Abstract
Neighborhood context might influence the risk of chronic kidney disease (CKD), a condition that impacts approximately 10% of the United States population and is associated with significant morbidity, mortality, and costs. We included a sample of 23,692 individuals in Philadelphia, Pennsylvania, who were seen in a large academic primary care practice between January 1, 2016 and December 31, 2017. We used generalized linear equations to estimate the associations between indicators of neighborhood context (e.g., proximity to healthy foods stores, neighborhood walkability, social capital, crime rate, socioeconomic status) and CKD, adjusted for age, sex, race/ethnicity, and insurance coverage. Among those with CKD, secondary outcomes were poor glycemic control (hemoglobin A1c ≥ 6.5%) and uncontrolled blood pressure (systolic ≥ 140 mm Hg and/or diastolic ≥ 90 mm Hg). The cohort represented residents from 97% of Philadelphia census tracts. CKD prevalence was 10%. When all neighborhood context metrics were considered collectively, only lower neighborhood socioeconomic index (a composite assessment of neighborhood income, educational attainment, and occupation) was associated with a higher risk of CKD (lowest tertile vs. highest tertile: adjusted relative risk [aRR] 1.46 [1.25, 1.69]; mid-tertile vs. highest-tertile: aRR 1.35 [1.25, 1.52]). Among those with CKD, compared to residence in the most walkable neighborhoods (i.e., where most essential resources are accessible by foot), residence in neighborhoods with mid-level WalkScore® (i.e., where only some essential neighborhood resources are accessible by foot) was independently associated with poor glycemic control (aRR 1.20, 95% CI 1.01–1.42). These findings suggest a potential role for measures of neighborhood socioeconomic status in identifying communities that would benefit from screening and treatment for CKD. Studies are also needed to determine mechanisms to explain why residence in neighborhoods not easily navigated by foot or car might hinder glycemic control among people with CKD.
Keywords: Kidney disease, Urban health, Health disparities, Social context
Introduction
Chronic kidney disease (CKD) affects approximately 10% of the United States (US) population, or more than 30 million people, and is its ninth leading cause of death (Statistics CfaCfH. 20, 2013; Coresh et al., 2007; Saran et al., 2019). However, despite the high prevalence and health consequences of CKD, less than 10% of Americans with CKD are aware of their condition (Hoerger et al., 2015). Among those who progress to end-stage kidney disease (ESKD), 30% initiate dialysis with little-or-no pre-dialysis care from a kidney disease specialist (Saran et al., 2019). Given the large human and financial costs of CKD to the US, the U.S. Department Health and Human Services launched the Advancing American Kidney Health Initiative in 2019. This initiative has called for improved identification of US populations at risk for CKD and has set a goal for a 25% decrease in the incidence of ESKD in the US by 2030 (Bieber & Gadegbeku, 2019; Government US, 2019).
An individual's socioeconomic status (SES) is typically defined by income, educational attainment, and occupation. An individual's SES can affect their health by influencing lifestyle, social support, and access to resources and services, such as healthcare. A host of observational studies document the association between individual-level SES and a variety of CKD outcomes, including disease progression, dialysis modality selection, and kidney transplant access (Banerjee et al., 2017; Barker-Cummings et al., 1995; Bruce et al., 2010; Crews et al., 2010; Hall, 2018; Maziarz et al., 2015; Morton et al., 2016; Purnell et al., 2013; Vart et al., 2015; Young et al., 1994). However, health status is not only influenced by one's own attributes, but by the attributes of their area of residence, or neighborhood context. Neighborhood context encompasses the social, economic, and physical features of the residential community (Lapidis et al., 2020). Specifically, it includes the neighborhood's collective SES (e.g., median household income), access to nutritious food, crime rate, transportation, space for recreational activity (sidewalks and parks), racial and ethnic composition, and shared culture (i.e, social capital – the strength of social bonds between resident and accepted behavioral norms) (Lapidis et al., 2020). Neighborhood context can impact an individual's health by affecting access to health-promoting resources and influencing health-related behaviors. It has been associated with increased risk of hypertension, cardiovascular disease, obesity, and diabetes (Christine et al., 2015; Diez Roux, Merkin et al., 2019; Foster et al., 2008; Kershaw et al., 2013; Mujahid et al., 2011).
Compared with individual-level SES, less is known about the independent associations between neighborhood context and CKD. Previous studies have identified associations between median neighborhood household income, educational level, and racial composition with CKD, CKD progression, dialysis-dependence, and kidney transplantation (Byrne et al., 1994; Hall et al., 2008; Hao et al., 2015; Karter et al., 2002; Klag et al., 1997; Patzer et al., 2015; Rodriguez et al., 2007; Shoham et al., 2007; Volkova et al., 2008). However, many of these investigations were geographically limited, and included individuals who were recruited from largely rural areas into prospective cohort studies (Merkin et al., 2005; Shoham et al., 2007). Furthermore, only a few studies have investigated the full breadth of neighborhood context features, which include built environment (e.g., transportation resources and walkability), social capital, and neighborhood safety in addition to traditional SES metrics (Bowe et al., 2017; Hicken et al., 2019). Therefore, due the heterogeneity of previous study populations and exposure variables, there remain substantial gaps in the understanding of how the broad range of neighborhood context features might influence CKD prevalence, especially within urban populations.
Philadelphia, Pennsylvania, is an important setting in which to examine the potential influence of neighborhood context on CKD. Philadelphia is the sixth most populous city in the US, with large population of non-Hispanic Blacks (41%) and Hispanics (14%), racial and ethnic groups that are known to be at higher risk for CKD than non-Hispanic Whites (Assessment PsCH, 2018; Desai et al., 2019; Hsu et al., 2003). Among 1.5 million residents, 25% of Philadelphians live below the federal poverty level (i.e., annual household income of <$25,000 for a family of four), and nearly 12% live in deep poverty (i.e., < $12,500 annually), the highest prevalence of deep poverty among the 10 largest US cities (The State of Philadelphians Living in Poverty, 2019). The city is also highly segregated, with one racial or ethnic group forming a majority in 84% of its 381 census tracts (Philadelphia TDoPHotCo, 2018).
The goal of this study was to examine the neighborhood context of a large, diverse cohort of adult Philadelphia-area residents who were seen for primary care in an academic health system. We sought to investigate the relationship between CKD and a broad range of social, economic and physical features of neighborhoods by census tract. We also examined the associations between neighborhood context, blood pressure control, and glycemic control among individuals with CKD. We hypothesized that low neighborhood-level SES, lack of neighborhood resources, and low neighborhood-level social capital would be associated with higher risk of CKD. Further, among those with CKD, we hypothesized that less affluent neighborhood characteristics would be linked with poor glycemic and blood pressure control, both of which are risk factors for CKD and CKD progression.
Methods
Study design and description of patient cohort and geocoding
Individuals were included in the study cohort if they were ≥18 years old and received primary care at one of 13 Philadelphia-based clinics affiliated with a large academic health system between January 1, 2016 and December 31, 2017. Individuals were excluded if they did not live within Philadelphia or if their address was not able to be geocoded. Geocoding of patients’ addresses was performed using ArcGIS 10.5 with the Business Analyst 2016 Composite Address Locator and assigned to the census tract of residence (ESRI, 2018). The protocol was submitted to the Institutional Review Board which determined that it was exempt from review.
Exposures – neighborhood characteristics
Using multiple data sources, we determined the following neighborhood characteristics of individuals in the cohort (Supplemental Table 1): 1) density of healthy food stores per km (Coresh et al., 2007) within 800 m of a 30 m grid (derived from the 2014 National Establishment Time Series Database); 2) census tract-level Walk Score® from 2015 (created by Walk Score Research Services); 3) violent crime rate per 10,000 population as recorded by Philadelphia Police Department 2016–2017 (i.e., homicides, rapes, aggravated assaults, robberies, other assaults; 4) median household income; 5) population density (km (Coresh et al., 2007)) based on five-year estimates for the census tract; 6) % Hispanic residents; 7) % non-Hispanic Black residents; 8) % residents >25 years-old with at least a high school diploma; 9) % residents living below the federal poverty level (per five-year estimate for census tracts from 2013 to 2017 from the American Community Survey); 10) perceived social capital (derived from the 2015 Southeastern Pennsylvania Household Health Survey); and 11) socioeconomic index (Diez Roux, Merkin et al., 2019; Rundle et al., 2009; Kaufman et al., 2015; Street Smart Walk Sc, 2010; Department PP; OpenDataPhilly, 2019; Diez-Roux, Kiefe et al., 2019; Buehler et al., 2019; Le-Scherban et al., 2019; Southeastern Pennsylvania, 2015; American Communities Survey, 2013). The SES index was developed from the 2013–2017 American Community Survey using factor analysis as described elsewhere (Diez Roux, Merkin et al., 2019). It incorporates the following variables: median value of occupied housing units, % persons ≥ 25 years-old with at least high school diploma, % persons > 25 years-old with at least a bachelor's degree, % with management, professional, and related occupation, median household income, and % of households with interest, dividends, or net rental income.
Outcomes
The primary outcome was CKD, defined as either an ambulatory eGFR of <60 ml/min/1.73 m2 and/or an ICD-9 code for kidney disease (see Supplemental Appendix for a list of codes). The secondary outcomes were ascertained among those with CKD, and were uncontrolled blood pressure (BP), defined as at least one occasion of systolic BP ≥ 140 mm Hg and/or diastolic BP ≥ 90 mm Hg, and poor glycemic control, defined as at least one hemoglobin A1c ≥ 6.5%.
Covariates
Models were adjusted for patients’ age, sex, race/ethnicity, and insurance type, as ascertained from the electronic health record. We categorized insurance as commercial, Medicare, Medicare Advantage, or Medicaid. We identified the following comorbidities through ICD-9 codes in the medical record: human immunodeficiency virus (HIV) status, coronary artery disease, hypertension, and hepatitis C virus (HCV) status. Individuals were considered to have active HCV if they had both an assay with detectable HCV RNA or a diagnostic code for hepatitis C. Individuals were classified as having diabetes if they had a diagnostic code for diabetes mellitus (see Supplemental Appendix) or a hemoglobin A1C ≥ 6.5%. We ascertained smoking status from clinical documentation in the medical record and categorized patients with documentation of either current or previous tobacco smoking as a smoker. We also captured body mass index (BMI) from the first encounter in the medical record during the study period.
Statistical analysis
In the primary analysis, we used generalized estimating equations with the log Poisson distribution and an exchangeable correlation structure to estimate adjusted risk ratios for associations between CKD and individual and neighborhood characteristics. Neighborhood characteristics were categorized in tertiles. First, we examined unadjusted models with each neighborhood characteristic modeled separately (Model 1 in Table 2). Then, we added age, sex (male or female), and race/ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, non-Hispanic Asian, non-Hispanic other) as adjustment to the previous models (Model 2 in Table 2). As a proxy for individual income, we then adjusted for individual insurance coverage (commercial, Medicaid, Medicare, Medicare Advantage) (Model 3 in Table 2). In our final model (Model 4 in Table 2), we included age, sex, race/ethnicity, insurance status and, for neighborhood characteristics, density of healthy food stores, Walk Score®, percentage non-Hispanic Black, percentage Hispanic, violent crime rate per 10,000 population, socioeconomic index, and social capital. Education, income and poverty were not included in the final model, as they were highly correlated (correlation coefficients ranged from 0.72 to 0.84), and were components of the socioeconomic index. We also examined models in which population density was included as an additional covariate in the fully adjusted model (Model 5 in the Supplemental Appendix). Of note, we did not adjust for comorbidities (e.g. obesity, diabetes and hypertension) in the multivariable models because these factors might lie along the causal pathway to CKD. In a sensitivity analysis, we examined whether results were similar if CKD was defined as either 1) eGFR <60 ml/min/1.73 m2 or 2) an ICD-9 code for CKD. We also examined whether CKD was an effect modifier of associations between neighborhood characteristics and blood pressure and blood glucose control, respectively. We used SAS® (v9.4) for statistical analyses. All statistical tests are 2-tailed, and risk ratio estimates are shown with 95% confidence intervals (CIs). We considered statistical tests to be statistically significant when p-values were <0.05.
Table 2.
Association between neighborhood characteristics and chronic kidney disease.
| Characteristic | Category | Model 1* RR (95% CI) | Model 2** aRR (95% CI) | Model 3*** aRR (95% CI) | Model 4*** aRR (95% CI) |
|---|---|---|---|---|---|
| Healthy food within 800 m | 1 (0) | 1.12 (0.9, 1.29) | 1.05 (0.95, 1.16) | 1.05 (0.95, 1.16) | 1.04 (0.94, 1.15) |
| 2 (0.5–0.99) | 1.06 (0.94, 1.20) | 1.00 (0.91, 1.10) | 1.00 (0.91, 1.10) | 0.99 (0.90, 1.08) | |
| 3 (1.49–5.97) | 1.00 | 1.00 | 1.00 | 1.00 | |
| Walk Score® | 1 (20.48–78) | 1.30 (1.12, 1.51) | 0.99 (0.90, 1.09) | 1.01 (0.92, 1.11) | 0.98 (0.89, 1.09) |
| 2 (78.09–86) | 1.48 (1.28, 1.71) | 1.08 (0.98, 1.19) | 1.07 (0.97, 1.17) | 0.98 (0.88, 1.08) | |
| 3 (86.25–99) | 1.00 | 1.00 | 1.00 | 1.00 | |
| % ≥ high school education | 1 (41.9%-81.85%) | 1.73 (1.48, 2.03) | 1.28 (1.14, 1.42) | 1.22 (1.10, 1.36) | |
| 2 (82.04%-91.36%) | 1.60 (1.36, 1.89) | 1.23 (1.11, 1.38) | 1.21 (1.08, 1.35) | ||
| 3 (91.51%-100%) | 1.00 | 1.00 | 1.00 | ||
| Median household income | 1 (9945–30786) | 1.90 (1.63, 2.21) | 1.35 (1.20, 1.51) | 1.27 (1.14, 1.43) | |
| 2 (31,124–57027) | 1.46 (1.25, 1.71) | 1.21 (1.08, 1.36) | 1.20 (1.08, 1.35) | ||
| 3 (57,039–149,211) | 1.00 | 1.00 | 1.00 | ||
| % below poverty level | 1 (0.76%-16.84%) | 1.00 | 1.00 | 1.00 | |
| 2 (16.84%-32.11%) | 1.16 (0.99, 1.35) | 1.06 (0.96, 1.16) | 1.03 (0.94, 1.14) | ||
| 3 (32.45%-67.96%) | 1.41 (1.23, 1.61) | 1.15 (1.04, 1.27) | 1.08 (0.98, 1.19) | ||
| % non-Hispanic Black | 1 (0–12.4%) | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 (12.73%-70.11%) | 1.25 (1.07, 1.47) | 1.05 (0.93, 1.18) | 1.06 (0.95, 1.19) | 0.96 (0.85, 1.07) | |
| 3 (70.19%-99.85%) | 1.76 (1.52, 2.03) | 1.06 (0.93, 1.20) | 1.08 (0.95, 1.23) | 0.92 (0.79, 1.06) | |
| % Hispanic | 1 (0–3.23%) | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 (3.28%-7.27%) | 0.81 (0.70, 0.94) | 0.95 (0.87, 1.03) | 0.94 (0.87, 1.02) | 0.93 (0.86, 1.01) | |
| 3 (7.27%-90.63%) | 0.78 (0.69, 0.88) | 1.03 (0.95, 1.12) | 1.02 (0.94, 1.11) | 1.00 (0.91, 1.11) | |
| Violent crime rates | 1 (7.56–144.18) | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 (144.4–305.96) | 1.28 (1.09, 1.51) | 1.10 (0.99, 1.22) | 1.07 (0.96, 1.19) | 0.99 (0.89, 1.11) | |
| 3 (306.63–1134.59) | 1.54 (1.34, 1.78) | 1.17 (1.06, 1.29) | 1.10 (0.99, 1.21) | 1.00 (0.89, 1.13) | |
| SES Index | 1 ((-10.66)-(-2.68)) | 2.00 (1.71, 2.34) | 1.41 (1.25, 1.58) | 1.36 (1.21, 1.52) | 1.46 (1.25, 1.69) |
| 2 ((-2.67)-4.46) | 1.62 (1.37, 1.92) | 1.30 (1.17, 1.46) | 1.30 (1.17, 1.45) | 1.35 (1.21, 1.52) | |
| 3 (4.47–14.1) | 1.00 | 1.00 | 1.00 | 1.00 | |
| Social capital | 1 (0.56–0.59) | 1.20 (1.04, 1.38) | 1.09 (0.99, 1.20) | 1.06 (0.97, 1.17) | 0.96 (0.87, 1.06) |
| 2 (0.59–0.63) | 1.30 (1.13, 1.50) | 1.14 (1.05, 1.24) | 1.12 (1.03, 1.22) | 1.03 (0.94, 1.13) | |
| 3 (0.63–0.72) | 1.00 | 1.00 | 1.00 | 1.00 |
Abbreviations: RR-relative risk; aRR-adjusted relative risk; CI-Confidence Interval; m—Meters; SES—Socioeconomic Status.
Model 1: Unadjusted.
Model 2: Adjusted for age, race/ethnicity, and sex.
Model 3: Adjusted for age, race/ethnicity, sex, and insurance type.
Model 4: All neighborhood measures modeled jointly in the same model. Also adjusted for age, race/ethnicity, sex, insurance type.
Results
Study cohort description
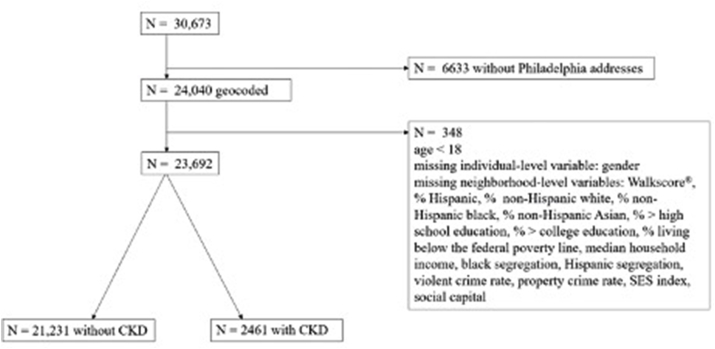
The final analytic cohort was comprised of 23,692 individuals (Fig. 1) from 13 primary care clinics. The cohort included residents of 369 of Philadelphia's 381 census tracts. In the overall cohort, 60% were female; 46% non-Hispanic Black; 33% non-Hispanic white; 5% Hispanic; 3% non-Hispanic Asian; and 11% non-Hispanic other. The majority (54%) had health insurance from a commercial plan, whereas 26% were Medicaid beneficiaries and 10% were Medicare beneficiaries. The average age was 43.5 ( ±16.6) years. The overall prevalence of diabetes in the cohort was 11%; hypertension, 27%; coronary artery disease, 4%; HIV, 6%; and hepatitis C, 4%. Nearly half (42%) had a documented history of tobacco use.
Fig. 1.
Assembly of the analytical cohort.
CKD prevalence in the cohort was 10%. Compared to those without CKD, those with CKD were older (63 vs. 41 years; p < 0.001); more likely to self-identify as non-Hispanic Black (68% vs. 44%; <0.001); and more likely to have Medicare for primary insurance coverage (33% vs. 7%; <0.001). In addition, those with CKD had a higher burden of all measured comorbidities than those without CKD. Patients with CKD had a mean of 7.6 clinic visits over the study period, while those without CKD had 3.5 visits (Table 1).
Table 1.
Baseline characteristics by chronic kidney disease status.
| Characteristic | Category | Total sample |
CKD Diagnosis |
P-value | |
|---|---|---|---|---|---|
| N = 23,692 | NO |
YES |
|||
| N = 21,231 (89.6%) | N = 2461 (10.4%) | ||||
| Person-level | |||||
| Age | Age at first visit in years (mean (std)) | 43.5 (16.6) | 41.3 (15.3) | 62.9 (14.9) | <0.001 |
| Race/ethnicity | Non-Hispanic Asian | 803 (3.39%) | 768 (3.62%) | 35 (1.42%) | <.0001 |
| Non-Hispanic Black | 11,013 (46.48%) | 9335 (43.97%) | 1678 (68.18%) | ||
| Hispanic or Latino | 1237 (5.22%) | 1108 (5.22%) | 129 (5.24%) | ||
| Non-Hispanic Other | 2716 (11.46%) | 2649 (12.48%) | 67 (2.72%) | ||
| Non-Hispanic White | 7923 (33.44%) | 7371 (34.72%) | 552 (22.43%) | ||
| Sex | Female | 14,289 (60.31%) | 12,843 (60.49%) | 1446 (58.76%) | 0.0958 |
| Male | 9403 (39.69%) | 8388 (39.51%) | 1015 (41.24%) | ||
| Insurance | Missing | 625 (2.64%) | 605 (2.85%) | 20 (0.81%) | <.0001 |
| Commercial | 12,779 (53.94%) | 12,281 (57.84%) | 498 (20.24%) | ||
| Medicaid | 6052 (25.54%) | 5547 (26.13%) | 505 (20.52%) | ||
| Medicare | 2401 (10.13%) | 1581 (7.45%) | 820 (33.32%) | ||
| Medicare Advantage | 1835 (7.75%) | 1217 (5.73%) | 618 (25.11%) | ||
| Clinic visits | Per subject during the study period (mean (std)) | 3.9 (3.8) | 3.5 (3.2) | 7.6 (6.1) | <0.001 |
| Hepatitis C | No | 22,847 (96.43%) | 20,626 (97.15%) | 2221 (90.25%) | <.0001 |
| Yes | 845 (3.57%) | 605 (2.85%) | 240 (9.75%) | ||
| HIV | No | 22,244 (93.89%) | 20,102 (94.68%) | 2142 (87.04%) | <.0001 |
| Yes | 1448 (6.11%) | 1129 (5.32%) | 319 (12.96%) | ||
| Coronary artery Disease | No | 22,811 (96.28%) | 20,769 (97.82%) | 2042 (82.97%) | <.0001 |
| Yes | 881 (3.72%) | 462 (2.18%) | 419 (17.03%) | ||
| Smoking status | Missing | 473 (2%) | 442 (2.08%) | 31 (1.26%) | <.0001 |
| No | 13,238 (55.88%) | 12,216 (57.54%) | 1022 (41.53%) | ||
| Yes | 9981 (42.13%) | 8573 (40.38%) | 1408 (57.21%) | ||
| Diabetes | No | 21,160 (89.31%) | 19,629 (92.45%) | 1531 (62.21%) | <.0001 |
| Yes | 2532 (10.69%) | 1602 (7.55%) | 930 (37.79%) | ||
| Hypertension | No | 17,230 (72.72%) | 16,471 (77.58%) | 759 (30.84%) | <.0001 |
| Yes | 6462 (27.28%) | 4760 (22.42%) | 1702 (69.16%) | ||
| Neighborhood-level | |||||
| Healthy food stores within 800 m | 1(0) | 6845 (28.89%) | 6118 (28.82%) | 727 (29.54%) | <.0001 |
| 2(0.5–0.9) | 9880 (41.70%) | 8777 (41.34%) | 1103 (44.82%) | ||
| 3(1.4–5.9) | 6967 (29.41%) | 6336 (29.84%) | 631 (25.64%) | ||
| Walk Score® | 1(20.4–77.9) | 7831 (33.05%) | 7008 (33.01%) | 823 (33.44%) | <.0001 |
| 2(78.0–85.9) | 7843 (33.10%) | 6890 (32.45%) | 953 (38.72%) | ||
| 3(86.0–99) | 8018 (33.84%) | 7333 (34.54%) | 685 (27.83%) | ||
| % > = high school education | 1 (41.9%-81.8%) | 7843 (33.10%) | 6814 (32.09%) | 1029 (41.81%) | <.0001 |
| 2 (82.0%-91.3%) | 7953 (33.57%) | 7057 (33.24%) | 896 (36.41%) | ||
| 3 (91.5%-100%) | 7896 (33.33%) | 7360 (34.67%) | 536 (21.78%) | ||
| Median household income (U.S. dollars) | 1(9945–30786) | 7901 (33.35%) | 6781 (31.94%) | 1120 (45.51%) | <.0001 |
| 2(31,124–57027) | 7894 (33.32%) | 7087 (33.38%) | 807 (32.79%) | ||
| 3(57,039–149,211) | 7897 (33.33%) | 7363 (34.68%) | 534 (21.7%) | ||
| % below poverty level | 1(0.7%-16.8%) | 7751 (32.72%) | 7112 (33.5%) | 639 (25.97%) | <.0001 |
| 2(16.8%-32.1%) | 8018 (33.84%) | 7251 (34.15%) | 767 (31.17%) | ||
| 3(32.4%-67.9%) | 7923 (33.44%) | 6868 (32.35%) | 1055 (42.87%) | ||
| SES Index | 1((-10.66)-(-2.68)) | 7894 (33.32%) | 6796 (32.01%) | 1098 (44.62%) | <.0001 |
| 2((-2.67)-4.46) | 8132 (34.32%) | 7277 (34.28%) | 855 (34.74%) | ||
| 3(4.47–14.1) | 7666 (32.36%) | 7158 (33.71%) | 508 (20.64%) | ||
| Social capital | 1(0.56–0.59) | 7905 (33.37%) | 6977 (32.86%) | 928 (37.71%) | <.0001 |
| 2(0.59–0.63) | 7937 (33.50%) | 7047 (33.19%) | 890 (36.16%) | ||
| 3(0.63–0.72) | 7850 (33.13%) | 7207 (33.95%) | 643 (26.13%) | ||
| % Hispanic | 1(0–3.23%) | 7900 (33.34%) | 6930 (32.64%) | 970 (39.41%) | <.0001 |
| 2(3.28%-7.27%) | 8099 (34.18%) | 7329 (34.52%) | 770 (31.29%) | ||
| 3(7.27%-90.63%) | 7693 (32.47%) | 6972 (32.84%) | 721 (29.3%) | ||
| % Non-Hispanic Black | 1(0–12.4%) | 7920 (33.43%) | 7359 (34.66%) | 561 (22.8%) | <.0001 |
| 2(12.73%-70.11%) | 7851 (33.14%) | 7086 (33.38%) | 765 (31.08%) | ||
| 3(70.19%-99.85%) | 7921 (33.43%) | 6786 (31.96%) | 1135 (46.12%) | ||
| Violent crime rates (per 10,000 persons) | 1(7.56–144.18) | 7965 (33.62%) | 7402 (34.86%) | 563 (22.88%) | <.0001 |
| 2(144.4–305.96) | 7812 (32.97%) | 6997 (32.96%) | 815 (33.12%) | ||
| 3(306.63–1134.59) | 7915 (33.41%) | 6832 (32.18%) | 1083 (44.01%) | ||
| Population density (persons per square km) | 1(113.53–6053.78) | 7888 (33.29%) | 7105 (33.47%) | 783 (31.82%) | <.0001 |
| 2(6055.46–9358.54) | 7946 (33.54%) | 7010 (33.02%) | 936 (38.03%) | ||
| 3(9370.49–32498.47) | 7858 (33.17%) | 7116 (33.52%) | 742 (30.15%) | ||
Abbreviations: CKD—Chronic Kidney Disease; std—Standard Deviation; HIV—Human Immunodeficiency Virus; m—meter; SES—Socioeconomic Status; km—kilometer; US—United States.
Neighborhood characteristics and prevalence of CKD by census tract
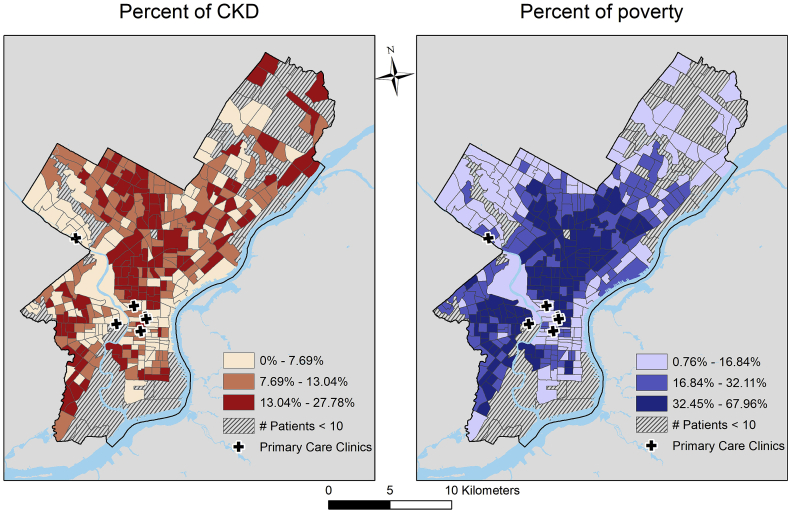
CKD prevalence varied substantially across census tracts and was 16–28% in 68 census tracts (Fig. 2). In general, the prevalence of CKD across census tracts in Philadelphia closely mirrored that of neighborhood poverty (Fig. 2), with higher (i.e., >13%) CKD prevalence in many of the census tracks in which 32% or more residents have incomes that are under the federal poverty level.
Fig. 2.
Distribution of chronic kidney disease and percentage of residents living below the federal poverty line across philadelphia census tracts.
Association between neighborhood characteristics and CKD
In unadjusted models, lower neighborhood walkability, lower % of high school graduates, lower median household income, higher % of residents living below the federal poverty line, higher % of non-Hispanic Black residents, higher violent crime rate, and lower neighborhood social capital were associated with higher CKD prevalence (p < 0.05 for all) (Table 2). Higher % of Hispanic residents was associated with lower prevalence of CKD in unadjusted models (highest tertile vs. lowest tertile: RR 0.78 [0.69, 0.88]; mid-tertile to lowest tertile: RR 0.81 [0.70, 0.94]). After adjustment for age, race/ethnicity, sex, and individual insurance type, % of high school graduates, median household income, and the SES index retained significant associations with CKD. In a fully adjusted model with all neighborhood characteristics as well as age, race/ethnicity, sex and insurance, only the SES index retained a statistically significant association with prevalent CKD (lowest tertile vs. highest tertile: aRR 1.46 [1.25, 1.69]; mid-tertile vs. highest-tertile: aRR 1.35 [1.21, 1.52]) (Table 2). Results were robust in sensitivity analyses that defined CKD by either eGFR <60 ml/min/1.73m2 or an ICD-9 code for CKD (Supplemental Tables 5 and 6).
Association between neighborhood characteristics and blood pressure and glycemic control in patients with CKD
Among those with CKD and documented BP measures (n = 2441), 66% (n = 1605) had an uncontrolled BP on at least one occasion during the study period (Table 3). Age was similar when comparing those with and without uncontrolled BP (mean 63 vs 62 years, p = 0.16). In unadjusted analyses, those with uncontrolled BP were more likely to live in neighborhoods with higher violent crime rates, lower median household income, lower educational attainment, lower social capital, lower SES index, and a higher percentage of residents who are non-Hispanic Blacks. In multivariable models adjusted for age, race/ethnicity, sex, insurance status, and all neighborhood characteristics, no neighborhood characteristics retained a significant association with uncontrolled BP among those with CKD.
Table 3.
Neighborhood characteristics of individuals with chronic kidney disease, stratified by blood pressure control status.
| Characteristic | Overall Cohort with CKD and >1 BP measurement Mean (SE) Median (IQR) |
Uncontrolled BPa Mean (SE) Median (IQR) |
P-value | |
|---|---|---|---|---|
| N = 2441 | NO N = 836 (34.3%) |
YES N = 1605 (65.7%) |
||
| Healthy food stores per sq km within 800 m | 0.83 (0.81) | 0.86 (0.85) | 0.82 (0.78) | 0.20 |
| 0.5 (0, 1.49) | 0.5 (0, 1.49) | 0.5 (0, 0.99) | ||
| Walk Score® | 79.68 (12.3) | 79.11 (14.31) | 79.97 (11.1) | 0.12 |
| 81.86 (74.79, 87) | 81.67 (74, 88.08) | 81.86 (75, 86.25) | ||
| % persons ≥ high school | 83.48% (9.61) | 84.3% (9.91) | 83.05% (9.43) | <0.01 |
| 84.04 (78.37, 90.58) | 85.01 (78.54, 92.23) | 83.27 (78.18, 89.82) | ||
| median household income | 39759.68 (22641.9) | 43603.69 (24154.09) | 37757.43 (21550.5) | <.0001 |
| 32,984 (21,750, 53,233) | 37,130 (24941.5, 59,063) | 31,332 (21,106, 50,208) | ||
| % persons below poverty level | 28.85% (15) | 26.46% (15.02) | 30.09% (14.84) | <.0001 |
| 30.19 (15.77, 38.93) | 26.53 (13.42, 36.27) | 31.29 (16.97, 40.96) | ||
| SES Index | −0.66 (5.51) | 0.12 (5.84) | −1.06 (5.29) | <.0001 |
| −2.11 (−4.85, 3.43) | −1.37 (−4.64, 4.74) | −2.36 (−5.09, 1.67) | ||
| Social capital | 0.61 (0.03) | 0.61 (0.03) | 0.61 (0.03) | 0.0001 |
| 0.6 (0.59, 0.63) | 0.61 (0.59, 0.64) | 0.6 (0.59, 0.62) | ||
| % Hispanic | 9.23% (14.5) | 9.39% (14.79) | 9.15% (14.35) | 0.70 |
| 4.53 (2.46, 8.4) | 4.51 (2.43, 8.45) | 4.53 (2.51, 8.34) | ||
| % Non-Hispanic Black | 54.87% (34.54) | 49.44% (36.01) | 57.71% (33.41) | <.0001 |
| 65.01 (15.34, 88.2) | 53.86 (10.19, 87.33) | 68.42 (24, 88.36) | ||
| Population Density in sq km | 8022.6 (4022.12) | 8124.55 (4379.12) | 7969.5 (3823.34) | 0.39 |
| Violence crime rates per 10,000 population | 322.25 (214.6) | 304.92 (220.37) | 331.27 (211.04) | 0.004 |
| 273.21 (156.65, 455.99) | 247.69 (136.47, 418.44) | 276.6 (165.89, 473.7) | ||
Abbreviations: CKD—chronic kidney disease; BP—blood pressure; SE−standard error; IQR—interquartile range; m—meters; SES—socioeconomic status; sq—square; km--kilometers.
Uncontrolled blood pressure defined as ≥ 140 mmHg systolic and/or ≥ 90 mm Hg diastolic on at ≥ 1 measurement.
Among those with CKD who had at least one measured hemoglobin A1c during the study period (n = 2008), poor glycemic control (i.e., hemoglobin A1c > 6.5%) was observed in 42% (n = 838) (Table 4). Age was similar when comparing those with and without poor glycemic control (mean 63 vs 63 years, p = 0.49). Unadjusted analyses showed a higher likelihood of poor glycemic control in non-Hispanic Blacks living in neighborhoods with high violent crime rates, lower median household income, lower educational attainment, lower social capital, and lower SES index. In models adjusted for age, race/ethnicity, sex, insurance status, and all neighborhood characteristics, residence in neighborhoods with mid-level WalkScore® (relative to the most walkable neighborhoods) was significantly associated with poor glycemic control (aRR 1.20, 95% CI 1.01–1.42) among those with CKD. Among those without CKD, lower neighborhood walkability defined by WalkScore® and lower neighborhood SES index were independently associated with poor blood pressure control in multivariable models adjusted for other neighborhood characteristics, whereas lower SES index was independently associated with poor blood glucose control (see Supplemental Appendix).
Table 4.
Neighborhood characteristics of individuals with chronic kidney disease, stratified by glycemic control status.
| Characteristic | Overall Cohort with CKD and ≥ Hemoglobin A1C Measurement Mean (SE) Median (IQR) |
Poor Glycemic Controla Mean (SE) Median (IQR) |
P-value | |
|---|---|---|---|---|
| N = 2008 | NO N = 1170 (58.2%) |
YES N = 838 (41.7%) |
||
| Healthy food stores per sq km within 800 m | 0.84 (0.81) | 0.85 (0.84) | 0.83 (0.78) | 0.62 |
| 0.5 (0, 1.49) | 0.5 (0, 1.49) | 0.5 (0, 0.99) | ||
| Walk Score® | 80.11 (11.5) | 80.25 (12.08) | 79.92 (10.63) | 0.52 |
| 82 (75, 87) | 82.5 (75, 87.86) | 81.67 (75, 86) | ||
| % persons ≥ high school | 83.06% (9.54) | 83.61% (9.5) | 82.29% (9.56) | <0.01 |
| 83.36 (78.09, 89.64) | 84.12 (78.09, 90.4) | 82.98 (77.88, 88.82) | ||
| Median household income | 38315.21 (21661.13) | 39555.83 (22496.95) | 36583.07 (20323.93) | <0.01 |
| 32265.5 (21,495, 50,556) | 33,026 (21,667, 52,465) | 30,638 (21,106, 45,610) | ||
| % persons below poverty level | 29.65% (14.82) | 29.21% (15.35) | 30.27% (14.03) | 0.10 |
| 30.85 (16.95, 40.55) | 30.2 (15.77, 40.66) | 31.49 (18.41, 40.38) | ||
| SES Index | −0.95 (5.38) | −0.56 (5.64) | −1.48 (4.95) | <0.001 |
| −2.23 (−5.08, 1.78) | −2.07 (−4.85, 3.43) | −2.42 (−5.35, 1.09) | ||
| Social capital | 0.61 (0.03) | 0.61 (0.03) | 0.61 (0.03) | 0.01 |
| 0.6 (0.59, 0.62) | 0.6 (0.59, 0.63) | 0.6 (0.59, 0.62) | ||
| % Hispanic | 9.36% (14.52) | 8.84% (14.13) | 10.07% (15.04) | 0.06 |
| 4.57 (2.46, 8.46) | 4.51 (2.42, 8.34) | 4.88 (2.51, 9.21) | ||
| % Non-Hispanic Black | 56.55% (33.81) | 55.13% (34.64) | 58.54% (32.55) | 0.02 |
| 66.76 (20.1, 88.2) | 65.01 (17.23, 88.2) | 68.85 (25.9, 88.2) | ||
| Violence crime rates per 10,000 population | 329.43 (213.69) | 329.36 (223.25) | 329.53 (199.71) | 0.98 |
| 276.6 (164.15, 466.81) | 272.63 (157.52, 466.81) | 287.94 (168.94, 466.81) | ||
| Population Density in sq km | 8107.09 (3929.34) | 8162.81 (3951.11) | 8029.3 (3899.77) | 0.45 |
Abbreviations: sq—square; km—kilometer; RR-relative risk; aRR-adjusted relative risk; CI-Confidence Interval; km—kilometer.
Poor Glycemic Control Defined as Hemoglobin A1C > 6.5% on ≥1 Measurement.
Discussion
In this study, we characterized Philadelphia neighborhoods by factors including access to healthy food stores, walkability, violent crime rate, social capital, and SES (median household income, education level, etc.) and found that several of these neighborhood indicators were associated with CKD prevalence. However, when these neighborhood context features were considered collectively in multivariable models, neighborhood SES (measured by the SES index) was the only one to retain a statistically significant association with CKD risk. Furthermore, among those with CKD, the middle-tier of neighborhood walkability (WalkScore®) was associated with worse glycemic control than neighborhoods with the lowest and highest walkability. Our findings indicate that in an urban setting, neighborhood-level SES and walkability may influence the risk of CKD and CKD progression.
The SES index is a composite of multiple SES factors, including median household income; median value of housing units; percentage of residents in executive/managerial/professional occupations; percentage of households with interest, dividend, or rental income; percentage of residents with a high school diploma; and percentage of residents with a college diploma. It was devised to circumvent the challenge of disentangling highly-correlated features of SES, such as education and income, on clinical outcomes, and has been associated with stroke and cardiovascular disease (Diez Roux, Merkin et al., 2019; Diez-Roux, Kiefe et al., 2019; Howard et al., 2016). We found an independent association between lower neighborhood SES index and CKD risk. Further, among those without CKD, lower SES was associated with poor blood pressure and glycemic control, respectively. One potential explanation for our findings is that the SES index might be a surrogate marker for access to health-promoting resources. The general educational attainment of a community influences its health literacy, which represents its members' ability to obtain, process, and understand basic health information and services to make appropriate health decisions (Parker et al., 2003). Specifically, activities inherent to health literacy can include communicating health history with medical providers, participating in self-care and chronic disease management, and understanding math concepts such as probability and risk, all of which may be determinants of CKD risk and outcomes (Parker et al., 2003; Healthy People 2020). The financial well-being of a community represents available capital for resources like healthy food, safe living conditions, and quality healthcare. Therefore, neighborhood-level socioeconomic characteristics, if favorable, might overcome other neighborhood-level barriers to health (e.g., violent crime and lack of proximity to healthy food). A similar phenomenon was observed by Bowe et al. using nationwide, county-level data from the Veteran's Administration to examine the association between a wide variety of neighborhood context measures (health-related behaviors, healthcare accessibility, socioeconomic characteristics, transit/housing availability, air and water quality, community safety) and rapid eGFR loss (>5 ml/min/1.73 m2/year). Veterans residing in counties with the least-favorable scores for all varieties of neighborhood context experienced significantly more rapid eGFR loss than residents in counties with the most-favorable scores, but healthcare accessibility and SES were the only measures to show a significant difference between mid-level and most-favorable scores (Bowe et al., 2017). As in our study, these findings underscore the relative importance of neighborhood-level SES compared to other neighborhood-level characteristics in CKD risk and outcomes.
The results of our study also suggest that there is an association between neighborhood walkability and glycemic control among CKD patients and blood pressure control among those without CKD. This finding is consistent with observations from cohorts that did not specify the subjects’ CKD status (Le-Scherban et al., 2019; Tabaei et al., 2018). Interestingly, in our study only mid-level WalkScore® (i.e., the middle of three tiers of walkability) retained statistical significance with worse glycemic control among CKD patients after adjustment for all neighborhood context factors. An explanation for this might be that a mid-level WalkScore® can be described as a “somewhat walkable” neighborhood. This contrasts with the highest tertile of WalkScore®, where residents can accomplish most or all errands by walking, and the lowest tertile, where residents require a car to accomplish nearly all activities. Conversely, among those without CKD, the lowest tertile of WalkScore® was associated with poor blood pressure control, relative to the most walkable neighborhoods. Often, there is residual confounding of WalkScore® by SES. That is, the most walkable neighborhoods tend to be in “downtown” areas with close proximity to shopping districts with higher real estate values, and the least walkable neighborhoods, suburban-like communities also characterized by higher real estate values. This was the case in our cohort. However, the associations we observed between WalkScore® and glycemic control and blood pressure control, respectively, remained statistically significant after adjustment for the SES index. Among those with CKD, our findings could suggest that the physical structure of somewhat walkable neighborhoods might be a barrier to accessing the resources needed to achieve glycemic control (e.g., medical facilities and pharmacies). Poor glycemic control is a risk factor for CKD progression, particularly in the early course of diabetes (de Boer & Group, 2014; Holman et al., 2008; Warren et al., 2018). Conversely, among those without CKD, the least walkable neighborhoods might promote a sedentary lifestyle, a risk factor for poor blood pressure control (Dempsey et al., 2018). The results of our study suggest that interventions to improve access to health care resources and neighborhood walkability might have measurable impacts on CKD risk factors, and that neighborhood context should be considered when evaluating factors that might influence CKD progression (Richardson, Ghosh-Dastidar, & Collins, 2020).
The social constructs of race and ethnicity are often examined as risk factors for CKD. Non-Hispanic Blacks experience higher rates of CKD progression and ESKD and lower rates of kidney transplantation than other groups (Klag et al., 1997; Mehrotra et al., 2008; Whittle et al., 1991). Reasons for these differences likely include both genetic predisposition as well as a long-term consequence of structural racism (Beydoun et al., 2017; Parsa et al., 2013). Previous studies have observed that residence in predominantly Black neighborhoods is associated with lower rates of transplantation wait-listing (Arriola, 2017; Peng et al., 2018). However, less is understood about the relationship of Hispanic ethnicity and CKD, at both an individual and neighborhood level. Some studies have shown similar incidence of CKD progression among Hispanics as non-Hispanic Blacks, but the risk is attenuated by adjustment for important comorbidities and SES factors (Fischer et al., 2016). We found evidence of a significant unadjusted association between non-Hispanic Black neighborhood racial composition and CKD. However, this association was no longer statistically significant after adjustment for individual-level age, sex, race, ethnicity, insurance, and other neighborhood characteristics. A reverse relationship was observed for residents of predominantly Hispanic neighborhoods, but this association was also substantially attenuated after multivariable adjustment. In combination with our other findings, these results suggest that neighborhood SES might be a more reliable marker of CKD risk than neighborhood racial composition.
To meet the ambitious goals of the Advancing American Kidney Health Initiative, US health care providers are in need of tools to identify more people with CKD in its earlier stages (Bieber & Gadegbeku, 2019; Government US, 2019). Our findings suggest that consideration of social determinants of health, at a neighborhood level, should be fundamental to the redesign of CKD care. Metrics such as the SES index might be a useful tool for both policymakers and physicians when creating programs to addresskidney health. Indeed, experts recommend that integrating neighborhood-level social determinants of health into electronic health records could provide physicians with insights on typically unmeasured drivers of health status (Cantor & Thorpe, 2018). Our results suggest that integration of the SES index into electronic health records could provide a quick snapshot of the collective health literacy and financial well-being of an individual patient's neighborhood of residence and therefore, serve as another tool to stratify CKD risk. In addition, low SES neighborhoods should be the targets of on-site CKD screening and awareness programs, particularly to reach residents who might have difficulty accessing the healthcare system.
The study has several strengths, including its large sample size and its granular data on neighborhood social context in a contemporary urban population struggling with the highest deep poverty rate of all large UScities. Our cohort characteristics largely mirrored those of the overall Philadelphia population. CKD prevalence in our cohort was similar to the US national CKD prevalence. However, there are also limitations to this study. For example, due to its cross-sectional design, we could not assess casual relationships between neighborhood context and CKD progression. We were also unable to adjust for individual-level SES beyond insurance type. Generalizability of our findings might also be a concern given our focus on a single academic health system in Philadelphia. There is also the potential for selection bias given that our study only included Philadelphia residents who utilized primary care, as well as susceptibility to misclassification bias from the use of billing codes to identify some comorbid conditions and potentially, errors in BP measurement. However, our approach was well-suited to examine the potential utility of neighborhood metrics, and specifically the SES index, as tools to screen for at-risk individual patients in the primary care setting and to design interventions for diagnosing and treating CKD in at-risk neighborhoods in Philadelphia.
In conclusion, in a large cohort of adults from Philadelphia, we found that the SES of a neighborhood might influence the risk of CKD. The SES index, in particular, could be a valuable tool to help achieve the goals of the Advancing American Kidney Health Initiative if it can identify which US communities are at highest risk for CKD. Additionally, our findings that neighborhood WalkScore® is independently associated with poor glycemic control in CKD patients and poor blood pressure control in those without CKD indicate that interventions to enhance neighborhood walkability should be tested to improve CKD and related outcomes. Further investigation in longitudinal urban cohorts is necessary to examine whether SES index and other neighborhood characteristics might influence CKD progression.
Author statement
Suzanne Boyle: Funding acquisition, Conceptualization, Investigation, Methodology, Data Curation, Writing-Original Draft Preparation, Review, and Editing; Yuzhe Zhao: Methodology, Formal Analysis, Visualization, Writing-Original Draft Preparation, Review, and Editing; Edgar Chou: Conceptualization, Writing-Original Draft Preparation, Review, and Editing; Kari Moore: Conceptualization, Methodology, Formal Analysis, Writing-Original Draft Preparation, Review, and Editing, Supervision; Meera Harhay: Funding acquisition, Conceptualization, Methodology, Writing-Original Draft Preparation, Review, and Editing, Supervision
Ethics statement
The authors declare that there are no financial or personal relationships with other people or organizations that could have inappropriately influenced or biased their work.
Financial conflicts of interest
The authors declare no financial conflicts of interest.
Acknowledgements
This research was supported by the Drexel University Urban Health Collaborative pilot award program. MNH is supported by NIH grants - K23DK105207 and R01DK124388.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2020.100646.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- American Communities Survey Population density, 2013-2017. https://www.census.gov/programs-surveys/acs
- Arriola K.J. Race, racism, and access to renal transplantation among African Americans. Journal of Health Care for the Poor and Underserved. 2017;28(1):30–45. doi: 10.1353/hpu.2017.0005. [DOI] [PubMed] [Google Scholar]
- Assessment PsCH Health of the city. 2018. https://www.phila.gov/media/20181220135006/Health-of-the-City-2018.pdf 2018.
- Banerjee T., Crews D.C., Wesson D.E. Food insecurity, CKD, and subsequent ESRD in US adults. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2017;70(1):38–47. doi: 10.1053/j.ajkd.2016.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker-Cummings C., McClellan W., Soucie J.M., Krisher J. Ethnic differences in the use of peritoneal dialysis as initial treatment for end-stage renal disease. Jama. 1995;274(23):1858–1862. [PubMed] [Google Scholar]
- Beydoun M.A., Poggi-Burke A., Zonderman A.B., Rostant O.S., Evans M.K., Crews D.C. Perceived discrimination and longitudinal change in kidney function among urban adults. Psychosomatic Medicine. 2017;79(7):824–834. doi: 10.1097/PSY.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber S.D., Gadegbeku C.A. A call to action for the kidney community: Nephrologists' perspective on advancing American kidney health. Clinical Journal of the American Society of Nephrology. 2019;14(12):1799–1801. doi: 10.2215/CJN.10470919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer I.H., Group D.E.R. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):24–30. doi: 10.2337/dc13-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B., Xie Y., Xian H., Lian M., Al-Aly Z. Geographic variation and US county characteristics associated with rapid kidney function decline. Kidney Int Rep. 2017;2(1):5–17. doi: 10.1016/j.ekir.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M.A., Beech B.M., Crook E.D. Association of socioeconomic status and CKD among African Americans: The jackson heart study. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2010;55(6):1001–1008. doi: 10.1053/j.ajkd.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler J.W., Castro J.C., Cohen S., Zhao Y., Melly S., Moore K. Personal and neighborhood attributes associated with cervical and colorectal cancer screening in an urban African American population. Preventing Chronic Disease. 2019;16:E118. doi: 10.5888/pcd16.190030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C., Nedelman J., Luke R.G. Race, socioeconomic status, and the development of end-stage renal disease. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 1994;23(1):16–22. doi: 10.1016/s0272-6386(12)80806-7. [DOI] [PubMed] [Google Scholar]
- Cantor M.N., Thorpe L. Integrating data on social determinants of health into electronic health records. Health Affairs. 2018;37(4):585–590. doi: 10.1377/hlthaff.2017.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christine P.J., Auchincloss A.H., Bertoni A.G. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: The multi-ethnic study of Atherosclerosis (MESA) JAMA Intern Med. 2015;175(8):1311–1320. doi: 10.1001/jamainternmed.2015.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coresh J., Selvin E., Stevens L.A. Prevalence of chronic kidney disease in the United States. Jama. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- Crews D.C., Charles R.F., Evans M.K., Zonderman A.B., Powe N.R. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2010;55(6):992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey P.C., Larsen R.N., Dunstan D.W., Owen N., Kingwell B.A. Sitting less and moving more: Implications for hypertension. Hypertension. 2018;72(5):1037–1046. doi: 10.1161/HYPERTENSIONAHA.118.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department Pp Philadelphia Police department. Crime maps and stats. https://www.phillypolice.com/crime-maps-stats/
- Desai N., Lora C.M., Lash J.P., Ricardo A.C. CKD and ESRD in US Hispanics. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2019;73(1):102–111. doi: 10.1053/j.ajkd.2018.02.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux A.V., Merkin S.S., Arnett D. Neighborhood of residence and incidence of coronary heart disease. New England Journal of Medicine. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- Diez-Roux A.V., Kiefe C.I., Jacobs D.R., Jr. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Annals of Epidemiology. 2001;11(6):395–405. doi: 10.1016/s1047-2797(01)00221-6. [DOI] [PubMed] [Google Scholar]
- Esri ArcGIS for desktop: Creating a composite address locator (ArcMap 10.5) 2018. http://desktop.arcgis.com/en/arcmap/10.5/manage-data/geocoding/creatinga- composite-address-locator.htm
- Fischer M.J., Hsu J.Y., Lora C.M. CKD progression and mortality among Hispanics and non-hispanics. Journal of the American Society of Nephrology : Journal of the American Society of Nephrology. 2016;27(11):3488–3497. doi: 10.1681/ASN.2015050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M.C., Hwang S.J., Larson M.G. Overweight, obesity, and the development of stage 3 CKD: The framingham heart study. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2008;52(1):39–48. doi: 10.1053/j.ajkd.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government US Executive order on advancing American kidney health. 2019. https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/
- Hall Y.N. Social determinants of health: Addressing unmet needs in nephrology. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2018;72(4):582–591. doi: 10.1053/j.ajkd.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Hall Y.N., O'Hare A.M., Young B.A., Boyko E.J., Chertow G.M. Neighborhood poverty and kidney transplantation among US Asians and Pacific Islanders with end-stage renal disease. American Journal of Transplantation. 2008;8(11):2402–2409. doi: 10.1111/j.1600-6143.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- Hao H., Lovasik B.P., Pastan S.O., Chang H.H., Chowdhury R., Patzer R.E. Geographic variation and neighborhood factors are associated with low rates of pre-end-stage renal disease nephrology care. Kidney International. 2015;88(3):614–621. doi: 10.1038/ki.2015.118. [DOI] [PubMed] [Google Scholar]
- Healthy people 2020: Health literacy. 2019. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-health/interventions-resources/health-literacy#1 2019.
- Hicken M.T., Katz R., Crews D.C., Kramer H.J., Peralta C.A. Neighborhood social context and kidney function over Time: The multi-ethnic study of atherosclerosis. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2019;73(5):585–595. doi: 10.1053/j.ajkd.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerger T.J., Simpson S.A., Yarnoff B.O. The future burden of CKD in the United States: A simulation model for the CDC CKD initiative. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2015;65(3):403–411. doi: 10.1053/j.ajkd.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. New England Journal of Medicine. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Howard V.J., McClure L.A., Kleindorfer D.O. Neighborhood socioeconomic index and stroke incidence in a national cohort of blacks and whites. Neurology. 2016;87(22):2340–2347. doi: 10.1212/WNL.0000000000003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.Y., Lin F., Vittinghoff E., Shlipak M.G. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. Journal of the American Society of Nephrology : Journal of the American Society of Nephrology. 2003;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- J K. Street Smart Walk score. 2010. https://www.redfin.com/blog/2010/08/street-smart-walk-score.html 2019.
- Karter A.J., Ferrara A., Liu J.Y., Moffet H.H., Ackerson L.M., Selby J.V. Ethnic disparities in diabetic complications in an insured population. Jama. 2002;287(19):2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- Kaufman T.K., Sheehan D.M., Rundle A. Measuring health-relevant businesses over 21 years: Refining the national establishment time-series (NETS), a dynamic longitudinal data set. BMC Research Notes. 2015;8:507. doi: 10.1186/s13104-015-1482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw K.N., Albrecht S.S., Carnethon M.R. Racial and ethnic residential segregation, the neighborhood socioeconomic environment, and obesity among Blacks and Mexican Americans. American Journal of Epidemiology. 2013;177(4):299–309. doi: 10.1093/aje/kws372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klag M.J., Whelton P.K., Randall B.L., Neaton J.D., Brancati F.L., Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. Jama. 1997;277(16):1293–1298. [PubMed] [Google Scholar]
- Lapidis C.M.L., Jang B., Hodgin J., Hicken M. Understanding the link between neighbhorhoods and kidney disease. Kidney. 2020;1(6) doi: 10.34067/KID.0001202019. 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Scherban F., Ballester L., Castro J.C. Identifying neighborhood characteristics associated with diabetes and hypertension control in an urban African-American population using geo-linked electronic health records. Prev Med Rep. 2019;15:100953. doi: 10.1016/j.pmedr.2019.100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziarz M., Black R.A., Fong C.T., Himmelfarb J., Chertow G.M., Hall Y.N. Evaluating risk of ESRD in the urban poor. Journal of the American Society of Nephrology : Journal of the American Society of Nephrology. 2015;26(6):1434–1442. doi: 10.1681/ASN.2014060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra R., Kermah D., Fried L., Adler S., Norris K. Racial differences in mortality among those with CKD. Journal of the American Society of Nephrology : Journal of the American Society of Nephrology. 2008;19(7):1403–1410. doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkin S.S., Coresh J., Diez Roux A.V., Taylor H.A., Powe N.R. Area socioeconomic status and progressive CKD: The atherosclerosis risk in communities (ARIC) study. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2005;46(2):203–213. doi: 10.1053/j.ajkd.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Morton R.L., Schlackow I., Staplin N. Impact of educational attainment on health outcomes in moderate to severe CKD. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2016;67(1):31–39. doi: 10.1053/j.ajkd.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujahid M.S., Diez Roux A.V., Cooper R.C., Shea S., Williams D.R. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis) American Journal of Hypertension. 2011;24(2):187–193. doi: 10.1038/ajh.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OpenDataPhilly. 2019. https://www.opendataphilly.org/group/public-safety-group
- Parker R.M., Ratzan S.C., Lurie N. Health literacy: A policy challenge for advancing high-quality health care. Health Affairs. 2003;22(4):147–153. doi: 10.1377/hlthaff.22.4.147. [DOI] [PubMed] [Google Scholar]
- Parsa A., Kao W.H., Xie D. APOL1 risk variants, race, and progression of chronic kidney disease. New England Journal of Medicine. 2013;369(23):2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer R.E., Plantinga L.C., Paul S. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. Jama. 2015;314(6):582–594. doi: 10.1001/jama.2015.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R.B., Lee H., Ke Z.T., Saunders M.R. Racial disparities in kidney transplant waitlist appearance in Chicago: Is it race or place? Clinical Transplantation. 2018;32(5) doi: 10.1111/ctr.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philadelphia TDoPHotCo . 2018. Philadelphia's community health assessment: Health of the city. 2018. [Google Scholar]
- Purnell T.S., Xu P., Leca N., Hall Y.N. Racial differences in determinants of live donor kidney transplantation in the United States. American Journal of Transplantation. 2013;13(6):1557–1565. doi: 10.1111/ajt.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A.S., Ghosh-Dastidar M., Collins R.L. Improved Street walkability, incivilities, and esthetics are associated with greater park use in two low-income neighborhoods. Journal of Urban Health. 2020;97(2):204–212. doi: 10.1007/s11524-019-00416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R.A., Sen S., Mehta K., Moody-Ayers S., Bacchetti P., O'Hare A.M. Geography matters: Relationships among urban residential segregation, dialysis facilities, and patient outcomes. Annals of Internal Medicine. 2007;146(7):493–501. doi: 10.7326/0003-4819-146-7-200704030-00005. [DOI] [PubMed] [Google Scholar]
- Rundle A., Neckerman K.M., Freeman L. Neighborhood food environment and walkability predict obesity in New York City. Environmental Health Perspectives. 2009;117(3):442–447. doi: 10.1289/ehp.11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saran R., Robinson B., Abbott K.C. US renal data system 2018 annual data report: Epidemiology of kidney disease in the United States. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2019;73(3S1):A7–A8. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham D.A., Vupputuri S., Diez Roux A.V. Kidney disease in life-course socioeconomic context: The atherosclerosis risk in communities (ARIC) study. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2007;49(2):217–226. doi: 10.1053/j.ajkd.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 2015 southeastern Pennsylvania household health Survey. 2015. https://www.phmcresearch.org/52-project-spotlight/248-southeastern-pennsylvania-household-health-survey 2019.
- Statistics CfDCaPNCfH. 2013. https://www.cdc.gov/nchs/fastats/kidney-disease.htm 2019.
- Tabaei B.P., Rundle A.G., Wu W.Y. Associations of residential socioeconomic, food, and built environments with glycemic control in persons with diabetes in New York city from 2007-2013. American Journal of Epidemiology. 2018;187(4):736–745. doi: 10.1093/aje/kwx300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The State of Philadelphians Living in Poverty . 2019. The pew charitable trusts. 2019. [Google Scholar]
- Vart P., Gansevoort R.T., Crews D.C., Reijneveld S.A., Bultmann U. Mediators of the association between low socioeconomic status and chronic kidney disease in the United States. American Journal of Epidemiology. 2015;181(6):385–396. doi: 10.1093/aje/kwu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkova N., McClellan W., Klein M. Neighborhood poverty and racial differences in ESRD incidence. Journal of the American Society of Nephrology : Journal of the American Society of Nephrology. 2008;19(2):356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren B., Rebholz C.M., Sang Y. Diabetes and trajectories of estimated glomerular filtration rate: A prospective cohort analysis of the atherosclerosis risk in communities study. Diabetes Care. 2018;41(8):1646–1653. doi: 10.2337/dc18-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle J.C., Whelton P.K., Seidler A.J., Klag M.J. Does racial variation in risk factors explain black-white differences in the incidence of hypertensive end-stage renal disease? Archives of Internal Medicine. 1991;151(7):1359–1364. [PubMed] [Google Scholar]
- Young E.W., Mauger E.A., Jiang K.H., Port F.K., Wolfe R.A. Socioeconomic status and end-stage renal disease in the United States. Kidney International. 1994;45(3):907–911. doi: 10.1038/ki.1994.120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.