Abstract
Aortic dissection (AD) is a fatal disease characterized by a ruptured intima that leads to the complete rupture of the aorta. The aim of this study is to examine the immunohistochemical expression of inflammation/fibrosis-associated chemical mediators in AD patients. Surgical specimens of aortic tissues were obtained from 37 patients who underwent an open thoracic aortic repair. AD was detected with histological staining. Local congestion and hemorrhage as well as chronic inflammatory cells infiltrations were observed at the dissection. Moreover, extensive disarrangement and disruption of elastic fibers were observed in the medial layer of the aorta with dissection. In summary, our study revealed that the apoptotic rate of vascular SMCs (VSMCs) in the vascular middle layer is higher in the dissected aortas than in the control aortas, suggesting that abnormally elevated apoptosis is correlated with AD pathogenesis. Functional studies of key genes identified in the apoptotic pathways as well as in extracellular matrix would be critical in thoroughly understanding the underlying mechanisms of AD development. Targeting the mediators related to TGF-β1, the Smad family proteins, and caspase 3 or anti-apoptotic agents may provide diagnostic markers and therapeutic targets that could be used to prevent AD.
Keywords: Aortic dissection, vascular smooth muscle cells (VSMCs), apoptosis, fibrosis
Introduction
Aortic dissection (AD) is a potentially fatal disease characterized by a ruptured intima through which blood flows into the middle layer of the aorta, leading to the formation of a dual cavity in the aorta or to its complete rupture [1,2]. Its onset involves the separation of the aortic wall and the entry of blood into the aortic wall through this intimal tear [3]. AD is one of the most severe aortic diseases, and approximately 20% of AD patients die before reaching the hospital and 30% die during hospital admission [4,5]. The classical clinical presentation of AD is the sudden onset of severe back pain, although it is sometimes mild or even clinically silent [6].
Thoracic AD is associated with laceration and degeneration of the aortic media [7]. Structural changes in aortic wall include aortic wall degeneration, focal or zonal necrosis of the media, fragmentation of elastic fibers, and changes of smooth muscle cells (SMCs) [8]. In vascular diseases, vascular SMCs (VSMCs) in the aortic media display degenerative changes and abnormal proliferation [9,10]. Many studies revealed the critical role of VSMCs in the middle layer in AD pathogenesis [11,12]. Various risk factors including hypertension, atherosclerosis, drinking, smoking, obesity, mental stress, and age are involved in AD pathogenesis [13]. The molecular mechanism underlying AD is not well understood. Hence, its prevention remains pharmacologically impossible [14-17].
AD pathogenesis occurs due to collagen deposition and elastin degradation, along with the dysfunctions of the elastic lamina layer of the aorta wall. Transforming growth factor (TGF)-β is a major regulator of the production of extracellular matrix (ECM) components, mostly collagen [18,19]. Dysregulated TGF-β signaling is thus crucial in AD pathogenesis [20]. Mutations in genes encoding the TGF-β-signaling components, including Smad2 and Smad3, have been detected in thoracic AD [21]. Smads are a family of proteins that are among the important intracellular effectors of TGF-β1 [22]. A TGF-β1 ligand binds to TGF-βRII/TGF-βRI receptors, leading to the phosphorylation of Smad2/3. The phosphorylated Smad2/3 binds to Smad4 to form a protein complex that undergoes nuclear translocation and regulates the expression of ECM components. Smad3 mutations are reported in families with thoracic AD, which is inherited in an autosomal dominant manner [23]. These findings indicated that a dissected aorta had an upregulated expression of Smad in compared with non-AD tissues.
Many studies have attempted to understand the pathogenesis of AD [24,25]. These studies put forward important disease mechanisms, but most of them focused on genetic heterogeneity, clinical pathology, and hemodynamics of AD. This study aims to examine the immunohistochemical expression levels of inflammation/fibrosis-associated chemical mediators in AD patients. Moreover, this study describes the detailed immunophenotypical changes in AD and investigates the pathogenesis of the development of AD.
Materials and methods
Patients and samples
Surgical specimens of aortic tissues were obtained from 37 patients who underwent an open thoracic aortic repair. Control aortic tissues were collected from autopsy samples without aortic aneurysm or dissection. Patients with heritable connective tissue diseases, such as Marfan syndrome, trauma-related thoracic AD, and infection, were excluded from this study. Samples of the ascending aorta were obtained during the surgery of 37 patients with acute dissections (17 male patients aged 26-85 years; mean age: 61.2 years; median age: 63.5 years) at the Daegu Catholic University Hospital. The study protocol was approved by the Institutional Review Board of Daegu Catholic University Hospital (IRB No. CR-19-057).
The Institutional Review Board exempted this work from obtaining written informed consent because of the retrospective nature of this study, wherein the analysis is based on medical records.
Tissue microarray (TMA)
Surgical specimens were fixed in buffered neutral formalin (10%) for at least 24 h. The representative areas were embedded in paraffin blocks. All available hematoxylin and eosin (H&E)-stained slides were re-examined in order to obtain areas suitable for preparing TMA blocks. Two cores (5 mm) of the selected aortic tissues were obtained from one or two paraffin blocks of each patient and transplanted onto recipient TMA blocks.
Histopathological analysis
Histological evaluation was carried out on formalin-fixed and paraffin-embedded specimens. All tissue samples were stained with H&E, Verhoeff’s elastic, and Masson’s trichrome stains according to standard procedures. Elastic fibers or elastin in AD patients was examined through elastic Verfoeff’s elastic staining according to the manufacturer’s instructions. In brief, 4 μm paraffin sections were deparaffinized, hydrated in water, and stained in elastin stain solution for 8-20 h, followed by counterstaining with a staining solution for 1 min. The sections were mounted with a mounting medium and examined under a light microscope. Aortic fibrosis was assessed by detecting collagen fibers within the aortic wall by staining the paraffin-embedded sections with Masson’s trichrome followed by examination under a light microscope.
Immunohistochemistry (IHC)
The aortic tissues collected from the diseased part of a thoracic AD were embedded in paraffin for IHC validation using monoclonal antibodies according to the manufacturer’s instructions [24]. After 24-72 h of fixation in normal buffered formalin, the samples underwent standard histological processing and then embedded in paraffin. Immunoperoxidase reactions were performed in 4 μm-thick sections according to laboratory standards. Immunohistochemical staining for TGF-β1, Smad3+Smad4 (phospho T8), SNAIL+SLUG, α-smooth muscle actin (SMA), and phospho-Smad4 on TMA blocks with a BOND III autostainer (Leica biosystems, Nussloch, Germany) in accordance with the manufacturer’s protocol.
Primary antibodies for TGF-β1 (1:100, Abcam, Cambridge, United Kingdom), Smad3+Smad4 (1:50, Abcam, Cambridge, United Kingdom), SNAIL+SLUG (1:100, Abcam, Cambridge, United Kingdom), α-SMA (1:1000, Abcam, Cambridge, United Kingdom), and Phospho-Smad4 (1:200, AP3251a, ABGENT, San Diego, United States) were used to investigate protein expression. The immunohistochemical staining results were evaluated using a scoring system based on staining intensity and on the proportion of stained cells. Staining intensity was graded as follows: (0) negative, (1) weak, (2) moderate, or (3) strong (Y: 19). We considered the result as positive when the staining intensity was moderate or strong and when the proportion of stained cells was more than 10%. The immunohistochemical staining results were obtained through light microscopic examination and were independently evaluated by two pathologists.
Results
H&E/elastic staining
The aorta consists of three layers, namely, intima, media, and adventitia. The intima was thickened due to an atherosclerotic change. Adventitial structures were loose and showed mild hemorrhage and scattered inflammatory cell infiltrations. Thoracic AD was detected with histological staining. Local congestion and hemorrhage and chronic inflammatory cells infiltration could be observed in the dissection (Figure 1). No breakage or dissection was observed in the aortic wall of the control group (data not shown). In the aorta with dissection, extensive disarrangement and disruption of elastic fibers at the medial layer were observed (Figure 2).
Figure 1.
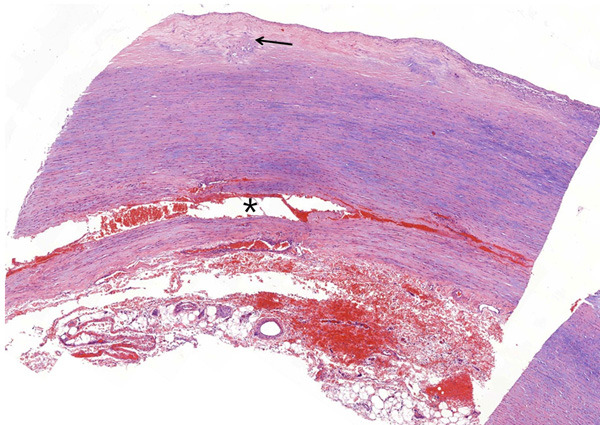
Aortic media dissected (asterisk) with congestion and hemorrhage. The intima is thickened (arrow) due to the atherosclerotic change (hematoxylin & eosin stain, ×100).
Figure 2.
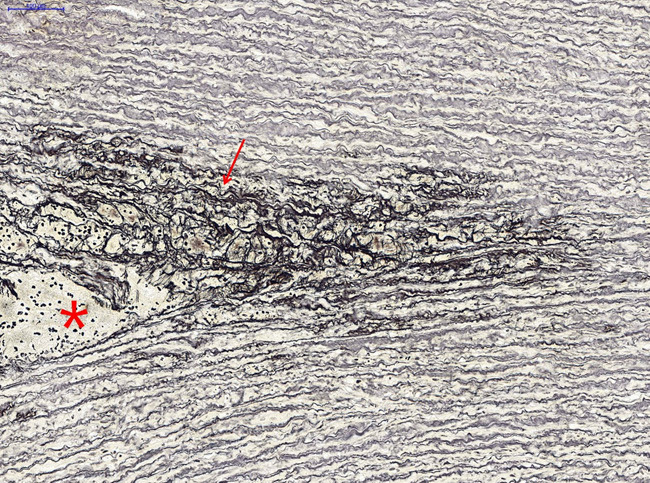
Extensive disarrangement and disruption (arrow) of elastic fibers located close to the dissected lesion (asterisk) of the aortic media (elastic stain, ×400).
IHC results
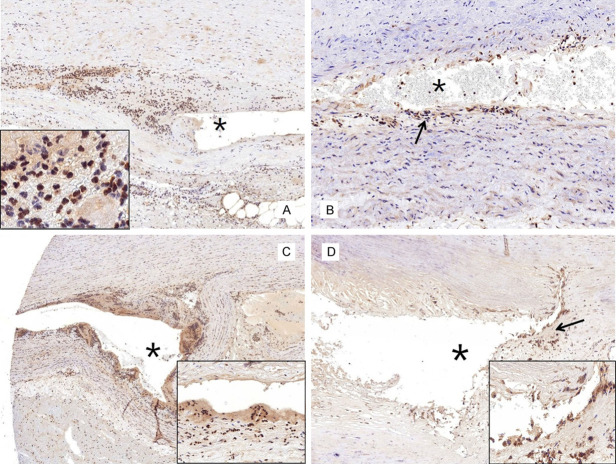
IHC showed a mild to moderately positive α-SMA expression in the aortic media of the control patients with atherosclerosis (Figure 3A). The expressions levels of α-SMA in the aorta with atherosclerosis were significantly reduced at the myxoid lesion of the media (Figure 3B). Additionally, the α-SMA expression in the aortic media of patients with AD did not differ with that in atherosclerotic aorta (Figure 4). Caspase 3, which is a biological marker for apoptosis, was negatively expressed in the normal and atherosclerotic blood vessels (Figure 5A and 5B). By contrast, caspase 3 showed strongly positive expression levels in the cytoplasm of SMCs in the dissected lesion (Figure 5C and 5D). P-Smad3/4 immunostaining was weakly positive in the cytoplasm and nuclei of SMCs in the dissected lesion (Figure 6A and 6B). p-Smad3/4 also showed positive immunostaining in the inflammatory cells near the dissected lesion, and the p-Smad3/4 expression levels were slightly stronger in the fibrotic area near the dissected lesion than in the control atherosclerotic vessel. The expression levels of Snail/Slug, which are the transcription factors related to fibrosis, showed a weakly positive staining at the nucleus near the dissected lesion (Figure 6C). They were also stained in the nuclei of inflammatory cells and SMCs of the atherosclerotic vessels. The results for Snail/Slug expression were similar to those for Smad3/4. As for TGF-β1, its expression was slightly stronger in the dissected lesion than in the atherosclerotic and control vessels (Figure 6D).
Figure 3.
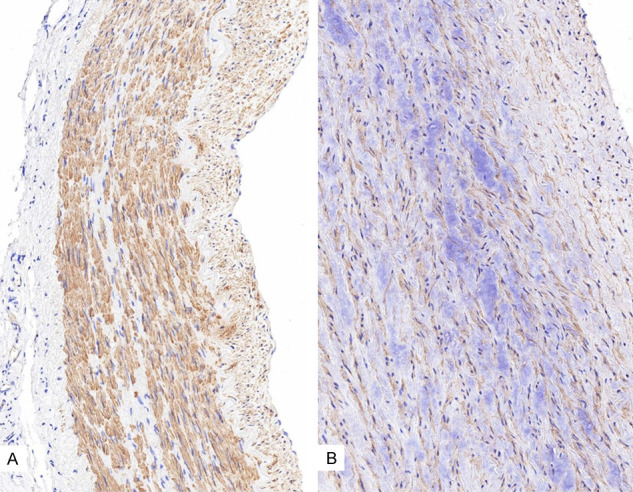
Immunohistochemistry results for α-SMA showing its mild to moderately positive expression levels in the aortic media of the control patients (A. ×200) and its decreased expression levels in the control aorta of atherosclerotic patient (B. ×400).
Figure 4.
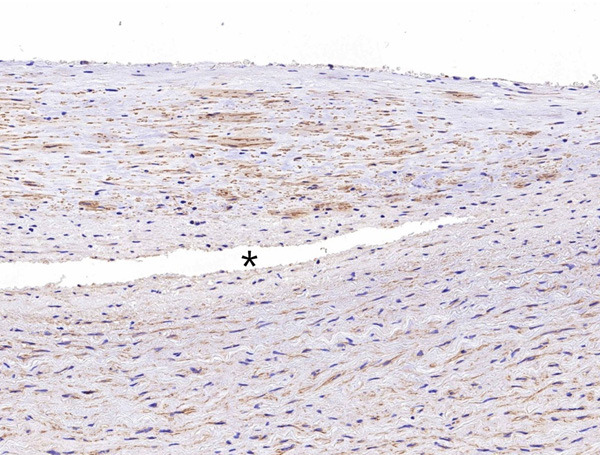
No difference in α-SMA expression was observed between the region close to the dissected lesion (asterisk) and the other regions of the aortic media (×400).
Figure 5.
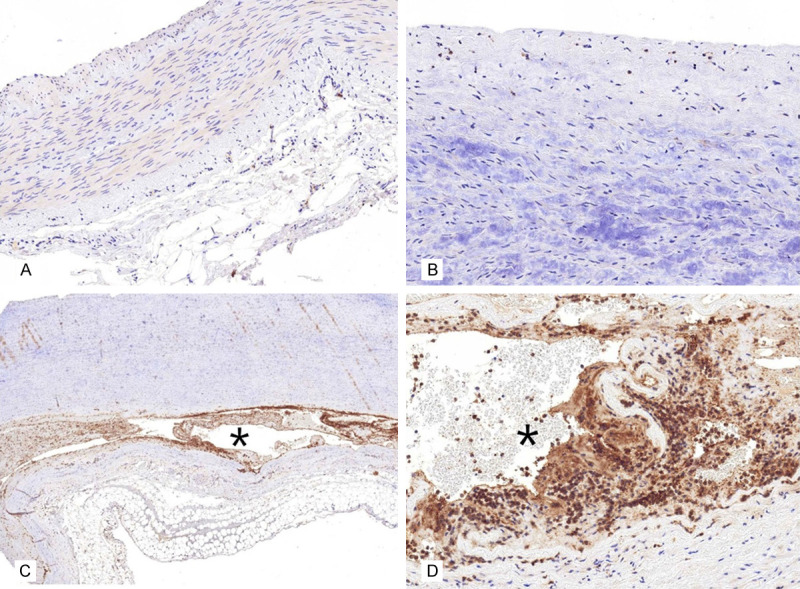
Caspase 3 showed negative immunohistochemical staining result in the normal (A. ×200) and atherosclerotic (B. ×400) aorta. By contrast, caspase 3 showed strongly positive expression levels (C. ×100) in the region close to the dissected lesion (asterisk) and in the cytoplasm of smooth muscle cells (D. ×400) near the dissected lesion (asterisk).
Figure 6.
Immunohistochemistry for p-Smad3/4, Snail/Slug, and TGF-β1. A. The expression levels of p-Smad3/4 are weakly positive near the dissected lesion (asterisk). The inset shows the inflammatory and smooth muscle cells (SMCs) stained with p-Smad3/4 near the dissected lesion (×200). B. The p-Smad3/4 expression levels are slightly stronger in the fibrotic area (arrow) near the dissected lesion than in the control atherosclerotic aorta (×400). C. The expression levels of Snail/Slug are weakly positive in the nuclei of the inflammatory cells and SMCs (inset, ×100) near the dissected lesion (asterisk). D. The TGF-β1 expression levels are slightly stronger (arrow and inset, ×100) in the region near the dissected lesion (asterisk) than in other regions of the aortic media.
Discussion
AD can be caused by trauma; moreover, pulsatile flow and high blood pressure contribute to the occurrence of AD [26]. Aortic aneurysms and dissections have been investigated together because they share not only a frequent relationship with underlying conditions, such as arterial hypertension, but they also share histopathological features, such as fragmentation of elastic fibers, at the medial layer of an artery [27]. Elastic fiber fragmentation and disrupted collagen production seem to be involved in the pathogenesis of AD [28-30].
Previous findings indicated the vital role of VSMCs in aortic wall degeneration, which initiates the pathological changes in AD [31]. VSMCs are major components of the medial layer of aorta and they play a significant role in maintaining wall structure, thereby mediating the vascular dilation and constriction function [32]. Our study showed the prominent signs of aortic medial degeneration, such as myxoid change, in AD and the participation of VSMCs and inflammatory cells in the development of AD. In the aortic medial layer, almost all cells are SMCs. They produce ECM components, including collagen and enzymes, that degrade these components [33]. In a normal arterial media, VSMCs express SMC markers, including SMC myosin and actin. α-SMA, encoded by the acyl-coenzyme A (CoA): cholesterol acyltransferase 2 (ACTA2) gene, is an SMCs. It is typically expressed in VSMCs [34]. The SMCs of atherosclerotic vessels showed a reduced SMC expression, probably due to the degenerative change in the aortic media, as shown in our study, and due to their increased capacity for cell proliferation, migration, and ECM protein generation [35].
Tanaskovic et al. found through their immunohistochemical study that α-SMA was positively stained in SMCs of the media and in the margins of AD [36]. The other study used western blot analysis and immunohistochemical to investigate α-SMA expression in dissected thoracic aorta and found that α-SMA expressions level were significantly downregulated in medial SMCs compared with those in a normal aorta. Similarly, Zhang et al. noted a reduced aortic α-SMA positivity in patients with dissected aortic media compared with that in a control without dissected media [34]. Our study also showed decreased α-SMA expression levels in the atherosclerotic vessel wall. These results are probably due to the degenerative or myxoid change in the aortic media. Moreover, no difference in SMA expression was observed between the atherosclerotic vessel and the dissecting aneurysmal wall.
Some studies showed that apoptosis of VSMCs plays a critical role in AD pathogenesis [11,12]. Das et al. reported the enrichment of proinflammatory cytokines in Ads; moreover, they found that the genetic depletion of S100A12 or its receptor resulted in suppressed caspase 3 activation [37]. Caspase 3 is an important protein for regulating cell apoptosis in caspase protein family, and it can effectively activate the signal pathway for cell apoptosis and inhibit cell proliferation [38]. A key apoptotic protein, caspase 3 was significantly increased in AD, and its association with increased apoptotic VSMC depletion is probably caused by the disrupted equilibrium between antiapoptotic and proapoptotic proteins [11]. Yuan et al. reported a significantly higher VSMC apoptotic rate in vascular tissues of AD patients than in the control group [12]. Our study also showed increased caspase 3 expression levels in the dissected lesion of the aorta. Therefore, either apoptosis have resulted from AD or AD have resulted from apoptosis in the aortic wall, due to any reason. Thus, detection of AD-related apoptotic agents, such as caspase 3, from the blood or body fluid could help in the early diagnosis and prevent AD.
Among the intracellular effectors of TGF-β1, Smads are pivotal molecules. They regulate various biological activities, including cell proliferation, apoptosis, migration, differentiation, and adhesion [22]. Yuan et al. found higher Smad4 expression levels in AD patients than in AD-free patients [39]. Gomez et al. have showed an increase in phosphorylated Smad3/4 in AD [40]. Our study also showed higher phosphorylated Smad3/4 expression levels in dissections than in atherosclerotic or control vessels. This result demonstrated that fibrosis is more severe in dissected lesion than in other areas. Moreover, it is necessary to study whether fibrosis is caused by AD or whether AD results in fibrosis. Another possibility is that TGF-β1, mostly through non-Smad pathways, causes matrix damage [41]. TGF-β1 is known to exhibit a profibrotic action [42]; thus, an increase in TGF-β1 should be expected based on the Smad3/4 expression levels.
In summary, our study revealed that the apoptotic rate of VSMCs in vascular middle layer is significantly higher in the dissected aortas than in the control aortas, suggesting that abnormally elevated apoptosis is correlated with AD pathogenesis, similar to other findings [12,14]. Functional studies of other important genes that are identified to be involved in fibrosis, as well as in the apoptotic pathway, would also be critical in understanding the underlying mechanisms of AD. Targeting the mediators related to apoptotic pathways involved in AD development may provide diagnostic markers and therapeutic targets that are useful in preventing AD. New therapies targeting the TGF-β1, the Smad family of proteins, and possibly caspase 3 or anti-apoptotic agents could also help to diminish the weakness of the aortic wall in patients with AD.
Acknowledgements
This work was supported by the National Research Foundation of Korea grant funded by the Korean Government (Ministry of Science and ICT) (No. 2017R1C1B5017117).
Disclosure of conflict of interest
None.
References
- 1.Wang L, Liu S, Yang W, Yu H, Zhang L, Ma P, Wu P, Li X, Cho K, Xue S, Jiang B. Plasma amino acid profile in patients with aortic dissection. Sci Rep. 2017;7:40146. doi: 10.1038/srep40146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye W, Liu CW, Song XJ, Li YJ, Liu B, Zheng YH, Zeng R. Endovascular repair for acute type B aortic dissection. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:715–719. doi: 10.3881/j.issn.1000-503X.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 3.El-Hamamsy I, Ouzounian M, Demers P, McClure S, Hassan A, Dagenais F, Chu MW, Pozeg Z, Bozinovski J, Peterson MD, Boodhwani M, McArthur RG, Appoo JJ Canadian Thoracic Aortic Collaborative (CTAC) State-of-the-art surgical management of acute type A aortic dissection. Can J Cardiol. 2016;32:100–9. doi: 10.1016/j.cjca.2015.07.736. [DOI] [PubMed] [Google Scholar]
- 4.Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–8. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 5.Huang W, Huang C, Ding H, Luo J, Liu Y, Fan R, Xiao F, Fan X, Jiang Z. Involvement of miR-145 in the development of aortic dissection via inducing proliferation, migration, and apoptosis of vascular smooth muscle cells. J Clin Lab Anal. 2020;34:e23028. doi: 10.1002/jcla.23028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visonà SD, de Boer OJ, Mackaaij C, de Boer HH, Pertiwi KR, de Winter RW, Osculati A, van der Wal AC. Immunophenotypic analysis of the chronological events of tissue repair in aortic medial dissections. Cardiovasc Pathol. 2018;34:9–14. doi: 10.1016/j.carpath.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Wu D, Shen YH, Russell L, Coselli JS, LeMaire SA. Molecular mechanisms of thoracic aortic dissection. J Surg Res. 2013;184:907–24. doi: 10.1016/j.jss.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesauskaite V, Tanganelli P, Sassi C, Neri E, Diciolla F, Ivanoviene L, Epistolato MC, Lalinga AV, Alessandrini C, Spina D. Smooth muscle cells of the media in the dilatative pathology of ascending thoracic aorta: morphology, immunoreactivity for osteopontin, matrix metalloproteinases, and their inhibitors. Hum Pathol. 2001;32:1003–11. doi: 10.1053/hupa.2001.27107. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93:1317–542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- 10.Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–53. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 11.Durdu S, Deniz GC, Balci D, Zaim C, Dogan A, Can A, Akcali KC, Akar AR. Apoptotic vascular smooth muscle cell depletion via BCL2 family of proteins in human ascending aortic aneurysm and dissection. Cardiovasc Ther. 2012;30:308–16. doi: 10.1111/1755-5922.12007. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Y, Wang C, Xu J, Tao J, Xu Z, Huang S. BRG1 overexpression in smooth muscle cells promotes the development of thoracic aortic dissection. BMC Cardiovasc Disord. 2014;14:144. doi: 10.1186/1471-2261-14-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coady MA, Davies RR, Roberts M, Goldstein LJ, Rogalski MJ, Rizzo JA, Hammond GL, Kopf GS, Elefteriades JA. Familial patterns of thoracic aortic aneurysms. Arch Surg. 1999;134:361–7. doi: 10.1001/archsurg.134.4.361. [DOI] [PubMed] [Google Scholar]
- 14.Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372:55–66. doi: 10.1016/S0140-6736(08)60994-0. [DOI] [PubMed] [Google Scholar]
- 15.Clouse WD, Hallett JW Jr, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, Melton LJ 3rd. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79:176–80. doi: 10.4065/79.2.176. [DOI] [PubMed] [Google Scholar]
- 16.Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, Szép L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–8. doi: 10.1378/chest.117.5.1271. [DOI] [PubMed] [Google Scholar]
- 17.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: final data for 2009. Natl Vital Stat Rep. 2011;60:1–116. [PubMed] [Google Scholar]
- 18.Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell. 2006;124:929–42. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 19.August P, Suthanthiran M. Transforming growth factor beta signaling, vascular remodeling, and hypertension. N Engl J Med. 2006;354:2721–3. doi: 10.1056/NEJMcibr062143. [DOI] [PubMed] [Google Scholar]
- 20.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–16. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H, Vriend G, Pattynama PM, Collée M, Majoor-Krakauer D, Poldermans D, Frohn-Mulder IM, Micha D, Timmermans J, Hilhorst-Hofstee Y, Bierma-Zeinstra SM, Willems PJ, Kros JM, Oei EH, Oostra BA, Wessels MW, Bertoli-Avella AM. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–6. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 22.Brown KA, Pietenpol JA, Moses HL. A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling. J Cell Biochem. 2007;101:9–33. doi: 10.1002/jcb.21255. [DOI] [PubMed] [Google Scholar]
- 23.Regalado ES, Guo DC, Villamizar C, Avidan N, Gilchrist D, McGillivray B, Clarke L, Bernier F, Santos-Cortez RL, Leal SM, Bertoli-Avella AM, Shendure J, Rieder MJ, Nickerson DA NHLBI GO Exome Sequencing Project. Milewicz DM. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res. 2011;109:680–6. doi: 10.1161/CIRCRESAHA.111.248161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou S, Liao M, Yang J, Huang T, Green M, Wu J, Qu L. Heat shock protein 27 plays a protective role in thoracic aortic dissection by promoting cell proliferation and inhibiting apoptosis. Cell Mol Biol Lett. 2017;22:24. doi: 10.1186/s11658-017-0056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley TJ, Bowdin SC, Morel CF, Pyeritz RE. The expanding clinical spectrum of extracardiovascular and cardiovascular manifestations of heritable thoracic aortic aneurysm and dissection. Can J Cardiol. 2016;32:86–99. doi: 10.1016/j.cjca.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Gao F, Watanabe M, Matsuzawa T. Stress analysis in a layered aortic arch model under pulsatile blood flow. Biomed Eng Online. 2006;5:25. doi: 10.1186/1475-925X-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirst AE, Gore I. Editorial: is cystic medionecrosis the cause of dissecting aortic aneurysm? Circulation. 1976;53:915–6. doi: 10.1161/01.cir.53.6.915. [DOI] [PubMed] [Google Scholar]
- 28.de Figueiredo Borges L, Jaldin RG, Dias RR, Stolf NA, Michel JB, Gutierrez PS. Collagen is reduced and disrupted in human aneurysms and dissections of ascending aorta. Hum Pathol. 2008;39:437–43. doi: 10.1016/j.humpath.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Curci JA. Digging in the “soil” of the aorta to understand the growth of abdominal aortic aneurysms. Vascular. 2009;17(Suppl 1):S21–9. doi: 10.2310/6670.2008.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dingemans KP, Teeling P, van der Wal AC, Becker AE. Ultrastructural pathology of aortic dissections in patients with Marfan syndrome: comparison with dissections in patients without Marfan syndrome. Cardiovasc Pathol. 2006;15:203–12. doi: 10.1016/j.carpath.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–9. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Xu J, Guo JL, Lin CY, Luo WM, Yuan Y, Liu H, Zhang J. YAP1 up-regulation inhibits apoptosis of aortic dissection vascular smooth muscle cells. Eur Rev Med Pharmacol Sci. 2017;21:4632–9. [PubMed] [Google Scholar]
- 33.Aparecida-Silva R, Borges LF, Kessler K, Dias RR, Moreira LFP, Kalil J, Gutierrez PS. Transforming growth factor-beta1 SMAD effectors and medial cell number in ascending aorta diseases. Cardiovasc Pathol. 2016;25:240–6. doi: 10.1016/j.carpath.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Yuan SM, Wu N. Aortic alpha-smooth muscle actin expressions in aortic disorders and coronary artery disease: an immunohistochemical study. Anatol J Cardiol. 2018;19:11–6. doi: 10.14744/AnatolJCardiol.2017.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanasković I, Mladenović-Mihailović A, Usaj-Knezević S, Stanković V, Aleksić A, Kastratović T, Aleksić A, Lazić Z, Mladenović-Bogdanović Z, Zivanović A, Djurić J, Jovicić U, Sorak M. Histochemical and immunohistochemical analysis of ruptured atherosclerotic abdominal aortic aneurysm wall. Vojnosanit Pregl. 2010;67:959–64. doi: 10.2298/vsp1012959t. [DOI] [PubMed] [Google Scholar]
- 37.Das D, Gawdzik J, Dellefave-Castillo L, McNally EM, Husain A, Raman J, Hofmann Bowman MA. S100A12 expression in thoracic aortic aneurysm is associated with increased risk of dissection and perioperative complications. J Am Coll Cardiol. 2012;60:775–85. doi: 10.1016/j.jacc.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen H, Lu S, Dong L, Xue Y, Yao C, Tong C, Wang C, Shu X. hsa-miR-320d and hsa-miR-582, miRNA biomarkers of aortic dissection, regulate apoptosis of vascular smooth muscle cells. J Cardiovasc Pharmacol. 2018;71:275–82. doi: 10.1097/FJC.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 39.Yuan SM, Wang J, Hu XN, Li DM, Jing H. Transforming growth factor-beta/Smad signaling function in the aortopathies. Rev Bras Cir Cardiovasc. 2011;26:393–403. doi: 10.5935/1678-9741.20110014. [DOI] [PubMed] [Google Scholar]
- 40.Gomez D, Al Haj Zen A, Borges LF, Philippe M, Gutierrez PS, Jondeau G, Michel JB, Vranckx R. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J Pathol. 2009;218:131–42. doi: 10.1002/path.2516. [DOI] [PubMed] [Google Scholar]
- 41.Jones JA, Spinale FG, Ikonomidis JS. Transforming growth factor-beta signaling in thoracic aortic aneurysm development: a paradox in pathogenesis. J Vasc Res. 2009;46:119–37. doi: 10.1159/000151766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakerakanti S, Trojanowska M. The role of TGF-beta receptors in fibrosis. Open Rheumatol J. 2012;6:156–62. doi: 10.2174/1874312901206010156. [DOI] [PMC free article] [PubMed] [Google Scholar]