Abstract
Preserving the antigen effectiveness and DNA when bleaching melanin from melanin-containing tissues is an important part of medical diagnosis. Some prior studies focused excessively on the speed of bleaching neglecting the preservation of antigen and DNA, especially the nucleic acids in the long-archived tissues. The approach of this study was to determine the optimal bleaching conditions by increasing the H2O2 concentration and to compare that with the high temperature and potassium-permanganate bleaching methods. The comparisons involve immunohistochemical staining, HE staining, and gel electrophoresis, and setting the blank control (tissues without bleaching). The results demonstrated that bleaching using strong oxidizers or at high temperatures destroyed the antigen and DNA. Incubation with 30% H2O2 for 12 h at 24°C leaves only a small amount of melanin, preserving both the antigen effectiveness and the quality of the nucleic acids, and the target bands are clearly visible after PCR amplification. In conclusion, bleaching by increasing the concentration is a simple method, and it satisfies the requirements of clinical pathology and molecular pathology for the diagnosis and differential diagnosis of melanin-containing tissues.
Keywords: Hydrogen peroxide, bleaching melanin, immunohistochemical staining, gel electrophoresis
Introduction
Melanin is a stable product of the oxidation and tautomerization of dihydroxyphenylalanine (DOPA). It is derived from the amino acid tyrosine [1] and cannot be removed after fixation. Most malignant melanomas and most moles may contain excessive amounts of melanin, but the extent varies by region. During immunohistochemical staining, melanin may hamper the histopathological assessment of melanocytic lesions by obscuring the cellular morphology [2]. Melanin pigment is brown-black in tissues, so it physical masks the antigen-antibodies directly [3]. 3-3-diaminobenzidene (DAB), which is used for demonstrating antigen-antibody reactions, also has a brown color, making it hard to distinguish from the pigment. Therefore, the removal of melanin is a key step in the staining of tissues containing melanin.
Some studies have reported the use of warm hydrogen peroxide (H2O2) for depigmentation. The higher the temperature, the less time it takes to remove the pigment. 55°C is the most commonly used temperature, and it takes 2 h for depigmentation [2]. However, the results of our repeated experiments were inconsistent under this condition, and the integrity of some cells was destroyed. Meanwhile, depigmentation at a higher temperature seriously affected the quality of the DNA, resulting in the disappearance of the target bands in the gel electrophoresis results. The potassium permanganate-oxalic acid (KMnO4/oxalate) method is another traditional method for removing pigments. Due to the method’s strong oxidation effect, melanin is completely removed after this treatment, but some antigenicity is lost. These changes can lead to false positive and false negative results like SOX-10 and MELANE staining. This method cannot preserve the protein and nucleic acids, leading to the disappearance of the target band in the gel electrophoresis results. Some studies recommend the use of methyl green or azure B to cover the melanin, to make it green or blue, so it can be distinguished from DAB chromogen. However, the physical masking still remains because of the excess melanin granules, and it also affects the PCR result.
Therefore, it is important to seek an effective and simple manual melanin bleaching method that can preserve antigenicity and DNA and is suitable for most laboratory conditions.
Materials and methods
Samples and materials
Forty-five archived wax blocks of malignant melanoma and melanocytic nevi from the Department of Pathology of the First Affiliated Hospital of Fujian Medical University collected from October 2015 to March 2018 were utilized. The melanin-rich areas were cut to form a microarray block with 5 rows and 9 lines, with a diameter of 3 mm. Serial 4-μm-thick sections were sliced from the microarray block and adhered to the antistripping slides for preparation.
Reagents and antibodies
① Hydrogen peroxide solution, 30% (SanYuan, Sanming, China), potassium permanganate (XingTa, Shanghai, China), and oxalic acid (XingTa, Shanghai, China) were used for the depigmentation treatment. ② SOX-10: Nuclear positive (Maixin, China), Melan-A: cytomembrane positive (Zhongshan, China), HMB45: cytomembrane positive (Zhongshan, China), S-100: nuclear and cytoplasm positive (Zhongshan, China), K8002 (DAKO, Denmark), and DAB (DAKO, Denmark) were used for the IHC. ③ The DNA extraction reagent (AmoyDx, China) was used for Gel the electrophoresis.
Optimum conditions for H2O2 depigmentation
The sections were incubated in 3%, 10%, 20% or 30% H2O2 for 2 h, 6 h, 12 h, or 24 h respectively at room temperature (24°C), followed by the HE staining (Figure 1). We selected the optimum concentration and time which were able to remove the maximum amount of melanin pigment without destroying the integrity of the tissues.
Figure 1.
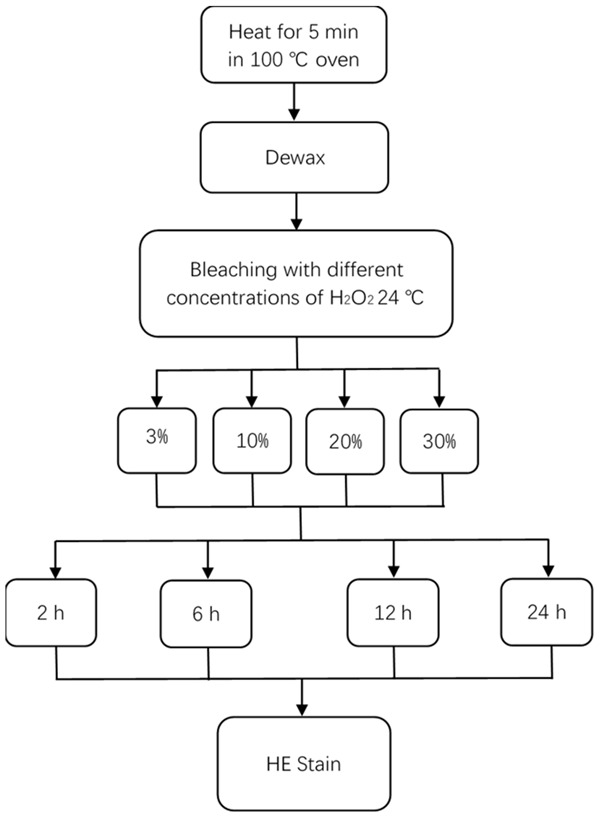
Finding the optimum conditions for the H2O2 depigmentation.
Comparison of the four methods of depigmentation
Method A: Blank control, the slides were not treated with bleaching melanin. Method B: The repaired sections were incubated in 0.25% KMnO4 at room temperature for 5 minutes and washed 3 times with 1% oxalic acid for 10 s. Method C: The sections were incubated in 3% H2O2 in a 55°C oven for 2 h. Method D: The sections were incubated in 30% H2O2 at 24°C for 12 h.
Immunohistochemical staining, HE staining, and gel electrophoresis were performed after each depigmentation method.
IHC staining
(1) The sections were heated in a 65°C oven overnight. (2) The sections were dewaxed twice with xylene for 10 minutes. (3) The sections were successively incubated in 100%, 95%, 80%, and 70% alcohol for 10 minutes each time, then in distilled water for 5 minutes. (4) Acid repair was performed for 2 minutes for the SOX-10 and S-100, and alkaline repair for 20 minutes for the Melan-A and HMB45. (5) The sections were cooled, subjected to melanin bleaching, and placed in distilled water for 5 minutes. (6) The sections were blocked with 3% H2O2 for 10 minutes, then placed in distilled water for 5 minutes. (7) The sections were incubated with a primary antibody for 60 minutes, then placed in distilled water for 5 minutes. (8) The sections were incubated with the second antibody for 30 minutes, then placed in distilled water for 5 minutes. (9) The sections were stained with DAB for 5 minutes, then placed in distilled water for 5 minutes. (10) The sections were stained with hematoxylin for 2 minutes, then placed in distilled water for 10 minutes. (11) The sections were successively dehydrated in 70%, 80%, 95%, and 100% alcohol for 5 minutes each time. (12) The sections were incubated in xylene for 10 minutes. (13) The sections were mounted.
HE staining
(1) The sections were heated in a 100°C oven for 30 minutes. (2) The sections were dewaxed using xylene for 10 minutes 2 times. (3) The sections were successively placed in 100%, 95%, 80%, and 70% alcohol for 10 minutes each time, then in distilled water for 5 minutes. (4) The melanin was bleached, and the sections were placed in distilled water for 5 minutes. (5) The sections were stained with hematoxylin for 2 minutes, then placed in distilled water for 10 minutes. (6) The sections were placed in 1% HCl for 10 seconds or distilled water for 10 minutes. (7) The sections were stained with eosin for 1 minute. (8) The sections were successively placed in 70%, 80%, 95%, and 100% alcohol for 5 minutes each time. (9) The sections were placed in xylene for 10 minutes. (10) Mounting was performed.
Gel electrophoresis experiment
(1) The sections were heated in a 65°C oven overnight. (2) The sections were dewaxed twice with xylene for 10 minutes. (3) The sections were successively incubated in 100%, 95%, 80%, and 70% alcohol for 10 minutes each time, then in distilled water for 5 minutes. (4) DNA extraction. (5) PCR amplificated C-Kit 17 exon. (6) The amplified products were analyzed using gel electrophoresis.
Interpretation of the results and the statistical analysis
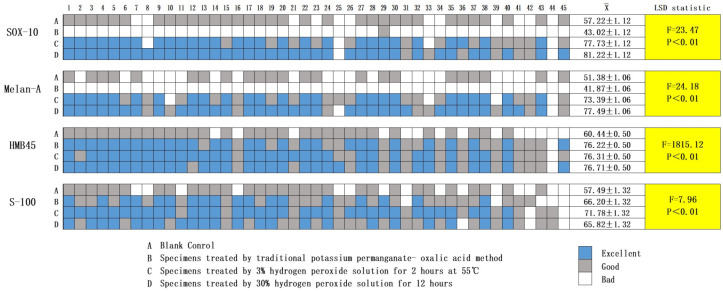
The IHC staining was scored under a microscope by two pathologists, and the average scores of the two were taken as the final result. The criteria included the integrity of the tissue structure, depigmentation effect, IHC antigen presentation, and staining effect (Table 1). The total possible score for each sample was 100, with three grades: excellent (80-100), good (50-80), and bad (< 50). According to the score of each sample, 45 of the samples were divided into the corresponding grades and displayed using an interval distribution diagram.
Table 1.
Scoring criteria for the IHC staining of the tissues after melanin bleaching
| Standard | Full mark (score) | Scoring criteria |
|---|---|---|
| Structural integrity | 30 | The structure is slightly incomplete, but does not affect the diagnosis: 25, |
| The structure is incomplete and begins to affect the diagnosis: 15, | ||
| Most or all of the tissue is lost: 0 | ||
| Melanin bleaching effect | 30 | Very little melanin remains and does not covers the cells: 25, |
| Melanin remains and covers part of the cells, but it can be distinguished from the positive presentation: 20, | ||
| Much melanin remains and cannot be distinguished from the positive presentation: 5 | ||
| Antigen presentation | 20 | Malposition presentation: 0 |
| Staining effect | 20 | Slight background stain: 15, |
| Deep background stain: 10, | ||
| Week antigen presentation: 10 | ||
| Full mark and level | 100 | Excellent: 80-100 |
| Good: 50-80 | ||
| Bad: 0-50 |
SPSS 19.0 was used for the data processing. A statistical description was used to show the expression of the four methods on the four different indicators, and a univariate randomized block square analysis was used to show the differences in the expressions of the four methods on the four different indicators. The pairwise comparisons among the four methods were conducted using the LSD method.
Results
Results of the H2O2 depigmentation
Melanin decreases with increasing H2O2 concentration and exposure time, but the destruction of cellular structure also increases. At least 24 h is needed to remove the melanin completely. However, tissue soaked with H2O2 at any concentration for more than 12 h will be significantly destroyed. Within 12 hours, however, the structure is almost undamaged. Therefore, within 12 h, the choice of a hydrogen peroxide solution with a concentration of 30% can maximize the removal of melanin and ensure the integrity of the cellular structure.
According to the results, incubation in 30% H2O2 for 12 h is the best condition for bleaching at 24°C.
The structure after different methods of depigmentation
Unbleached tissue retains its structural integrity after HE and IHC staining (Figure 2A1, 2A2). Part of the tissue subjected to IHC staining after the potassium permanganate decolorization was destroyed (Figure 2B1), but the structure subjected to the HE staining was intact (Figure 2B2). Part of the tissue subjected to IHC staining after the H2O2 decolorization at 55°C was destroyed (Figure 2C1), but the structure subjected to the HE staining was intact (Figure 2C2). A very small amount of tissue subjected to the IHC staining after the H2O2 decolorization at 24°C was destroyed (Figure 2D1), but the structure subjected to the HE staining was intact (Figure 2D2). Compared with the incubation in potassium permanganate (Figure 2B1) and H2O2 at 55°C (Figure 2C1), good structural integrity was preserved when the tissue was incubated in H2O2 at 24°C (individual incomplete sections were caused by continuous slicing).
Figure 2.
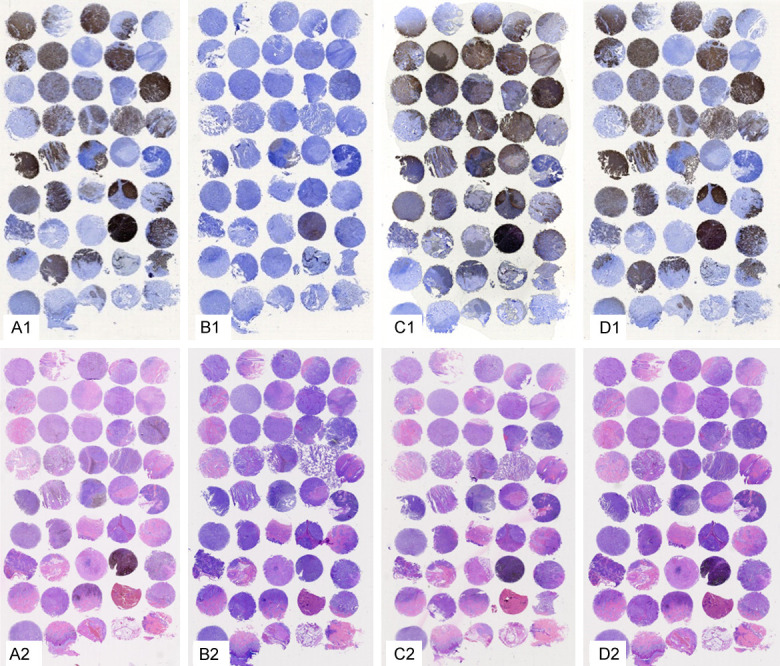
The structure after the different methods of depigmentation (Sox-10 was selected for the IHC staining, as shown in the figure). A1, A2: Blank control, the specimens were not treated with bleaching melanin; B1, B2: The specimens were treated with potassium permanganate; C1, C2: The specimens were treated with 3% H2O2 for 2 h at 55°C; D1, D2: The specimens were treated with 30% H2O2 for 12 h at 24°C. A1-D1: SOX-10 staining; A2-D2: HE staining.
Comparison and statistical analysis of the staining results
Some tissues not treated with depigmentation were covered with melanin, which interfered with the observation of the positive expression (Figure 3A1-A5). The melanin was completely removed with potassium permanganate, but the SOX-10 and melan-A expression results were almost all negative (Figure 3B1 and 3B2), but the results of the HMB45 and S-100 expressions were accurate (Figure 3B3 and 3B4) in contrast to the results of the HE staining with no melanin coverage (Figure 3B5). There was only a small amount of melanin remaining after the treatment with 3% H2O2 at 55°C, the antigen expression was accurate (Figure 3C1-C4), and the HE staining showed a sharp contrast (Figure 3C5). The melanin pigment was completely eliminated or at least minimized after treatment with 30% H2O2 at 24°C. An accurate positive expression was observed (Figure 3D1-D4), and the HE staining showed a sharp contrast (Figure 3D5). However, the stain intensity was more moderate than it was after the treatment (Figure 3C1-C5), and the background was clearer.
Figure 3.
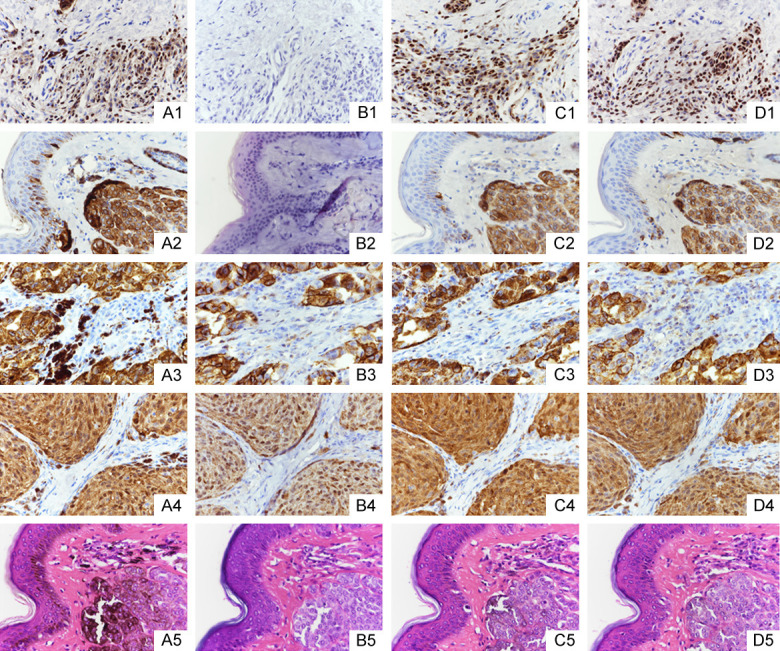
The results of the staining with different methods of depigmentation (samples selected from the first line and eighth row in the tissue microarray, as shown in the figure). A1-A5: Blank control, the specimens were not treated to bleach melanin; B1-B5: The specimens were treated with potassium permanganate; C1-C5: The specimens were treated with 3% H2O2 for 2 h at 55°C; D1-D5: The specimens were treated with 30% H2O2 for 12 h at 24°C. A1-D1: SOX-10 staining; A2-D2: Melan-A staining; A3-D3: HMB45 staining; A4-D4: S-100 staining; A5-D5: HE staining.
Among the four IHC staining methods, methods C and D had the highest rate of excellence. As shown in Figure 4, four results were statistically significant. In this test, we conclude that the potassium permanganate depigmentation was suitable only for the HMB45 and S-100 staining. However, the hydrogen peroxide method was suitable for all the indicators in these experiments, so we recommend its use in the IHC staining of melanin-containing tissues.
Figure 4.
Interval distribution diagram and statistical analysis. The IHC staining results of the 45 samples after the removal of the melanin by different methods.
Comparison of the DNA (C-Kit 17) electrophoresis results
To test the preservation of the nucleic acids, we chose 5 samples from these cases, and used the four methods for nucleic acid extraction, PCR amplification, and electrophoresis. The target bands were completely invisible in the tissues depigmented with potassium permanganate or tissues the depigmented with warm H2O2. In the tissues treated with H2O2 for 12 h at 24°C, all the target bands were clearly visible. Otherwise, the result of the blank control showed that 3 target bands were visible, but the others were lost after the PCR amplification (Figure 5).
Figure 5.
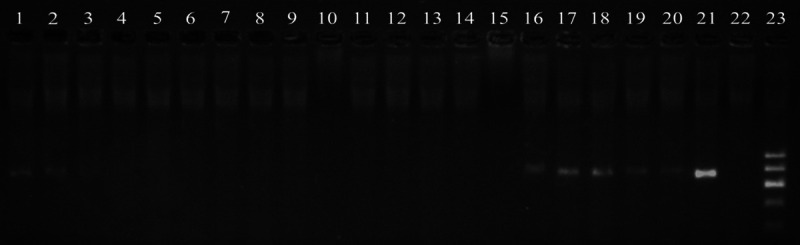
The Results of the DNA electrophoresis with different methods of depigmentation. 1-5: The samples were not treated to bleach melanin; 6-10: The samples were treated with potassium permanganate; 11-15: The samples were treated with 3% H2O2 for 2 h at 55°C; 16-20: The samples were treated with 30% H2O2 for 12 h at 24°C. 21-23: Positive control, negative control and marker.
Discussion
There are many methods to remove melanin, among which the potassium permanganate-oxalic acid method is the most commonly used. Potassium permanganate is a strong oxidant; in its oxidation-reduction reaction with oxalic acid, the electron absorption ability of the carboxyl group breaks the carbon-carbon bond, effecting the bleaching treatment. This is the mechanism by which the bleaching effect is achieved by breaking the stability of the protein.
Foss and Orchard tested potassium permanganate/oxalate depigmentation with 13 commonly used antibodies, and the results showed that potassium permanganate would change the specificity or sensitivity of some antigens, creating false negative or false positive staining results [4,5]. Potassium permanganate could also efficiently bleach DAB [6]. In this experiment, false negative SOX-10 and Melan-A staining results occurred after treatment with potassium permanganate, but HMB45 and S-100 showed accurate results. It has been pointed out that S-100 is a low-molecular-weight acidic calcium-binding protein with a molecular weight of 10-12 kDa, and its amino acid sequence is highly conserved [7]. The S-100 protein family belongs to the EF-hand superfamily, and a large number of hydrophobic residues are distributed on the N-terminal and C-terminal helix of the EF-hand, so S-100 forms homologous or heterologous dimers with noncovalent bonds in most states [8]. HMB45 is a specific monoclonal antibody against 10 kDa cytoplasmic glycoprotein and is considered to be one of the components of the promelanin complex [9]. Melan-A is a melanosomal differentiation antigen recognized by autosomal cytotoxic T cells [10]. SOX-10 is a key transcription factor that promotes the malignant transformation of melanocytes [11], but its protein structure is unknown. The protein structure of the latter two is unstable, and the carboxyl group in each is oxidized and decomposed in the potassium permanganate-oxalic acid reaction, hindering subsequent antigen and antibody reactions.
Hydrogen peroxide contains a peroxide bond, which easily generates peroxide free radicals when broken, and it is a much weaker oxidant than the potassium permanganate solution. When pigments interact with these radicals, the pigment molecules are oxidized and lose their original color. This effect is suitable for most antibodies [12,13].
A number of experiments tried to achieve rapid bleaching by raising the temperature of the hydrogen peroxide. Although the results of the HE staining and IHC staining were satisfactory, they often ignored the experimental results of the DNA testing. In this experiment, we found the optimal condition for melanin bleaching at room temperature by increasing the concentration of hydrogen peroxide. The results of the HE and IHC staining were similar to the warm hydrogen peroxide results, the IHC staining showed accurate positioning and strong positive expression, and HE staining was bright, with no melanin coverage. But the results of the DNA test were different. When treating with 30% hydrogen peroxide for 12 h at 24°C, after the PCR amplification, the target band was clearly visible, and the results of the repeated experiments were consistent. But when bleaching with warm hydrogen peroxide, the gel electrophoresis of the target bands disappeared after the PCR amplification. The reason may be that high temperatures tend to degrade DNA fragments. Potassium permanganate bleaching, also a comparative experiment, showed that the target bands disappeared possibly because the strong acid destroyed the structure of the protein. It is worth mentioning that the electrophoresis results of the blank control group were not ideal either. Because the melanin was not removed, the physical masking affected the extraction of the DNA, resulting in the disappearance of some target bands in the electrophoresis experiment.
To date, genetic testing plays an important role in the pathological diagnosis of malignant melanoma. While preserving antigenicity, the quality and quantity of nucleic acids should also be ensured. Treatment with 30% hydrogen peroxide for 12 h at 24°C could meet all the above. It should be noted that different tissues contain different amounts of melanin, and the depigmentation reaction time can be adjusted according to the melanin content in the tissues. In addition, we usually use 3% H2O2 to block the endogenous peroxidase after repair, so the blocking step can be omitted after depigmentation with 30% H2O2.
The reaction time was as long as 12 h, but it avoided the risk of repeated staining caused by structure destruction and prevented false negatives. The maximum removal of melanin was achieved while preserving the integrity of the tissue, and the antigen-antibody binding remained accurate, preventing the DNA fragments from being degraded, making this approach an efficient method to improve differential diagnosis in clinicopathology.
The limitation of this method is that the depigmentation time is longer than it is in the traditional method, there are only a few published studies on antibody structure, and there may be errors in the experiments performed under different conditions in different laboratories. At present, we have not found a fast and reliably effective manual method, so we need to further improve and explore this experimental method and perform tests with additional antibodies.
Conclusion
Compared with the traditional method, this experimental method reduces the probability of erroneous diagnosis and provides a degree of stability in staining with the above four antibodies, so the results of the gel electrophoresis will not be biased. Therefore, this method can be recommended as an easy depigmentation method.
Acknowledgements
This work was supported by the Natural science foundation of Fujian province (2018J01155) and Funds of Fujian Provincial Research Project on the Reform of Undergraduate Education and Teaching (FBJG20170072).
Disclosure of conflict of interest
None.
References
- 1.Seagle BL, Rezai KA, Kobori Y, Gasyna EM, Rezaei KA, Norris JR Jr. Melanin photoprotection in the human retinal pigment epithelium and its correlation with light-induced cell apoptpsis. Proc Natl Acad Sci U S A. 2005;102:8978–8983. doi: 10.1073/pnas.0501971102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Momose M, Ota H, Hayama M. Re-evaluation of melanin bleaching using warm diluted hydrogen peroxide for histopathological analysis. Pathol Int. 2011;61:345–350. doi: 10.1111/j.1440-1827.2011.02667.x. [DOI] [PubMed] [Google Scholar]
- 3.Orchard GE. Use of heat provides a fast and efficient way to undertake melanin bleaching with dilute hydrogen peroxide. Br J Biomed Sci. 2007;64:89–91. doi: 10.1080/09674845.2007.11978097. [DOI] [PubMed] [Google Scholar]
- 4.Foss AJ, Alesander RA, Jeffereies LW, Lightman S. Immunohistochemical techniques; the effect of melannin bleaching. Br J Biomed Sci. 1995;52:22–25. [PubMed] [Google Scholar]
- 5.Orchard GE, Calonje E. The effect of melanin bleaching on immunohistochemical staining in heavily pigmented melanocytic neoplasms. Am J Dermatopathol. 1998;20:357–361. doi: 10.1097/00000372-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Shen HW, Wu WQ. Study of melanin bleaching after immunohistochemistry of melanin-containing tissues. Appl Immunohistochem Mol Morphol. 2014;23:303–307. doi: 10.1097/PAI.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moroz OV, Antson AA, Murshudov GN, Maitland NJ, Dodson GG, Wilson KS, Skibshøj I, Lukanidin EM, Bronstein IB. The three-dimensional structure of human S-100A12. Acta Crystallogr D Biol Crystallogr. 2001;57:20–29. doi: 10.1107/s090744490001458x. [DOI] [PubMed] [Google Scholar]
- 8.Heizmann CW, Cox JA. New perspectives on S100 proteins: a multi-functional Ca(2+)-, Zn(2+)- and Cu(2+)-binding protein family. Biometals. 1998;11:383–397. doi: 10.1023/a:1009212521172. [DOI] [PubMed] [Google Scholar]
- 9.Gown AM, Vogel AM, Hoak D, Gough F, McNutt MA. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986;123:195–203. [PMC free article] [PubMed] [Google Scholar]
- 10.Satzger I, Völker B, Meier A, Schenck F, Kapp A, Gutzmer R. Prognostic significance of isolated HMB45 or Melan A positive cells in Melanoma sentinel lymph nodes. Am J Surg Pathol. 2007;31:1175–1180. doi: 10.1097/PAS.0b013e3180341ebc. [DOI] [PubMed] [Google Scholar]
- 11.Shakhova O, Zingg D, Schaefer SM, Hari L, Civenni G, Blunschi J, Claudinot S, Okoniewski M, Beermann F, Mihic-Probst D, Moch H, Wegner M, Dummer R, Barrandon Y, Cinelli P, Sommer L. SOX10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat Cel Biol. 2012;14:882–890. doi: 10.1038/ncb2535. [DOI] [PubMed] [Google Scholar]
- 12.McGovern J, Crocker J. The effect of melanin pigment removal on the peroxidase-antiperoxidase immunoperoxidase technic. Am J pathol. 1987;88:480–483. doi: 10.1093/ajcp/88.4.480. [DOI] [PubMed] [Google Scholar]
- 13.Orchard GE. Heavily pigmented melanocytic neoplasma: comparison of two melanin bleaching techniques and subsequent immunohistochemical staining. Br J Biomed Sci. 1999;56:188–193. [PubMed] [Google Scholar]