Abstract
During heavy and severe constant-load exercise, VO2 displays a slow component (VO2sc) typically interpreted as a loss of efficiency of locomotion. In the ongoing debate on the underpinnings of the VO2sc, recent studies suggested that VO2sc could be attributed to a prolonged shift in energetic sources rather than loss of efficiency. We tested the hypothesis that the total cost of cycling, accounting for aerobic and anaerobic energy sources, is affected by time during metabolic transitions in different intensity domains. Eight active men performed 3 constant load trials of 3, 6, and 9 min in the moderate, heavy, and severe domains (i.e., respectively below, between, and above the two ventilatory thresholds). VO2, VO2 of ventilation and lactate accumulation ([La−]) were quantified to calculate the adjusted oxygen cost of exercise (AdjO2Eq, i.e., measured VO2 − VO2 of ventilation + VO2 equivalent of [La−]) for the 0–3, 3–6, and 6–9 time segments at each intensity, and compared by a two-way RM-ANOVA (time × intensity). After the transient phase, AdjO2Eq was unaffected by time in moderate (ml*3 min−1 at 0–3, 0–6, 0–9 min: 2126 ± 939 < 2687 ± 1036, 2731 ± 1035) and heavy (4278 ± 1074 < 5121 ± 1268, 5225 ± 1123) while a significant effect of time was detected in the severe only (5863 ± 1413 < 7061 ± 1516 < 7372 ± 1443). The emergence of the VO2sc was explained by a prolonged shift between aerobic and anaerobic energy sources in heavy (VO2 − VO2 of ventilation: ml*3 min−1 at 0–3, 0–6, 0–9 min: 3769 ± 1128 < 4938 ± 1256, 5091 ± 1123, [La−]: 452 ± 254 < 128 ± 169, 79 ± 135), while a prolonged metabolic shift and a true loss of efficiency explained the emergence of the VO2sc in severe.
Electronic supplementary material
The online version of this article (10.1007/s00424-020-02437-7) contains supplementary material, which is available to authorized users.
Keywords: Oxidative metabolism, VO2 kinetics, Exercise physiology, Oxygen consumption, Excess VO2, Loss of efficiency
Introduction
After the cardio-dynamic phase, oxygen consumption (VO2) during constant heavy and severe intensity exercise, is better fitted by a two-component rather than a single-component model. Based on this observation, it has been suggested, that a slow component of VO2 exists (VO2sc), that does not start at exercise onset but rather appears later in exercise (time delay ~ 120–180 s) [9]. Furthermore, it has been assumed, but not proven, that in association with VO2sc, the muscle displays an increasing energy demand as a function of time [10].
VO2sc represents an increased O2 cost of locomotion when exercise is protracted more than 3 min at a constant workload above the lactate threshold (LT) [9]. Typically, when exercise is performed between LT and the critical power, (i.e. heavy intensity domain) VO2sc tends to a delayed steady-state (i.e. compared to moderate). On the contrary, when effort rises above critical power (i.e. severe exercise domain) a steady-state is not achievable and VO2 increases tending to the maximal oxygen consumption (VO2max) [9]. The magnitude of VO2sc is considered to be associated with exercise intolerance and fatigue [7], and the study of this phenomenon is particularly important in diseased/frail populations (e.g. [19, 24]). Nevertheless, despite the efforts of the past 40 years to clarify the exact physiological underpinnings of the VO2sc, these remain debated even in healthy subjects. [9].
A key finding from the past studies was that roughly 85% of the VO2sc originates from the contracting muscles, while the remaining 15% corresponds to the increased VO2 cost of ventilation [20]. Focusing on the muscular component of the VO2sc, subsequent investigations proposed that the recruitment of less efficient type II fibres necessary to maintain a specific power output (PO) [9] or metabolic instability due to metabolites accumulation occurring within the working fibres [26, 28] could elicit an increased cost of locomotion. Nevertheless, a satisfactory theory explaining the mechanisms underpinning the VO2sc is still missing.
In this context, a recent study [17] refuted that the energy demand of a constant, high-intensity exercise changes over time. By subtracting the VO2 cost of ventilation and accounting for the contribution of the glycolytic energy sources [17, 21, 22] O’Connell et al. quantified the total, adjusted metabolic cost of muscle exercise over time during a constant load trial in the severe domain. The authors concluded that the overall cost of locomotion does not increase over time, other than what required by the augmenting cost of ventilation. The apparent VO2 increases over time would be the result of the prolonged shift in metabolic sources beyond the first 3 min of exercise (i.e. an increased contribution of the aerobic metabolism to ATP resynthesis, mirrored by a decreased contribution of anaerobic ATP resynthesis over time). In other words, these findings suggest that VO2sc may in fact not represent a true loss of efficiency as a function of time but rather a prolonged adjustment of the oxidative metabolism. However, while this new hypothesis could explain why a satisfying explanatory theory of VO2sc is still missing, the amount of literature that reported VO2sc in different exercise modalities (i.e. whole-body vs isolated muscles) and models (i.e. humans vs animals) calls for further investigation [9]. In particular, the intensity domain in which O’Connell measures were obtained is not fully clear; while most studies in the field of VO2 kinetics define intensity domains based on gas exchange thresholds, O’Connell used lactate-based measures. Yet, the correspondence between the above methods remains a controversial issue [3, 12, 13]. In addition, loss of efficiency, the metabolic shift among substrates and excess ventilation manifest and increase differently over time in the three domains of exercise (e.g. no loss of efficiency is present in the moderate domain while an elevated steady state is reached in the heavy yet not in the severe domain) [10]. Therefore, O’Connell’s findings need to be confirmed and broadened with particular consideration to the three intensity domains of exercise.
Accordingly, by calculating the energy cost of ventilation, the glycolytic contribution to exercise, and directly measuring the aerobic cost of locomotion over time, we tested if and to what extent a true loss of efficiency during cycling explains the emergence of the slow component of VO2 in different intensity domains. Specifically, we hypothesised that (i) the overall cost of locomotion would not be affected by the time during metabolic transitions in the moderate and heavy exercise domains (i.e. no loss of efficiency over time exists at these intensities but rather a prolonged metabolic shift is responsible for the observed VO2sc); (ii) the overall cost of locomotion would be affected by the time during metabolic transitions in the severe exercise domain (i.e. a prolonged metabolic shift is not sufficient to explain the emerge loss the observed VO2sc and a true loss of efficiency over time will manifest at this intensity).
Methods
Ethical approval
The study was conducted according to the Declaration of Helsinki and all procedures were approved by the University of Verona Ethics Committee for Research on Human Subjects (CARU no 15/2019). Procedures and risks were explained to each subject, and all participants volunteered and gave informed written consent to participate before the start of the study.
Participants
Eight active men were recruited in the study (age 25 ± 2 years, body mass 74 ± 10 kg, height 181 ± 5 cm, VO2max 49.3 ± 3.4 ml*kg−1*min−1, performing recreational aerobic activity 3–4 times × week). Inclusion criteria were male sex and age between 20 and 35 years; exclusion criteria were smoking and any condition that could influence the physiological responses during testing. All participants were instructed to avoid caffeine consumption and physical activity respectively for at least 8 h and 24 h before each testing session. Moreover, they received a standard and individualized food intake prescription before all the testing sessions to minimize variability of glycogen stores and glucose oxidation (i.e. 2 g of low glycemic index carbohydrates per kg of body weight, 2 h before testing; 0.5 L of water in the 90 min before testing; restriction from caffeine during the 8 h before testing).
Experimental protocol
After medical clearance, participants visited the laboratory on ten occasions within a maximum of 4 weeks. All subjects completed: (i) a preliminary maximal ramp incremental exercise test to exhaustion for the determination of gas exchange threshold (GET), respiratory compensation point (RCP) and the peak of oxygen uptake (VO2peak); (ii) three constant load trials (CLT) respectively of 3, 6 and 9 min in the “moderate” exercise intensity domain (iii) three CLT respectively of 3, 6 and 9 min in the “heavy” exercise intensity domain (iv) three CLT trials respectively of 3, 6 and 9 min in the “severe” exercise intensity domain. Tests of (ii), (iii) and (iv) aimed at determining VO2 response and blood lactate ([La−]) accumulation as a function of time in the three exercise intensity domains in order to evaluate the overall energetic contribution of the aerobic and anaerobic metabolisms [22] to ATP turnover. Moreover, these tests were executed in randomized order with the only exception of the longest CLT in the “severe” exercise domain, that was completed as first to assure that subjects were able to sustain the PO for the required time. All exercise tests were conducted on an electromagnetically braked cycle ergometer (Sport Excalibur, Lode, Groningen, Netherlands), at a similar time of the day in an environmentally controlled laboratory (18 °C, 55–65% relative humidity).
Ramp incremental test
The ramp incremental test consisted of a 3-min baseline cycling at 50 W, followed by a 30-W*min−1 increase in PO until volitional exhaustion. Participants were asked to pick a self-selected cadence in the range of 70–90 rpm and to maintain it throughout all subsequent tests. Failure to maintain the indicated cadence within 5 rpm (for longer than 5 s) during testing despite strong verbal encouragement was considered as the criterion for exhaustion. Breath-by-breath pulmonary gas exchange and ventilation were continuously measured using a metabolic cart (Jaeger Oxycon Pro, Viasys Healtcare GmbH, Höchberg, Germany) as previously described [23]. Heart rate (HR) was monitored continuously (H7 Sensor, Polar, Kempele, Finland).
Constant load trials
After the preliminary ramp incremental test, subjects completed 3 CLT within each exercise intensity domain (i.e. moderate, heavy, and severe [18]) in a randomized order:
-
i)
Moderate: 3 CLT respectively of 3,6 and 9 min at 80% of GET.
-
ii)
Heavy: 3 CLT respectively of 3,6 and 9 min at 50%Δ between GET and RCP.
-
iii)
Severe: 3 CLT respectively of 3,6 and 9 min at 60%Δ between GET and VO2peak.
Each CLT was preceded by a 3-min warm-up at 20 W. Throughout the test, subjects kept the same, constant rpm and bike position as selected during the ramp incremental test.
VO2 and HR data were measured with the same method described for the ramp incremental test. Moreover, capillary blood samples (65 μl) were drawn from the fingertip in the last 30 s of warm-up and at the 1st, 3rd, 5th, and 7th min after each test and were immediately analysed (Radiometer ABL90 FLEX, Radiometer Medical ApS, Brønshøj, Denmark) to measure [La−]. The highest value was considered as the peak of blood lactate concentration and used for further analysis to calculate anaerobic energetic contribution.
Data analysis
Ramp incremental test
For the gas exchange variables, aberrant data-points that lay 3 SD from the local mean were removed, and trials were linearly interpolated on a 1-s basis and then averaged every 5 s. VO2peak was determined as the highest VO2 obtained over a 10-s interval [6]. GET and RCP were determined with the standard technique from gas exchange variables by three blinded expert reviewers as detailed elsewhere [6]. Briefly, GET was determined by visual inspection as the VO2 at which CO2 output began to increase out of proportion in relation to VO2, with a systematic rise in the ventilation (VE)-to-VO2 relation and end-tidal PO2 whereas the ventilatory equivalent of VCO2 (VE/VCO2) and end-tidal PCO2 is stable [2]. RCP was determined as the point where end-tidal PCO2 began to fall after a period of isocapnic buffering [27]. This point was confirmed by examining VE/VCO2 plotted against VO2 and by identifying the sec breakpoint in the VE-to-VO2 relation. Finally, we determined the constant workload equivalent to the specific moderate (80% of GET), heavy (50% Δ between GET and RCP), and severe (60%Δ between GET and VO2peak) VO2 targets. To this aim, the VO2/W relationship identified with the incremental test was left-shifted to account for the mean response time [6].
Constant load trials
VO2, VCO2, and VE during CLT were sampled breath-by-breath and interpolated using the same procedure described for the ramp incremental test. Interpolated data from different CLT performed at the same exercise intensity were mediated in order to reduce breath-by-breath signals variability (data from 3 tests were mediated in the time segment from 0 to 3 min and data from 2 tests were mediated between 3 and 6 min) [11]. The sum of the oxygen consumed during each 3-min time segment was then considered as the aerobic energetic contribution to exercise.
VO2sc was calculated as the sum of the amount of oxygen exciding the VO2 reached at the end of the 0-to-3-min time segment [25].
Work of breathing (WB) was calculated based on VE using the equation by Coast et al.:
WB = − 0.430 + 0.050 * VE + 0.00161 VE2
Then, the WB was used to calculate the amount of VO2 requested by ventilatory muscles (VO2VM):
VO2VM = 34.9 + 7.45 *WB [4]
Anaerobic (glycolytic) contribution to exercise was calculated from the amount of [La−] accumulation over time calculated as follows:
0–3 min segment [La−] accumulation = 3-min CLT peak [La−] − warm-up [La−]
3–6 min segment [La−] accumulation = 6-min CLT peak [La−] − 3-min CLT peak [La−]
6–9 min segment [La−] accumulation = 9-min CLT peak [La−] − 6-min CLT peak [La−]
The so obtained values were utilized to estimate the energy contribution from anaerobic glycolysis in each time segment, based on the oxygen equivalent for lactate of Di Prampero (i.e. 1 mmol*L−1 [La−] accumulation = 3.0 ml*kg−1 VO2) [22].
Overall energetic cost of the activity (expressed as ml of oxygen) was calculated as described by O’Connell et al. and defined as “adjusted oxygen equivalent” (AdjO2Eq): o.
VO2 gain (VO2gain, i.e. the amount of oxygen equivalent utilized to sustain each W during cycling) was calculated as the ratio between the amount of AdjO2Eq required to sustain exercise during a specific time segment and the number of W imposed by the test: VO2gain = (3 min AdjO2Eq − warm-up AdjO2Eq) / (test PO (W) − warm-up PO (W)).
Statistics
After assumptions verification (i.e., normality, homogeneity of variance), the within-subject coefficient of variation and a two-way repeated-measure ANOVA (trial × intensity domain) were used to evaluate VO2 data repeatability measured at the end of the third min of exercise of each CLT.
Two-way repeated-measure ANOVA was also performed to assess differences over time between different intensities domains (time segment × intensity domain) for VO2, VO2VM, O2 equivalent of [La−], AdjO2Eq, VO2gain. The amplitude of the VO2sc in different intensity domains was compared using a one-way repeated measures ANOVA (intensity domain). Post-hoc analyses were performed using the Holm-Sidak method. Moreover, partial eta squares (ηp2) were calculated to quantify the effects sizes of different independent variables during the constant load trials [14]. Based on an expected standard deviation of breath-by-breath VO2 measurements for steady-state exercise equal to 2.5%, and a minimum detectable change in VO2 of 100–170 ml·min−1 at a VO2 of 2.1 to 3.5 L·min−1 [12], the minimum sample size to obtain a power of 0.8 was 6 individuals.
Data are presented as means ± SD. All statistical analyses were performed using Sigmaplot version 12 and α was set in advance at the 0.05 level. Statistical significance was accepted when p < α.
Results
Subjects’ anthropometrical and functional characteristics obtained during the ramp incremental test are reported in Table 1.
Table 1.
Subjects’ anthropometrical and functional characteristics obtained during the ramp incremental test
| Subjects characteristics | Ramp incremental test | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| # | Weight (kg) | Height (cm) | Age (years) | PPO (W) | VO2peak (ml*min−1) | VO2peak (ml*min−1*kg) | MRT (sec) | GET (ml*min−1) | RCP (ml*min−1) |
| 8 ♂ | 74 ± 9 | 180 ± 5 | 25 ± 1 | 376 ± 36 | 3643 ± 457 | 49 ± 3 | 41 ± 12 | 2418 ± 385 | 3093 ± 377 |
Values are means ±SD. PPO, peak PO; VO2peak: peak of oxygen consumption; MRT, mean response time; GET, gas exchange threshold; RCP, respiratory compensation point
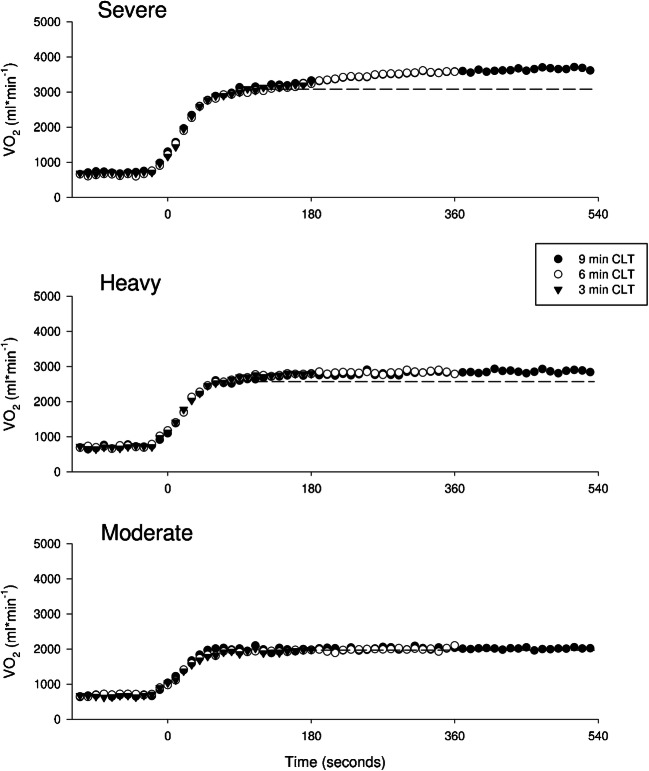
Repeatability of group mean VO2 data (10-s averages) is displayed in Fig. 1. Average measured VO2 displays a complete overlap among the three durations trials (3, 6, 9 min) at the three intensities (moderate, heavy, severe). Furthermore, mean VO2 values of the last 10 s of the third min of exercise for the 9, 6, and 3-min CLT were respectively: 3328 ± 470, 3231 ± 434, 3285 ± 443 ml*min−1 (severe) 2804 ± 408, 2783 ± 492, 2793 ± 470 ml*min−1 (heavy), and 1979 ± 281, 1966 ± 330, 2095 ± 258 ml*min−1 (moderate), with a mean within-subject coefficient of variation of 3.3 ± 2.4% (severe), 4.3 ± 1.4% (heavy), 3.7 ± 1.4% (moderate). As expected, ANOVA on these VO2 values revealed a significant main effect of the intensity domain at which exercise was performed (p ≤ 0.001, ηp2: 0.95) but no significant main effect among the three trials performed at the same intensity (p = 0.437, ηp2: 0.08).
Fig. 1.
Repeatability of VO2: group mean VO2 data are displayed as 10s-averages respectively for the “severe” (top panel), “heavy” (medium panel), and “moderate” (bottom panel) exercise intensity domain. Symbols represent the three duration trials: black dots = 9-min CLT, white dots = 6-min CLT, black triangles = 3-min CLT
Mean values of PO and per-min measures of VO2, VCO2, VE, and HR in the last 30 s of each time segment are displayed in Table 2, along with measures of [La−] at the end of the warm-up and at the end of each time segment (peak [La−]). ANOVA revealed a significant “time segment” × “intensity domain” interaction for VO2 (p ≤ 0.001, ηp2: 0.57), VCO2 (p = 0.014, ηp2: 0.35), VE (p ≤ 0.001, ηp2: 0.73), HR (p = 0.011, ηp2: 0.30), and [La−] (p ≤ 0.001, ηp2: 0.29). In detail, HR and [La−] reached a steady-state within the 3rd min only in the moderate and heavy-intensity exercise trials, while they increased over time in the severe exercise trials. VO2, VCO2, and VE reached a steady-state within the 3rd min in the moderate-intensity and within the 6th min of the heavy-intensity exercise trials. On the contrary, these parameters continued to increase significantly over time in the severe exercise trials. Finally, a significant main effect of “intensity” on the magnitude of VO2sc was detected (p ≤ 0.001, ηp2: 0.77), and VO2sc amplitude was increasing going from moderate (12.7 ± 45.6 ml*min−1) to heavy (136.7 ± 99.3 ml*min−1) and severe (416.7 ± 165.9 ml*min−1; post-hoc: moderate vs heavy p = 0.026 moderate vs severe p ≤ 0.001, heavy vs severe p = 0.021).
Table 2.
Metabolic response in the moderate, heavy and severe exercise domains
| Load | VO2 | VCO2 | VE | HR | [La−] | |
|---|---|---|---|---|---|---|
| Time Segment | (W) | (ml*min−1) | (ml*min−1) | (L*min−1) | (b*min−1) | (mmol*L) |
| Severe | ||||||
| Warm-up | 20.0 ± 0.0 | 1034.5 ± 51.4 | 743.8 ± 479.1 | 17.7 ± 2.2 | 73.2 ± 9.6 | 1.7 ± 0.6 |
| 0 to 3 min | 267.0 ± 37.5 | 3198.7 ± 435.6 | 3585.8 ± 599.2 | 92.9 ± 26.9 | 157.3 ± 4.7 | 6.7 ± 1.6 |
| 3 to 6 min | 267.0 ± 37.5 # | 3489.1 ± 498.8 # | 3792.3 ± 597.9 # | 109.9 ± 31.1 # | 169.5 ± 5.5 | 9.2 ± 2.4 # |
| 6 to 9 min | 267.0 ± 37.5 #* | 3615.5 ± 521.9 #* | 3888.1 ± 634.2 #* | 119.1 ± 31.0 #* | 175.2 ± 4.8 # | 10.7 ± 2.5 #* |
| Heavy | ||||||
| Warm-up | 20.0 ± 0.0 | 1067.7 ± 57.6 | 605.8 ± 58.6 | 18.6 ± 2.6 | 74.2 ± 8.4 | 1.8 ± 0.8 |
| 0 to 3 min | 208.9 ± 28.7 | 2771.6 ± 429.3 | 2748.8 ± 438.2 | 66.5 ± 14.4 | 141.5 ± 11.2 | 3.4 ± 1.1 |
| 3 to 6 min | 208.9 ± 28.7 # | 2851.5 ± 425.4 # | 2818.3 ± 400.8 # | 70.9 ± 13.4 # | 146.3 ± 15.3 | 3.9 ± 1.4 |
| 6 to 9 min | 208.9 ± 28.7 # | 2822.7 ± 395.7 # | 2741.1 ± 367.5 # | 71.0 ± 13.1 # | 151.1 ± 15.2 | 4.1 ± 1.4 |
| Moderate | ||||||
| Warm-up | 20.0 ± 0.0 | 1026.3 ± 65.3 | 566.4 ± 60.7 | 17.1 ± 1.7 | 73.3 ± 8.5 | 1.7 ± 0.6 |
| 0 to 3 min | 128.7 ± 27.0 | 1951.6 ± 261.7 | 1731.0 ± 275.5 | 42.9 ± 8.1 | 117.5 ± 23.6 | 2.4 ± 0.8 |
| 3 to 6 min | 128.7 ± 27.0 | 1987.1 ± 277.4 | 1841.5 ± 275 | 45.3 ± 7.2 | 122.0 ± 27.0 | 1.8 ± 0.6 |
| 6 to 9 min | 128.7 ± 27.0 | 1992.3 ± 298.7 | 1871.7 ± 332.7 | 47.6 ± 9.8 | 122.9 ± 29.0 | 1.3 ± 0.3 |
Values are means ±SD. Values of PO and per-min measures of VO2, VCO2, VE, and HR in the last 30s of each time segment are displayed, along with measures of lactate concentration ([La−]) at the end of warm-up and at the end of each time segment. ANOVA revealed a significant “intensity” × “time” interaction for VO2 (p ≤ 0.001), VCO2 (p = 0.014), VE (p ≤ 0.001), HR (p = 0.011) and [La−] (p ≤ 0.001). Multiple comparisons are also displayed: # represents significant statistical difference with 0 to 3 min segment; * represents significant statistical difference with 3 to 6 min segment. For greater clarity were omitted: comparisons vs warm-up (always significantly different, with the only exception of [La−] in the moderate exercise intensity domain) and between exercise domains (always significantly different, with the only exception of HR between “severe” and “heavy” during the 0–3 segment)
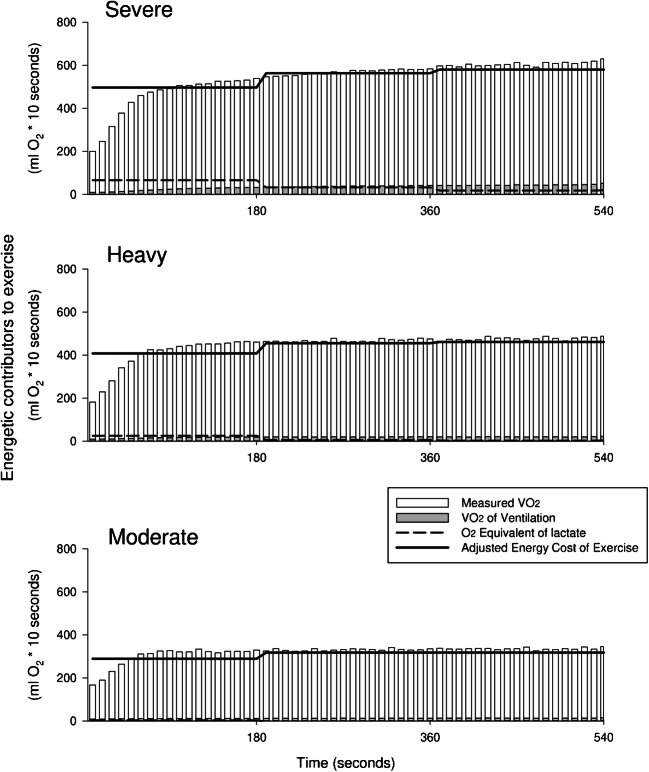
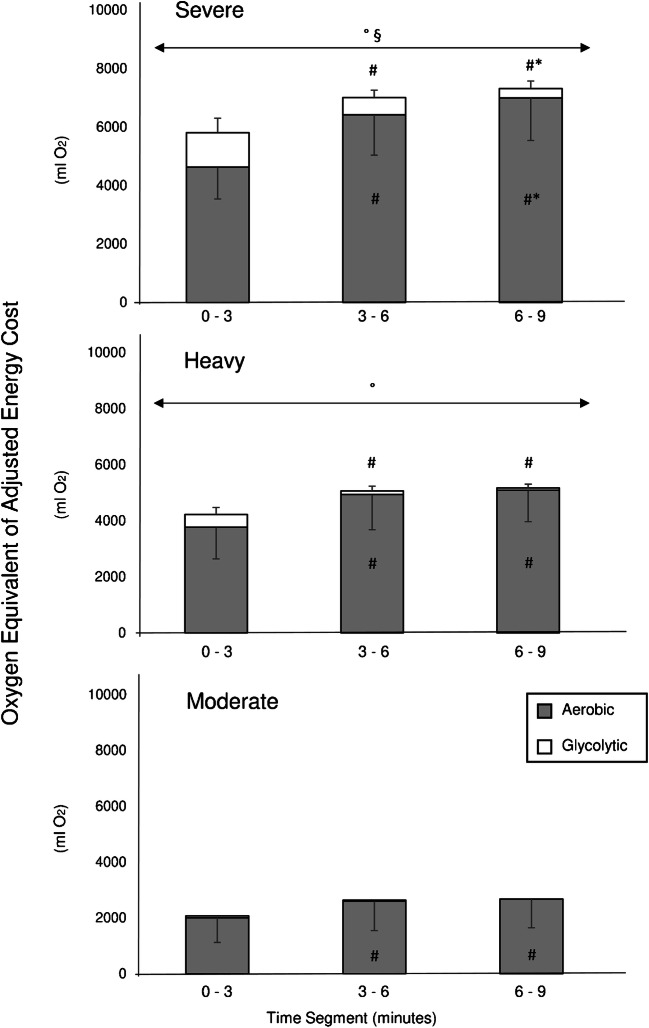
An overview of the energetic contributors to exercise (i.e., measured VO2, VO2VM, [La−] equivalent of O2, and AdjO2Eq) is reported as 10-s averages and over 3-min time segments in Fig. 2 and in Table 3. For all time segments, the contribution of anaerobic glycolysis (as represented by [La−] equivalent) was significantly increased going from moderate to heavy to severe intensity trails (significant main effect of intensity, p ≤ 0.001, ηp2: 0.91). Furthermore, the contribution of anaerobic glycolysis in the three domains was significantly affected by time (significant main effect of time segment p ≤ 0.001, ηp2: 0.67). In particular, the post-hoc analysis revealed a limited and unvaried contribution of anaerobic glycolysis for the moderate-intensity domain (0–3 vs 3–6 time segments p = 0.772, 3–6 vs 6–9 p = 0.947); in heavy, a decreased contribution of anaerobic glycolysis from 0 to 3 to 3–6 time segments, yet no further after that (0–3 vs 3–6 p = 0.014, 3–6 vs 6–9 p = 0.429); finally, a continuous decrease in the contribution of anaerobic glycolysis over time in the severe exercise trials (0–3 vs 3–6 p ≤ 0.001, 3–6 vs 6–9 p = 0.024; Fig. 3).
Fig. 2.
An overview of the energetic contributors to exercise is reported in 10-s averages for the “severe” (top panel), “heavy” (medium panel), and “moderate” (bottom panel) exercise intensity domain. White columns represent directly measured VO2. Grey columns indicate the O2 cost requested by ventilation. The black dashed line displays the energy provided by glycolytic sources over 3 min segments. Finally, the black solid line represents the adjusted cost of exercise accounting for both aerobic and glycolytic energy sources. Please note that during all the first 3 min segments, the contribution of immediate energy sources (O2 and phosphocreatine) was not accounted and was probably the cause of the lower adjusted VO2 between the first and the successive time segments [8, 22]
Table 3.
Energetic contributors to exercise in the moderate, heavy and severe exercise domains
| Time segment | VO2 (ml O2*3min−1) | VO2VM (ml O2*3min−1) | [La−] Equivalent (ml O2*3min−1) | AdjO2Eq (ml O2*3min−1) | VO2gain (ml O2*min−1 * W) |
|---|---|---|---|---|---|
| Severe | |||||
| 0 to 3 min | 5037 ± 1155 | 356 ± 113 | 1182 ± 492 | 5863 ± 1413 | 7.9 ± 1.2 |
| 3 to 6 min | 7096 ± 1509 # | 623 ± 234 # | 588 ± 256 # | 7061 ± 1516 # | 9.5 ± 1.2 # |
| 6 to 9 min | 7780 ± 1573 #* | 729 ± 270 #* | 321 ± 259 #* | 7372 ± 1443 #* | 9.9 ± 1.1 # |
| Heavy | |||||
| 0 to 3 min | 4062 ± 1184 | 216 ± 62 | 452 ± 254 | 4278 ± 1074 | 7.5 ± 1.1 |
| 3 to 6 min | 5289 ± 1321 # | 295 ± 77 # | 128 ± 169 # | 5121 ± 1268 # | 9.0 ± 1.3 # |
| 6 to 9 min | 5450 ± 1180 # | 304 ± 77 # | 79 ± 135 # | 5225 ± 1123 # | 9.2 ± 1.0 # |
| Moderate | |||||
| 0 to 3 min | 2200 ± 906 | 140 ± 25 | 67 ± 94 | 2126 ± 939 | 6.4 ± 1.8 |
| 3 to 6 min | 2823 ± 1083 # | 169 ± 33 | 34 ± 58 | 2687 ± 1036 # | 8.1 ± 1.7 # |
| 6 to 9 min | 2910 ± 1067 # | 189 ± 40 | 0 ± 0 | 2731 ± 1035 # | 8.3 ± 1.8 # |
Values are means ±SD. Values are reported over 3-min time segments. VO2: directly measured VO2; VO2VM: VO2 requested by ventilatory muscles; [La−] equivalent: oxygen equivalent of lactate; AdjO2Eq: energy cost of exercise expressed as a sum of aerobic and glycolytic sources (VO2 + [La−] equivalent) and subtracted by VO2VM. ANOVA revealed a significant “intensity” × “time” interaction for VO2 (p ≤ 0.001), VO2VM (p ≤ 0.001), [La−] equivalent (p ≤ 0.001), and AdjO2Eq (p ≤ 0.001). Multiple comparisons are displayed in the table: # represents significant statistical difference with 0 to 3 min segment; * represents significant statistical difference with 3 to 6 min segment. Please note that values measured during warm-up were subtracted. For greater clarity comparisons between intensity domains were omitted (always significantly different, with the exceptions of (i) [La−] equivalent between “heavy” and “moderate” during the 3–6 and 6–9 time segments and (ii) VO2gain between “severe” and “heavy” in the 0–3 segment (iii) VO2gain between “severe” and “heavy” and “heavy” and “moderate” in the 3–6 segment)
Fig. 3.
Three-min mean oxygen equivalents of aerobic (grey, i.e. VO2 subtracted by VO2VM) and glycolytic (white, i.e. [La−] equivalent) cost of exercise are represented respectively for the “severe” (top panel), “heavy” (medium panel) and “moderate” (bottom panel) exercise intensity domain. ANOVA revealed a significant “intensity” × “time” interaction both for aerobic (p ≤ 0.001, ηp2: 0.81) and anaerobic (p ≤ 0.001, ηp2: 0.58) cost of exercise. Multiple comparisons are displayed as: # significant statistical difference with 0 to 3 min segment; * significant statistical difference with 3 to 6 min segment. ° and § represent respectively statistical difference with the “moderate” and “heavy” exercise intensity domain. Please note that values measured during warm-up were subtracted
Discussion
By comprehensively quantifying aerobic and glycolytic energy sources used during exercise, deprived of the VO2 cost of ventilation, this study tested the hypothesis that the overall cost of cycling (i.e. AdjO2Eq) is affected by the time during metabolic transitions in the moderate, heavy and severe intensity domains. In agreement with our hypothesis, our findings suggest that (i) the overall cost of locomotion does not increase over time in the moderate domain after the 3rd min of exercise; (ii) in the heavy intensity domain, the emergence of a VO2 slow component is ascribable to a “metabolic shift” between aerobic and anaerobic metabolisms that protracted beyond the 3rd min of exercise and to a small yet significant increase in the VO2 of ventilation, rather than to an actual increased cost of locomotion after the 3rd min; (iii) finally, only in the severe domain the raise of the VO2 slow component deprived of its ventilatory part was not completely explained by a prolonged metabolic shift between energy sources, and the further increase in AdjO2Eq was possibly indicating that a true loss of efficiency over time occurred. This investigation provides new insight of the mechanisms underpinning VO2sc and exercise tolerance.
The fitness level of our sample measured during the ramp incremental tests indicated that the participants of this investigation were active subjects. (VO2peak: 49 ± 3 ml*min−1*kg, see Table 1 for the other characteristics) [1], and presented an overall metabolic response to constant load exercise consistent with previous studies (e.g. no VO2sc in the moderate domain; VO2sc in heavy +4.2 ± 3.4% tending to steady-state; VO2sc in severe domain +11.4 ± 4.9% tending to VO2max) [17, 25, 29].
By applying an approach previously proposed by O’Connell et al. [17], our study quantified the individual contributors to the overall net VO2 cost of exercise (i.e. AdjO2Eq) and their interplay during the metabolic transition and steady-state. While O’Connell et al. studied the VO2 of active muscles, the VO2 associated with ventilation and the VO2 equivalent of [La−] in the severe domain only, we extended the analysis to the three exercise intensity domains.
As expected, based on the changes in ventilation described above, the VO2 associated with ventilation increased going from moderate (3 ± 3% of the VO2sc), heavy (7 ± 3%), to severe (14 ± 7%) intensity. In agreement with previous work [20], this finding confirms that ~ 85% of the VO2sc occurs within the working muscles. In addition, our study demonstrated that the VO2 associated with ventilation increased over time in the severe domain only. Furthermore, for all the exercise domains, the O2 equivalent of [La−] accumulation decreased after the transition phase (i.e. 0-to-3-time segment), compatible with a progressive decrease of the contribution of glycolysis to ATP resynthesis over time [22]. Interestingly, in the domains below RCP, this decreased contribution of glycolysis to the energy provision was “mirrored” by an increased contribution of oxidative metabolism, to satisfy an invariant energetic demand (Table 3 and Fig. 3). In other words, the VO2sc that is observed in the heavy domain may be related to a prolonged “metabolic shift” between anaerobic and aerobic energy sources of ATP resynthesis rather than to an augmented cost of locomotion over time. Such a view is in agreement with the findings and interpretations of previous human studies, performed in the heavy/severe domain of exercise [16, 17] as well as with studies based on computer modelling of the skeletal muscle bioenergetics [15]. For the exercise in the severe domain, however, we found that accounting for the total energetic cost of locomotion did not completely explain VO2sc. We were the first to demonstrate that, in this domain, the AdjO2Eq continued to increase showing an augment energy required to sustain the same PO, compatible with a true loss of efficiency over time.
Relative to the severe intensity domain, the partial discrepancy between our data and O’Connell’s may be explained by methodological differences between both studies: (i) as we did, most studies in the field of VO2 kinetics define intensity domains based on gas exchange thresholds [10, 23]; on the contrary, O’Connell used lactate-based measures. Yet, the correspondence between the above methods remains controversial [3, 12, 13] and the difference may have led to unmatched intensity domains among our studies. (ii) O’Connell incremental protocol for VO2peak detection was performed after 10 min of recovery from a ~ 20-min protocol with 3-min steps used for LT determination. This approach could be responsible for an underestimation of peak-PO and consequently of ∆60% and may not have guaranteed an exercise intensity corresponding to the severe domain for all participants. In summary, while a direct comparison between different studies may be difficult, our data provides the first, comprehensive, domain-specific characterization of the contributors to the observed VO2sc.
Traditionally, VO2sc has been attributed to an increased cost of locomotion when exercise is protracted more than 3 min at a constant workload in the heavy and severe exercise intensity domains, in relation to either fatigue or recruitment of higher-order motor units or both [9]. Interestingly, while a clear distinction between exercises performed above and below RCP is a common concept in exercise physiology [10], VO2sc measured in the heavy and in the severe exercise domains is usually considered as the expression of a single phenomenon [7, 9]. In this context, a recent paper based on mathematical modelling of the muscle bioenergetics, proposed that a metabolic shift between the aerobic and the anaerobic energy systems, caused by a progressive inhibition of the glycolytic ATP supply by cytosol acidification, may also contribute to VO2sc [15]. The authors also suggest that the size of the VO2sc can increase when the contribution of glycolysis to ATP resynthesis (and in turn proton accumulation) is higher; a lower VO2 in the initial stages of exercise rather than an increased VO2 after 3–6 min into exercise may explain the larger VO2sc at the higher exercise intensities [15].
This is the first experimental study to determine the contributors of VO2sc in the three intensity domains of exercise. Our findings (i.e. a prolonged metabolic shift in the heavy domain and a true loss of efficiency over time in the severe intensity domain only) appear to support the theory proposed by Korzeniewski & Zoladz in 2015. We speculate that, with increasing exercise intensity, the recruitment of bigger, preferentially glycolytic muscle fibres could explain a higher contribution of glycolysis to ATP resynthesis at exercise onset and therefore a delayed VO2 steady state. Furthermore, the recruitment of these intrinsically less efficient and fatigable type II fibres could explain the true loss of efficiency that appears over time in the severe domain of exercise [5, 7, 9]. The novelty and the practical implications of this, if confirmed by further studies, would be: (i) in the heavy domain, the VO2sc is the expression of a delayed adjustment of VO2 rather than of a loss of efficiency developing over time; (ii) contrary to what is currently accepted, the adjustment of VO2 in the heavy domain may be described by a slow primary component rather than by the summation of a primary plus a slow component; (iii) in the severe domain the VO2sc may be explained by both a prolonged metabolic shift and a true loss of efficiency over time; (iv) VO2sc likely has different physiological underpinnings in the heavy and severe domain of exercise; therefore, we should be mindful of this when the two intensity paradigms are used in the context of VO2sc as different interventions may produce different effects in the two domains.
In conclusion, the innovative methodological approach applied in this study allowed to discriminate three contributors to the VO2sc: increased VO2 cost of ventilation, prolonged shifting between aerobic and glycolytic metabolism, and loss of efficiency over time. How these mechanisms contribute to the VO2sc depends on relative exercise intensity, with a true loss of efficiency over time occurring only in the severe domain.
Limitations
It should be acknowledged that data interpretation in this investigation depends upon estimates of the energetic yield of lactate accumulation and of the VO2 cost of ventilation and ignores the contribution of oxygen stores and anaerobic alactic mechanism of ATP resynthesis. These estimates rely on some assumptions (e.g. fingertip capillary lactate reflects whole-body lactate accumulation) and systematic biases in applying the same values/equations to different subjects. In the belief that these limitations should not have altered the interpretation of our results; we provided a deep discussion on these issues for the interested reader as supplementary material of this article.
Electronic supplementary material
(DOCX 18 kb)
Acknowledgements
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. All authors declare that there are no financial and personal relationships with third parties or organizations that could have inappropriately influenced the present work. The results of the study are presented clearly, honestly and without fabrication, falsification or inappropriate data manipulation.
Authors contributions
ALC and SP concepted the study methodology. ALC and KC collected the data. All the authors contributed to the analysis of the results. ALC, JB and SP drafted and revised the manuscript. All the authors approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (CARU no 15/2019) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American College of Sports Medicine . ACSM’S guidelines for exercise testing and prescription, 10th ed. Wolters: Kluwer; 2017. [DOI] [PubMed] [Google Scholar]
- 2.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 3.Broxterman RM, Craig JC, Richardson RS. The respiratory compensation point and the deoxygenation break point are not valid surrogates for critical power and maximum lactate steady state. Med Sci Sports Exerc. 2018;50:2379–2382. doi: 10.1249/MSS.0000000000001699. [DOI] [PubMed] [Google Scholar]
- 4.Coast JR, Rasmussen SA, Krause KM, O’Kroy JA, Loy RA, Rhodes J. Ventilatory work and oxygen consumption during exercise and hyperventilation. J Appl Physiol. 1993;74:793–798. doi: 10.1152/jappl.1993.74.2.793. [DOI] [PubMed] [Google Scholar]
- 5.Colosio AL, Baldessari E, Basso E, Pogliaghi S (2019) Respiratory and muscular response to acute non-metabolic fatigue during ramp incremental cycling. Respir Physiol Neurobiol 270. doi: 10.1016/j.resp.2019.103281 [DOI] [PubMed]
- 6.Fontana FY, Keir DA, Bellotti C, De Roia GF, Murias JM, Pogliaghi S. Determination of respiratory point compensation in healthy adults: can non-invasive near-infrared spectroscopy help? J Sci Med Sport. 2015;18:590–595. doi: 10.1016/j.jsams.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Grassi B, Rossiter HB, Zoladz JA (2015) Skeletal muscle fatigue and decreased efficiency : two sides of the same coin? Exerc Sport Sci Rev 75–83. doi: 10.1249/JES.0000000000000043 [DOI] [PubMed]
- 8.Hill DW. Energy system contributions in middle-distance running events. J Sports Sci. 1999;17:477–483. doi: 10.1080/026404199365786. [DOI] [PubMed] [Google Scholar]
- 9.Jones AM, Grassi B, Christensen PM, Krustrup P, Bangsbo J, Poole DC. Slow component of VO2 kinetics: mechanistic bases and practical applications. Med Sci Sports Exerc. 2011;43:2046–2062. doi: 10.1249/MSS.0b013e31821fcfc1. [DOI] [PubMed] [Google Scholar]
- 10.Jones AM, Poole DC. Oxygen uptake kinetics. Compr Physiol. 2005;2:933–996. doi: 10.1002/cphy.c100072. [DOI] [PubMed] [Google Scholar]
- 11.Keir DA, Murias JM, Paterson DH, Kowalchuk JM. Breath-by-breath pulmonary O2 uptake kinetics: effect of data processing on confidence in estimating model parameters. Exp Physiol. 2014;99:1511–1522. doi: 10.1113/expphysiol.2014.080812. [DOI] [PubMed] [Google Scholar]
- 12.Keir DA, Fontana F, Robertson TC, Murias JM, Paterson DH, Kowalchuk JM, Pogliaghi S. Exercise intensity thresholds: identifying the boundaries of sustainable performance. Med Sci Sports Exerc. 2015;47:1932–1940. doi: 10.1249/MSS.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 13.Keir DA, Pogliaghi S, Murias JM. The respiratory compensation point and the deoxygenation break point are valid surrogates for critical power and maximum lactate steady state. Med Sci Sports Exerc. 2018;50:2375–2378. doi: 10.1249/MSS.0000000000001698. [DOI] [PubMed] [Google Scholar]
- 14.Keppel G. Design and analysis: a researcher’s handbook, r3rd ed. Englewood Cliffs: Prentice Hall; 1991. [Google Scholar]
- 15.Korzeniewski B, Zoladz JA. Possible mechanisms underlying slow component of VO2 on-kinetics in skeletal muscle. J Appl Physiol. 2015;118:1240–1249. doi: 10.1152/japplphysiol.00027.2015. [DOI] [PubMed] [Google Scholar]
- 16.Krustrup P, Soderlund K, Mohr M, Bangsbo J (2004) The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch - Eur J Physiol 855–866. doi: 10.1007/s00424-003-1203-z [DOI] [PubMed]
- 17.O’Connell J, Weir J, Macintosh B. Blood lactate accumulation decreases during the slow component of oxygen uptake without a decrease in muscular efficiency. Pflugers Arch - Eur J Physiol. 2017;469:1257–1265. doi: 10.1007/s00424-017-1986-y. [DOI] [PubMed] [Google Scholar]
- 18.Poole D, Jones AM (2012) Oxygen uptake kinetics. Compr Physiol. 10.1002/cphy.c100072 [DOI] [PubMed]
- 19.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise ( in ) tolerance. Am J Physiol Heart Circ Physiol. 2012;302:1050–1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, Prediletto R, Wagner PD. Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol. 1991;71:1245–1260. doi: 10.1152/jappl.1991.71.4.1245. [DOI] [PubMed] [Google Scholar]
- 21.di Prampero PE. The energy cost of human locomotion on land and in water. Int J Sports Med. 1986;7:55–72. doi: 10.1055/s-2008-1025736. [DOI] [PubMed] [Google Scholar]
- 22.di Prampero PE, Ferretti G, Enrico P, Ferretti G. The energetics of anaerobic muscle metabolism: a reappraisal of older and recent concepts. Respir Physiol. 1999;118:103–115. doi: 10.1016/S0034-5687(99)00083-3. [DOI] [PubMed] [Google Scholar]
- 23.De Roia G, Pogliaghi S, Adami A, Papadopoulou C, Capelli C. Effects of priming exercise on the speed of adjustment of muscle oxidative metabolism at the onset of moderate-intensity step transitions in older adults. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1158–R1166. doi: 10.1152/ajpregu.00269.2011. [DOI] [PubMed] [Google Scholar]
- 24.Salvadego D, Lazzer S, Busti C, Galli R, Agosti F, Lafortuna C, Sartorio A, Grassi B. Gas exchange kinetics in obese adolescents. Inferences on exercise tolerance and prescription. Am J Phys Regul Integr Comp Phys. 2010;299:1298–1305. doi: 10.1152/ajpregu.00038.2010. [DOI] [PubMed] [Google Scholar]
- 25.Santana MG, Tufik S, Passos GS, Santee DM, Denadai BS, Mello MT. Comparison between different methods of analysis of slow component of oxygen uptake; a view in severe exercise domain. Rev Bras Med Esporte. 2007;13:217–220. doi: 10.1590/S1517-86922007000400006. [DOI] [Google Scholar]
- 26.Vanhatalo A, Poole DC, DiMenna FJ, Bailey SJ, Jones AM (2011) Muscle fiber recruitment and the slow component of O 2 uptake: constant work rate vs. all-out sprint exercise. Am J Physiol Integr Comp Physiol. 10.1152/ajpregu.00761.2010 [DOI] [PubMed]
- 27.Whipp BJ, Davis JA, Wasserman K. Ventilatory control of the “isocapnic buffering” region in rapidly-incremental exercise. Respir Physiol. 1989;76:3657–3667. doi: 10.1016/0034-5687(89)90076-5. [DOI] [PubMed] [Google Scholar]
- 28.Zoladz JA, Gladden LB, Hogan MC, Nieckarz Z, Grassi B. Progressive recruitment of muscle fibers is not necessary for the slow component of V̇O2 kinetics. J Appl Physiol. 2008;105:575–580. doi: 10.1152/japplphysiol.01129.2007. [DOI] [PubMed] [Google Scholar]
- 29.Zuccarelli L, Porcelli S, Rasica L, Marzorati M, Grassi B. Comparison between slow components of HR and VO2 kinetics. Med Sci Sports Exerc. 2018;50:1649–1657. doi: 10.1249/MSS.0000000000001612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18 kb)