Abstract
Objective: To explore the significance of a DNA quantitative analysis of lung cancer cells with different pathological types taken from bronchial brush specimens and its relationship with the clinicopathological features. Methods: 903 bronchial brush cytological specimens taken in the Cytology Department of the Fourth Hospital of Hebei Medical University were collected from March 2017 to December 2019 and divided into three groups: the squamous carcinoma (SC) group, the adenocarcinoma (AC) group, and the small-cell carcinoma (SCC) group. The maximum DNA index (DI) value, the percentage of cells with a DI larger than 2.5, the mean DI, and the peaks of the aneuploid cells of the three groups were compared. A single factor analysis was used to analyze the relationship between the DNA content, aneuploidy, and the clinico pathological features of the patients who had surgery. Results: The peaks of the aneuploid cells in the SC group, the AC group, and the SCC group had no significant differences (P=0.57). The maximum DI, the percentage of cells with a DI larger than 2.5, and the mean DI of the three groups showed statistically significant differences (P<0.001). The clinicopathological features of the AC patients and the SC patients, such as gender, age, tumor type, the maximum tumor diameter, clinical stage, vascular invasion, nerve invasion, pleural invasion, tracheal spread, and lymph node metastasis were not independent factors that influence the DNA content and have no statistical significance (P > 0.05). Conclusion: The reason why the DNA content of small-cell lung cancer is lower than SC and AC remains to be further studied.
Keywords: DNA content, squamous cell carcinoma, adenocarcinoma, small-cell carcinoma, clinicopathological characteristics
Introduction
Lung cancer is the malignant tumor with the highest morbidity and mortality, ranking first in China and even in the world [1,2]. Most patients are diagnosed too late, so early diagnosis is key to treating the disease. A method of diagnosing of lung cancer early, bronchoscopy has the advantages of causing little trauma, convenient specimen preparation, and high repeatability [3,4]. Using the brush specimens obtained from bronchoscopies, a better diagnostic rate can be obtained. As an ancillary method of cytological diagnosis, DNA quantitative analysis is widely used for early diagnoses because of its repeatability and sensitivity [5,6]. However, there are few studies on the relationship between the DNA content and the biological behavior of different pathological types of lung cancer. Therefore, in this study, the correlation of the DNA content with the different pathological types of lung cancer cells and its clinical significance were investigated.
Materials and methods
Materials
903 cases of bronchial brush smears in from the Cytology Department of the Fourth Hospital of Hebei Medical University were collected from March 2017 to December 2019. Each participant filled out an informed consent form, and this study was approved by the hospital ethics committee. Their clinicopathological information was collected. Among the patients, 143 underwent surgical operations and had surgical specimens taken.
Methods
Using bronchoscopy, all the patients underwent a brush biopsy after lesions were found. The brushed cells were smeared on six slides, four of which were used for routine cytological diagnosis and classification using HE staining and immunocytochemistry. The remaining two were dyed with Feulgen staining for an automatic DNA quantitative analysis. The histological specimens obtained through bronchoscopy bite biopsies and percutaneous transthoracic biopsies under CT guidance were sent to the histopathology department for examination.
Diagnosis
The cytological and histopathological diagnoses were made by a team of two experienced cytologists and two experienced pathologists. The cytological and histological specimens with matching results were included, and those with conflicting results were excluded.
DNA quantitative analysis
LD DNA image cytometry (LD DNA-ICM, Landing Med Tech, Wuhan) was used for the DNA quantitative analysis. The DNA-ICM included a Wave NP370D2 server, an Olympus BX41 microscope, a ueye M2240 camera, and an automatic control platform. The DNA-ICM was used to determine the DNA content by measuring the integrated optical density (IOD) of the stained nucleus DNA, using normal lymphocytes on the same slide as the control. The DNA content was expressed using the DNA Index (DI=DNA IOD of the tested cell/DNA IOD average of normal cell). The DNA content was usually measured in c (content). 1c was half of the DNA content of normal G0/G1 cells. When the tested cell was in the G0/G1 phase, its IOD value was very close to the average IOD value of normal cells, so its DNA index was 1, namely 2c. When the DNA content of the tested cells formed peaks between 2c~4c, 4c~8c and 8c~16c; or when there were more than 3 cells with a DI larger than 2.5 (5c), tumors should be highly suspected.
Aneuploid cell peaks included: ① type I no peak, which shows aneuploid cells of DI > 2.5 are scattered everywhere; ② type II single peak, which can appear in different positions, DI mostly between 1-2; ③ type III double peaks, which can appear in a variety of tumors, and the DI of the second peak is often twice that of the first peak; ④ type IV multi-peaks, and the histogram shows different heights like Manhattan houses, and the appearance of multiple peaks indicates that the chromosome structure of the tumor is very unstable (Figure 1). The maximum DI, the percentage of cells with DI > 2.5, mean DI (mean DI of the first 20 representative tumor cells) and the peaks of the aneuploid cells were used as the statistical indicators for the DNA quantitative analysis. The number of cells on the slides used for the DNA quantitative analysis should be no less than 2000.
Figure 1.
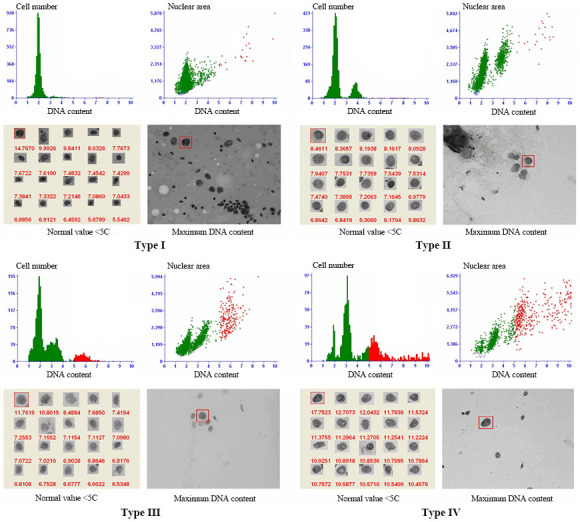
DNA quantitative analysis.
Clinicopathological features
Age, gender, maximum tumor diameter, tumor type, lymph node metastasis, and other relevant information about the patients whose surgical specimens were collected.
Statistical methods
SPSS 26.0 software was used for the statistical analysis. The DNA quantitative analysis indexes were tested using Kruskal-Wallis and Bonferroni tests, and the relationships between the DNA content and the clinico pathological features were tested using Mann-Whitney U tests. P<0.05 was considered a statistically significant difference.
Results
Clinicopathological features of the patients
A total of 903 patients were enrolled, with a median age of 63, and ranging in age from 31 to 87. Among them, 682 were men and 221 were women. The diagnoses determined that 337 cases were SC, 268 were AC, and 298 were SCC. Among the 143 patients with surgical specimens, 121 were male and 22 were female, ranging in age from 39 to 78, with a median age of 62. The diagnoses indicated that 95 cases were SC and 48 were AC.
Results of the DNA quantitative analysis
There were no significant differences in the SC, AC and SCC aneuploid cell peaks (P=0.057). But the maximum DI value, the percentage of cells with a DI > 2.5, and the mean DI value in the three groups showed a significant difference by comparison (Table 1). The maximum DI and, the percentage of SC and AC cells with a DI > 2.5 were higher than the corresponding SCC values (P<0.001), and the above two indicators in SC and AC showed no significant differences (P=1). The average DI value of AC was higher than the average DI value of SC and SCC (P<0.001), and the average DI value of SC was slightly higher than the average DI value of SCC, but the differences was not statistically significant (P=0.156) (Table 2).
Table 1.
The DNA analysis results of SC, AC, and SCC
| Pathology type | the maximum DI | percentage of cells with DI larger than 2.5 | ||||||
|
|
|
|||||||
| Median | Average rank | statistical quantity | p | Median | Average rank | statistical quantity | p | |
|
| ||||||||
| SC | 5.35 | 478.98 | 33.779 | <0.001 | 1.78 | 484.75 | 40.492 | <0.001 |
| AC | 5.42 | 479.14 | 2.20 | 497.91 | ||||
| SCC | 4.81 | 380.90 | 0.94 | 373.67 | ||||
|
| ||||||||
| Pathology type | mean DI | the peaks of aneuploid cells | ||||||
|
|
|
|||||||
| median | Average rank | statistical quantity | p | Average rank | statistical quantity | p | ||
|
| ||||||||
| SC | 3.50 | 459.75 | 29.74 | <0.001 | 197.60 | 1.04 | 0.60 | |
| AC | 3.64 | 509.93 | 210.85 | |||||
| SCC | 3.48 | 391.14 | 202.39 | |||||
Table 2.
The DNA analysis results of SC, AC ,and SCC using pairwise comparisons
| Pathology type | the maximum DI | percentage of cells with DI larger than 2.5 | Content mean DI | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| statistical quantity | p | statistical quantity | p | statistical quantity | p | |
| SC-SCC | 98.082 | <0.001 | 111.079 | <0.001 | 40.290 | 0.156 |
| AC-SCC | 116.239 | <0.001 | 124.232 | <0.001 | 118.508 | <0.001 |
| SC-AC | -18.157 | 1.000 | -13.153 | 1 | -78.219 | <0.001 |
The relationship between the DNA quantitative analysis and the clinicopathological features
The patients’ clinicopathological features, such as gender, age, maximum tumor diameter, type of tumor, pleural invasion, vascular invasion, nerve invasion, lymph node metastasis, and stage were not independent influencing factors of the DNA content (P > 0.05) (Table 3).
Table 3.
A single factor analysis of the DNA content, aneuploidy, and clinico pathological features
| clinico pathological features | NO. | the maximum DI | percentage of cells with DI larger than 2.5 | Content mean DI | the peaks of aneuploid cells | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Z | P | Z | p | Z | p | Z | p | ||
| Gender | -1.007 | 0.314 | -0.490 | 0.624 | -0.257 | 0.797 | -0.952 | 0.341 | |
| men | 121 | ||||||||
| women | 22 | ||||||||
| Age | -0.413 | 0.680 | -0.668 | 0.504 | -1.630 | 0.103 | -1.191 | 0.234 | |
| <62 | 66 | ||||||||
| ≥62 | 77 | ||||||||
| Tumor type | -0.325 | 0.745 | -0.543 | 0.587 | -0.675 | 0.499 | -0.910 | 0.363 | |
| SC | 95 | ||||||||
| AC | 48 | ||||||||
| Tumor diameter | -0.591 | 0.555 | -2.104 | 0.035 | -0.340 | 0.734 | -0.608 | 0.543 | |
| <4 cm | 62 | ||||||||
| ≥4 cm | 81 | ||||||||
| Stage | 4.050 | 0.132 | 1.736 | 0.420 | 0.722 | 0.697 | 2.750 | 0.253 | |
| I | 44 | ||||||||
| II | 44 | ||||||||
| III | 55 | ||||||||
| Vascular Invasion | -0.597 | 0.550 | -0.360 | 0.719 | -0.457 | 0.648 | -0.051 | 0.960 | |
| yes | 8 | ||||||||
| no | 135 | ||||||||
| Nerve invasion | -0.884 | 0.377 | -0.744 | 0.457 | -1.993 | 0.46 | -2.040 | 0.41 | |
| yes | 7 | ||||||||
| no | 136 | ||||||||
| Pleura invasion | -0.627 | 0.531 | -0.468 | 0.640 | -0.181 | 0.857 | -0.199 | 0.842 | |
| yes | 25 | ||||||||
| no | 118 | ||||||||
| Tracheal spread | -0.600 | 0.549 | -0.258 | 0.797 | -0.050 | 0.960 | -1.387 | 0.165 | |
| yes | 30 | ||||||||
| no | 113 | ||||||||
| Lymph node metastasis | -0.854 | 0.393 | -0.968 | 0.333 | -0.213 | 0.832 | -0.105 | 0.916 | |
| yes | 66 | ||||||||
| no | 77 | ||||||||
Discussion
Lung cancer is one of the most common malignant tumors in China. The incidence of lung cancer (35/100,000) in China is comparable to that in US, but the five-year survival in China is lower, and the death rate is 1.4 times that of the United States [7,8]. The reason is that with most patients in China the disease is found in the advanced stages, so early diagnosis is the key to treating the disease. In the clinic, CT, x-rays, and other imaging examinations play important roles in detecting pulmonary diseases. However, for the diagnosis of lung tumors, the pathological results are the gold standard. Bronchoscopy, a common method of testing for lung cancer, has a high diagnosis rate for central lung cancer and peripheral lung cancer invading bronchi. The lesion can be directly observed, and cyto- and histopathological specimens can be obtained using brush and bite biopsies. Bronchial brush biopsies can brush the lesion site with a wider range, so they can acquire diseased specimens more easily and successfully than bite biopsies [9-11].
DNA, a carrier of genetic information, plays an important role in cell growth, differentiation and reproduction [12]. When a malignant tumor occurs, the tumor genes undergo point mutation, fusion and amplification, and as the tumor progresses, the DNA content increases significantly. An automatic DNA image analysis system is used to measure the DNA content of the detected cells. This technique over comes missed diagnoses and overdiagnosis that occur due to fatigue and humans’ strong subjectivity, so it is more objective. It has been widely used in the auxiliary diagnosis of cytology because of its objectivity, repeatability, and high sensitivity [13-19]. Many studies have shown that the DNA content level and the number of aneuploid cells are related to tumor occurrence, proliferation, invasion, and prognosis [20-35]. The poorer the differentiation of the tumor, the higher the DNA content and the more aneuploid cells [36]. However, there are few studies comparing the DNA content and the aneuploid cells among different pathological types of the same organ tumor.
Our study was designed to determine whether the DNA content and the aneuploid cells were different in different pathological types of lung cancer, and whether they can predict the biological behavior of lung cancer.
As early as 1982, Hirsch et al. [37] proposed that small-cell lung cancer could spread widely at the early stage. Yokota et al. [38] believed that small-cell lung cancer progressed faster than lung AC and SC. Byrd et al. [39] analyzed the imaging manifestations of common lung tumors and found that hilar lymph nodes of small-cell lung cancer tend to be early and heavily involved compared with other types of cancer. Later, Chen et al. [40] also mentioned that small-cell lung cancer is the most invasive subtype, with rapid growth, high proliferation, early transmission, and a wide spread, and even brain metastasis can occur in more than half of the patients. Pfeifer et al. [41] and Dai et al. [42] found that P53 gene mutations were more common in small-cell lung cancer and suggested a poor prognosis. According to previous studies and the observations of clinical cases, SCC is more malignant. Our study compared the DNA content of lung SC, AC, and SCC and found that the maximum DI and the mean DI of lung AC and SC were higher than the corresponding SCC values. Therefore, DNA content cannot be used separately to evaluate the malignancy of different pathological types of lung cancer, especially small cell cancer. Kogan’s research [43] showed that the higher the DNA content, the more aneuploid cells and the less differentiated the lung squamous carcinoma and adenocarcinoma, but they found that smallcell lung carcinoma, a special type of tumor unlike other types of carcinoma, is characterized by a low, sometimes diploid DNA content which does not correlate with its malignancy, and this finding is similar to those of our study.
Our study also investigated the correlation of DNA content and aneuploidy with the clinicopathological features of lung cancer, like gender, age, tumor type, maximum tumor diameter, clinical stage, vascular invasion, nerve invasion, pleural invasion, tracheal spread, and lymph node metastasis, and found no statistically significant differences, so perhaps the patients we studied who had surgery were too small a cohort, and our research question needs further study.
Conclusion
(1) Although the DNA content is different in different pathological types of lung cancer, it cannot be used alone to compare the levels of malignancy among the different pathological types of lung cancer, especially small-cell lung cancer. (2) The reason why the DNA content of small-cell lung cancer is lower than SC and AC remains to be further studied. (3) The correlation between DNA content and aneuploidy and the clinicopathological features of lung cancer was not determined in this study, so further research is needed.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Du Y, Zhao Y, Sidorenkov G, de Bock GH, Cui X, Huang Y, Dorrius MD, Rook M, Groen HJM, Heuvelmans MA, Vliegenthart R, Chen K, Xie X, Liu S, Oudkerk M, Ye Z. Methods of computed tomography screening andmanagement of lung cancer in Tianjin: design of a population-based cohort study. Cancer Biol Med. 2019;16:181–188. doi: 10.20892/j.issn.2095-3941.2018.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, Fu R, Shi P, Qin S, Chen C. Latest progress of intraoperative pleural lavage cytology in lung cancer surgery. Zhongguo Fei Ai Za Zhi. 2018;21:719–726. doi: 10.3779/j.issn.1009-3419.2018.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabatabai ZL, Auger M, Kurtycz DF, Laucirica R, Souers RJ, Nayar R, Khalbuss WE, Moriarty AT, Fraig M. Do liquid-based preparations of pulmonary bronchial brushing specimens perform differently from classically prepared cases for the diagnosis of malignancies? Observations from the college of American pathologists interlaboratory comparison program in nongynecologic cytology. Arch Pathol Lab Med. 2015;139:178–83. doi: 10.5858/arpa.2013-0282-CP. [DOI] [PubMed] [Google Scholar]
- 5.Macey R. DNA-image cytometry and computer-assisted brush biopsy have potential as diagnostic tools for clinically suspected oral precancer and oral cancer. J Evid Based Dent Pract. 2016;16:113–4. doi: 10.1016/j.jebdp.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Abt E. DNA-image cytometry has promise for oral cancer detection. Evid Based Dent. 2015;16:106–107. doi: 10.1038/sj.ebd.6401130. [DOI] [PubMed] [Google Scholar]
- 7.Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics. Cancer Commun (Lond) 2019;39:22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higginson J. International agency for research on cancer. WHO Chronicle. 1969;22:517–522. [PubMed] [Google Scholar]
- 9.Liu C, Wen Z, Li Y, Peng L. Application of ThinPrep bronchial brushing cytology in the early diagnosis of lung cancer: a retrospective study. PLoS One. 2014;9:e90163. doi: 10.1371/journal.pone.0090163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao H, Guo H, Zhang C, Zhao L, Cao J, Pan Q. Value of liquid-based cytology of brushing specimens obtained via fiberoptic bronchoscopy for the diagnosis of lung cancer. Zhonghua Zhong Liu Za Zhi. 2015;37:431–435. [PubMed] [Google Scholar]
- 11.Liu C, Wen Z, Li Y, Peng L. Application of ThinPrep bronchial brushing cytology in the early diagnosis of lung cancer: a retrospective study. PLoS One. 2014;9:e90163. doi: 10.1371/journal.pone.0090163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillooly JF, Hein A, Damiani R. Nuclear DNA content varies with cell size across human cell types. Cold Spring Harb Perspect Biol. 2015;7:a019091. doi: 10.1101/cshperspect.a019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yildirim-Assaf S, Coumbos A, Hopfenmüller W, Foss HD, Stein H, Kühn W. The prognostic significance of determining DNA content in breast cancer by DNA image cytometry: the role of high grade aneuploidy in node negative breast cancer. J Clin Pathol. 2007;60:649–55. doi: 10.1136/jcp.2005.035550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Högmo A, Kuylenstierna R, Lindholm J, Nathansson A, Auer G, Munck-Wikland E. Nuclear DNA content and p53 overexpression in stage I squamous cell carcinoma of the tongue compared with advanced tongue carcinomas. Mol Pathol. 1998;51:268–72. doi: 10.1136/mp.51.5.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aghmesheh M, Saxena A, Niknam F. BRCA1 mutation site may be linked with nuclear DNA ploidy in BRCA1-mutated ovarian carcinomas. Asia Pac J Clin Oncol. 2015;11:135–141. doi: 10.1111/ajco.12310. [DOI] [PubMed] [Google Scholar]
- 16.Njølstad TS, Trovik J, Hveem TS, Kjæreng ML, Kildal W, Pradhan M, Marcickiewicz J, Tingulstad S, Staff AC, Haugland HK, Eraker R, Oddenes K, Rokne JA, Tjugum J, Lode MS ENITEC Network/MoMaTEC Study Group. Amant F, Werner HM, Salvesen HB, Danielsen HE. DNA ploidy in curettage specimens identifies high-risk patients and lymph node metastasis in endometrial cancer. Br J Cancer. 2015;112:1656–1664. doi: 10.1038/bjc.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi A, Min W, Xiang L, Xu W, Jiang T. Value of automatic DNA image cytometry for diagnosing lung cancer. Oncol Lett. 2018;16:915–923. doi: 10.3892/ol.2018.8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G, Guillaud M, Follen M, MacAulay C. Double staining cytologic samples with quantitative Feulgen-thionin and anti-Ki-67 immunocytochemistry as a method of distinguishing cells with abnormal DNA content from normal cycling cells. Anal Quant Cytopathol Histpathol. 2012;34:273–284. [PMC free article] [PubMed] [Google Scholar]
- 19.Garner D. Clinical application of DNA ploidy to cervical cancer screening: a review. World J Clin Oncol. 2014;5:931–65. doi: 10.5306/wjco.v5.i5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaino RJ, Davis AT, Ohlsson-Wilhelm BM, Brunetto VL. DNA content is an independent prognostic indicator in endometrial adenocarcinoma. A gynecologic oncology group study. Int J Gynecol Pathol. 1998;17:312–319. doi: 10.1097/00004347-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Mangili G, De Marzi P, Viganò R, Rabaiotti E, Sassi I, Taccagni GL, Garancini P, Frigerio L. Identification of high risk patients with endometrial carcinoma. Prognostic assessment of endometrial cancer. Eur J Gynaecol Oncol. 2002;23:216–220. [PubMed] [Google Scholar]
- 22.Pradhan M, Abeler VM, Danielsen HE, Trope CG, Risberg BA. Image cytometry DNA ploidy correlates with histological subtypes in endometrial carcinomas. Mod Pathol. 2006;19:1227–1235. doi: 10.1038/modpathol.3800641. [DOI] [PubMed] [Google Scholar]
- 23.Maounis NF, Chorti M, Apostolakis E, Ellina E, Blana A, Aggelidou M, Dritsas I, Markidou S. Prognostic impact of Deoxyribonucleic acid (DNA) image analysis cytometry and immunohistochemical expression of Ki67 in surgically resected non-small cell lung cancers. Cancer Detect Prev. 2006;30:507–514. doi: 10.1016/j.cdp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Yildirim-Assaf S, Coumbos A, Hopfenmuller W, Foss HD, Stein H, Kuhn W. The prognostic significance of determining DNA content in breast cancer by DNA image cytometry: the role of high grade aneuploidy in node negative breast cancer. J Clin Pathol. 2007;60:649–655. doi: 10.1136/jcp.2005.035550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janisson-Dargaud D, Durlach A, Lorenzato M, Grange F, Bernard P, Birembaut P. Aneuploidy, but not Ki-67 or EGFR expression, is associated with recurrences in basal cell carcinoma. J Cutan Pathol. 2008;35:916–921. doi: 10.1111/j.1600-0560.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- 26.Baba H, Korenaga D, Okamura T, Saito A, Watanabe A, Sugimachi K. Prognostic significance of DNA content with special reference to age in gastric cancer. Cancer. 1989;63:1768–1772. doi: 10.1002/1097-0142(19900501)63:9<1768::aid-cncr2820630919>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 27.Song T, Lee JW, Choi CH, Kim TJ, Bae DS, Sung CO, Song SY, Kim BG. Ploidy and S-phase fraction are correlated with lymphovascular space invasion that is predictive of outcomes in endometrial cancer. Int J Clin Oncol. 2012;17:590–597. doi: 10.1007/s10147-011-0329-9. [DOI] [PubMed] [Google Scholar]
- 28.Proctor L, Pradhan M, Leung S, Cheng A, Lee CH, Soslow RA, Gilks CB, Talhouk A, McAlpine JM, Danielsen HE, Hoang LN. Assessment of DNA Ploidy in the ProMisE molecular subgroups of endometrial cancer. Gynecol Oncol. 2017;146:596–602. doi: 10.1016/j.ygyno.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Kamphues C, Al-Abadi N, Bova R, Rademacher S, Al-Abadi H, Klauschen F, Bahra M, Neuhaus P, Pratschke J, Seehofer D. The DNA index as a prognostic tool in hilar cholangiocarcinoma. J Surg Oncol. 2015;112:214–218. doi: 10.1002/jso.23977. [DOI] [PubMed] [Google Scholar]
- 30.Kamphues C, Al-Abadi H, Dürr A, Al-Abadi N, Schricke D, Bova R, Müller V, Stenzinger A, Klauschen F, Seehofer D, Neuhaus P, Bahra M. DNA index as a strong prognostic factor in patients with adenocarcinoma of the pancreatic head: results of a 5-year prospective study. Pancreas. 2013;42:807–812. doi: 10.1097/MPA.0b013e3182773eb6. [DOI] [PubMed] [Google Scholar]
- 31.Hellman K, Johansson H, Andersson S, Pettersson F, Auer G. Prognostic significance of cell cycle- and invasion-related molecular markers and genomic instability in primary carcinoma of the vagina. Int J Gynecol Cancer. 2013;23:41–51. doi: 10.1097/IGC.0b013e31827670c4. [DOI] [PubMed] [Google Scholar]
- 32.Habermann J, Lenander C, Roblick UJ, Krüger S, Ludwig D, Alaiya A, Freitag S, Dümbgen L, Bruch HP, Stange E, Salo S, Tryggvason K, Auer G, Schimmelpenning H. Ulcerative colitis and colorectal carcinoma: DNA-profile, laminin-5 gamma2 chain and cyclin A expression as early markers for risk assessment. Scand J Gastroenterol. 2001;36:751–758. doi: 10.1080/003655201300192021. [DOI] [PubMed] [Google Scholar]
- 33.Lundgren C, Frankendal B, Silfverswärd C, Nilsson B, Tryggvason K, Auer G, Nordström B. Laminin-5 gamma2-chain expression and DNA ploidy as predictors of prognosis in endometrial carcinoma. Med Oncol. 2003;20:147–156. doi: 10.1385/MO:20:2:147. [DOI] [PubMed] [Google Scholar]
- 34.Auer GU, Heselmeyer KM, Steinbeck RG, Munck-Wikland E, Zetterberg AD. The relationship between aneuploidy and p53 overexpression during genesis of colorectal adenocarcinoma. Virchows Arch. 1994;424:343–347. doi: 10.1007/BF00190554. [DOI] [PubMed] [Google Scholar]
- 35.Forsslund G, Esposti PL, Nilsson B, Zetterberg A. The prognostic significance of nuclear DNA content in prostatic carcinoma. Cancer. 1992;69:1432–1439. doi: 10.1002/1097-0142(19920315)69:6<1432::aid-cncr2820690621>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Sun XR, Wang J. DNA quantitative cytology. Hubei Science and Technology Press; 2018. [Google Scholar]
- 37.Hirsch FR, Paulson OB, Hansen HH, Larsen SO. Intracranial metastases in small cell carcinoma of the lung. Correlation of clinical and autopsy findings. Cancer. 1982;50:2433–2437. doi: 10.1002/1097-0142(19821201)50:11<2433::aid-cncr2820501131>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 38.Yokota J, Kohno T. Molecular footprints of human lung cancer progression. Cancer Sci. 2004;95:197–204. doi: 10.1111/j.1349-7006.2004.tb02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrd RB, Carr DT, Miller WE, Payne WS, Woolner LB. Radiographic abnormalities in carcinoma of the lung as related to histological cell type. Thorax. 1969;24:573–575. doi: 10.1136/thx.24.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Li J, Zhang Y, Hu Y, Zhang G, Yan X, Lin Z, Zhao Z, Jiao S. Early versus late prophylactic cranial irradiation in patients with extensive small cell lung cancer. Strahlenther Onkol. 2018;194:876–885. doi: 10.1007/s00066-018-1307-1. [DOI] [PubMed] [Google Scholar]
- 41.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 42.Dai Y, Morishita Y, Mase K, Sato N, Akaogi E, Mitsui T, Noguchi M. Application of the p53 and K-ras gene mutation patterns for cytologic diagnosis of recurrent lung carcinomas. Cancer. 2015;90:258–263. doi: 10.1002/1097-0142(20000825)90:4<258::aid-cncr10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 43.Kogan EA, Rodionov KV, Trishkina NV, Dubrovskaia MI. The DNA content in precancer, peripheral cancer and carcinoids of the lung (histospectrophotometric research) arkhiv patologii. 1991;53:17–24. [PubMed] [Google Scholar]


