Abstract
Background
Strong preclinical evidence suggests that exenatide, a glucagon-like peptide-1 (GLP-1) receptor agonist used for treating type 2 diabetes, is neuroprotective and disease-modifying in Alzheimer’s Disease (AD).
Objective
We performed an 18-month double-blind randomized placebo-controlled Phase II clinical trial to assess the safety and tolerability of exenatide and explore treatment responses for clinical, cognitive, and biomarker outcomes in early AD.
Method
Eighteen participants with high probability AD based on cerebrospinal fluid (CSF) biomarkers completed the entire study prior to its early termination by the sponsor; partial outcomes were available for twenty-one.
Results
Exenatide was safe and well-tolerated, showing an expectedly higher incidence of nausea and decreased appetite compared to placebo and decreasing glucose and GLP-1 during Oral Glucose Tolerance Tests. Exenatide treatment produced no differences or trends compared to placebo for clinical and cognitive measures, MRI cortical thickness and volume, or biomarkers in CSF, plasma, and plasma neuronal extracellular vesicles (EV) except for a reduction of Aβ42 in EVs.
Conclusion
The positive finding of lower EV Aβ42 supports emerging evidence that plasma neuronal EVs provide an effective platform for demonstrating biomarker responses in clinical trials in AD. The study was underpowered due to early termination and therefore we cannot draw any firm conclusions. However, the analysis of secondary outcomes shows no trends in support of the hypothesis that exenatide is disease-modifying in clinical AD, and lowering EV Aβ42 in and of itself may not improve cognitive outcomes in AD.
Keywords: GLP-1 agonist, exenatide, memory, diabetes, placebo, Alzheimer’s disease
1. INTRODUCTION
Alzheimer’s Disease (AD) is currently the fifth leading cause of death in Americans aged over 65 years and its worldwide incidence and socioeconomic impact are expected to increase dramatically over the coming decades [1]. Developing a disease-modifying treatment for AD is of critical importance for the aging population and a public health emergency. Unfortunately, the four medications that are currently approved for the disease only offer symptomatic relief, disease-modifying treatments do not exist, and a disappointingly large number of Phase III multi-center clinical trials have failed to show clinical benefit [2]. Given that the mechanisms of most medications that failed in clinical trials were based on the “amyloid hypothesis” of AD, which posits that accumulation of oligomeric beta-amyloid (Aβ) is the key etiopathogenic event that triggers a downstream cascade resulting in neuroinflammation, tau misfolding and neurodegeneration [3], there has been progressive disenchantment with the amyloid hypothesis and a drive to target alternative pathogenic mechanisms [2]. A prominent alternative target that is broadly compatible with versions of the amyloid hypothesis is brain insulin resistance [4–6].
Insulin Resistance (IR), broadly defined as a reduced cellular response to insulin [7], has multiple links with the two main etiopathogenic cascades in AD involving Aβ and tau (reviewed extensively by Mullins et al. [4]), and direct effects on brain glucose metabolism and neurodegeneration. Briefly, temporoparietal brain regions that are preferentially vulnerable to combined Aβ and Tau deposition show decreased expression of glucose transporters and insulin signaling genes in healthy individuals compared to brain regions resilient to AD pathology. These vulnerable regions are energetically-reliant on glycolytic metabolism, which in turn promotes Aβ production, especially in physiological states of impaired glucose homeostasis [8, 9]. The resulting accumulation of Aβ promotes serine phosphorylation on Insulin Receptor Substrate 1 (IRS-1) [6], which further impairs downstream insulin signaling and marks the development of regional brain insulin resistance, which in turn promotes tau hyperphosphorylation and tangle formation [4]. For these reasons and based on a litany of epidemiological studies, brain insulin resistance has been identified as a therapeutic target in AD, with a variety of pharmacological approaches, such as intranasal insulin [10, 11] and pioglitazone [12–14], already the subject of clinical trials. An intriguing possibility for reversing brain insulin resistance has been the targeting of glucagon-like peptide-1 (GLP-1) receptors (GLP-1R) [5; 15].
GLP-1 and glucose-dependent insulinotropic peptide (GIP) are peptide hormones that belong to a class of gut hormones called incretins. These are secreted from specialized enteroendocrine cells of the gut in response to food and enhance the secretion, production and cellular actions of insulin [16]. GLP-1, in particular, is a powerful agent for lowering blood glucose in type 2 diabetes mellitus (T2DM) and consequently, its biological effects have been the subject of intense study. Given that endogenous GLP-1 has a short half-life of 1–2 minutes necessitating continuous infusions in order to maintain its biological effects, long-acting GLP-1 analogs and GLP-1R agonists were developed for clinical use [16]. Among these, exendin-4 is a 39-amino acid peptide produced in nature only in the salivary glands of Gila monsters. It is a potent and specific agonist of GLP-1R [17], with effects in all tissues, such as β cells in islets of Langerhans [16] that express GLP-1Rs while its half-life is approximately 2.4 hours. Its biological effects are therefore protracted compared to those of GLP-1. Exendin-4 is manufactured for human use as exenatide, it is delivered via subcutaneous (SC) injection and is the first-in-class of the GLP-1R agonists in routine use.
GLP-1 and exenatide rapidly enter the brain, [18] and G-protein coupled GLP-1Rs are abundantly expressed in neurons throughout the brain [19, 20]. Of relevance to AD, in vitro studies suggest that GLP-1R stimulation is neurotrophic / neuroprotective, by inducing differentiation and neurite outgrowth [21], and providing protection against glutamate-induced apoptotic neuronal cell death. Additionally, it decreases Aβ levels in neuronal cultures, and protects against oxidative stress and membrane lipid peroxidation caused by brain iron accumulation [22]. Apart from these neuroprotective actions, GLP-1R stimulation promotes subventricular zone neurogenesis [23, 24], and enhances cortical and hippocampal synaptic plasticity [25, 26] as well as learning and memory in rodents [27, 28]. GLP-1 agonists including exenatide have shown reductions in Aβ plaques alongside improvements in synaptic function and behavior in multiple transgenic mouse models of AD [24, 29, 30]. In addition, exenatide administration in transgenic AD mice decreases brain IR, which in turn decreases the expression of pS312-IRS-1 and increases pY-IRS-1[6]. Reversal of brain IR is associated with decreased Aβ plaque deposition and may constitute a major mechanism for beneficial actions of exenatide in AD. Because of their multiple neurotrophic, neuroprotective pro-insulinergic and anti-amyloid effects, GLP-1R agonists including exenatide have been proposed as potential disease-modifying therapies for AD [5, 22]. In parallel and based on similar preclinical evidence, GLP1R agonists were identified as potential disease-modifying treatments in Parkinson’s disease (PD) and exenatide was recently found to have beneficial effects on motor function in a randomized, placebo-controlled double-blind Phase II trial in PD [31].
Here we report results from an 18-month double-blind randomized placebo-controlled clinical trial assessing safety and tolerability of FDA-approved doses of exenatide in early AD, during which we explored treatment responses for clinical, cognitive, Magnetic Resonance Imaging (MRI) and biochemical biomarkers’ outcomes.
2. METHODS
2.1. Regulatory Framework, Participants and Study Design
This study was performed under the National Institute of Health CNS Institutional Review Board-approved protocol and an Investigational New Drug exemption as determined by the Food and Drug Administration. The study was registered in clinicaltrials.gov before enrollment started (NCT01255163). The conduct of the trial was supervised by a Data Safety Monitoring Board (DSMB) which met at least bi-annually. Moreover, periodic quality assessments were performed by an NIA official, who was not involved with the study and secured reliability of study procedures and data. The trial was conducted under a Clinical Trial Agreement between the National Institute on Aging (NIA) and the manufacturer of commercially available exenatide (Byetta®), AstraZeneca, Inc. The study was terminated prematurely when AstraZeneca, Inc. informed NIA Investigators of its business decision to withdraw support for the study; early termination was not related to safety considerations. All study procedures took place at the NIA Clinical Research Unit (Baltimore, MD) between December 2010 and September 2016.
Prospective participants were accompanied by a caregiver and underwent an assessment of consent capacity followed by informed consent. Participants were required to appoint a Durable Power of Attorney for research that would be activated in case they lost consent capacity during the study. Clinical investigations were conducted as per the principles expressed in the Declaration of Helsinki.
The screening/baseline visit was used to determine eligibility and provide baseline secondary measures for those subsequently randomized and included History and Physical (HP) with neurological examination, detailed neuropsychological assessment, a Lumbar Puncture (LP) for Collection of Cerebrospinal Fluid (CSF), blood draws for clinical laboratory tests and biomarkers, Oral Glucose Tolerance Test (OGTT), and brain MRI/Magnetic Resonance Spectroscopy (MRS). Caregivers participated in a structured interview to determine Clinical Dementia Rating (CDR) and an unstructured interview as part of the HP.
Eligibility criteria can be found in detail at https://clinicaltrials.gov/ct2/show/NCT01255163, and include age > 60 years, absence of DM by established fasting blood glucose and OGTT criteria, clinical diagnosis of amnestic Mild Cognitive Impairment (MCI) or (mild) probable AD, CSF Aβ42< 192 pg/ml, absence of other neurological disorders or significant neuroimaging abnormalities on MRI, and absence of significant depression (Hamilton Depression Scale (HDS) < 12). Notably, all participants that were randomized fulfill criteria for high probability AD based on clinical diagnosis of amnestic MCI or probable AD, low CSF Aβ42, high CSF total tau and/or p181-tau, although their enrollment preceded the development of recent biomarker-based diagnostic criteria [32, 33] and the recently developed A/T/N framework [34]. All participants had a Clinical Dementia Rating (CDR) global score of 0.5 (corresponding to MCI) or 1 (corresponding to mild dementia). In exploratory models, participants with CDR 0.5 or 1 showed no differences in results and were therefore combined into one group. All participants were treated with acetylcholinesterase inhibitors (on stable doses for at least six weeks) prior to enrollment and remained on them for the duration of the trial. There were no participants on memantine prior to enrollment, although 3 participants were made to start memantine by their treating physicians at some point during their participation.
Eligible participants were randomized to exenatide or placebo treatment and both the participants and caregivers were trained to administer the injections. All the participants were given 5 mcg exenatide or placebo SC (indicated by dialing 2 on the injection pen), twice daily. After 1 week, the dose was augmented to 10 mcg exenatide or placebo SC (indicated by dialing 4 on the injection pen), twice daily. Two participants who did not tolerate the higher dose because of nausea were switched back to the lower dose which was continued for the duration of the study. Compliance was assessed by keeping a medication diary. Safety and tolerability and compliance assessment visits took place at 1 week, 2 weeks and 3 months after the onset of the treatment. Participants underwent visits for assessment of safety, tolerability and compliance, but also a collection of exploratory secondary outcomes at 6, 12, and 18 months. The 6 and 12-month visits included a neuropsychological assessment, blood biomarkers, and MRI/MRS. The final 18-month visit also included an LP and an OGTT.
A total of 57 participants were enrolled after signing an informed consent to undergo screening procedures. Of those, 28 met all inclusion criteria, 27 were randomized to exenatide or placebo, 21 had at least one follow-up visit with a collection of outcome measures at 6 months, and 18 completed the full 18 months of the study. Investigators, patients and caregivers were blinded to participant group assignment until 1/31/2017, at which point, all study procedures were finalized and data was saved. Participants’ demographics and key variables of interest for the 21 participants with outcome data are included in Table 1. The analysis for safety and tolerability was based on data taken from the 27 participants who ever received the experimental drug, whereas the analysis for secondary outcomes was based on the 21 participants for whom we had any outcome data.
Table 1.
Demographic information and key variables at baseline.
| - | Placebo Group | Exenatide Group | P-value |
|---|---|---|---|
| n | 10 | 11 | |
| Age (years) | 74.0 ± 6.4 | 71.7 ± 6.9 | .445 |
| Sex | 4M/6F (60% F) | 7M/4F (36% F) | .395 |
| BMI | 26.9 ± 5.9 | 27.1 ± 3.4 | .941 |
| Fasting Glucose (mg/dl) | 93.8 ± 8.4 | 89.6 ± 7.4 | .258 |
| Fasting Insulin (μIU/ml) | 7.83 ± 4.8 | 8.1 ± 4.6 | .915 |
| HOMA2 %B | 85.0 ± 36.2 | 97.7 ± 37.9 | .477 |
| HOMA2 %S | 134.5 ± 68.3 | 122.5 ± 57.3 | .694 |
| HOMA2 IR | 1.0 ± 0.7 | 1.0 ± 0.6 | .914 |
| MMSE | 26.0 ± 3.5 | 25.5 ± 4.2 | .751 |
| CDR global 0.5/1.0 (n/n) | 9/1 | 8/3 | .586 |
| CDR sob | 2.5 ± 1.5 | 2.9 ± 1.7 | .564 |
| HDS | 4.2 ± 3.1 | 3.1 ± 2.6 | .435 |
| Plasma Aβ42 (pg/ml) | 9.7 ± 0.5 | 8.4 ± 0.5 | .057 |
| Plasma Aβ40 (pg/ml) | 38.7 ± 3.7 | 39.6 ± 3.5 | .921 |
| Plasma Aβ42 /Aβ40 (pg/ml) | 0.27 ± 0.07 | 0.24 ± 0.10 | .505 |
| CSF Aβ42 (pg/ml) | 77.6 ± 12.9 | 98.6 ± 12.3 | .809 |
| CSF Total tau (pg/ml) | 148.3 ± 13.9 | 152.4 ± 13.3 | .251 |
| CSF p181-tau (pg/ml) | 44.5 ± 8.7 | 60.3 ± 8.3 | .208 |
| EV p181-tau (AU) | 2017.9 ± 294.7 | 1841.0 ± 294.7 | .705 |
| EV pS-IRS1(AU) | 3219.5 ± 668.0 | 1962.0 ± 636.9 | .322 |
| EV pY-IRS1(AU) | 726.7 ± 449.9 | 350.2 ± 429.0 | .372 |
| EV Aβ42 (pg/ml) | 5.0 ± 0.5 | 4.1 ± 0.5 | .238 |
| EV Aβ40 (pg/ml) | 137.3 ± 16.5 | 78.0 ± 14.2 | .061 |
Demographic, metabolic, cognitive and plasma/CSF/EV biomarker variables are presented as mean ± standard deviation. Significances (P-value) were calculated using an independent samples t-test for continuous variables and a two-sided Fisher’s exact test for Sex and CDR global. Body Mass Index (BMI), HOMA2 %B/%S/%R: Homeostasis Model Assessment 2 beta-cell function / insulin sensitivity / insulin resistance, MMSE: Mini-Mental State Exam, CDR: clinical dementia rating, CDR sob: CDR sum-of-boxes, Aβ42: B-amyloid-42; Aβ40: B-amyloid-40, p181 tau: tau phosphorylated on threonine 181, pS-IRS1: insulin receptor substrate-1 phosphorylated on serine residues, pY-IRS1: insulin receptor substrate-1 phosphorylated on tyrosine residues. AU: arbitrary units, percent electrochemoluminescence signal of undiluted standard.
2.2. MRI Acquisitions and Data Processing
T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) images were acquired on a 3T Phillips Achieva scanner with an 8-channel SENSE head coil. A Turbo Field Echo acquisition sequence was used with the following parameters: TR = 6.803 ms, TE = 3.19 ms, number of excitations = 1, flip angle = 8°, acquisition matrix = 256 × 256 × 170, resolution = 1 × 1 × 1.2 mm. Using SPM12 (http:// www.fil.ion.ucl.ac.uk/spm/software/spm12/), the MPRAGE structural images were pre-processed by: 1). using the "Serial Longitudinal Registration" toolbox, saving the midpoint average for each subject and Jacobian for each visit; 2) using the "Segment" toolbox to segment the midpoint average for each subject; 3) multiplying the Gray Matter (GM) (c1) and White Matter (WM) (c2) segmented images by each visit’s Jacobian; 4) using the "Run DARTEL (create templates)" toolbox to create templates from the image produced in step 3; 5) running the Dartel Tools: Normalizing to MNI space" toolbox on the 6th iteration template from step 4, to acquire flow fields from each subject and input the images produced in step 3. This resulted in a smoothed and normalized VBM-ready GM image for each subject and visit. CAT-12 (http://www.neuro.uni-jena.de/cat/) was used to acquire measures of cortical thickness, using the "CAT-12: Segment Longitudinal Data" toolbox, and "Surface Tools" for added measures of gyrification, sulcal depth, and cortical complexity.
Junctional Point-Resolved Spectroscopy (J-PRESS) was acquired within an anisotropic voxel (25 × 18 × 20 mm3) placed at midline over bilateral posteromedial cortex (PMC) as previously described [35]. Line widths for water resonance were monitored for intra-subject scan reliability and were (mean ± SD) 7.3 ± 1.7 Hz. Prior-Knowledge Fitting (ProFit) [36] software was used to determine relative metabolite ratios to creatine, as described [35].
2.3. Neuropsychological Assessment
Neuropsychological assessment included determination of the Clinical Dementia Rating (CDR) based on both participant and caregiver input (which were used to derive combined CDR global score and sum of boxes (CDR-sob)), Alzheimer’s Disease Cooperative Study - Activities of Daily Living (ADCS-ADL), and performance on the Mini-Mental State Exam (MMSE), the 70-point Alzheimer’s Disease Assessment Scale cognitive subscale (ADAS-Cog), the Frontal Assessment Battery (FAB), Verbal Fluency, the California Verbal Learning Task (CVLT), Boston Naming Test, Wechsler Memory Scale Logical Memory Subtest, Trail-making Test Parts A & B, American National Adult Reading Test (ANART), Clock drawing, Wechsler Adult Intelligence Scale Digit-Symbol and Digit Span Forward & Backward Subtests, the Benton Visual Retention Task (BVRT) and the University of Pennsylvania Smell Identification Test (UPSIT). The battery largely overlaps with the battery employed in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study, allowing for comparing the study results to that of a larger cohort.
2.4. Blood and CSF Biomarkers of AD
Baseline values for established CSF biomarkers of AD and metabolic parameters are provided in Table 1. Blood samples were collected by venipuncture into vacutainer EDTA tubes incubated for 10 min at room temperature and centrifuged for 15 min at 2500 g at 25°C, then re-aliquoted in 0.25-ml aliquots and stored at −80°C. Samples were thawed to room temperature once before assaying. The levels (pg/ml) of Aβ42 and Aβ40 in the soluble phase of plasma were determined at the University of Pennsylvania Biomarker
Research Laboratory using Luminex xMAP technology with INNO-BIA plasma Aβ forms kits provided by Fujirebio. Cerebrospinal fluid was stored at −80 °C immediately after the LP until thawed for sample processing. The levels (pg/ml) of Aβ42, p181-tau and total tau were also determined at the University of Pennsylvania Biomarker Research Laboratory using Luminex xMAP technology with INNO-BIA Alz Bio3 kits provided by Fujirebio. Exenatide concentrations in plasma and CSF were quantified in duplicate from a subset of samples collected at 18 months (N=7), using a Chemiluminescent EIA Kit (CEK-070–94; from Phoenix Pharmaceutical, Burlingame, CA, USA) at the National Institute on Aging (Baltimore, MD, USA).
2.5. Metabolic Parameters
For the OGTT, which was conducted at baseline and 18 months, participants drank a 75-gram orange-flavored glucose drink (296 ml) within 5 minutes, after fasting for at least 12 hours. At 18 months, the subjects received exenatide or placebo injections in the morning of the OGTT, 1 – 1.5 hours prior to the test. Blood samples were collected into EDTA-coated tubes containing DPP 4 inhibitor and aprotinin. Sampling times were 0 (before consuming the beverage) and 10, 20, 30, 40, 60, 90, 120, 150, and 180 minutes for measurements of plasma glucose, insulin, active GLP-1 (7–36) and total GIP, which were quantified as previously described [37]. Homeostatic Model Assessment (HOMA2) values were calculated by entering the fasting glucose and insulin values in the HOMA2 Calculator (https://www.dtu.ox.ac.uk/homacalculator/).
2.6. Plasma-based Extracellular Vesicle (EV) Biomarkers
Pre-analytical factors for blood collection and storage comply with guidelines for EV biomarker analysis [38]. The methodology for isolating plasma EVs enriched for neuronal origin has been published previously [39–41] and was detailed in a methods paper [42]. Briefly, plasma samples were defibrinated with thrombin by adding protease and phosphatase inhibitors. A pellet of total EVs was precipitated with Exoquick® (System Biosciences, Inc., Mountainview, CA). EVs were resuspended in Ultra-pure distilled water with protease and phosphatase inhibitors and incubated for 1 hour at 4°C with 4 micrograms of mouse anti-human CD171 (or else L1CAM) biotinylated antibody (clone 5G3, Thermo Scientific, Inc.), followed by incubation with 25 μl of Pierce™ Streptavidin Plus UltraLink™ Resin (Thermo Scientific, Inc.) for 30 min at 4°C. After centrifugation, pellets were resuspended in 0.1M glycine-HCl to detach EVs followed by centrifugation to remove beads. Supernatants containing EVs were transferred to clean tubes, pH was neutralized, and EVs were either lysed with detergent solution MPER and two freeze-thaw cycles or diluted 1: 200 to permit quantification in the range of 3–15 × 108/ml with nanoparticle tracking analysis (NTA) by Nanosight NS500 (Malvern, Amesbury, UK). Five exposures of 20 seconds each were recorded from fields chosen randomly by NanoSight software (NanoSight NTA 3.2), which also calculated average EV concentration and diameter. We performed electrochemiluminescence assays for p181-tau (N45CB-1), pSer312-IRS-1 (K150HLD) and pY-IRS-1 (panTyr) (N45CA-1) using the Mesoscale Discovery (MSD®, Meso Scale Diagnostics, Rockville, MD) platform. We performed the SIMOA® assay for Aβ42 (Simoa™ Aβ42 2.0 Kit, # 101664) and for Aβ40 (Simoa™ Aβ40 2.0 Kit, # 101672). All the assays were conducted in duplicate and the average Coefficients of Variance (CVs) were 10.5% for pS312-IRS-1, 11.1% for pY-IRS-1, 8.5% for p181-tau, and 9.8% for Aβ42.
2.7. Statistical Analysis
All the analyses were performed in SPSS 23.0 (Chicago, IL) except for MRI/MRS. Safety and tolerability of Exenatide were determined by comparing the number of AEs to placebo and historical data, using Fisher’s exact test. All exploratory outcomes were analyzed using repeated measures linear mixed models with Visit (Baseline, 6 months, 12 months, and 18 months) as the repeated measures variable with a first-order autoregressive [AR(1)] repeated covariance and Group (Exenatide or Placebo) and visit as constant factors. The model included the Group*Visit interaction term to assess group differences in within-subject differences over time. Multiple time-point OGTT data (insulin, glucose, GLP-1, GIP) were first log-transformed and then inputted as dependent variables into a linear mixed model with Visit (Baseline or Outcome) and Timepoint (0–180 minutes) as repeated measures with a AR(1) heterogeneous repeated covariance type, with Group and Visit as constant factors.
For analysis of both the CAT-12 cortical thickness and SPM-12 VBM images, we conducted a whole-brain flexible factorial analysis with Group and Visit as factors and the Group*Visit interaction term. The VBM analysis used age, sex, and total intracranial volume (TIV) from the CAT-12 toolbox as covariates. An uncorrected p-value threshold of 0.001 was used with an extent threshold of p = 0.001, resulting in 1500 voxels for the SPM-12 VBM images and 300 vertices for the CAT-12 cortical thickness. A family-wise error (FWE) correction at cluster-level p < 0.05 and false-discovery rate (FDR) correction at cluster-level p < 0.05 were also applied to the reported results in the SPM outputs as shown in supplemental figures 1 and 2.
3. RESULTS
3.1. Safety & Tolerability
The primary outcome of this study was safety and tolerability, and adverse events (AEs) of all grades were recorded as part of the HP session in each visit. Safety data are reported for the 27 participants who ever received exenatide or placebo. There were possibly or probably no related serious AEs, or AEs Grade 3 or above as determined by the study investigators and the DSMB. Nausea (Grade 1 or 2) is the main expected adverse event with exenatide and the most usual dose-limiting AE. There was a significantly higher incidence of nausea in the exenatide group, with 38% (5/13) of exenatide-treated participants reporting nausea compared to none among placebo-assigned participants (p = 0.016, two-sided Fisher’s exact test). According to the investigators’ brochure provided by the manufacturer, in clinical trials, nausea occurred in about 40% of participants treated with exenatide twice daily compared to about 10% for placebo-assigned participants. Symptoms of upper gastrointestinal (GI) infection (including nausea, symptoms of Gastroesophageal Reflux Disease (GERD), and other non-specific complaints, but not pain) were more common in the exenatide group (p = .004, two-sided Fisher’s exact test), as was the loss of appetite and weight loss (p = .041, two-sided fisher’s exact test). Table 2 shows the incidence of nausea and other reported adverse events.
Table 2.
Adverse events reported by participants.
| Adverse Event | Exenatide (N=13) | Placebo (N=14) | P-value (2s) |
|---|---|---|---|
| Nausea | 5 (38%) | 0 | 0.016 |
| GERD symptoms | 2 (15%) | 1 (7%) | 0.596 |
| Upper GI upset (nausea + GERD + non-specific complaints) | 8 (62%) | 1 (7%) | 0.004 |
| Loss of appetite/weight loss | 4 (31%) | 0 | 0.041 |
| Changes in taste (dysgeusia) | 0 | 1 (7%) | 1.000 |
| Lower GI upset (Diarrhea) | 3 (23%) | 1 (7%) | 0.326 |
| Transient abdominal pain (non-specific) | 1 (8%) | 0 | 0.481 |
| Transient asymptomatic elevation in pancreatic enzymes (amylase, lipase) | 2 (15%) | 2 (14%) | 0.673 |
| Dizziness/lightheadedness/diaphoresis (symptoms suspicious for hypoglycemia) | 3 (23%) | 2 (14%) | 0.648 |
| Injection site ecchymosis/pain | 1 (8%) | 1 (7%) | 1.000 |
| Abnormal kidney function tests (BUN, Creatine) | 2 (15%) | 2 (14%) | 1.000 |
| Other (fatigue, allergic conjuctivitis, etc.) | 3 (23%) | 1 (7%) | 0.326 |
Adverse events incidence and percentage in the placebo and exenatide groups. P-values are for a two-sided Fisher’s exact test, significant events are in bold.
3.2. Neuropsychological Assessment
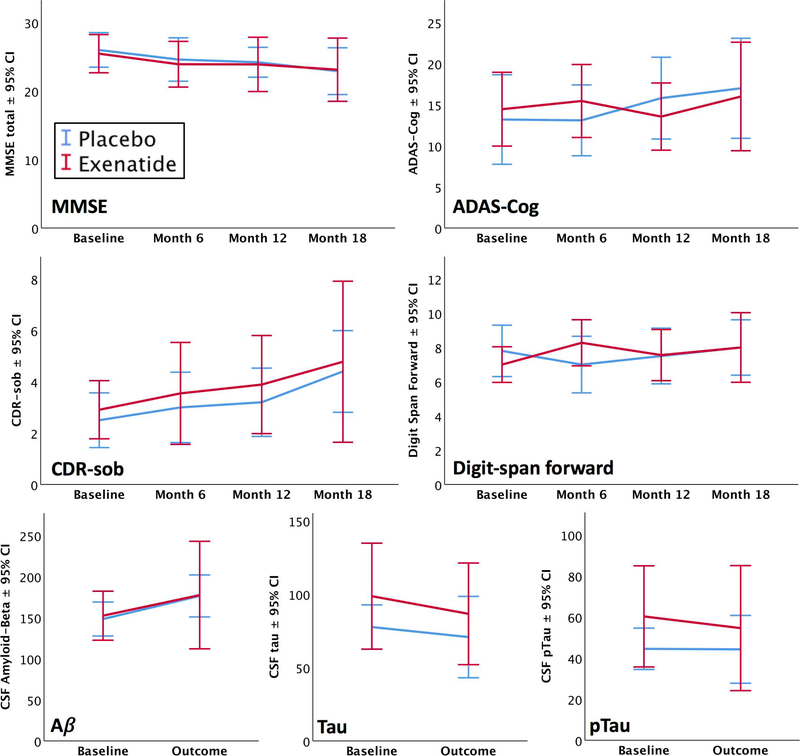
In the cognitive tests, significant Group*Visit interactions were observed only for Digit-Span forward total score (F[3,36.2] = 4.804, p = .006) and maximum digit span forward (F[3,35] = 3.889, p = .017)), reflecting better performance in the exenatide group at 6 months only. The other ratings and tests in the battery (including CDR-sob and ADAS-Cog) showed no significant treatment effects. The Visit factor, however, revealed significant changes over the course of the study in both the groups, including decreases in the MMSE (F[3,52.0]=3.738, p = .017); verbal fluency (F[3,52.1] = 3.008, p = .038); Boston naming correct after stimulus cue, (F[3,53.0] = 3.973, p = .013); and Digit-symbol total (F[3,45.0] = 2.918, p = .044). Increases were evident in CDR-sob, (F[3,50.9]=4.422, p = .008) Trails B time (F[3,49.4 = 3.023, p = .038); ADCS (F[3, 49.7] = 5.47, p = .003); and Digit-symbol total (F[3,45.0] = 2.918, p = .044). (See Fig. 1 below and Supplemental Table 1 for details).
Fig. (1). Select Outcomes.
Mean line graphs with 95% confidence intervals displaying the longitudinal results for the Mini-mental state exam (MMSE) total, Alzheimer’s Disease Assessment Scale-cognitive (ADAS-Cog) total, Clinical Dementia Rating (CDR) sum of boxes, Digit-span forward score, CSF amyloid-beta, CSF total tau, and CSF p181-tau. Blue lines indicate placebo and red indicate exenatide.
3.3. Metabolic Parameters
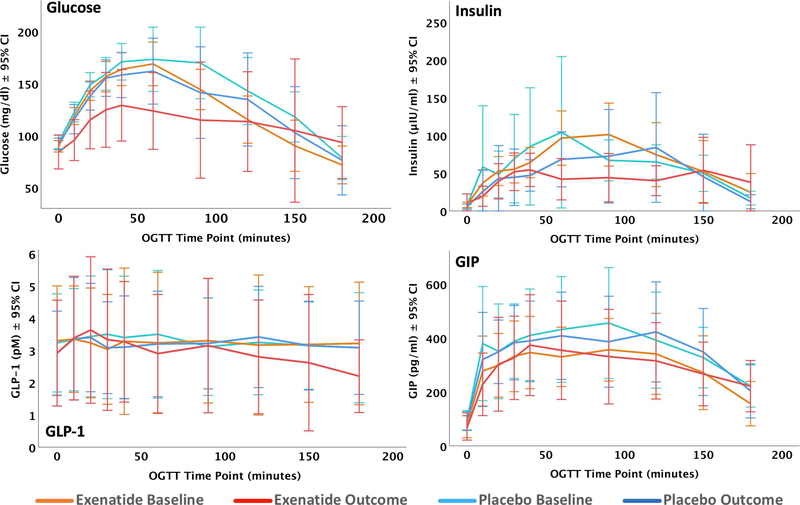
Body Mass Index (BMI) in the exenatide group showed a significant decrease over the 18 months of the study (F[3,52.9] = 3.203, p = .031). There were no significant treatment effects for fasting glucose, fasting insulin, HOMA2 %B, HOMA2 %S or HOMA2 IR. Among the biomarkers of glucose metabolism and systemic insulin resistance determined by the OGTT, there was a significant treatment effect for glucose (Group*Visit interaction F[3,82.8] = 39.8, p = 1.3 × 10−8) and GLP-1 (F[3,95] = 5.496, p = .021). Using post-hoc least squared differences (LSD) tests between the groups, exenatide-treated participants displayed lower levels of glucose in their OGTT results compared to their baseline OGTT (p < 0.001) and placebo-assigned participants at baseline (p < 0.001) or outcome (p = 0.001). The GLP-1 differences were characterized by a reduction in GLP-1 in exenatide-treated participants compared to their baseline (p = 0.002). Line graphs for these comparisons are shown in (Fig. 2), below.
Fig. (2).
Line graphs with 95% confidence intervals over the course of each oral glucose tolerance test (OGTT). Orange indicates the exenatide group at baseline, red at outcome. Light blue indicates the placebo group at baseline, dark blue at outcome. Glucose, Insulin, active GLP-1, and total GIP were measured as described in the methods.
3.4. CSF, Plasma and EV Biomarkers
There were no significant treatment effects on biomarkers in CSF (Aβ42, total tau, p181-tau), plasma (Aβ42, Aβ40) and plasma neuronal EVs (p-181tau, IRS-1, Aβ40), except for Aβ42 in EVs, which showed a significant Group*Visit interaction effect decreasing over time in exenatide-treated group compared to placebo-assigned participants (F[1,16.3] = 4.71, p = 0.045). The Visit factor revealed significant decreases over the course of the study in both the groups for CSF levels of Aβ42 (F[1, 14.9] = 9.218, p = 0.008) and plasma Aβ40 (F[3,50.2] = 3.998, p = .013) (see Supplemental Table 2 for details).
3.5. J-PRESS MRS Results
There was a nominally significant Group*Visit interaction for Aspartate (F[3, 26.7] = 3.69, p =.024), but this was strongly influenced by a downward deviation in the placebo group at 6 months. No other precuneal J-PRESS MRS metabolites showed significant group changes over time.
3.6. Structural MRI Results
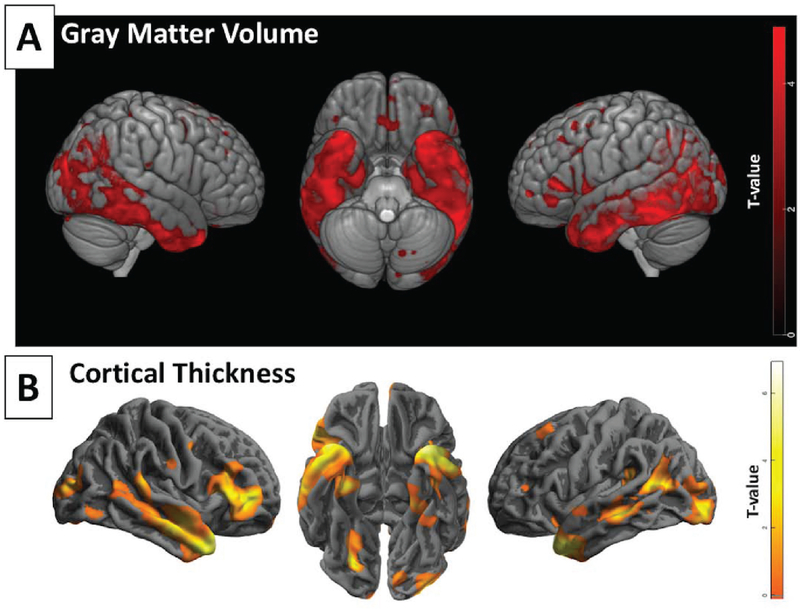
Using the SPM flexible factorial model detailed in the methods with Group and Visit as main effects and Group*Visit as the interaction term of interest, only the main effect of Visit was significant (Fig. 3A) indicating progression of GM atrophy in precuneal and medial temporal-parietal regions in both the groups over 18 months of follow-up (See Supplemental Fig. 1 for coordinates). Using CAT-12 to derive cortical thickness, the same model also revealed significant cortical thinning in both the groups in 18 months (Fig. 3B). There were however no significant voxels for the Group*Visit interaction that would imply an effect of exenatide on preserving GM volume or cortical thickness. Similarly, there were no significant treatment effects on cortical complexity, gyrification, or sulcal depth. Fig. (3) shows an overview of volumetric loss and cortical thinning in 18 months in both the groups combined, and Supplemental (Figs. 1S & 2S) for detailed output of the volumetric and cortical thickness analyses.
Fig. (3).
Rendered volumetric images of progressive GM atrophy and cortical thinning in both groups combined (areas negatively associated with factor Visit). A) brain regions with lower GM volume over 18 months, figure created in MRIcroGL. B) brain regions with lower cortical thickness over 18 months, figure created in CAT12. Clusters are displayed at p < 0.001 uncorrected with no extent threshold.
3.7. Exenatide CSF and Plasma Concentrations
Exenatide levels were quantified in CSF and plasma samples obtained from 7 subjects receiving exenatide at 18 months. Within an hour after the 10 mcg exenatide dosing, mean CSF and plasma concentrations were 10.2 ± 1.1 pg/ml and 739.6 ± 111.6 pg/ml, respectively. This provided a CSF/plasma ratio = 0.014 ± 0.002.
4. DISCUSSION
This small and prematurely terminated randomized double-blinded placebo-controlled study of exenatide aimed primarily at assessing tolerability in an MCI/early AD population and secondarily to assess treatment responses for clinical, cognitive, MRI, and biomarker outcomes. The lack of serious AEs or AEs above Grade 3 suggests that exenatide is safe and reasonably well tolerated in this population. Exenatide-treated participants experienced a higher incidence of Grade 1 or 2 nausea, upper GI upset, loss of appetite and decreased BMI compared to placebo-assigned participants. The incidence of nausea was similar to the incidence observed in clinical trials in subjects with DM despite the older age of our cohort. For instance, in a multi-center clinical trial of exenatide 5–10 mcg SC injections involving participants with DM with a mean age of 59 ± 9 years, the incidence of nausea was 41% with exenatide compared to 8% with placebo [43]. Interestingly, in our study, BMI decreased in exenatide-treated participants in the middle visits (6–12 months), but the two groups had similar BMIs by the end of the study. This is perhaps attributable to the fact that nausea, upper gastrointestinal complaints and loss of appetite were more prominent earlier in the course of exenatide treatment. Exenatide is known to slow gastric emptying and reduce food intake [44]. Importantly, treatment-associated differences in the expected side-effects of exenatide suggest that subjects were compliant with the regimen, as also evidenced by their medication logs.
The CSF and plasma concentrations determined for exenatide in the present study (739.6 pg/ml and 10.2 pg/ml, respectively) yield a CSF/plasma ratio of 0.014 within an hour of 10 mcg exenatide SC administration (as part of chronic twice-daily administration of 10 mcg exenatide immediate-release SC). While these ratios reveal a much lower concentration in CSF, this is not unusual for CNS-active compounds [45]. Comparatively, these findings closely match the level recently reported in PD subjects (543·3 pg/mL and 11·4 pg/mL, respectively), which showed a CSF/plasma ratio of 0.021 following exenatide 2 mg once weekly SC (Bydureon® delayed-release dosing) [43]. This does, however, raise the pertinent question of whether these reported CSF levels are actually sufficient to yield therapeutic effects in the brain, and if longer-acting by larger studies of this and similar compounds.
In terms of expected metabolic effects of exenatide, the 3-hour OGTT results at 18 months showed a relative attenuation of the blood glucose rise after ingestion of oral glucose in the exenatide group compared to baseline and the placebo group, whereas no differences were observed for plasma insulin. If blood glucose changes were the only driver of blood insulin levels during the OGTT, one would expect them to be decreased compared to baseline and placebo following the limited rise in glucose. The fact that insulin levels were similarly high compared to baseline and placebo is attributable to direct effects of GLP-1R-mediated, exenatide-stimulated insulin secretion, i.e. exenatide which led to increased insulin-to-glucose ratio. It is notable that the GLP-1 levels registered an overall decrease, most prominently at the 180 min time point, compared to baseline and placebo. While the glucose attenuation is an expected pattern based on prior studies [44], the GLP-1 decrease is a novel finding that we attribute to decreased secretion due to entrainment by lower blood glucose levels after eating. GLP-1 does not suppress its own secretion [46] and therefore exenatide is also not likely to suppress its secretion. Given that participants received an exenatide or placebo injection in the morning of the test day, changes in OGTT parameters can be considered acute (induced by the morning dose on that day), not chronic (induced by persistent changes in post-prandial glucose and insulin).
Neuropsychological measures (including scores for MMSE, ADAS-cog, and CDR-sob, which constitute common cognitive outcomes in clinical trials in AD) were largely similar between exenatide and placebo groups at 18 months, with the possible exception of an improvement in the exenatide group in the digit-span forward task at 6 months only. This test assesses attention and short-term memory [47], and is considered to be sensitive to mild cognitive impairment [48], but is not specific for dementia [49]. This finding was not shown for the other cognitive measures and could be a spurious result. Therefore, we do not wish to over-interpret this finding. Similarly, CSF and plasma classic AD biomarkers (Aβ42, total tau, p181-tau) did not show any treatment-associated changes or changes over time. It is worth noting that both the groups did show significant deficits over time in the MMSE, CDR-sob, temporal lobe volume by MRI volumetrics, and other AD-related measures, suggesting that cognitive decline did occur over the course of the study period. Thus, while they were sensitive to cognitive decline, the overall cognitive scores, clinical ratings and classic biomarkers do not provide trends supporting the hypothesis that exenatide is disease-modifying in early clinical AD.
While the anatomical MRI imaging revealed that enrolled participants showed progression of GM atrophy and cortical thinning in the canonical AD pattern (mainly involving medial and lateral temporal and parietal areas), it did not show any differences or trends in the progression of atrophy between the two groups that could be attributable to neurotrophic and neuroprotective exenatide actions. In respect to J-PRESS MRS, we recently showed that certain MRS metabolites are significantly different within the precuneus in AD patients compared to cognitively normal older adults, such as higher glucose and lactate and lower NAA [35]. In the present study, we did not observe any changes over time in these metabolites or any treatment associated differences or trends. However our limited MRI and MRS findings may be due to low numbers, they provide no support to the hypothesis that exenatide is disease-modifying in early AD.
In a series of publications, we have demonstrated that EVs enriched for neuronal origin by immunoprecipitation against neural cell adhesion molecule L1 (L1CAM) provide diagnostic biomarkers for AD [40, 42] and have been advocating the view that EV biomarkers may also be used to show target engagement and molecular-level responses to experimental treatments [42]. Based on the study by Talbot et al. [6] who showed higher levels of pSer-IRS1 and lower levels of pY-IRS1 in the brains of patients with MCI and AD, we hypothesized that these finding would be reflected in IRS1 phosphotypes in plasma EVs enriched for neuronal origin in patients with AD, a hypothesis that was tested in a case control study [39]. Subsequently, we found that IRS1 phosphotypes in EVs are related to brain atrophy in AD [50] and the change in response to a diet intervention [51]. Given that exenatide reverses brain IR in animals [5], we hypothesized that pSer-IRS1 in plasma EVs enriched for neuronal origin would decrease and pY-IRS1 would increase in exenatide-treated participants. This hypothesis is not supported by our findings, with the caveat that the small sample size renders the study underpowered to test for modest effect sizes. In contrast, levels of Aβ42, in plasma EVs enriched for neuronal origin showed a decrease in exenatide-treated participants at 18 months compared to their baseline levels and placebo-assigned participants. Whereas we do not wish to over-interpret this isolated finding, it does suggest a decrease in the intensity of brain amyloidosis, as reflected in plasma exosomes enriched for neuronal origin. This tentative EV Aß42 finding is an excellent trigger for replication attempts in further studies with a more sound basis.
The study was successfully conducted until its early termination and showed a retention rate 21 out of 27 comparable to other AD trials [52]. Its strengths include a well-characterized study population with high-probability early AD, which is the main target of disease-modifying treatment trials. The study was a pioneer in requiring evidence for a biomarker of brain amyloidosis (low CSF Aβ42) as a criterion for enrollment, which subsequently became commonplace among clinical trials in AD. The fact that enrolled subjects also showed high p181-tau and total tau and characteristic atrophy means that they also meet criteria for high probability AD [32; 33] and can be classified as A+/T+/N+ by current classification schemes [34]. In addition, enrolled participants showed the expected progression of GM atrophy and cognitive decline over 18 months. Therefore, it is unlikely that participants’ misclassification may account for the negative results of the trial. Another strength is the fact that it employed a large number of multifaceted exploratory outcomes in order to generate generalizable knowledge that might be used to determine the strength for larger studies of GLP-1 agonists in AD. Study limitations, besides the small N and its premature termination, include the fact that it was conducted at a single-center, which limits its generalizability.
CONCLUSION
In conclusion, exenatide was safe and reasonably well-tolerated in MCI/early AD participants. Despite its early termination and small N, the study proved to be sufficiently sound enough to demonstrate priori hypothesized metabolic effects: (slight) reduction in BMI and improved glucose tolerance. Unfortunately, that was not the case for AD-related outcomes with the possible exception of a decrease in Aβ42 in neuronal-origin enriched EVs, and a marginal improvement in one measure of attention and memory. While underpowered due to early termination, our results agree with the largely negative results of a recently published small Phase II double-blind placebo-controlled clinical trial of another GLP-1 analogue (liraglutide) in MCI/early AD [53]. Collectively these two small studies cast some doubt on the therapeutic potential of GLP-1 analogues in AD, although a more definitive answer will be awaited until the ongoing large multi-center UK-based trial of liraglutide (ELAD, https://clinicaltrials.gov/ct2/show/NCT01843075) is completed.
Supplementary Material
Acknowledgments
FUNDING
This research was supported by the Intramural Research Program of the National Institute on Aging (NIA/NIH). This study was also supported by AstraZeneca Pharmaceuticals, LP according to the terms of a Clinical Trial Agreement (“U.S. Clinical Contracts and Grants Office Agreement # 22851”).
LIST OF ABBREVIATIONS
- AD
Alzheimer’s Disease
- ADAS-cog
Alzheimer’s disease assessment scale-cognitive subscale
- AE
Adverse Event
- ANART
American National Adult Reading Test
- Aβ
Amyloid-Beta
- BID
Twice Daily
- BLSA
Baltimore Longitudinal Study of Aging
- BMI
Body Mass Index
- BVRT
Benton Visual Retention Test
- CDR
Clinical Dementia Rating
- CSF
Cerebral Spinal Fluid
- CVLT
California Verbal Learning Task
- DM
Diabetes Mellitus
- EV
Extracellular Vesicle
- GLP-1
Glucagon-like peptide-1
- GLP-1R
G-Protein coupled receptor of GLP-1
- IRB
Institutional Review Board
- LP
Lumbar Puncture
- mcg
microgram
- MCI
Mild Cognitive Impairment
- mL
Milliliters
- MMSE
Mini Mental Status Exam
- NAA
N-Acetyl Aspartate
- NIA
National Institute on Aging
- NIH
National Institute of Health
- NTA
Nanoparticle Tracking Analysis
- OGTT
Oral Glucose Tolerance Test
- SAE
Serious Adverse Event
- SC
Subcutaneous
- SD
Standard Deviation
- UPSIT
University of Pennsylvania Smell Identification Test
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Combined Neuroscience Institutional Review Board, (USA), Chair Barbara Karp, M.D. approved this protocol (Approval number: 10-AG-0423) as part of continuing review on 4/25/2018, in accordance with 45 CFR 46.110 and 21 CFR 56. 110. The study was registered in clinicaltrials.gov (NCT01255163).
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. All humans research procedures were followed in accordance with the guidelines of the Declaration of Helsinki of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Informed consent was obtained from patients.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analysed during the current study are available at https://clinicaltrials.gov/ct2/show/NCT01255163 or the corresponding author on reasonable request.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- [1].Alzheimer’s A. Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12(4): 459–509 (2016). [ 10.1016/j.jalz.2016.03.001] [DOI] [PubMed] [Google Scholar]
- [2].Becker RE, Kapogiannis D, Greig NH. Does traumatic brain injury hold the key to the Alzheimer’s disease puzzle? Alzheimers Dement 14(4): 431–43 (2018). [ 10.1016/j.jalz.2017.11.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8(6): 595–608 (2016). [ 10.15252/emmm.201606210] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mullins RJ, Diehl TC, Chia CW, Kapogiannis D. Insulin resistance as a link between amyloid-beta and tau pathologies in Alzheimer’s disease. Front Aging Neurosci 9: 118 (2017). [ 10.3389/fnagi.2017.00118] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J Clin Invest 122(4): 1339–53 (2012). [ 10.1172/JCI57256] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122(4): 1316–38 (2012). [ 10.1172/JCI59903] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol 90(5A): 3G–10G (2002). [ 10.1016/S0002-9149(02)02553–5] [DOI] [PubMed] [Google Scholar]
- [8].Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci 14(6): 750–6 (2011). [ 10.1038/nn.2801] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R, et al. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J Clin Invest 125(6): 2463–7 (2015). [ 10.1172/JCI79742] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, et al. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain 131(Pt 12): 3311–34 (2008). [ 10.1093/brain/awn288] [DOI] [PubMed] [Google Scholar]
- [11].Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 5(6): 514–6 (2002). [ 10.1038/nn0602-849] [DOI] [PubMed] [Google Scholar]
- [12].De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, et al. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA 106(6): 1971–6 (2009). [ 10.1073/pnas.0809158106] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller BW, Willett KC, Desilets AR. Rosiglitazone and pioglitazone for the treatment of Alzheimer’s disease. Ann Pharmacother 45(11): 1416–24 (2011). [ 10.1345/aph.1Q238] [DOI] [PubMed] [Google Scholar]
- [14].Landreth G, Jiang Q, Mandrekar S, Heneka M. PPARgamma agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics 5(3): 481–9 (2008). [ 10.1016/j.nurt.2008.05.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Talbot K, Wang HY. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement 10(1)(Suppl.): S12–25 (2014). [ 10.1016/j.jalz.2013.12.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60(4): 470–512 (2008). [ 10.1124/pr.108.000604] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 268(26): 19650–5 (1993). [PubMed] [Google Scholar]
- [18].Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 18(1–2): 7–14 (2002). [ 10.1385/JMN:18:1-2:07] [DOI] [PubMed] [Google Scholar]
- [19].Alvarez E, Martínez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem 92(4): 798–806 (2005). [ 10.1111/j.1471-4159.2004.02914.x] [DOI] [PubMed] [Google Scholar]
- [20].Hamilton A, Hölscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport 20(13): 1161–6 (2009). [ 10.1097/WNR.0b013e32832fbf14] [DOI] [PubMed] [Google Scholar]
- [21].Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther 300(3): 958–66 (2002). [ 10.1124/jpet.300.3.958] [DOI] [PubMed] [Google Scholar]
- [22].Perry T, Greig NH. Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer’s disease. Curr Alzheimer Res 2(3): 377–85 (2005). [ 10.2174/1567205054367892] [DOI] [PubMed] [Google Scholar]
- [23].Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res 86(2): 326–38 (2008). [ 10.1002/jnr.21483] [DOI] [PubMed] [Google Scholar]
- [24].Hamilton A, Patterson S, Porter D, Gault VA, Holscher C. Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J Neurosci Res 89(4): 481–9 (2011). [ 10.1002/jnr.22565] [DOI] [PubMed] [Google Scholar]
- [25].Hölscher C, Li L. New roles for insulin-like hormones in neuronal signalling and protection: new hopes for novel treatments of Alzheimer’s disease? Neurobiol Aging 31(9): 1495–502 (2010). [ 10.1016/j.neurobiolaging.2008.08.023] [DOI] [PubMed] [Google Scholar]
- [26].McClean PL, Gault VA, Harriott P, Hölscher C. Glucagon-like peptide-1 analogues enhance synaptic plasticity in the brain: a link between diabetes and Alzheimer’s disease. Eur J Pharmacol 630(1–3): 158–62 (2010). [ 10.1016/j.ejphar.2009.12.023] [DOI] [PubMed] [Google Scholar]
- [27].During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 2003; 9(9): 1173–9. [ 10.1038/nm919] [DOI] [PubMed] [Google Scholar]
- [28].Isacson R, Nielsen E, Dannaeus K, Bertilsson G, Patrone C, Zachrisson O, et al. The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur J Pharmacol 650(1): 249–55 (2011). [ 10.1016/j.ejphar.2010.10.008] [DOI] [PubMed] [Google Scholar]
- [29].LiY Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis 19(4): 1205–19 (2010). [ 10.3233/JAD-2010-1314] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gengler S, McClean PL, McCurtin R, Gault VA, Hölscher C. Val(8)GLP-1 rescues synaptic plasticity and reduces dense core plaques in APP/PS1 mice. Neurobiol Aging 33(2): 265–76 (2012). [ 10.1016/j.neurobiolaging.2010.02.014] [DOI] [PubMed] [Google Scholar]
- [31].Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Choudhary K, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 390(10103): 1664–75 (2017). [ 10.1016/S0140-6736(17)31585-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3): 280–92 (2011). [ 10.1016/j.jalz.2011.03.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3): 263–9 (2011). [ 10.1016/j.jalz.2011.03.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jack CR Jr, Bennett DA, Blennow K, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016; 87(5): 539–47. [ 10.1212/WNL.0000000000002923] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mullins R, Reiter D, Kapogiannis D. Magnetic resonance spectroscopy reveals abnormalities of glucose metabolism in the Alzheimer’s brain. Ann Clin Transl Neurol 5(3): 262–72 (2018). [ 10.1002/acn3.530] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schulte RF, Boesiger P. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed 19(2): 255–63 (2006). [ 10.1002/nbm.1026] [DOI] [PubMed] [Google Scholar]
- [37].Chia CW, Carlson OD, Kim W, et al. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes 58(6): 1342–9 (2009). [ 10.2337/db08-0958] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research J Extracell Vesicles 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kapogiannis D, Boxer A, Schwartz JB, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J 29(2): 589–96 (2015). [ 10.1096/fj.14-262048] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement 11(6): 600–7 (2015). [ 10.1016/j.jalz.2014.06.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Goetzl EJ, Boxer A, Schwartz JB, et al. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 2015; 85(1): 40–7. [ 10.1212/WNL.0000000000001702] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mustapic M, Eitan E, Werner JK Jr, et al. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front Neurosci 11: 278 (2017). [ 10.3389/fnins.2017.00278] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 154(2): 103–12 (2011). [ 10.7326/0003-4819-154-2-201101180-00300] [DOI] [PubMed] [Google Scholar]
- [44].Kyriacou A, Ahmed AB. Exenatide Use in the Management of Type 2 Diabetes Mellitus. Pharmaceuticals (Basel) 3(8): 2554–67 (2010). [ 10.3390/ph3082554] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Maurer TS, Debartolo DB, Tess DA, Scott DO. Relationship between exposure and nonspecific binding of thirty-three central nervous system drugs in mice. Drug Metab Dispos 33(1): 175–81 (2005). [ 10.1124/dmd.104.001222] [DOI] [PubMed] [Google Scholar]
- [46].Elahi D, Ruff DA, Carlson OD, Meneilly GS, Habener JF, Egan JM. Does GLP-1 suppress its own basal secretion? Endocr Res 41(1): 16–20 (2016). [ 10.3109/07435800.2015.1038353] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Spaan PE, Raaijmakers JG, Jonker C. Early assessment of dementia: the contribution of different memory components. Neuropsychology 19(5): 629–40 (2005). [ 10.1037/0894-4105.19.5.629] [DOI] [PubMed] [Google Scholar]
- [48].Lortie JJ, Remington R, Hoffmann H, Shea TB. Lack of Correlation of WAIS Digit Span with Clox 1 and the Dementia Rating Scale in MCI. Int J Alzheimers Dis 2012: 829743 (2012). [ 10.1155/2012/829743] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kiewel NA, Wisdom NM, Bradshaw MR, Pastorek NJ, Strutt AM. A retrospective review of digit span-related effort indicators in probable Alzheimer’s disease patients. Clin Neuropsychol 26(6): 965–74 (2012). [ 10.1080/13854046.2012.694478] [DOI] [PubMed] [Google Scholar]
- [50].Mullins RJ, Mustapic M, Goetzl EJ, Kapogiannis D. Exosomal biomarkers of brain insulin resistance associated with regional atrophy in Alzheimer’s disease. Hum Brain Mapp 38(4): 1933–40 (2017). [ 10.1002/hbm.23494] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Eitan E, Tosti V, Suire CN, et al. In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell 16(6): 1430–3 (2017). [ 10.1111/acel.12657] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer’s disease clinical trials. Alzheimers Res Ther, 2(6): 34 (2010). [ 10.1186/alzrt58] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gejl M, Gjedde A, Egefjord L, et al. In Alzheimer’s Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front Aging Neurosci 8: 108 (2016). [ 10.3389/fnagi.2016.00108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.