Abstract
Aims/hypothesis
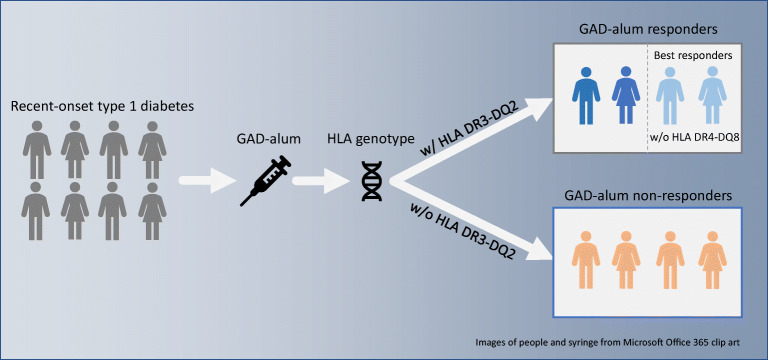
The aim of this study was to determine if retention of C-peptide following immunotherapy using recombinant GAD65 conjugated to aluminium hydroxide (GAD-alum) is influenced by HLA risk haplotypes DR3-DQ2 and DR4-DQ8.
Methods
HLA-dependent treatment effect of GAD-alum therapy on C-peptide retention in individuals with recent-onset type 1 diabetes was evaluated using individual-level patient data from three placebo-controlled, randomised clinical trials using a mixed repeated measures model.
Results
A significant and dose-dependent effect was observed in individuals positive for the genotypes that include HLA-DR3-DQ2 but not HLA-DR4-DQ8 and in the broader subgroup of individuals positive for all genotypes that include HLA-DR3-DQ2 (i.e. including those also positive for HLA-DR4-DQ8). Higher doses (three or four injections) showed a treatment effect ratio of 1.596 (95% CI 1.132, 2.249; adjusted p = 0.0035) and 1.441 (95% CI 1.188, 1.749; adjusted p = 0.0007) vs placebo for the two respective HLA subgroups.
Conclusions/interpretation
GAD65-specific immunotherapy has a significant effect on C-peptide retention in individuals with recent-onset type 1 diabetes who have the DR3-DQ2 haplotype.
Graphical abstract
Keywords: Antigen-specific, Autoimmune diabetes, C-peptide, GAD, Glutamic acid decarboxylase, HLA, Immunotherapy, Type 1 diabetes, Vaccine
Introduction
Recombinant human GAD65 conjugated to aluminium hydroxide (GAD-alum) is an antigen-specific immunotherapy intended to induce specific immunological tolerance to preserve the pancreatic beta cells that are targeted in type 1 diabetes by autoreactive cytotoxic T cells. The active ingredient, recombinant human GAD65, is a pancreatic beta cell protein, GAD65 being one of the most frequent autoantigens associated with type 1 diabetes. The effect of GAD-alum on preserving endogenous insulin production has been evaluated in several placebo-controlled, randomised trials in individuals recently diagnosed with type 1 diabetes, albeit with inconclusive results [1–4]. It is becoming increasingly clear that factors such as genetic background in the form of HLA genotype affect both the risk of diabetes affliction and the pathogenesis of the disease [5–13]. It is conceivable that HLA may also influence the effect of antigen-specific immunotherapies like GAD-alum and while this possibility has been considered incidentally in prior clinical trials [2, 3], this hypothesis has not been extensively evaluated.
The aim of this study was to estimate, using individual-level patient data from previous randomised, placebo-controlled trials, whether the efficacy of GAD-specific immunotherapy depends on the presence of the GAD and insulin antibody-associated HLA haplotypes DR3-DQ2 and DR4-DQ8.
Methods
We combined individual-level data from three published randomised, controlled clinical trials [2–4], clinical trial identifiers NCT00435981, NCT00529399 and NCT00723411, that evaluated subcutaneous GAD-alum therapy (compared with alum) in GAD autoantibody-positive individuals with recent-onset type 1 diabetes.
The similarities and differences between the clinical studies have been described in detail elsewhere [1]. Table 1 summarises the number of participants eligible for the analysis, treatment schedules and HLA genotype distribution. Briefly, all trials evaluated treatment with two injections of 20 μg GAD-alum or alum only (placebo). Two trials [2, 3] also evaluated three or four injections of 20 μg GAD-alum. GAD-alum or placebo was administered at days 1, 30, 90 and 270, respectively. For the analysis, the treatment was coded as placebo, low dose (two injections) and high dose (three or four injections).
Table 1.
Summary of data, HLA genotype distribution and treatment regimens as evaluated in the analysis
| Ludvigsson et al, 2008 (NCT00435981) [4] | Wherrett et al, 2011 (NCT00529399) [3] | Ludvigsson et al, 2012 (NCT00723411) [2] | |
|---|---|---|---|
| Participants eligible for analysis, n | 69 | 139 | 313 |
| All participants | 70 | 145 | 334 |
| With baseline and at least one post-baseline value missing | 1 | 4 | 7 |
| With missing HLA information | 0 | 2 | 14 |
| HLA distribution, n (%) | |||
| HLA-DR3-DQ2 | 34 (49%) | 71 (50%) | 161 (50%) |
| HLA-DR3-DQ2/not HLA-DR4-DQ8 | 17 (24%) | 36 (25%) | 74 (23%) |
| Treatment schedulea | |||
| Low dose | Two doses of 20 μg GAD-alum (days 1 and 30) | Two doses of 20 μg GAD-alum (days 1 and 30), one dose of alum (day 90) | Two doses of 20 μg GAD-alum (days 1 and 30), two doses of alum (days 90 and 180) |
| High dose | - | Three doses of 20 μg GAD-alum (days 1, 30 and 90) | Four doses of 20 μg GAD-alum (days 1, 30, 90 and 180) |
| Placebo | Two doses of alum (days 1 and 30) | Three doses of alum (days 1, 30 and 90) | Four doses of alum (days 1, 30, 90 and 180) |
aFor Wherrett et al 2011 the treatment schedule was defined as baseline, week 4 and week 12, while Ludvigsson 2008 and 2012 defined the schedule based on days from baseline (1, 30, 90 and 180). For consistency, days from baseline are used in the table. For a detailed description on the similarities and differences between the clinical trials, please see [1]
A total of 521 participants (of 549) were included in the final analysis. Patients that did not have a baseline and at least one post-baseline C-peptide value (n = 12 out of 549) were excluded as well as individuals with missing HLA information (n = 16 out of 537 remaining). The HLA subgroup term was coded as either presence or absence of genotypes that include HLA-DR3-DQ2 and, in a second model, as either presence or absence of genotypes that include HLA-DR3-DQ2 but not HLA-DR4-DQ8.
The treatment effect on C-peptide retention (loge of the ratio of C-peptide AUC at 15 months/C-peptide AUC at baseline) was estimated using the restricted maximum likelihood approach in a mixed repeated measures model. All measurements from baseline to the primary endpoint readout (12 months for NCT00529399, 15 months for NCT00435981 and NCT00723411) were used. The model was adjusted for the fixed effects of baseline C-peptide, study, treatment, HLA subgroup, visit, country, sex and age, as well as the interaction of baseline C-peptide by visit and treatment by HLA subgroup by visit. Baseline value, age and visit were treated as continuous variables. Study, treatment, HLA subgroup, country and sex were treated as categorical variables. Patient identification number and country were included as categorical random effects to yield a variance components structure. The treatment effect ratios at 15 months were based on least square means with adjusted p values using the Bonferroni–Šidák correction reported along with two-sided 95% confidence intervals. A p value of <0.05 was considered significant. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
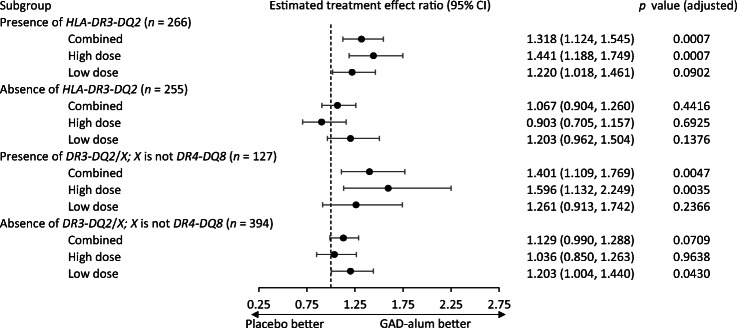
A significant interaction was observed in the main statistical model between treatment, C-peptide retention (loge of the C-peptide AUC ratio 15 months/baseline), and the HLA-DR3-DQ2 (p = 0.02) or DR3-DQ2/not DR4-DQ8 genotype groups (p = 0.03), indicating that the treatment effect of GAD-alum differs depending on the patient’s HLA haplotype. Post hoc tests showed that the estimated treatment effect ratio (Fig. 1) of GAD-alum compared with placebo at 15 months from baseline was 1.318 (95% CI 1.124, 1.545; adjusted p = 0.0007) in individuals positive for DR3-DQ2 (n = 266) genotype and 1.401 (95% CI 1.109, 1.769; adjusted p = 0.0047) in individuals positive for the DR3-DQ2/ not DR4-DQ8 (n = 127) genotypes. A higher dose (three or four injections of GAD-alum) showed a treatment effect ratio of 1.441 (95% CI 1.188, 1.749; adjusted p = 0.0007) and 1.596 (95% CI 1.132, 2.249; adjusted p = 0.0035) vs placebo for the two HLA subgroups. No significant effect after adjusting for multiple testing was seen in these subgroups following a lower dose (two injections).
Fig. 1.
Estimated treatment effect ratio (C-peptide retention; active vs placebo) at 15 months post baseline in patients with or without genotypes that include HLA-DR3-DQ2 (n = 266 and 255), or HLA-DR3-DQ2 but not HLA-DR4-DQ8 (n = 127 and n = 394). Adjusted p values using the Bonferroni- Šidák correction are reported along with two-sided 95% CIs
In individuals negative for the DR3-DQ2/not DR4-DQ8 genotypes no significant effect was seen for any dose or for the combination of doses. For individuals negative for DR3-DQ2 genotypes, a treatment effect ratio of 1.203 (95% CI 1.004, 1.440; adjusted p = 0.043) for lower dose was seen. No significance was seen for higher dose or for the combined dose regimen.
Discussion
This study demonstrates that the efficacy of GAD-alum immunotherapy may depend on specific HLA risk haplotypes. The concept that HLA molecules play an important role in guiding antigen-specific autoimmunity in type 1 diabetes is not new as previous studies demonstrate that the first appearance of either GAD or insulin autoantibodies was associated with HLA-DR3-DQ2 and DR4-DQ8, respectively [7]. However, this is the first report that we are aware of identifying the ability of specific HLA types to determine the efficacy of GAD-based therapeutics.
Specifically, based on this analysis where we combine individual patient-level data from three previous placebo-controlled clinical trials, we show that GAD-alum immunotherapy has a significant and dose-dependent effect on C-peptide retention in individuals positive for genotypes that include the HLA-DR3-DQ2 haplotype. We further show that the effect is most pronounced in individuals who are simultaneously negative for genotypes that include the HLA-DR4-DQ8 haplotype. Interestingly, recent research highlights the existence of different phenotypes of type 1 diabetes based on autoantibody seroconversion that, in turn, are linked to HLA risk haplotypes [7, 9, 10]. More specifically and as noted above, as HLA-DR3-DQ2 has been associated with seroconversion to GAD65 autoantibodies and DR4-DQ8 with seroconversion to insulin autoantibodies [7, 10], our results suggest that the best efficacy of antigen-specific immunotherapy may be achieved when targeting individuals that show a specific HLA type that is linked to the tolerising antigen.
These findings are in alignment with recent discussions highlighting the importance of considering disease heterogeneity in type 1 diabetes when evaluating therapeutic strategies [10–13]. Importantly, HLA genotyping is a straightforward and clinically feasible strategy to prospectively identify individuals with type 1 diabetes that have a higher likelihood of responding to the GAD-alum treatment.
Going forward, HLA information will be an integral part of any type 1 diabetes trial with GAD-alum. The current retrospective analyses involve comprehensive data from three randomised controlled trials conducted both in Europe and in the USA, with slightly different inclusion criteria. As these analyses were performed post hoc, it will be important to prospectively verify the findings presented here with regards to the interaction of HLA and GAD-alum therapy in ongoing trials. It will also be important to understand how HLA affects the immunological response to the therapy. Interestingly, preliminary findings (U. Hannelius, M. Danelljan, unpublished results) indicate that HLA does affect the GAD antibody response. There is also a clear rationale to retrospectively analyse the interaction between HLA and treatment in other antigen-specific trials, especially where insulin has been used as the tolerising antigen given the association between insulin autoimmunity and HLA-DR4-DQ8.
Acknowledgements
We thank M. Atkinson, University of Florida, and Å. Lernmark, Lund University, for valuable comments on the manuscript.
Duality of interest
UH is an employee of and owns stock in Diamyd Medical. CAB has served in a consulting role for Diamyd Medical. JL has received an unrestricted research grant from Diamyd Medical.
Abbreviation
- GAD-alum
Recombinant human GAD65 conjugated to aluminium hydroxide
- rhGAD65
Recombinant human GAD65
Contribution statement
UH designed the study, analysed the data and wrote the manuscript. CAB contributed to design of the study, analysed the data and reviewed/edited the manuscript. JL contributed to the design of the study and reviewed/edited the manuscript. All authors approved the final version of the manuscript. UH is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. UH attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
Open access funding provided by Linköping University. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Diamyd Medical was involved in study design and data collection only and did not impose any restrictions regarding the publication of the report.
Data availability
The data that support the findings of this study are available on reasonable request from the corresponding author (UH).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ulf Hannelius, Email: ulf.hannelius@diamyd.com.
Johnny Ludvigsson, Email: Johnny.Ludvigsson@liu.se, Email: Johnny.Ludvigsson@regionostergotland.se.
References
- 1.Beam CA, MacCallum C, Herold KC, Wherrett DK, Palmer J, Ludvigsson J. GAD vaccine reduces insulin loss in recently diagnosed type 1 diabetes: findings from a Bayesian meta-analysis. Diabetologia. 2017;60(1):43–49. doi: 10.1007/s00125-016-4122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludvigsson J, Krisky D, Casas R, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366(5):433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 3.Wherrett DK, Bundy B, Becker DJ, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378(9788):319–327. doi: 10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludvigsson J, Faresjo M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359(18):1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity. 2010;32(4):468–478. doi: 10.1016/j.immuni.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krischer JP, Liu X, Lernmark A, et al. The influence of type 1 diabetes genetic susceptibility regions, age, sex, and family history on the progression from multiple autoantibodies to type 1 diabetes: A TEDDY study report. Diabetes. 2017;66(12):3122–3129. doi: 10.2337/db17-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krischer JP, Lynch KF, Lernmark A, et al. Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: The TEDDY study. Diabetes Care. 2017;40(9):1194–1202. doi: 10.2337/dc17-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenker M, Hummel M, Ferber K, et al. Early expression and high prevalence of islet autoantibodies for DR3/4 heterozygous and DR4/4 homozygous offspring of parents with type I diabetes: the German BABYDIAB study. Diabetologia. 1999;42(6):671–677. doi: 10.1007/s001250051214. [DOI] [PubMed] [Google Scholar]
- 9.Krischer JP, Lynch KF, Schatz DA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980–987. doi: 10.1007/s00125-015-3514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care. 2020;43(1):5–12. doi: 10.2337/dc19-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes. 2014;63(11):3835–3845. doi: 10.2337/db14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claessens LA, Wesselius J, van Lummel M, et al. Clinical and genetic correlates of islet-autoimmune signatures in juvenile-onset type 1 diabetes. Diabetologia. 2020;63(2):351–361. doi: 10.1007/s00125-019-05032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roep BO, Wheeler DCS, Peakman M. Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol. 2019;7(1):65–74. doi: 10.1016/S2213-8587(18)30109-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author (UH).