Abstract
Our aim in this study was to evaluate the effect of low-level laser therapy (LLLT) by means of diode laser bio-stimulation compared to Teriparatide in induced osteoporosis in rats. A total of 45 adult female Egyptian albino rats were used. Rats were divided into five groups: normal control, osteoporotic control, Teriparatide (TPTD) group (T), laser group (L), and laser and teriparatide (T+L) combination group. Osteoporosis was induced by performing double ovariectomy in rats. Lower jaws and left femurs were dissected. The specimens were tested using a Computed tomography unit, scanning EM (SEM) equipped with Energy Dispersive X-Ray Analyzer, and Rat PINP ELISA Kit. The histopathologic examination of experimental rat jaws and femurs revealed changes in bone architecture among the various groups throughout the experiment. CT examination showed a noticeable difference in radiodensity between jaw and femur bones. By SEM, bones of the Normal Control (NC) group showed normal bone porosity. However, bones of the Osteoporotic Control (OC) group showed a great difference as bone pores were large and numerous with irregular outlines. The ELISA test for PINP concentration showed a steady rise in the PINP concentrations in OC, T, L and T+L groups. We concluded that TPTD has osteogenic potential and is capable to enhance bone architecture by inducing the formation of new well-organized bone with narrower bone pore diameter. LLLT can be used as a good alternative local treatment strategy with minimal side effects and superior outcomes.
Keywords: Osteoporosis, teriparatide, low level laser therapy, amino terminal propeptide of type I procollagen, bone porosity
Introduction
Osteoporosis (OP) is a global problem and one of the most common bone diseases in humans. Its significance is rapidly increasing as the population in the world is both growing and aging. Unfortunately, the main side effect of OP is pathologic bone fracture which results in great suffering, disability, as well as loss of productivity and quality of life. Fractures represent an enormous burden on healthcare systems, especially in older people who suffer long-term disability, resulting in loss of independence and a higher risk of death [1,2].
Concerning our community, it is projected that by 2050 Egypt will have the largest population in the region with approximately 130 million inhabitants. More than 30% of the population age will be over 50 years old [3]. Therefore, an increase in osteoporotic patients is predicted. Furthermore, to date, access to diagnostics which allow early diagnosis of OP is extremely limited in the Middle East and African regions [4]. Accordingly, the development of novel therapeutic agents is necessary to improve patient outcome, and the availability of effective therapies for people at high risk of OP and related fractures is mandatory.
In 2002, the Food and Drug Administration (FDA) has approved Teriparatide (TPTD), the first anabolic agent that stimulates osteoblastic bone formation to improve bone quality and bone mass. To date, it is the only currently available therapeutic agent that increases the formation of new bone tissue and can provide some remediation of the architectural defects in the osteoporotic skeleton. However, it is restricted to second-line use for OP treatment due to its higher cost than first-line less effective agents such as alendronate [5].
During the process of OP treatment, biochemical markers of bone turnover showed potential to provide early feedback to patients and prescribers [6]. Exhibiting a positive biochemical marker response could confirm an anabolic biologic response in the bone. Contrarily, patients without a significant change in biochemical marker concentration could have certain problems in the treatment plan. If such problems are identified, addressed, and corrected, this type of early assessment could improve patient outcome [7].
Among the most accepted biochemical markers of bone formation, procollagen type I N propeptide (PINP) appeared to be beneficial [8]. Detection of PINP levels is an appealing approach, as it is a byproduct of type I collagen, which is the most prevalent protein in bone. PINP concentrations have reflected the integrated amount of skeletal new bone formation [7]. Thus, diseases involving high bone turnover would be expected to be associated with high concentrations of PINP [9].
In the past few decades, low-level laser therapy (LLLT) has gained acceptance in both medical and dental practices. The application of low intensity influences the cellular behavior through photophysical, photochemical, and photobiological effects on the irradiated cell tissues. Various studies showed that LLLT could be an alternative, co-adjuvant, and noninvasive treatment method with a significant effect on the healing process with fewer side effects. Due to the several benefits of LLLT, researchers have attempted to determine its effect on bone. However, scarce studies were accomplished to investigate the osteogenic potential of LLLT on OP [10].
Accordingly, it seems interesting to study the effect of LLLT on OP and compare it to the widely approved TPTD, as well as, to study their combined effect, using a state of the art techniques like computed tomography (CT), ordinary light microscope, scanning electron microscope (SEM), energy dispersive x-ray analyzer (EDAX) and enzyme-linked immunosorbent assay (ELISA). While SEM was used to detect bone pores, ELISA was used to detect PINP concentrations. This study is looking forward to widening the variety of the less invasive treatment options to improve the disease prognosis, and enhance the quality of life for humans, especially elderly patients.
Materials and methods
Experimental animals
A total of 45 adult female Egyptian albino rats, maintained in an animal house as an inbred colony, were obtained from the Faculty of Medicine, Cairo University. Rats were selected with an average age range of 3 to 4 months and a weight range of 130 to 200 gm for carrying out this experiment.
Materials
Laser unit (Quanta system-Italy)
Laser photon unit, manufactured in China and specially assembled at the National Institute of Laser Science, Cairo University.
Teriparatide
FORTEO (teriparatide [rDNA origin] injection), purchased from Egy-Drug Company, Cairo, Egypt and manufactured by Eli Lilly, LLC Company, USA.
Methods
Study design
The experimental protocol used was approved by Institutional Animal Care and Use Committee (CU-IACUC), Faculty of Science, Cairo University, with the approval number: CU III S 9 16. The study design was a randomized animal study. Sample size was based on the previous work of Diniz et al. [11]; a minimum of 6 rats per group was enough. This number was increased to 9 rats to compensate for deaths and several euthanizations during the experiment (45 total).
A. Animal Grouping.
The 45 rats were randomly distributed into 5 groups (n = 9) as follows:
I. Control groups: Normal Control (NC) Group was used as a negative control, where osteoporosis was not induced in the rats. Osteoporotic Control (OC) Group was used as a positive control, where osteoporosis was induced but without the introduction of any treatment.
II. Experimental groups: Teriparatide group (T): these are osteoporotic rats which were treated by subcutaneous injection of TPTD. Laser group (L): these are osteoporotic rats which were treated by LLLT application. Laser and teriparatide combination group (T+L): where the osteoporotic rats were subjected to both treatments.
Experimental procedure is summarized in Table 1.
Table 1.
Experimental procedure
| Procedure | Groups | ||||
|---|---|---|---|---|---|
|
| |||||
| NC | OC | T | T+L | L | |
| Induction of osteoporosis | No ovariectomy | Double ovariectomy was done in the beginning of the experiment | |||
| Subcutaneous TPTD injection (12th week) | No injection | Subcutaneous injections 6 days a week | No injection | ||
| LLLT application (12th week) | No application | LLLT application on the lower jaw and left femur every 48 hours (3 times a week). | |||
| Euthanization dates &dissection of animals | One third of each group were euthanized at the end of weeks: 16, 19 and 24. | ||||
| Lower jaws (divided into right and left halves) and left femurs were dissected. | |||||
| Bone density measurement | Done using CT at the end of weeks: 16 and 24. Assessment was done on lower jaws and left femurs. | ||||
| Histopathological examination | Done using ordinary light microscope at the end of weeks: 16, 19 and 24. Assessment was done on right halves of jaws and femur shafts. | ||||
| Measurement of average size of bone pores | Done using SEM at the end of weeks: 16, 19 and 24. Assessment was done on left halves of jaws. | ||||
| Calcium & phosphorus elemental analysis | Done using EDAX at the end of weeks: 16, 19 and 24. Assessment was done on left halves of jaws. | ||||
| Measurement of PINP levels | Done using an ELISA kit at the end of weeks: 16, 19 and 24. Assessment was done on femur head and neck. | ||||
Experimental animals
Housing and allocating animals to experimental groups
The animals were housed with the technician help in a controlled environment (temperature 25±2°C and 12 hr dark/light cycles). The rats were given their basic diet of regular rat chow and distilled water which was formulated to meet the nutrients needs of rodents for days of the experiment. The rats were housed in cages with stainless steel covers (3 rats/cage) under the same conditions of temperature and humidity. The rats were randomly distributed by a sequence generation computerized program (random.org).
Induction of osteoporosis
Osteoporosis was induced by performing double ovariectomy in rats. The operation was carried out by the veterinarian in the animal house, under local anesthesia. Ketamine (ketamar (eg), Amoun pharmaceutical company) was used as an anesthetic agent. Intramuscular 90/5-10 mg/kg dose was given half an hour before surgery, according to the procedure adopted by Idris [12]:
1. The anesthetized animal was placed on the operating table with its ventral side exposed and its tail towards the vet.
2. A single midline dorsal incision (2 cm) penetrating the skin using small scissors. An incision was done, directly below the bottom of the rib cage.
3. Using blunt forceps, freeing the subcutaneous connective tissue from the underlying muscle was done on each side.
4. Each ovary was located at a time under the thin muscle layer and a small incision (less than 1 cm) was made on each side to gain entry to the peritoneal cavity.
5. The edge of the incision was held securely with tooth forceps and the ovarian fat pad surrounding ovaries was retracted with blunt forceps to expose the oviduct.
6. A single ligature was performed around the oviduct (2 cm from the ovary) to prevent bleeding following the removal of the ovary.
7. Ovaries were removed by gently severing the oviduct, using sterile, small scissors. Then the surgical wound was sutured.
8. The animal was turned over to lay on its ventral surface and its tail pointing away from the vet.
Treatment application
At the 12th week (3 months) of ovariectomy:
A. Teriparatide: Experimental rats in Tand T+L groups were injected with TPTD subcutaneous injections of 6 µg/Kg per day (6 days a week). This dose was recommended by Takakura et al. [13].
B. Laser: Experimental rats in L and T+L groups were exposed to LLLT sessions with Wavelength = 807 nm, energy output = 350 mW, for 3 minutes on the lower jaw and 3 minutes on the left femur every 48 hours (3 times a week) [14]. Sessions were given at the animal house, Faculty of Medicine, Cairo University. The rats were not anesthetized to avoid deaths or undesired side effects. The technician was responsible to hold the rat with one hand and limit its limb movement by the other. While the researcher applied the laser beam with one hand, the other hand was used to control the application time and the switch control of the machine.
Euthanization of rats & dissection
Euthanization was achieved by an overdose of anesthetic solution (1 ml/100 gm) according to The Research Animal Guidelines of Euthanasia [15]. Animal euthanization was done at the end of weeks 16 (after one month), 19 (One month and three weeks) and 24 (three months) calculated from the beginning of the experiment, respectively. One-third of each group was euthanized at a time. Bone dissection took place immediately after euthanization, where the lower jaws and left femurs were dissected. Then the lower jaw was divided into right and left halves, whereas the femurs were divided into head, neck, and shaft. The specimens were tested using computed tomography unit (Bright Speed CT machine, GE Healthcare, Wisconsin, USA), SEM equipped with Energy Dispersive X-Ray Analyzer (EDAX) (QUANTA 250 FEG, SEM) and Rat PINP (Procollagen I N-Terminal Peptide) ELISA Kit (Sunny Southern California, San Diego, USA).
Computed tomography (CT)
Assessment of BMD was done on dissected lowers jaws and left femurs using CT, where, three random points were measured on each bone then the average was calculated. BMD was measured in HU. The assessment was done twice (weeks 16 and 24).
Histopathological examination
The right half of the jawbone and femur shaft were routinely prepared to obtain H&E stained sections. They were examined under the ordinary light microscope to detect and compare the histopathologic changes among different experimental groups after treatment application throughout the study.
SEM and EDAX examination
Left halves of the jaws were prepared to be examined under SEM. Calcium and phosphorus levels were measured through the comparative energy dispersion of minerals using the EDAX system equipped with the SEM. Then, calcium to phosphorus ratio was calculated. Carbon level was also measured to ensure electrical conductivity during data acquisition [16].
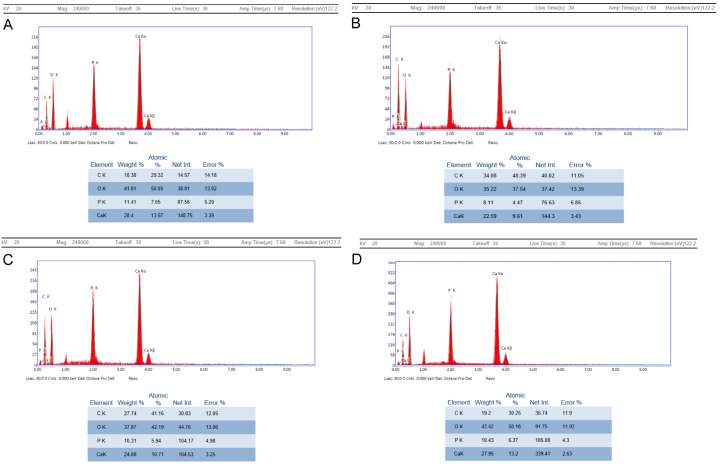
The calcium level was represented by a graph together with the levels of other elements (phosphorus, carbon and oxygen) in an EDAX report. A table at the end of each report illustrated the values recorded by the energy dispersion. The levels were represented by weight %.
SEM preparation and assessment
The specimens were prepared according to the procedure adopted by Canillioglu et al. [17]. Bone tissue was fixed in 2.5% glutaraldehyde in 0.1M phosphate buffer (pH 7.2-7.4) for 24-48 hours at 4°C. Then the tissue was rinsed in distilled water. Then, the specimen was immersed in a 5% NaOH solution for 3-5 days at room temperature to remove the adherent tissues. It was rinsed in distilled water for 12 h at 4°C. After that, the specimen was post-fixed in 1% osmium tetroxide for 2 h at 4°C. It was then dehydrated with ascending graded ethanol (70%, 80%, 90%, 95%, 100% ethanol) and dried with CO2 liquid in a critical point dryer. The specimen was then mounted on metal stubs then, coated with gold using an ion sputter.
The diameter of bone pores was measured in 3 random fields for each specimen. Ten different pore diameters were measured in each field and the average was calculated for the minimum and maximum values.
ELISA
The femur’s head and neck were dissected and wrapped with labeled aluminum foil then stored at -80°C. At the analysis time, the specimens were homogenized in phosphate buffer saline and then centrifuged for 20 min at 1000×g at 2~8°C. Then, the supernatant was collected to carry out the assay.
The supernatant was divided into samples of 100 μL. Each was added to a well in the micro ELISA plate. Then incubated for 90 min at 37°C. Then, the liquid was removed. 100 μL Biotinylated Detection Ab was added and incubated for 1 hour at 37°C. Then, aspirated and washed 3 times. A 100 μL HRP Conjugate was added and the sample was incubated for 30 min at 37°C. Then, it was aspirated and washed 5 times. A 90 μL Substrate Reagent was added and the sample was incubated for 15 min at 37°C. Then, 50 μL Stop Solution was added. Finally, readings were recorded at 450 nm immediately and the results were calculated.
Assessment
The optical density which was proportional to the concentration of PINP, was measured spectrophotometrically, using a Microtiter Plate Reader (Stat Fax 2100, Awareness Technology Inc, 6511 Bunker Lake Blvd. Ramsey, Minnesota, USA) at a wavelength of 450 nm within 15 min after adding the stop solution. The optical density value of the blank control well was set as zero.
Known concentrations of rat PINP standard and its corresponding reading optical density was plotted on the log scale (x-axis) and the log scale (y-axis), respectively. The concentration of rat PINP in samples was determined by plotting the sample’s optical density on the Y-axis.
Statistical methods
All data were collected, tabulated, and subjected to statistical analysis. Statistical analysis was performed by SPSS (Statistical Package for the Social Sciences) (version 17) in general. Microsoft Office Excel was used for data handling and graphical presentation. Quantitative variables were described by the Mean, Standard Deviation (SD), the Range (Minimum-Maximum), Standard Error (SE) and 95% confidence interval of the mean. Qualitative categorical variables were described by proportions and percentages. Shapiro-Wilk test of normality was used to test the normality hypothesis of all quantitative variables for further choice of appropriate parametric and non-parametric tests.
Results
Most of the variables were found normally distributed, allowing the use of parametric tests. Independent samples t-test was used for comparing the means of the two groups. On comparing more than two groups at the same time point, One Way Analysis of Variance (ANOVA) test was applied. At the end of the experiment (24 weeks), pairwise comparisons were carried out by applying Bonferroni test, whenever ANOVA was significant.
Histopathologic examination
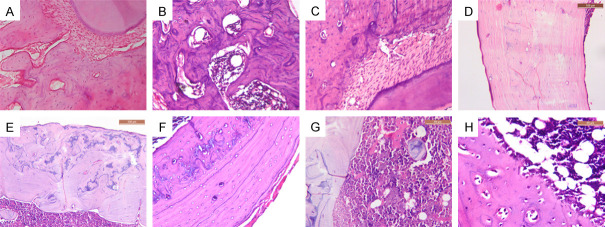
Histopathologic examination of experimental rat jaws and femurs revealed changes in the bone architecture among the various groups throughout the experiment. On comparing the control groups, a marked difference was observed between them. In the NC group, bone trabeculae were thick, dense, connected, and enclosing narrow cellular marrow spaces (Figure 1A and 1B). Also, various osteocytes were seen in their lacunae (Figure 1A and 1C). On the contrary, the OC group showed thinner bone trabeculae enclosing wide marrow spaces. Presence of thin separate bony spicules was also observed (Figure 2A and 2B).
Figure 1.
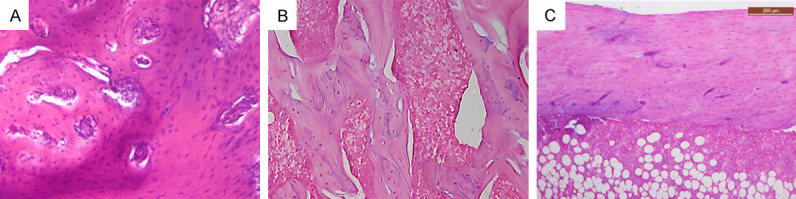
Photomicrographs of NC group. A. Jawbone showing thick connected network of bone trabeculae enclosing narrow marrow spaces (×200). B. Femur bone near the neck area showing thick connected network of bone trabeculae enclosing hematopoietic (×200). C. Femur bone showing thick cortical bone. Note, red marrow showing hematopoietic cells in addition to fat cells (×100).
Figure 2.
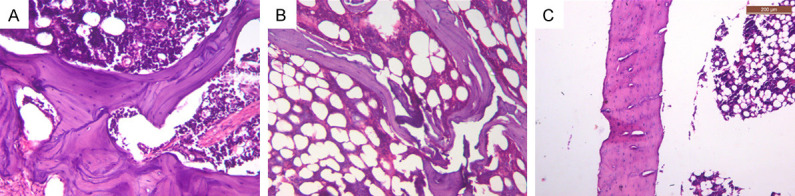
Photomicrographs of OC group. A. Jawbone showing thin bone trabeculae enclosing wide marrow spaces with many reversal lines (×200). B. Femur bone near the neck area showing thin discontinuous bone trabeculae, and wide marrow spaces filled with fibrofatty marrow (×200). C. Femur bone showing thin cortical bone and fibrofatty marrow with presence of irregular cavities within bone (×100).
Reduction of bone thickness was more evident in femur than in jawbones. A noticeable reduction in the cortical thickness and density was noted in the OC group (Figure 2C). While fibrofatty marrow was mainly detected in the OC group (Figure 2B and 2C), NC groups showed red bone marrow which was highly vascular and rich in hematopoietic cells (Figure 1A-C).
Concerning the experimental groups, thicker bone trabeculae and narrower marrow spaces were observed throughout the experiment (weeks 16 and 19), in comparison to the OC group. However, dramatic positive changes of bone were detected in week 24 in all the groups, especially T+L group. By the end of the experiment (week 24), the 3 experimental groups showed increased cortical thickness, thick network of bone trabeculae and narrower marrow spaces. Numerous reversal lines, disorganized blue bone as well as osteoid tissue were mainly observed in the lased groups (L and T+L). On the other hand, T group showed fewer reversal lines and well-organized bone. Osteoblastic rimming was detected in T+L group (Figure 3).
Figure 3.
Photomicrographs of experimental groups. A. Jawbone from T group showing thick connected bone trabeculae. Note some reversal lines (×200). B. Jawbone from L group showing thick connected bone trabeculae with numerous reversal lines (×200). C. Jawbone from T+L group showing sheets of bone enclosing extremely narrow marrow spaces with reversal lines at the bone periphery (×200). D. Femur bone from T group showing thick well-organized cortical bone (×200). E. Femur bone from L group showing thick cortical bone with reversal lines and blue bone (×200). F. Femur bone from T+L group showing thick cortical bone with reversal lines and blue bone (×200). G. Femur bone from L group showing osteoid bone in the highly cellular bone marrow (×400). H. Osteoblastic rimming in T+L group (×400).
Statistical analysis
Using t-test, statistical analysis (week 12) showed a highly significant difference in BMD mean values between NC and OC groups, where NC values were higher than OC groups. In both groups, the BMD values were found higher in the jawbones compared to femurs (Table 2).
Table 2.
Difference in BMD between NC and OC groups in week 12 using T test
| BMD | Group | Mean ± SD | P Value |
|---|---|---|---|
| Jaws | NC | 1506.82±135.23 | 0.00000** |
| OC | 778.90±205.57 | ||
| Femurs | NC | 1051.69±116.00 | 0.00000** |
| OC | 566.16±13.05 |
P < 0.001 HS.
In week 16, on comparing the 3 treated groups with the control groups, while the mean values of BMD of jawbones showed high statistical significance (Table 3), whereas those of femurs showed a significant difference (Table 4). Contrarily, minor changes in BMD mean values for both jaws and femurs were detected at week 24 and considered as statistically non-significant. On comparing changes in BMD within the same experimental group throughout the experiment, a non-significant difference was obtained in all treated groups for both jaw and femur bones (Tables 3 and 4).
Table 3.
Differences in jaw bone mineral density (BMD) among experimental groups using ANOVA
| T | L | T+L | P value | |
|---|---|---|---|---|
| Week 16 | 1110.61±106.32 | 1145.11±91.52 | 861.06±92.57 | 0.00000** |
| Week 24 | 1119.34±79.59 | 1195.63±110.11 | 1166.03±84.92 | 0.23156NS |
| P value | 0.84604NS | 0.30555NS | 0.0000** |
P < 0.001, HS;
P > 0.05, NS.
Table 4.
Difference in femur bone mineral density (BMD) among experimental groups using ANOVA
| T | L | T+L | P value | |
|---|---|---|---|---|
| Week 16 | 757.26±89.38 | 689.61±50.54 | 639.94±103.26 | 0.02329* |
| Week 24 | 717.14±103.81 | 694.58±78.41 | 663.15±78.78 | 0.43650NS |
| P value | 0.39263NS | 0.87493NS | 0.59930NS |
P < 0.05 = S, P > 0.05 = NS.
Computed tomography (CT)
In the current work, CT examination showed a noticeable difference in radio-density between jaw and femur bones. This was obvious in all 5 groups throughout the whole experiment. However, BMD differences in jawbones among experimental groups were negligible and the same was found when comparing femur bones together (Figure 4).
Figure 4.
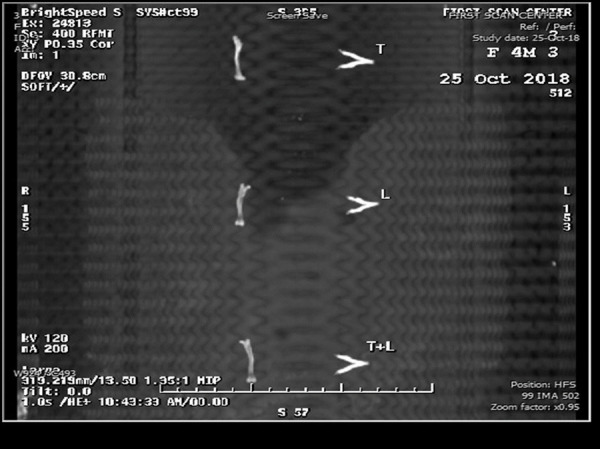
Copy of display showing jaw and femur bones of 3 different rats from the 3 treatment groups.
Concerning the 3 experimental treated groups, they showed an increase in the mean values of BMD, which was observed in jaw rather than femur bones. The highest mean values of BMD in jaw and femur bones were detected in L group and T group, respectively.
Scanning electron microscopy (SEM)
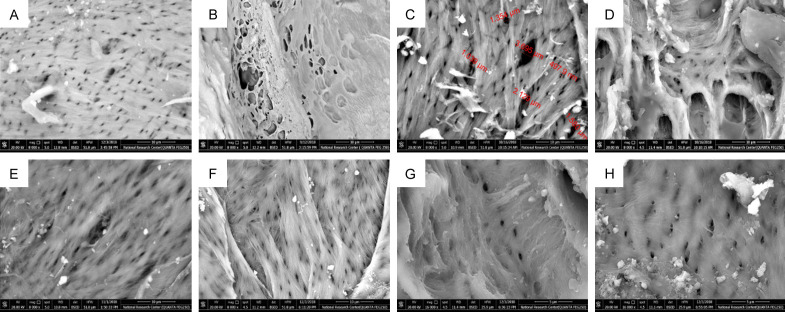
By examining jawbone specimens under SEM, bones of the NC group showed normal bone porosity (BP), where most of the pores were small, uniform and approximately equal (Figure 5A). On the other hand, bones of OC group showed a great difference when compared to NC group, as bone pores were large, numerous and had an irregular outline (Figure 5B). After treatment application, an improvement was noticed in all experimental groups regarding the size and uniformity of bone pores (Figure 5C-E).
Figure 5.
SEM images. A. NC group showing normal jawbone architecture with equal and uniform porosity. B. OC group showing increased BP, where large and irregular bone pores were observed. C. T group showing measurement of bone pores at week 16 with variation in size of bone pores. D. L group showing BP at week 16 with some bone pores started to get narrower. E. T+L group showing numerous small regular pores with few large ones at week 19. F. T group showing numerous uniform bone pores, G. L group showing uniform regular bone pores at week 24. H. T+L group showing uniform regular bone pores at week 24 with some pores are getting completely obscured.
At weeks 16 and 19, narrowing of bone pores was observed. However, some large irregular ones remained in week 16, and were reduced greatly at week 19. By the end of the experiment (week 24), BP in the treatment groups showed a marked decrease and was comparable to that of the NC group (Figure 5F-H). This was obviously revealed in T+L groups.
Energy dispersive X-ray analyzer (EDAX)
The calcium weight % showed higher levels in the NC group when compared to the OC group. After treatment application, there was a linear increase in the calcium weight % together with the phosphorus weight %. By the end of the experiment, the T+L group showed peak values which nearly approached those of NC group. It was followed by the L group and then T group. However, calcium/phosphorus ratio was constant for NC and all the treatment groups throughout the experiment. On the contrary, it gradually decreased in the OC group throughout the experiment (Table 5; Figure 6).
Table 5.
Calcium/Phosphorus ratio throughout the experiment
| Week 12 | Week 16 | Week 19 | Week 24 | |
|---|---|---|---|---|
| Ca/ph | Ca/ph | Ca/ph | Ca/ph | |
| NC | 2.49 | 2.50 | 2.45 | 2.52 |
| OP | 1.86 | 1.45 | 1.02 | 0.63 |
| T | 2.36 | 2.38 | 2.79 | |
| L | 2.63 | 2.72 | 2.34 | |
| T+L | 2.44 | 2.45 | 2.68 |
Figure 6.
EDAX reports showing values of calcium. A. NC group at week 24. B. T group at week 24. C. L group at week 24. D. T+L group at week 24.
ELISA for PINP concentration
Our results showed a steady rise in the PINP concentrations in OC, T, L, and T+L groups throughout the experiment. During weeks 16 and 19, the T+L group showed the highest PINP value followed by T group and finally L group. However, at week 24, T and L groups showed equal values, while the maximum increase was seen in T+L group. The minimum PINP concentration value was seen in OC group during the same week.
Statistical analysis
At the beginning of the experiment (week 12), a difference in the mean values of PINP concentrations between NC and OC groups was observed. The OC group recorded a higher value than the NC group. This difference was statistically significant (Table 6). Throughout the treatment weeks (16, 19 and 24), there was a statistically highly significant increase in the PINP concentration mean values in OC, T, L and T+L groups. The maximum increase was observed between week 19 and 24 in the mentioned groups. The maximum value for PINP concentration recorded was found in T+L group in week 24 (Table 7).
Table 6.
Difference between NC and OC groups for PINP levels mean values at week 12 using T test
| Group | Mean ± SD | P Value |
|---|---|---|
| NC | 3.10±0.77 | 0.01942* |
| OC | 4.00±0.69 |
P < 0.05, S.
Table 7.
Difference in PINP mean among experimental groups using ANOVA
| NC | OC | T | L | T+L | P value | |
|---|---|---|---|---|---|---|
| Week 16 | 3.10±0.77 | 4.90±0.70 | 13.10±0.84 | 10.60±0.89 | 17.20±0.82 | 0.00000** |
| Week 19 | 3.10±0.77 | 5.40±0.72 | 15.90±0.64 | 12.40±1.00 | 17.30±0.84 | 0.00000** |
| Week 24 | 3.10d±0.77 | 6.30c±0.73 | 21.30b±1.22 | 21.30b±0.76 | 34.90a±1.01 | 0.00000** |
| P value | 0.00000** | 0.00000** | 0.00000** |
Superscripts with different letters are statistically significant, while superscripts with the same letters are statistically non-significant
is the highest value;
is the second value;
is the third value;
is the lowest value.
P < 0.001, HS.
All multiple pairwise comparisons (week 24), showed a highly significant difference except for NC versus T+L groups and T versus L groups, which were non-significant (Table 7).
Discussion
Osteoporosis (OP) is a worldwide health problem; if left untreated, it can have a profound impact on day-to-day life. Although up to date, there is no full cure for OP. Early detection and proper selection of adequate treatment can shift the course of the disease. As with appropriate management, the progression of OP can be slowed, stopped, or even reversed in some cases [18]. For the above-mentioned points, it seemed interesting to examine the therapeutic effect of a combination of systemic and local treatment options.
Being inevitable, post-menopausal OP ranks top among the major health issues. It is mainly caused by estrogen withdrawal [19]. Despite the intense research and the relevant progress achieved in the last decades, the pathogenic mechanism underlying post-menopausal OP is still poorly understood. Such disorder and its related complications remain difficult to be effectively cured and prevented [18]. Because of this gap in knowledge, our study was carried out aiming to add to the current literature regarding merging tools for OP assessment.
Thus, the current study was carried out to compare the anabolic effect of TPTD to the LLLT non-pharmaceutical biostimulatory effect on bone. Furthermore, their combined effect was investigated. To the best of our knowledge, this is the first study in the available literature about the combined effect of TPTD and LLLT. Accordingly, there was a compelling need for novel techniques rather than the widely used ones and merging several assessment tools together to show the full picture of the effect of proposed treatments on bone.
The assessment methods were carefully chosen to include histopathologic examination which was considered as the ‘gold-standard’ by Du [20], and BMD assessment using CT. Despite DXA being currently used, it determines BMD in two dimensions only. Interestingly, CT allows measurement of volumetric trabecular BMD without superimposition of cortical bone and other tissues making it more sensitive over DXA [21]. To widen our field of research, SEM for bone porosity quantitative examination was also used as a novel technique, as most of previous studies used SEM to examine lamellar topography, resorption spaces and microcracks of bone rather than bone porosity [20,22,23]. To the best of our knowledge, the current work is among scarce studies concerned about monitoring treatment effect using bone porosity quantitative examination.
Moreover, EDAX system equipped SEM was used to investigate the chemical composition of the studied bone samples, in order to evaluate bone crystalline structure which reflects bone quality in terms of the amount of calcium by weight % [24]. For more validation of our work, the research was further extended to cover bone turnover markers. PINP was chosen since its concentration is equimolar to the amount of collagen incorporated into the bone matrix. This is consistent with Szulc et al. [25] who pointed out that PINP levels correlated significantly with histomorphometric measures of bone formation.
To accomplish the current work, rats were the experimental model of choice. Similarities in pathophysiologic responses between the human and rat skeleton, combined with husbandry and financial advantages, have made the rat a valuable model in our study [26]. In accordance with Sheu et al. [27], female rats were particularly selected over male ones because women are at higher risk for OP and osteoporotic fractures than men. Moreover, using them allowed us to elicit an estrogen-deprived state by ovariectomy, to serve our aim of tackling post-menopausal OP. Finally, the present experiment agreed with Lelovas et al. [26], to choose female rats of approximately 4 months old to guarantee their sexual maturity that is usually estimated at 2.5 months.
In order to expand the variety of the research outcome, studying osteoporotic changes was not limited to jawbones, but femur bones were included as well. In the same context, Johnston et al. [28] claimed that postmenopausal OP was commonly associated with increased risk of tooth loss, which may lead to compromised food choices resulting in a poor diet that can contribute to chronic disease risk. On the other hand, Lelovas et al. [26] pointed out that the analysis of cortical bone in the femur shaft was a very sensitive index for OP assessment because the bulk of bone loss occurs at this site. Therefore, investigation of both jaw and femur bones was done for better understanding of OP and the therapeutic outcomes of the proposed treatments.
Ovariectomy was the method of choice for OP induction in the present study, because it was found that hormonal changes resulting from ovary removal led to unevenness of bone remodeling, which caused an imbalance between bone resorption and bone formation leading to bone loss and high bone fracture risk [29]. Interestingly, our pilot study revealed osteoporotic changes in bones of experimental animals 12 weeks after ovariectomy; it was well documented using different assessment tools. This agrees with Lelovas et al. [26] and Sophocleous et [29]. They agreed that although osteoporotic changes in femur bones were detected after 30 days of the surgery, they were difficult to detect prior to 12 weeks in the jawbone of rats.
Results of the current work proved the capability forovariectomy to induce osteoporosis successfully in the OC group; this was evident by the different assessment methods used. Decreased BMD of either jaw or femur bones which was detected in the OC group, using CT, is in agreement with Kuroda et al. [30]. Regarding histopathologic findings, they are in line with those of Lorentzon et al. [31], who reported cortical thinning, increased cortical porosity as well as enlargement of marrow cavities in osteoporotic bone. In addition, the increase in the fat content of the marrow spaces is consistent with Li et al. [32]; they observed that increased marrow fat content has occurred in synchrony with the deterioration of trabecular microarchitecture in ovariectomized rabbits.
Regarding bone porosity using SEM, a significant increase in average size of bone pores in the OC group, is supported by the results of Ahmed et al. [33] and Ayana et al. [34], where the latter detected roughening and increased porosity in diabetic osteopenia in rat jaws. Moreover, EDAX analysis detected a drop in calcium level of OC group, reaching about half that of NC group; this also agreed with Ayana et al. [34] and Paolillo et al. [16].
Unexpectedly, PINP concentrations appeared to be higher in the OC group and reached more than double that of the NC group. This suggests an anabolic natural body response to counteract the deteriorating osteoporotic effect on bone. However, the body cannot depend totally on this response; this can be explained by the gap in values obtained by the OC group and all treatment groups, by the end of the experiment. This finding is in accordance with the results of Yan et al. [35]. Generally, all the findings of the OC group indicate the advancement of OP, with physicochemical and structural deterioration in bone. This directly affects bone strength and quality in addition to increasing the amount of deformation, creating and/or propagating cracks, which in turn may lead to greater bone fracture risk.
Throughout the experiment, a constant Ca/P ratio was observed in normal control and all experimental groups, which is consistent with Perdikouri et al. [36] and reflects normal bone health and active remodeling. Contrarily, the Ca/P ratio in the OP group continued to decrease. This finding is in line with Kourkoumelis et al. [24], who showed a strong relationship between induced bone loss and lowered Ca/P ratio. However, in disagreement with our results, Paolillo et al. [16] stated that the Ca/P ratio was constant during the experiment for both control and ovariectomized groups.
Despite acceptance of the idea that merging all accessible assessment tools was a wise decision for research work and patient welfare, it was interesting to identify the most reliable and reproducible used techniques. Cohen d effect size showed that a maximum change was seen in the detection of PINP concentrations using ELISA, followed by EDAX analysis. This finding suggests that these techniques have better precision and higher accuracy than bone porosity and BMD measurement using SEM and CT, respectively.
Although the assessment of BMD by CT was very beneficial in the identification of osteoporotic changes in either jaw or femur bones, it appeared to be unreliable for monitoring treatment progress in the experimental groups. In the same context, Li et al. [32] concluded that BMD changes were constant for a relatively longer time and lagged behind the pathologic course of OP. Krege et al. [7] added that BMD testing was not valuable before 1 to 2 years of treatment. Our results were further supported by Nguyen et al. [37] and Langdahl et al. [38] as both studies agreed that measuring BMD has its own limitations. About 50% of patients who had sustained osteoporotic fracture had BMD above the WHO definition of OP. This was contradictory to Kwon et al. [39] who stated that sequential monitoring of femoral BMD using QCT could be useful for OP assessment.
Fortunately, the results of the current study revealed that all the proposed treatments showed favorable outcomes, especially the combined group. Concerning TPTD, its positive impact on bone was consistent with Caffarelli et al., [40] and Yao et al., [41]; they believed that TPTD is the only bone anabolic drug which increased BMD with proven anti-fracture efficacy. However, its high cost and daily injection course make it an economic burden. That is why antiresorptive agents are still considered to be the first-line pharmaceutical treatment for OP [42]. Moreover, International Osteoporosis Foundation (2011) reported that although TPTD is well-known worldwide, it was available only in half of Arabic countries.
Histopathologic changes resulted from TPTD administration are in accordance with the findings of Fahrleitner-Pammer et al., [43] who concluded that TPTD was able to induce trabecular connectivity and increase cortical bone formation. In the same context, Larsson et al., [44] stated that TPTD increased calcium concentrations, which led to a rise in the degree of mineralization in the fracture callus which was accompanied by accelerated natural fracture healing and a faster remodeling process. Furthermore, the observed increase in PINP levels in association with TPTD is in line with Szulc et al., [25]. In conflict with our results, Roschger et al. [45] detected lower calcium concentrations in bones treated with TPTD.
On the other hand, the beneficial effect of LLLT on bone is attributed to its biostimulatory action, where it is capable of increasing mitochondrial activity, bone formation, osteocalcin and osteopontin gene expression, as well as alkaline phosphate activity [46]. This is supported by Torstrick et al., [47] who pointed out that LLLT could be utilized to prevent fracture, improve healing, and accelerate implant fixation. Accordingly, the results of the present study propose the possibility of using LLLT as local treatment strategy that could act as a good alternative with minimal side effects and superior outcome.
The osteogenic potential of LLLT is in harmony with the results of Scalize et al., [10] and Fallahnezhad et al. [48] as they observed new bone formation and improved cell viability of the ovariectomized rat bone marrow mesenchymal stem cells, respectively. The previous study of Khadra et al. [49] confirmed the rise in calcium levels, which was observed in the L group, since they found more calcium and phosphorus in bones of experimental animals in comparison to their controls. Concerning increased PINP concentrations, Tim et al. [50] and Ma et al. [51] agreed that LLLT induced a significant increase in collagen synthesis and expression of collagen genes. On the contrary, the results of Pereira et al. [52] showed no effect of LLLT on procollagen synthesis.
It is worth noting that observation of numerous reversal lines and blue bone formation in lased groups reflects the capability of LLLT to induce rapid bone formation and high bone turnover. In accordance with our results, Matsumoto et al. [53] highlighted that the experimental group irradiated by LLLT was able to induce woven bone formation faster than the control group. Moreover, the recent study of Suzuki et al. [54] demonstrated that LLLT could independently accelerate the bone remodeling process, and in turn accelerate tooth movement.
Despite the favorable therapeutic effects of both TPTD and LLLT on induced OP, all the assessed parameters revealed that their combination showed superior results, including the highest Cohen d size effect. Logically, the combination of TPTD and LLLT was in favor of maximizing anabolic bone turnover and getting the benefits of each of them. This elucidated the positive synergism between both TPTD and LLLT, which can possibly overcome systemic side effects of a high dose of TPTD, and the limitations of LLLT application. Finally, the results of the present study were very satisfactory, reflecting the osteogenic potential and capability of TPTD and LLLT to treat OP either solely or in combination. Furthermore, our work sheds light on the therapeutic importance of using TPTD and LLLT together as a novel combination of systemic and local treatment options.
Conclusion
TPTD has osteogenic potential and is capable to enhance bone architecture by inducing the formation of new well-organized bone with narrower bone pore diameter.
LLLT can possibly be used as a good alternative local treatment strategy with minimal side effects and superior outcome, as it can improve bone strength by faster bone deposition and higher calcium content.
Combination of both TPTD and LLLT has a synergistic beneficial effect on bones of experimental rats with induced OP. This can possibly overcome systemic side effects of a high dose of TPTD, and the limitations of LLLT application, as well as giving maximum benefit of uniform and speedy new bone formation.
Disclosure of conflict of interest
None.
References
- 1.Mohammed OAE, Shaheen HM, Kaoud YEG. Role of family medicine in the early detection and management of osteoporosis. Menoufia Med J. 2014;27:833. [Google Scholar]
- 2.Abimanyi-Ochom J, Watts JJ, Borgström F, Nicholson GC, Shore-Lorenti C, Stuart AL, Zhang Y, Iuliano S, Seeman E, Prince R, March L, Cross M, Winzenberg T, Laslett LL, Duque G, Ebeling PR, Sanders KM. Changes in quality of life associated with fragility fractures: Australian arm of the International Cost and Utility Related to Osteoporotic Fractures Study (AusICUROS) Osteoporos Int. 2015;26:1781–90. doi: 10.1007/s00198-015-3088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Tawab SS, Saba EKA, Elweshahi HMT, Ashry MH. Knowledge of osteoporosis among women in Alexandria (Egypt): a community based survey. Egypt Rheumatol. 2016;38:225–31. [Google Scholar]
- 4.El-Hajj Fuleihan G, Adib G, Nauroy L. The middle east & Africa regional audit, epidemiology, costs & burden of osteoporosis in 2011. Int Osteoporos Found. 2011 102011-5000. [Google Scholar]
- 5.Eastell R, Walsh JS. Anabolic treatment for osteoporosis: teriparatide. Clin Cases Miner Bone Metab. 2017;14:173. doi: 10.11138/ccmbm/2017.14.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer D, Krege J, Lane N, Leary E, Libanati C, Miller P, Myers G, Silverman S, Vesper HW, Lee D, Payette M, Randall S. National bone health alliance bone turnover marker project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos Int. 2012;23:2425–33. doi: 10.1007/s00198-012-2049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krege JH, Lane NE, Harris JM, Miller PD. PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos Int. 2014;25:2159–71. doi: 10.1007/s00198-014-2646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terreni A, Pezzati P. Biochemical markers in the follow-up of the osteoporotic patients. Clin Cases Miner Bone Metab. 2012;9:80. [PMC free article] [PubMed] [Google Scholar]
- 9.Vasikaran S, Cooper C, Eastell R, Griesmacher A, Morris HA, Trenti T, Kanis JA. International osteoporosis foundation and international federation of clinical chemistry and laboratory medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med. 2011;49:1271–4. doi: 10.1515/CCLM.2011.602. [DOI] [PubMed] [Google Scholar]
- 10.Scalize PH, de Sousa LG, Regalo SC, Semprini M, Pitol DL, da Silva GA, de Almeida Coelho J, Coppi AA, Laad AA, Prado KF, Siessere S. Low-level laser therapy improves bone formation: stereology findings for osteoporosis in rat model. Lasers Med Sci. 2015;30:1599–607. doi: 10.1007/s10103-015-1773-y. [DOI] [PubMed] [Google Scholar]
- 11.Diniz JS, Nicolau RA, de Melo Ocarino N, do Carmo Magalhaes F, de Oliveira Pereira RD, Serakides R. Effect of low-power gallium-aluminum-arsenium laser therapy (830 nm) in combination with bisphosphonate treatment on osteopenic bone structure: an experimental animal study. Lasers Med Sci. 2009;24:347–52. doi: 10.1007/s10103-008-0568-9. [DOI] [PubMed] [Google Scholar]
- 12.Idris AI. Ovariectomy/orchidectomy in rodents. Methods Mol Biol. 2012;816:545–51. doi: 10.1007/978-1-61779-415-5_34. [DOI] [PubMed] [Google Scholar]
- 13.Takakura A, Lee JW, Hirano K, Isogai Y, Ishizuya T, Takao-Kawabata R, Iimura T. Administration frequency as well as dosage of PTH are associated with development of cortical porosity in ovariectomized rats. Bone Res. 2017;5:17002. doi: 10.1038/boneres.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renno AC, de Moura FM, Dos Santos NS, Tirico RP, Bossini PS, Parizotto NA. Effects of 830-nm laser light on preventing bone loss after ovariectomy. Photomed Laser Ther. 2006;24:642–5. doi: 10.1089/pho.2006.24.642. [DOI] [PubMed] [Google Scholar]
- 15.Underwood W, Anthony R. AVMA guidelines for the euthanasia of animals. (2020th edition) 2013 [Google Scholar]
- 16.Paolillo FR, Romano RA, de Matos L, Martin AA, Guimarães FEG, de Castro Neto JC, Bagnato VS. Short-term and long-term effects of osteoporosis on incisor teeth and femoral bones evaluated by Raman spectroscopy and energy dispersive X-ray analysis in ovariectomized rats. J Bone Miner Metab. 2019;37:18–27. doi: 10.1007/s00774-018-0903-6. [DOI] [PubMed] [Google Scholar]
- 17.Canillioglu E, Bahcesehir ES. Preparation techniques of luminal and hard tissues for scanning electron microscopy. Microsc Adv Sci Res Educ Spain Formatex Res Cent. 2014:741–6. [Google Scholar]
- 18.Bonaccorsi G, Piva I, Greco P, Cervellati C. Oxidative stress as a possible pathogenic cofactor of post-menopausal osteoporosis: existing evidence in support of the axis oestrogen deficiency-redox imbalance-bone loss. Indian J Med Res. 2018;147:341–51. doi: 10.4103/ijmr.IJMR_524_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lizneva D, Yuen T, Sun L, Kim SM, Atabiekov I, Munshi LB, Epstein S, New M, Zaidi M. Emerging concepts in the epidemiology, pathophysiology, and clinical care of osteoporosis across the menopausal transition. Matrix Biol. 2018;71-72:70–81. doi: 10.1016/j.matbio.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Du Z. The effects of osteoporosis on osseointegration in the rat maxilla. Queensland University of Technology. 2015 [Google Scholar]
- 21.Celenk C, Celenk P. Bone density measurement using computed tomography. Comput Tomogr Appl. 2012:123–36. [Google Scholar]
- 22.Donnelly E. Methods for assessing bone quality: a review. Clin Orthop Relat Res. 2011;469:2128–38. doi: 10.1007/s11999-010-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Zhang M, Zhang D, Wang X, Cao H, Zhang Q, Yan C. Structural elucidation and anti-osteoporosis activities of polysaccharides obtained from Curculigo orchioides. Carbohydr Polym. 2019;203:292–301. doi: 10.1016/j.carbpol.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 24.Kourkoumelis N, Balatsoukas I, Tzaphlidou M. Ca/P concentration ratio at different sites of normal and osteoporotic rabbit bones evaluated by Auger and energy dispersive X-ray spectroscopy. J Biol Phys. 2012;38:279–91. doi: 10.1007/s10867-011-9247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 2017;28:2541–56. doi: 10.1007/s00198-017-4082-4. [DOI] [PubMed] [Google Scholar]
- 26.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–30. [PMC free article] [PubMed] [Google Scholar]
- 27.Sheu Y, Zmuda JM, Boudreau RM, Petit MA, Ensrud KE, Bauer DC, Gordon CL, Orwoll ES, Cauley JA Osteoporotic Fractures in Men MrOS Research Group. Bone strength measured by peripheral quantitative computed tomography and the risk of nonvertebral fractures: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2011;26:63–71. doi: 10.1002/jbmr.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston BD, Ward WE. The ovariectomized rat as a model for studying alveolar bone loss in postmenopausal women. Biomed Res Int. 2015;2015:635023. doi: 10.1155/2015/635023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sophocleous A, Idris AI. Rodent models of osteoporosis. Bonekey Rep. 2014;3:614. doi: 10.1038/bonekey.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuroda S, Mukohyama H, Kondo H, Aoki K, Ohya K, Ohyama T, Kasugai S. Bone mineral density of the mandible in ovariectomized rats: analyses using dual energy X-ray absorptiometry and peripheral quantitative computed tomography. Oral Dis. 2003;9:24–8. doi: 10.1034/j.1601-0825.2003.01810.x. [DOI] [PubMed] [Google Scholar]
- 31.Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. J Intern Med. 2015;277:650–61. doi: 10.1111/joim.12369. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Tang G, Liu Y, Tang R, Peng Y, Li W. MR spectroscopy and micro-CT in evaluation of osteoporosis model in rabbits: comparison with histopathology. Eur Radiol. 2012;22:923–9. doi: 10.1007/s00330-011-2325-x. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed LA, Shigdel R, Joakimsen RM, Eldevik OP, Eriksen EF, Ghasem-Zadeh A, Bala Y, Zebaze R, Seeman E, Bjørnerem Å. Measurement of cortical porosity of the proximal femur improves identification of women with nonvertebral fragility fractures. Osteoporos Int. 2015;26:2137–46. doi: 10.1007/s00198-015-3118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abu Ayana MA, Elmasry NA, Shehata FI, Khalil NM. Efficacy of quercetin on alveolar bone structure of rats with induced diabetes. Alexandria Dent J. 2017;42:141–6. [Google Scholar]
- 35.Yan HW, Xu LZ, Duan W. Calcaneal quantitative ultrasound-bone mineral density value for evaluating bone metabolism and bone turnover in patients with osteoporotic fracture. J Hainan Med Univ. 2017;23:150–3. [Google Scholar]
- 36.Perdikouri C, Tägil M, Isaksson H. Characterizing the composition of bone formed during fracture healing using scanning electron microscopy techniques. Calcif Tissue Int. 2015;96:11–7. doi: 10.1007/s00223-014-9930-z. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen TV, Meier C, Center JR, Eisman JA, Seibel MJ. Bone turnover in elderly men: relationships to change in bone mineral density. BMC Musculoskelet Disord. 2007;8:13. doi: 10.1186/1471-2474-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langdahl BL. Is there a place for bone turnover markers in the management of osteoporosis? J Bone Miner Res. 2018;33:1197–8. doi: 10.1002/jbmr.3478. [DOI] [PubMed] [Google Scholar]
- 39.Kwon D, Kim J, Lee H, Kim B, Han H, Oh H, Kim M, Yoon H, Lee B, Eom K. Quantitative computed tomographic evaluation of bone mineral density in beagle dogs: comparison with dual-energy x-ray absorptiometry as a gold standard. J Vet Med Sci. 2018:17–428. doi: 10.1292/jvms.17-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caffarelli C, Hayek J, Nuti R, Gonnelli S. Teriparatide in the treatment of recurrent fractures in a Rett patient. Clin Cases Miner Bone Metab. 2015;12:253. doi: 10.11138/ccmbm/2015.12.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao M, Shimo T, Ono Y, Obata K, Yoshioka N, Sasaki A. Successful treatment of osteonecrosis-induced fractured mandible with teriparatide therapy: a case report. Int J Surg Case Rep. 2016;21:151–3. doi: 10.1016/j.ijscr.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy DR, Smolen LJ, Klein TM, Klein RW. The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet Disord. 2012;13:213. doi: 10.1186/1471-2474-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fahrleitner-Pammer A, Burr D, Dobnig H, Stepan JJ, Petto H, Li J, Krege JH, Pavo I. Improvement of cancellous bone microstructure in patients on teriparatide following alendronate pretreatment. Bone. 2016;89:16–24. doi: 10.1016/j.bone.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Larsson S, Fazzalari NL. Anti-osteoporosis therapy and fracture healing. Arch Orthop Trauma Surg. 2014;134:291–7. doi: 10.1007/s00402-012-1558-8. [DOI] [PubMed] [Google Scholar]
- 45.Roschger P, Misof B, Paschalis E, Fratzl P, Klaushofer K. Changes in the degree of mineralization with osteoporosis and its treatment. Curr Osteoporos Rep. 2014;12:338–50. doi: 10.1007/s11914-014-0218-z. [DOI] [PubMed] [Google Scholar]
- 46.Bossini PS, Rennó AC, Ribeiro DA, Fangel R, Ribeiro AC, Lahoz Mde A, Parizotto NA. Low level laser therapy (830 nm) improves bone repair in osteoporotic rats: similar outcomes at two different dosages. Exp Gerontol. 2012;47:136–42. doi: 10.1016/j.exger.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Torstrick FB, Guldberg RE. Local strategies to prevent and treat osteoporosis. Curr Osteoporos Rep. 2014;12:33–40. doi: 10.1007/s11914-014-0191-6. [DOI] [PubMed] [Google Scholar]
- 48.Fallahnezhad S, Piryaei A, Darbandi H, Amini A, Ghoreishi SK, Jalalifirouzkouhi R, Bayat M. Effect of low-level laser therapy and oxytocin on osteoporotic bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2018;119:983–97. doi: 10.1002/jcb.26265. [DOI] [PubMed] [Google Scholar]
- 49.Khadra M, Kasem N, Haanaes HR, Ellingsen JE, Lyngstadaas SP. Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:693–700. doi: 10.1016/j.tripleo.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Tim CR, Bossini PS, Kido HW, Malavazi I, von Zeska Kress MR, Carazzolle MF, Rennó AC, Parizotto NA. Low-level laser therapy induces an upregulation of collagen gene expression during the initial process of bone healing: a microarray analysis. J Biomed Opt. 2016;21:88001. doi: 10.1117/1.JBO.21.8.088001. [DOI] [PubMed] [Google Scholar]
- 51.Ma H, Yang JP, Tan RK, Lee HW, Han SK. Effect of low-level laser therapy on proliferation and collagen synthesis of human fibroblasts in Vitro. J Wound Manag Res. 2018;14:1–6. [Google Scholar]
- 52.Pereira AN, Eduardo Cde P, Matson E, Marques MM. Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med. 2002;31:263–7. doi: 10.1002/lsm.10107. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto MA, Ferino RV, Monteleone GF, Ribeiro DA. Low-level laser therapy modulates cyclo-oxygenase-2 expression during bone repair in rats. Lasers Med Sci. 2009;24:195–201. doi: 10.1007/s10103-008-0544-4. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki SS, Garcez AS, Reese PO, Suzuki H, Ribeiro MS, Moon W. Effects of corticopuncture (CP) and low-level laser therapy (LLLT) on the rate of tooth movement and root resorption in rats using micro-CT evaluation. Lasers Med Sci. 2018;33:811–21. doi: 10.1007/s10103-017-2421-5. [DOI] [PubMed] [Google Scholar]