu B., Wang B., Wang Z., Liang Y. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncol. Rep. 2013;30(4):1976–1984. doi: 10.3892/or.2013.2633. [DOI] [PubMed] [Google Scholar]
- 86.Han H.B., Gu J., Zuo H.J., Chen Z.G., Zhao W., Li M. Let‐7c functions as a metastasis suppressor by targeting MMP11 and PBX3 in colorectal cancer. J. Pathol. 2012;226(3):544–555. doi: 10.1002/path.3014. [DOI] [PubMed] [Google Scholar]
- 87.Noguchi T., Toiyama Y., Kitajima T., Imaoka H., Hiro J., Saigusa S. miRNA-503 promotes tumor progression and is associated with early recurrence and poor prognosis in human colorectal cancer. Oncology. 2016;90(4):221–231. doi: 10.1159/000444493. [DOI] [PubMed] [Google Scholar]
- 88.Wang X.-W., Xi X.-Q., Wu J., Wan Y.-Y., Hui H.-X., Cao X.-F. MicroRNA-206 attenuates tumor proliferation and migration involving the downregulation of NOTCH3 in colorectal cancer. Oncol. Rep. 2015;33(3):1402–1410. doi: 10.3892/or.2015.3731. [DOI] [PubMed] [Google Scholar]
- 89.Zaharie F., Muresan M.-S., Petrushev B., Berce C., Gafencu G.-A., Selicean S. Exosome-carried microRNA-375 inhibits cell progression and dissemination via Bcl-2 blocking in colon cancer. J Gastrointestin Liver Dis. 2015;24(4):435–443. doi: 10.15403/jgld.2014.1121.244.375. [DOI] [PubMed] [Google Scholar]
- 90.Tsang W.P., Kwok T.T. The miR-18a* microRNA functions as a potential tumor suppressor by targeting on K-Ras. Carcinogenesis. 2009;30(6):953–959. doi: 10.1093/carcin/bgp094. [DOI] [PubMed] [Google Scholar]
- 91.Zheng K., Liu W., Liu Y., Jiang C., Qian Q. MicroRNA-133a suppresses colorectal cancer cell invasion by targeting Fascin1. Oncol. Lett. 2015;9(2):869–874. doi: 10.3892/ol.2014.2753. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Wang H., An H., Wang B., Liao Q., Li W., Jin X. miR-133a represses tumour growth and metastasis in colorectal cancer by targeting LIM and SH3 protein 1 and inhibiting the MAPK pathway. Eur. J. Canc. 2013;49(18):3924–3935. doi: 10.1016/j.ejca.2013.07.149. [DOI] [PubMed] [Google Scholar]
- 93.Wang S., Zeng Y., Zhou J.M., Nie S.L., Peng Q., Gong J. MicroRNA-1246 promotes growth and metastasis of colorectal cancer cells involving CCNG2 reduction. Mol. Med. Rep. 2016;13(1):273–280. doi: 10.3892/mmr.2015.4557. [DOI] [PubMed] [Google Scholar]
- 94.Li Y., Zhu X., Xu W., Wang D., Yan J. miR-330 regulates the proliferation of colorectal cancer cells by targeting Cdc42. Biochem. Biophys. Res. Commun. 2013;431(3):560–565. doi: 10.1016/j.bbrc.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 95.Sun J.-Y., Huang Y., Li J.-P., Zhang X., Wang L., Meng Y.-L. MicroRNA-320a suppresses human colon cancer cell proliferation by directly targeting β-catenin. Biochem. Biophys. Res. Commun. 2012;420(4):787–792. doi: 10.1016/j.bbrc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 96.Zhao L.-D., Zheng W.-W., Wang G.-X., Kang X.-C., Qin L., Ji J.-J. Epigenetic silencing of miR-181b contributes to tumorigenicity in colorectal cancer by targeting RASSF1A. Int. J. Oncol. 2016;48(5):1977–1984. doi: 10.3892/ijo.2016.3414. [DOI] [PubMed] [Google Scholar]
- 97.Wang M.-J., Li Y., Wang R., Wang C., Yu Y.-Y., Yang L. Downregulation of microRNA-124 is an independent prognostic factor in patients with colorectal cancer. Int. J. Colorectal Dis. 2013;28(2):183–189. doi: 10.1007/s00384-012-1550-3. [DOI] [PubMed] [Google Scholar]
- 98.Iwaya T., Yokobori T., Nishida N., Kogo R., Sudo T., Tanaka F. Downregulation of miR-144 is associated with colorectal cancer progression via activation of mTOR signaling pathway. Carcinogenesis. 2012;33(12):2391–2397. doi: 10.1093/carcin/bgs288. [DOI] [PubMed] [Google Scholar]
- 99.Feng Y., Zhu J., Ou C., Deng Z., Chen M., Huang W. MicroRNA-145 inhibits tumour growth and metastasis in colorectal cancer by targeting fascin-1. Br. J. Canc. 2014;110(9):2300–2309. doi: 10.1038/bjc.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.He X., Dong Y., Wu C.W., Zhao Z., Ng S.S., Chan F.K. MicroRNA-218 inhibits cell cycle progression and promotes apoptosis in colon cancer by downregulating BMI1 polycomb ring finger oncogene. Mol. Med. 2012;18(12):1491–1498. doi: 10.2119/molmed.2012.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li W., Chang J., Wang S., Liu X., Peng J., Huang D. miRNA-99b-5p suppresses liver metastasis of colorectal cancer by down-regulating mTOR. Oncotarget. 2015;6(27) doi: 10.18632/oncotarget.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun Y., Shen S., Liu X., Tang H., Wang Z., Yu Z. MiR-429 inhibits cells growth and invasion and regulates EMT-related marker genes by targeting Onecut2 in colorectal carcinoma. Mol. Cell. Biochem. 2014;390(1–2):19–30. doi: 10.1007/s11010-013-1950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang L., Dong Y., Zhu N., Tsoi H., Zhao Z., Wu C.W. microRNA-139-5p exerts tumor suppressor function by targeting NOTCH1 in colorectal cancer. Mol. Canc. 2014;13(1):124. doi: 10.1186/1476-4598-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abdelmaksoud-Dammak R., Chamtouri N., Triki M., Saadallah-Kallel A., Ayadi W., Charfi S. Overexpression of miR-10b in colorectal cancer patients: correlation with TWIST-1 and E-cadherin expression. Tumor Biol. 2017;39(3) doi: 10.1177/1010428317695916. 1010428317695916. [DOI] [PubMed] [Google Scholar]
- 105.Dai G., Yao X., Zhang Y., Gu J., Geng Y., Xue F. Colorectal cancer cell–derived exosomes containing miR-10b regulate fibroblast cells via the PI3K/Akt pathway. Bulletin du cancer. 2018;105(4):336–349. doi: 10.1016/j.bulcan.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J., Fei B., Wang Q., Song M., Yin Y., Zhang B. MicroRNA-638 inhibits cell proliferation, invasion and regulates cell cycle by targeting tetraspanin 1 in human colorectal carcinoma. Oncotarget. 2014;5(23) doi: 10.18632/oncotarget.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ji S., Ye G., Zhang J., Wang L., Wang T., Wang Z. miR-574-5p negatively regulates Qki6/7 to impact β-catenin/Wnt signalling and the development of colorectal cancer. Gut. 2013;62(5):716–726. doi: 10.1136/gutjnl-2011-301083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fu J., Tang W., Du P., Wang G., Chen W., Li J. Identifying microRNA-mRNA regulatory network in colorectal cancer by a combination of expression profile and bioinformatics analysis. BMC Syst. Biol. 2012;6(1):68. doi: 10.1186/1752-0509-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Falzone L., Scola L., Zanghì A., Biondi A., Di Cataldo A., Libra M. Integrated analysis of colorectal cancer microRNA datasets: identification of microRNAs associated with tumor development. Aging (Albany NY) 2018;10(5):1000. doi: 10.18632/aging.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ding T., Cui P., Zhou Y., Chen C., Zhao J., Wang H. Antisense oligonucleotides against miR-21 inhibit the growth and metastasis of colorectal carcinoma via the DUSP8 pathway. Mol. Ther. Nucleic Acids. 2018;13:244–255. doi: 10.1016/j.omtn.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sabry D., El-Deek S.E., Maher M., El-Baz M.A., El-Bader H.M., Amer E. Role of miRNA-210, miRNA-21 and miRNA-126 as diagnostic biomarkers in colorectal carcinoma: impact of HIF-1α-VEGF signaling pathway. Mol. Cell. Biochem. 2019;454(1–2):177–189. doi: 10.1007/s11010-018-3462-1. [DOI] [PubMed] [Google Scholar]
- 112.Zhang N., Lu C., Chen L. miR-217 regulates tumor growth and apoptosis by targeting the MAPK signaling pathway in colorectal cancer. Oncol. Lett. 2016;12(6):4589–4597. doi: 10.3892/ol.2016.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fasihi A., Soltani B M., Atashi A., Nasiri S. Introduction of hsa‐miR‐103a and hsa‐miR‐1827 and hsa‐miR‐137 as new regulators of Wnt signaling pathway and their relation to colorectal carcinoma. J. Cell. Biochem. 2018;119(7):5104–5117. doi: 10.1002/jcb.26357. [DOI] [PubMed] [Google Scholar]
- 114.Chen M., Lin M., Wang X. Overexpression of miR-19a inhibits colorectal cancer angiogenesis by suppressing KRAS expression. Oncol. Rep. 2018;39(2):619–626. doi: 10.3892/or.2017.6110. [DOI] [PubMed] [Google Scholar]
- 115.Hu W., Chan C.S., Wu R., Zhang C., Sun Y., Song J.S. Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol. Cell. 2010;38(5):689–699. doi: 10.1016/j.molcel.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hao H., Xia G., Wang C., Zhong F., Liu L., Zhang D. miR-106a suppresses tumor cells death in colorectal cancer through targeting ATG7. Med. Mol. Morphol. 2017;50(2):76–85. doi: 10.1007/s00795-016-0150-7. [DOI] [PubMed] [Google Scholar]
- 117.Jia L., Luo S., Ren X., Li Y., Hu J., Liu B. miR-182 and miR-135b mediate the tumorigenesis and invasiveness of colorectal cancer cells via targeting ST6GALNAC2 and PI3K/AKT pathway. Dig. Dis. Sci. 2017;62(12):3447–3459. doi: 10.1007/s10620-017-4755-z. [DOI] [PubMed] [Google Scholar]
- 118.Iida M., Hazama S., Tsunedomi R., Tanaka H., Takenouchi H., Kanekiyo S. Overexpression of miR-221 and miR-222 in the cancer stroma is associated with malignant potential in colorectal cancer. Oncol. Rep. 2018;40(3):1621–1631. doi: 10.3892/or.2018.6575. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y.-X., Chen Y.-R., Liu S.-S., Ye Y.-P., Jiao H.-L., Wang S.-Y. MiR-384 inhibits human colorectal cancer metastasis by targeting KRAS and CDC42. Oncotarget. 2016;7(51) doi: 10.18632/oncotarget.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu F., Di Wang X. miR-150-5p represses TP53 tumor suppressor gene to promote proliferation of colon adenocarcinoma. Sci. Rep. 2019;9(1):1–7. doi: 10.1038/s41598-019-43231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hwang W.-L., Yang M.-H. Numb is involved in the non-random segregation of subcellular vesicles in colorectal cancer stem cells. Cell Cycle. 2016;15(20):2697–2703. doi: 10.1080/15384101.2016.1218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fan D., Lin X., Zhang F., Zhong W., Hu J., Chen Y. Micro RNA 26b promotes colorectal cancer metastasis by downregulating phosphatase and tensin homolog and wingless‐type MMTV integration site family member 5A. Canc. Sci. 2018;109(2):354–362. doi: 10.1111/cas.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun W., Wang X., Li J., You C., Lu P., Feng H. MicroRNA-181a promotes angiogenesis in colorectal cancer by targeting SRCIN1 to promote the SRC/VEGF signaling pathway. Cell Death Dis. 2018;9(4):1–13. doi: 10.1038/s41419-018-0490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ji D., Chen Z., Li M., Zhan T., Yao Y., Zhang Z. MicroRNA-181a promotes tumor growth and liver metastasis in colorectal cancer by targeting the tumor suppressor WIF-1. Mol. Canc. 2014;13(1):86. doi: 10.1186/1476-4598-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liang L., Gao C., Li Y., Sun M., Xu J., Li H. miR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway. Cell Death Dis. 2017;8(8):e2968–e. doi: 10.1038/cddis.2017.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li J., Liang H., Bai M., Ning T., Wang C., Fan Q. miR-135b promotes cancer progression by targeting transforming growth factor beta receptor II (TGFBR2) in colorectal cancer. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0130194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nishida N., Nagahara M., Sato T., Mimori K., Sudo T., Tanaka F. Microarray analysis of colorectal cancer stromal tissue reveals upregulation of two oncogenic miRNA clusters. Clin. Canc. Res. 2012;18(11):3054–3070. doi: 10.1158/1078-0432.CCR-11-1078. [DOI] [PubMed] [Google Scholar]
- 128.Zhang G., Zhou H., Xiao H., Liu Z., Tian H., Zhou T. MicroRNA-92a functions as an oncogene in colorectal cancer by targeting PTEN. Dig. Dis. Sci. 2014;59(1):98–107. doi: 10.1007/s10620-013-2858-8. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y., Guo L., Li Y., Feng G.-H., Teng F., Li W. MicroRNA-494 promotes cancer progression and targets adenomatous polyposis coli in colorectal cancer. Mol. Canc. 2018;17(1):1–11. doi: 10.1186/s12943-017-0753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dong-Xu W., Jia L., Su-Juan Z. MicroRNA-185 is a novel tumor suppressor by negatively modulating the Wnt/β-catenin pathway in human colorectal cancer. Indian J. Canc. 2015;52(7):182. doi: 10.4103/0019-509X.186576. [DOI] [PubMed] [Google Scholar]
- 131.Chen Q., Xia H.-W., Ge X.-J., Zhang Y.-C., Tang Q.-L., Bi F. Serum miR-19a predicts resistance to FOLFOX chemotherapy in advanced colorectal cancer cases. Asian Pac. J. Cancer Prev. APJCP. 2013;14(12):7421–7426. doi: 10.7314/apjcp.2013.14.12.7421. [DOI] [PubMed] [Google Scholar]
- 132.Kalimutho M., Blanco G.D.V., Di Cecilia S., Sileri P., Cretella M., Pallone F. Differential expression of miR-144* as a novel fecal-based diagnostic marker for colorectal cancer. J. Gastroenterol. 2011;46(12):1391–1402. doi: 10.1007/s00535-011-0456-0. [DOI] [PubMed] [Google Scholar]
- 133.Zhang L., Meng L., Fan Z., Liu B., Pei Y., Zhao Z. Expression of plasma miR-106a in colorectal cancer and its clinical significance. J. Southern Med. Univ. 2014;34(3):354. [PubMed] [Google Scholar]
- 134.Wang Q., Huang Z., Ni S., Xiao X., Xu Q., Wang L. Plasma miR-601 and miR-760 are novel biomarkers for the early detection of colorectal cancer. PloS One. 2012;7(9) doi: 10.1371/journal.pone.0044398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yau T., Wu C., Dong Y., Tang C., Ng S., Chan F. microRNA-221 and microRNA-18a identification in stool as potential biomarkers for the non-invasive diagnosis of colorectal carcinoma. Br. J. Canc. 2014;111(9):1765–1771. doi: 10.1038/bjc.2014.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu C.W., Ng S.S., Dong Y.J., Ng S.C., Leung W.W., Lee C.W. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61(5):739–745. doi: 10.1136/gut.2011.239236. [DOI] [PubMed] [Google Scholar]
- 137.Yuan D., Li K., Zhu K., Yan R., Dang C. Plasma miR-183 predicts recurrence and prognosis in patients with colorectal cancer. Canc. Biol. Ther. 2015;16(2):268–275. doi: 10.1080/15384047.2014.1002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li J., Liu Y., Wang C., Deng T., Liang H., Wang Y. Serum miRNA expression profile as a prognostic biomarker of stage II/III colorectal adenocarcinoma. Sci. Rep. 2015;5:12921. doi: 10.1038/srep12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vychytilova-Faltejskova P., Radova L., Sachlova M., Kosarova Z., Slaba K., Fabian P. Serum-based microRNA signatures in early diagnosis and prognosis prediction of colon cancer. Carcinogenesis. 2016;37(10):941–950. doi: 10.1093/carcin/bgw078. [DOI] [PubMed] [Google Scholar]
- 140.Phua L.C., Chue X.P., Koh P.K., Cheah P.Y., Chan E.C.Y., Ho H.K. Global fecal microRNA profiling in the identification of biomarkers for colorectal cancer screening among Asians. Oncol. Rep. 2014;32(1):97–104. doi: 10.3892/or.2014.3193. [DOI] [PubMed] [Google Scholar]
- 141.Kanaan Z., Roberts H., Eichenberger M.R., Billeter A., Ocheretner G., Pan J. A plasma microRNA panel for detection of colorectal adenomas: a step toward more precise screening for colorectal cancer. Ann. Surg. 2013;258(3):400–408. doi: 10.1097/SLA.0b013e3182a15bcc. [DOI] [PubMed] [Google Scholar]
- 142.Sun Y., Liu Y., Cogdell D., Calin G.A., Sun B., Kopetz S. Examining plasma microRNA markers for colorectal cancer at different stages. Oncotarget. 2016;7(10) doi: 10.18632/oncotarget.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang S., Xiang J., Li Z., Lu S., Hu J., Gao X. A plasma microRNA panel for early detection of colorectal cancer. Int. J. Canc. 2015;136(1):152–161. doi: 10.1002/ijc.28136. [DOI] [PubMed] [Google Scholar]
- 144.Xu L., Li M., Wang M., Yan D., Feng G., An G. The expression of microRNA-375 in plasma and tissue is matched in human colorectal cancer. BMC Canc. 2014;14(1):714. doi: 10.1186/1471-2407-14-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zheng G., Du L., Yang X., Zhang X., Wang L., Yang Y. Serum microRNA panel as biomarkers for early diagnosis of colorectal adenocarcinoma. Br. J. Canc. 2014;111(10):1985–1992. doi: 10.1038/bjc.2014.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Toiyama Y., Imaoka H., Fujikawa H., Hiro J., Saigusa S., Tanaka K. AACR; 2016. Circulating microRNA-1290 as a Novel Diagnostic and Prognostic Biomarker in Human Colorectal Cancer. [DOI] [PubMed] [Google Scholar]
- 147.Cheng H., Zhang L., Cogdell D.E., Zheng H., Schetter A.J., Nykter M. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PloS One. 2011;6(3) doi: 10.1371/journal.pone.0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu H., Liang Y., Shen L., Shen L. MicroRNA-204 modulates colorectal cancer cell sensitivity in response to 5-fluorouracil-based treatment by targeting high mobility group protein A2. Biol. Open. 2016;5(5):563–570. doi: 10.1242/bio.015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mekenkamp L.J., Tol J., Dijkstra J.R., de Krijger I., Vink-Börger M.E., van Vliet S. Beyond KRAS mutation status: influence of KRAScopy number status and microRNAs on clinical outcome to cetuximab in metastatic colorectal cancer patients. BMC Canc. 2012;12(1):292. doi: 10.1186/1471-2407-12-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hansen T., Carlsen A., Heegaard N., Sørensen F.B., Jakobsen A. Changes in circulating microRNA-126 during treatment with chemotherapy and bevacizumab predicts treatment response in patients with metastatic colorectal cancer. Br. J. Canc. 2015;112(4):624–629. doi: 10.1038/bjc.2014.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nishida N., Yamashita S., Mimori K., Sudo T., Tanaka F., Shibata K. MicroRNA-10b is a prognostic indicator in colorectal cancer and confers resistance to the chemotherapeutic agent 5-fluorouracil in colorectal cancer cells. Ann. Surg Oncol. 2012;19(9):3065–3071. doi: 10.1245/s10434-012-2246-1. [DOI] [PubMed] [Google Scholar]
- 152.To K.K., Leung W., Ng S.S. Exploiting a novel miR-519c–HuR–ABCG2 regulatory pathway to overcome chemoresistance in colorectal cancer. Exp. Cell Res. 2015;338(2):222–231. doi: 10.1016/j.yexcr.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 153.Karaayvaz M., Zhai H., Ju J. miR-129 promotes apoptosis and enhances chemosensitivity to 5-fluorouracil in colorectal cancer. Cell Death Dis. 2013;4(6):e659–e. doi: 10.1038/cddis.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rasmussen M.H., Jensen N.F., Tarpgaard L.S., Qvortrup C., Rømer M.U., Stenvang J. High expression of microRNA-625-3p is associated with poor response to first-line oxaliplatin based treatment of metastatic colorectal cancer. Mol. Oncol. 2013;7(3):637–646. doi: 10.1016/j.molonc.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wilson G., Bryan J., Cranston K., Kitzes J., Nederbragt L., Teal T.K. Good enough practices in scientific computing. PLoS Comput. Biol. 2017;13(6) doi: 10.1371/journal.pcbi.1005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kjersem J., Ikdahl T., Lingjaerde O., Guren T., Tveit K.M., Kure E. Plasma microRNAs predicting clinical outcome in metastatic colorectal cancer patients receiving first-line oxaliplatin-based treatment. Mol. Oncol. 2014;8(1):59–67. doi: 10.1016/j.molonc.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Qian X., Yu J., Yin Y., He J., Wang L., Li Q. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle. 2013;12(9):1385–1394. doi: 10.4161/cc.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Svoboda M., Sana J., Fabian P., Kocakova I., Gombosova J., Nekvindova J. MicroRNA expression profile associated with response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients. Radiat. Oncol. 2012;7(1):195. doi: 10.1186/1748-717X-7-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Perez-Carbonell L., Sinicrope F.A., Alberts S.R., Oberg A.L., Balaguer F., Castells A. MiR-320e is a novel prognostic biomarker in colorectal cancer. Br. J. Canc. 2015;113(1):83–90. doi: 10.1038/bjc.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Borralho P.M., Kren B.T., Castro R.E., Moreira da Silva I.B., Steer C.J., Rodrigues C.M. MicroRNA‐143 reduces viability and increases sensitivity to 5‐fluorouracil in HCT116 human colorectal cancer cells. FEBS J. 2009;276(22):6689–6700. doi: 10.1111/j.1742-4658.2009.07383.x. [DOI] [PubMed] [Google Scholar]
- 161.Zhou Y., Wan G., Spizzo R., Ivan C., Mathur R., Hu X. miR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Mol. Oncol. 2014;8(1):83–92. doi: 10.1016/j.molonc.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hu J., Cai G., Xu Y., Cai S. The plasma microRNA miR-1914* and-1915 suppresses chemoresistant in colorectal cancer patients by down-regulating NFIX. Curr. Mol. Med. 2016;16(1):70–82. doi: 10.2174/1566524016666151222144656. [DOI] [PubMed] [Google Scholar]