Abstract
Introduction
Xerosis and pruritus are common manifestations of numerous dermatologic and systemic diseases. We evaluated the effectiveness of an emollient containing an Aquaphilus dolomiae extract (ADE-G1) for the management of pruritus and xerosis in patients of all age with a range of dermatologic and systemic diseases.
Methods
This open-label, real-world study involved 5910 patients from 33 European, South American, Asian, and North and South African countries, who applied the product for 7 days twice daily to the face and body after the skin had been cleansed and dried. The physician assessed xerosis severity and patients assessed pruritus severity, the duration of itch, sleep quality, and the impact of their skin disease on their quality of life, using scales derived from the SCORing Atopic Dermatitis (SCORAD) index and questionnaires, at inclusion and after 7 days of use.
Results
The 7-day care regimen resulted in 56% and 60% reductions in xerosis and pruritus severity, respectively, regardless of the underlying pathology (p < 0.0001), with the largest decreases observed for patients with ichthyosis for xerosis and for patients post scabies treatment for pruritus. The mean sleep disturbance and mean total Dermatology Life Quality Index (DLQI) scores were also reduced by 58% and 60% (p < 0.0001), respectively. The emollient was effective whether the product was used alone or in combination with topical or systemic treatments and was well tolerated.
Conclusion
Our study shows that the 7-day regimen with the emollient was a universally effective treatment for pruritus and xerosis, regardless of the underlying pathology.
Electronic supplementary material
The online version of this article (10.1007/s13555-020-00415-6) contains supplementary material, which is available to authorized users.
Keywords: Aquaphilus dolomiae, Emollient, Pruritus, Quality of life, Real-world study, Xerosis
Key Summary Points
| Why carry out this study? |
| Xerosis and pruritus are common manifestations of numerous dermatologic and systemic diseases and are associated with significant psychosocial morbidity and quality of life impairment. |
| An emollient containing an extract of Aquaphilus dolomiae (ADE-G1) has been shown to be efficacious at reducing the severity of xerosis and pruritus in children with atopic dermatitis. |
| This study aimed to investigate the effectiveness of this emollient in a real-world setting on xerosis and pruritus severity in a large population of all ages with a range of dermatologic and systemic diseases. |
| What was learned from the study? |
| This emollient was effective at reducing the severity of pruritus and xerosis, regardless of the underlying pathology, and whether it was used as a monotherapy or in combination with topical or systemic treatments. |
| It can be particularly useful for treating persistent itch, notably the intense pruritus associated with prurigo nodularis of Hyde. |
Introduction
Xerosis is a skin condition characterized by dry, rough, cracked, and fissured skin, often accompanied by itch (pruritus) [1]. Xerosis and pruritus are frequent manifestations of a range of dermatoses including atopic dermatitis, ichthyosis, psoriasis, contact dermatitis, and scabies [2, 3]. These skin conditions are also common in patients with systemic diseases, occurring in 10–50% of patients with underlying renal insufficiency, diabetes, liver disease and cholestasis, or hematologic diseases [3–5]. The likelihood of developing xerosis and pruritus increases with age as a result of physiological factors [6]. Contributing factors also include exposure to irritants (soaps, detergents, etc.), and environmental factors such as sun exposure, low temperature, humidity, and abrupt changes in conditions associated with the use of air conditioning and heating [1, 7]. In severe cases or when left untreated, xerosis and pruritus can lead to onset of lichen simplex chronicus and xerotic eczema (or eczema craquelé), and complications, such as inflammation and infection of lesions caused by repeated scratching [1]. Xerosis and pruritus are associated with significant psychosocial morbidity and impaired quality of life (QoL), mostly characterized by stigmatization and sleep disturbance [5, 8].
Moisturizers containing emollients, occlusive agents, and humectants play a major role in the management of both xerosis and pruritus [2, 9]. The aim of this study was to evaluate the effectiveness and safety of an emollient, containing an extract of Aquaphilus dolomiae (ADE-G1), for the management of xerosis and pruritus. ADE-G1 is a purified biomanufactured extract obtained from Aquaphilus dolomiae isolated from the deep aquifer of Avène thermal spring water (ATSW) in the Cévennes mountains (France) [10, 11]. The ADE-G1-containing emollient is referred to as an emollient “plus” (“topical formulations with vehicle-type substances and additional active, non-medicated substances”) in the 2018 consensus-based European guidelines for the treatment of atopic eczema in adults and children [12] and has already been shown to be effective at reducing the severity of xerosis and pruritus in children with atopic dermatitis in a randomized controlled study [13]. However, the beneficial effect of the emollient has not been tested in other xerosis and pruritus-associated pathologies. For this reason, we aimed to investigate the effectiveness of this emollient in a real-world setting by evaluating the impact of a 7-day care regimen with the study product on xerosis and pruritus severity in infants, children, and adults with a range of dermatologic and systemic diseases. The impact of the care regimen on itch duration and sleep quality, the impact of skin manifestations on patient QoL, and the safety and tolerance of the product were also studied.
Patients and Methods
Study Design
This open-label, international, real-world study was carried out by physicians practicing in multiple private, public, or hospital centers from 33 European, South American, Asian, and North and South African countries between October 2012 and January 2018. The study involved two visits, an inclusion visit on day 0 and a follow-up visit after about 7 days of product use. These visits were part of the systematic follow-up of the patient by the physician usually responsible for their management. No constraints were associated with this study and no additional invasive or specific examinations were carried out.
Compliance with Ethics Guidelines
As this was an observational real-life phase IV study conducted in a naturalistic setting where the choice of therapy was consistent with approved prescribing information and in line with the usual everyday practice of the physician at their office. The product was prescribed by the practitioner themselves, as per their routine practice. There was no systematic assignment of treatment. No constraints were associated with this study and no additional invasive or specific examinations were carried out. The protocol did not require approval by a local ethic committee or an institutional review board as, according to Article L1121-16-2 of the same code, Article L1121-4 does not apply to non-interventional studies evaluating cosmetic products, specifically when they are based on questionnaires or interviews. This is specified in Article 1 of the order of May 3, 2017 (https://www.legifrance.gouv.fr/eli/arrete/2017/5/3/AFSP1713710A/jo/article_1).
Each patient received an information leaflet translated into their native language and including a description of their rights with regard to the processing of their personal data, in accordance with Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons or with the local regulations in non-European countries. All patients, or their parent or guardian, provided signed consent before being enrolled in the study.
Participants
Physicians were invited by the sponsor to participate in the study and those agreeing to participate were asked to recruit at least five patients: adults or children (aged 3 months or above) with pruritus and xerosis that had persisted for a least 2 weeks before the consultation and which was associated with a skin condition, systemic disease, or persisted after successful scabies treatment. All patients enrolled in the study required prescription of an emollient to relieve the symptoms of their pruritic xerosis and had been prescribed the study product: an emollient in the form of either a cream or a balm (XeraCalm A.D, Eau Thermale Avène, France). Both the cream and the balm contained ADE-G1 as the active non-medicated substance, and both forms of the study product also contained ATSW and Cer-Omega. Variations in excipients resulted in differences in consistency between the two forms of the study product: the balm had an oilier consistency and was recommended for patients with very dry skin, whereas the cream was less oily and recommended for dry skin.
Non-prescription criteria were the presence of weeping lesions, cutaneous infections, and intolerance to any of the components of the test products. Patients receiving phototherapy and those who had applied a topical treatment (corticosteroid, calcineurin inhibitor, anesthetic, capsaicin, or antihistamine) within the 48 h preceding the consultation or taken a systemic treatment (corticosteroids, methotrexate, azathioprine, cyclosporine A, mycophenolate, or other immunosuppressant within 4 weeks of the consultation; doxepin within 1 week of the consultation; or antihistamines within 48 h of the consultation) were not eligible for prescription of the study product.
Study Procedure
At inclusion, physicians collected patient demographic and clinical data, including details of the associated skin or systemic disease and any concomitant topical or systemic treatments being used during the study period. They then prescribed the study product according to their usual practice in case of pruritus and xerosis, without any specific instructions or recommendations from the sponsor, with only patients fulfilling all other study criteria and for whom the study product was prescribed being eligible for the study. The physician instructed the patients to apply the product for 7 days according to the instructions for use, i.e., twice daily (morning and evening) to the face and body after the skin had been cleansed and dried. Participants were free to use any of their other usual toiletries (shampoos, soaps, cleansers, etc.) during the study period. The physician assessed xerosis severity at the inclusion and follow-up visits and evaluated the tolerance of the product and recorded any new topical or systemic treatments initiated during the study at the follow-up visit. Patients assessed pruritus severity, the duration of periods of itch, their sleep quality, and the impact of skin symptoms on their QoL at inclusion and at the follow-up visit.
Outcomes
The primary outcome was evaluation of the effectiveness of the study product on reducing xerosis and pruritus severity after 7 days of use in a real-life setting. Secondary outcomes were the effectiveness of the 7-day care regimen on reducing the duration of itch episodes and levels of sleep disturbance, and on lessening the impact of the skin manifestations on patient QoL. Both primary and secondary evaluation criteria were assessed at inclusion and at the end of the study period. Tolerance and compliance to the treatment regimen were also assessed at the end of the study period.
Assessment Methods
Xerosis and pruritus severity were assessed using visual analogue scales (VAS), ranging from 0 (absence), > 0 to < 4 (mild), ≥ 4 to < 7 (moderate), ≥ 7 to < 9 (severe), to ≥ 9 (very severe) [14], derived from the SCORing Atopic Dermatitis (SCORAD) index [15]. Itch duration was assessed using a questionnaire asking patients to record the number of days in the past week that itch occurred (0–7 days).
Levels of sleep disturbance were assessed using a VAS derived from the SCORAD index, ranging from 0 (no sleep disturbance) to 10 (very severe sleep disturbance).
The impact of skin manifestations on patient QoL was evaluated using the Dermatology Life Quality Index (DLQI) patient questionnaires adapted according to the age of the subject (a cartoon-based questionnaire for 0–7-year-old patients [16], an age-adapted written questionnaire for 7–17-year-old patients [17], and an adult questionnaire for patients aged > 17 years [18]. DLQI index scores were interpreted as described by Hongbo et al. [19].
Tolerance was assessed using a scale from 0 to 3 based on the following criteria: very poor—major functional and clinical signs of discomfort necessitating withdrawal of the treatment; poor—moderate or persistent functional or clinical signs of discomfort that did not necessitate interruption of the treatment; good—minimal or transient functional signs of discomfort that did not lead to interruption of treatment and no objective signs on clinical examination; and very good—no functional or clinical signs of discomfort. Details of any adverse events occurring during the course of the study were also recorded.
Patient use of the study product was evaluated at follow-up by asking patients to report the number of days and number of times per day they had applied the product during the study period.
Statistical Analyses
Descriptive data are expressed as the number and percentage, mean and standard deviation (SD), or median and range for each variable as appropriate. Differences in the changes from baseline of xerosis and pruritus severity, sleep disturbance, and DLQI scores were analyzed using an analysis of variance (ANCOVA) model. The ANCOVA was also used for comparisons of treatment groups: cream versus balm. Duration of itch was analyzed using the log-rank text and by Kaplan–Meier analysis. All statistical analyses were carried out using SAS®, version 9.4.
The same statistical methods were used to perform ad hoc analyses of the change in xerosis and pruritus severity, duration of itch, sleep disturbance, and DLQI scores over the study period in two subpopulations of the study cohort: patients prescribed no treatment other than the study product at inclusion and who were prescribed no new treatment during the study (study product only subgroup) and the remaining patients who were prescribed a topical or concomitant treatment at inclusion or during the study, or were not reported as being prescribed the study product only (concomitant treatment subgroup).
Results
Participant Flow
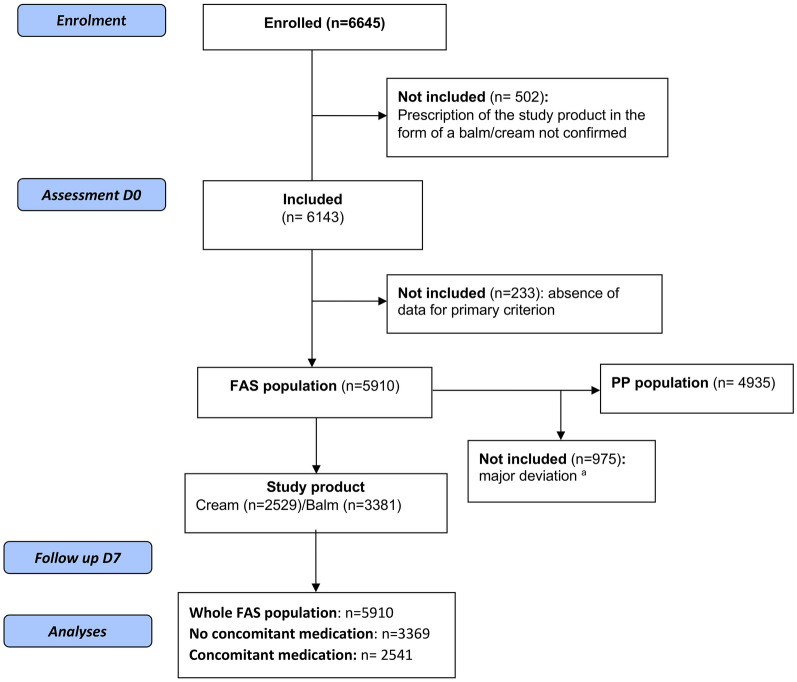
Participant flow through the study is illustrated in Fig. 1. In the FAS population (n = 5910), 2529 patients (42.7%) received the study product in the form of a cream and 3381 patients (57.2%) in the form of a balm. Unless otherwise stated, no significant differences in the effectiveness of the study product were observed between patients prescribed the cream and those prescribed the balm. Major deviations from the protocol were observed in 975 patients, resulting in a per protocol (PP) population consisting of 4935 patients. As no significant differences in outcomes were observed between the FAS population and the PP population, only data from analysis of the FAS population are presented.
Fig. 1.
Participant flow through the study. FAS full analysis set, PP per protocol. aFor the 975 patients who were excluded from the PP population, time to follow-up > 15 days (n = 214), absence of xerosis (n = 138), age missing or < 3 months (n = 109), and reason not specified (n = 449) were most common major deviations
Baseline Patient Demographics and Clinical Characteristics
Patient demographics and clinical characteristics at baseline are listed in Table 1. The median durations of xerosis and pruritus were 12 months and 3 months, respectively. Xerosis and pruritus were associated with a skin disease in 91.8% of the population, most commonly atopic dermatitis (65.2% of patients). A systemic disease was present in 12.1% of the FAS population, most commonly diabetes mellitus (3.9%) or thyroid disease (3.4%). The majority of patients (78.6%) were not receiving any other treatment for their underlying disorder at inclusion.
Table 1.
Demographic and clinical characteristics of the study population at baseline
| FAS N = 5910 |
|
|---|---|
| Demographics | |
| Gender, n (%) | n = 5718 |
| Female | 3507 (61.3) |
| Male | 2211 (38.7) |
| Age (years) | n = 5839 |
| Mean (SD) | 30.6 (25.8) |
| Median (min–max) | 25 (0–97) |
| Environment, n (%) | n = 5762 |
| Urban | 4812 (83.5) |
| Rural | 949 (16.5) |
| Both | 1 (0) |
| Clinical characteristics | |
| Duration of xerosis (months) | n = 4936 |
| Mean (SD) | 47.6 (89.0) |
| Median (Q1–Q3) | 12.0 (0–888) |
| Duration of pruritus | n = 4908 |
| Mean SD | 28.9 (68.8) |
| Median (min–max) | 3 (0–888) |
| Underlying skin diseases, n (%)a | n = 5862 |
| Skin disease, yes | 5384 (91.8) |
| Atopic dermatitis | 3511 (65.2) |
| Senile xerosis | 679 (12.6) |
| Prurigob | 363 (6.7) |
| Undetermined etiology | 250 (4.6) |
| Ichthyosis | 233 (4.3) |
| Psoriasis | 203 (3.8) |
| Scabies (pruritus after antiscabies treatment) | 156 (2.9) |
| Prurigo nodularis of Hyde | 38 (0.7) |
| Other pathology | 636 (11.8) |
| Underlying systemic diseases, n (%)a | n = 5043 |
| Systemic disease, yes | 610 (12.1) |
| Diabetes mellitus | 199 (3.9) |
| Thyroid disease | 170 (3.4) |
| Renal insufficiency | 64 (1.3) |
| Hepatic disease | 27 (0.5) |
| Hematologic disease | 25 (0.5) |
| Other disease | 273 (5.4) |
| Concomitant treatments, n (%)a | n = 5606 |
| Ongoing treatment prescription, yes | 1198 (21.4) |
| Topical treatments | 1016 (18.1) |
| Systemic treatments | 787 (13.3) |
FAS full analysis set, N number of patients in the whole study population, n number of patients for which data was collected, SD standard deviation, Q1–Q3 interquartile range
aPatients may have had more than one skin disease or systemic disease or may have been prescribed more than one concomitant treatment
bPatients with prurigo had lesions that did not show the specific characteristics of prurigo nodularis of Hyde, and had no diagnosis of any other underlying skin or systemic disease known to be associated with these lesions
The severity of the dermatologic symptoms and their impact on patient QoL and sleep quality as assessed at inclusion are summarized in Table 2. The severity of the xerosis and pruritus in the FAS population was moderate, with mean VAS scores of 5.7 (± 2.1) and 5.8 (± 2.4). During the week prior to inclusion, 63.4% of patients (n = 3718/5863) reported experiencing itch on each of the 7 days. Sleep disturbance was rated as mild to moderate with a mean VAS score of 3.3 (± 2.8). Total DLQI scores indicated that the dermatological symptoms had a moderate effect on patient QoL, with an average score of 9.7 (± 6.4).
Table 2.
Severity of dermatologic symptoms and their effect on patient quality of life and sleep quality at baseline
| FAS (N = 5910) |
|
|---|---|
| Dermatologic symptoms | |
| Xerosis severity (VAS scores) | |
| Mean (SD) | 5.7 (2.1) |
| Median (min–max) | 6 (0–10) |
| Pruritus severity (VAS scores) | |
| Mean (SD) | 5.8 (2.4) |
| Median (min–max) | 6 (0–10) |
| Itch | n = 5863 |
| Number of days on which itch occurred | n (%) |
| ≤ 6 | 2145 (36.6) |
| 7 | 3718 (63.4) |
| Sleep disturbance | n = 5856 |
| VAS scores | |
| Mean (SD) | 3.3 (2.8) |
| Median (min–max) | 3 (0–10) |
| Quality of life | n = 5179 |
| Total DLQI scores | |
| Mean (SD) | 9.7 (6.4) |
| Median (min–max) | 8 (0–30) |
FAS full analysis set, N number of patients in the whole study population, n number of patients for which data was collected, SD standard deviation, Q1–Q3 interquartile range, VAS visual analogue scale, DLQI Dermatology Life Quality Index
Effect of Study Product on Xerosis and Pruritus Severity
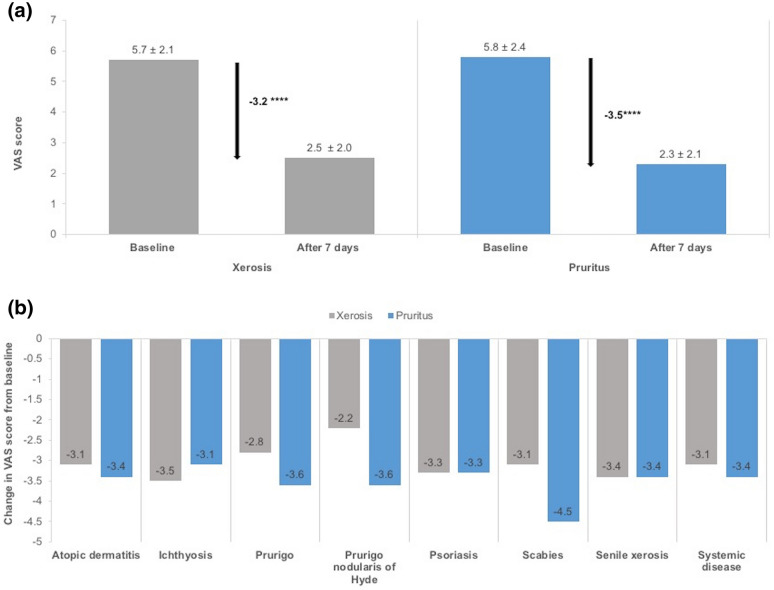
The 7-day care regimen with the study product led to significant decreases in VAS scores for xerosis and pruritus severity, with reductions of 56% (− 3.2; n = 5752) for xerosis and of 60% (− 3.5; n = 5766) for pruritus (p < 0.0001 for both; Fig. 2a). These significant decreases were observed regardless of the underlying treatment indication (p < 0.0001; Fig. 2b), with the largest decrease in VAS score for xerosis being observed for patients with ichthyosis (− 3.5) and that for pruritus being observed for patients post scabies treatment (− 4.5).
Fig. 2.
Effectiveness of the 7-day care regimen on xerosis and pruritus severity in the FAS population. a Mean visual analogue scale (VAS) scores (for xerosis and pruritus severity at baseline and after 7 days of treatment) and the mean change in VAS scores. Data presented are the mean ± standard deviation. ****p < 0.0001 (ANCOVA model) for the change in VAS score. b Mean change in VAS scores for xerosis and pruritus severity according to indication. p < 0.0001 according to the ANCOVA model for the change in VAS score for all treatment indications
Secondary Effectiveness Outcomes
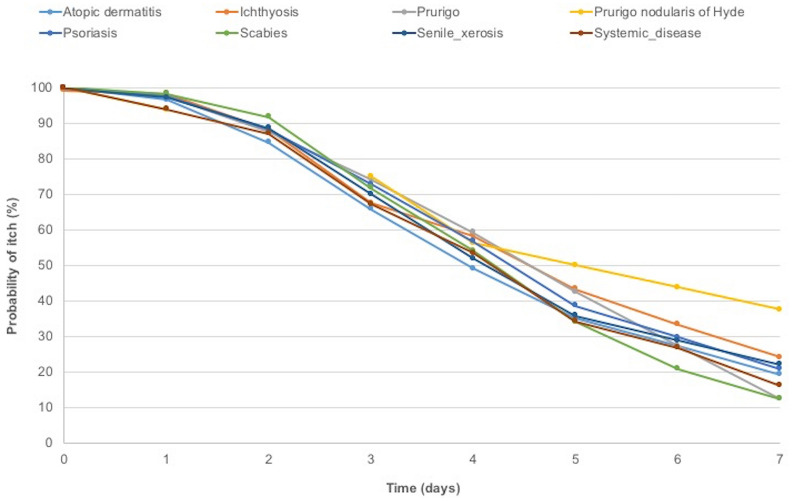
Kaplan–Meier estimates indicated that relief from itch occurred in over 25% of the patients by the third day of the care regimen, in over 50% by the fourth day, and in over 75% by the seventh day. The Kaplan–Meier curves by indication are shown in Fig. 3. When patients were grouped by indication, the mean duration of itch during treatment varied from 4.6 days for patients with a systemic disease to 5.9 days for those with atopic dermatosis, with median durations ranging from 4 days for patients with atopic dermatitis to 5.5 days for patients with prurigo nodularis of Hyde.
Fig. 3.
Duration of itch over the 7-day study period. Kaplan–Meier survival plot of itching duration according to indication
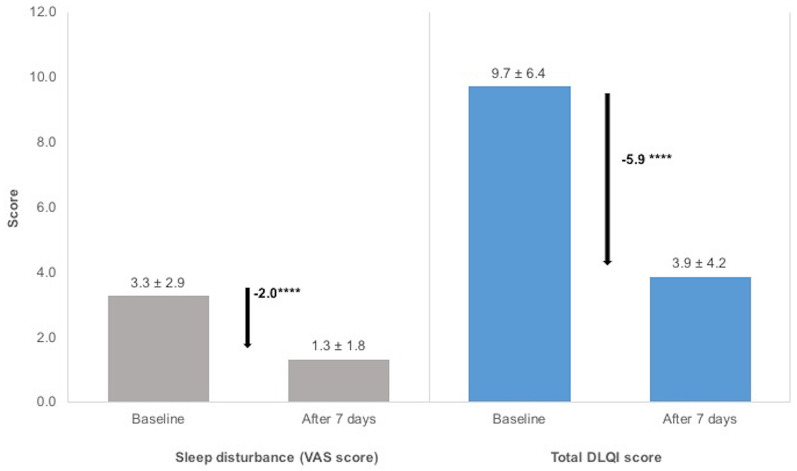
The VAS scores for sleep disturbance and DLQI scores also showed significant decreases over the study period (p < 0.0001 for both variables; Fig. 4). The mean sleep disturbance VAS score after 7 days of using the study product was 1.3 (± 1.8; n = 5600), a reduction of 58% (− 2.0 ± 2.33; n = 5566). A decrease of 60% (− 5.9 ± 5.2; n = 4838) was observed for the mean total DLQI score during the study period, leading to a mean total DLQI score of 3.9 (± 4.2; n = 5001) after 7 days.
Fig. 4.
Effectiveness of the 7-day care regimen on sleep quality and on the impact of dermatological symptoms on quality of life. Sleep disturbance was measured using a visual analogue scale (VAS) and the impact of skin symptoms on quality of life was assessed using Dermatology Life Quality Index (DLQI) questionnaires. Data presented are the mean ± standard deviation. ****p < 0.0001 (ANCOVA model) for the change in VAS and DLQI scores. aA small but significantly greater improvement in total DLQI scores was observed for patients prescribed the study product in the form of a cream (− 6.0) than for those prescribed the balm (− 5.8; p = 0.0169)
Tolerance and Safety
The majority of patients followed the skin care regimen recommended by their physician, with 86% of patients (n = 4235/4948) reporting that they applied the study product as directed for the duration of the study period, 4% patients (n = 13) reporting that they applied the study product more often than recommended, and around 10% (n = 522) reporting that they applied the study product less often than recommended. Tolerance was rated by physicians as good or very good in 97.2% (4673/5732) of cases (good in 15.9% of cases, n = 914, and very good in 81.3% of cases, n = 4659). The incidence of adverse events was 3.9% (n = 228/5910), with the majority of these events classed as being of mild (50.0%; n = 103/206) or moderate (40.8%; n = 84/206) severity. The most commonly reported adverse events were related to discomfort shortly after application of the cream, including skin irritation, itch, or burning sensations.
Concomitant Treatments During Study Period
A summary of the concomitant treatments used during the 7-day study period is shown in Table S1 (see electronic supplementary material). A new form of medication was prescribed during the study period for 550 patients. Topical corticosteroids were the most commonly prescribed additional treatment and were prescribed for 23.1% of the patients. Systemic antihistamines were the most frequently prescribed systemic treatment (9.4% of patients).
Subgroup Analyses: Effect of Concomitant Treatments on Study Product Effectiveness
A total of 3369 patients were included in the study product only subgroup and 2541 patients were included in the concomitant treatment subgroup. The demographic, clinical, and dermatological characteristics of the subpopulations at baseline are presented in Tables S2 and S3 (see electronic supplementary material). A total of 1497 patients (44.4%) in the study product only subpopulation and 1032 patients (40.6%) in the concomitant treatment subpopulation received the study product in the form of a cream, and 1872 (55.6%) and 1509 patients (59.4%) in each of the respective subpopulations received the product in the form of a balm.
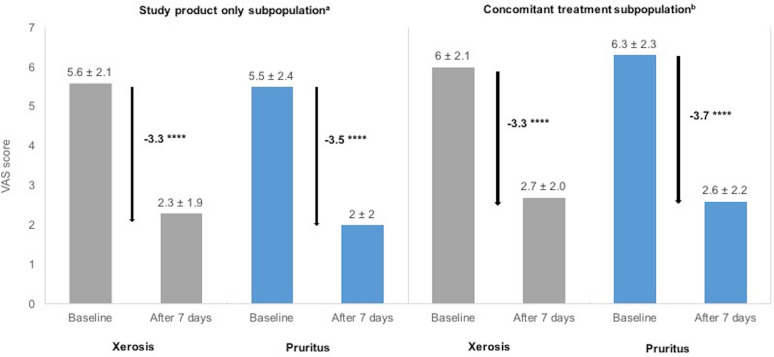
Significant decreases in VAS scores of 59% (− 3.3) for xerosis severity and of 62% (− 3.5) for pruritus severity were observed in the study product only subpopulation at the end of the study period (p < 0.0001 for both; Fig. 5). In the concomitant treatment subpopulation, significant decreases in VAS scores of 55% (− 3.3) and 58% (− 3.7) were observed for xerosis and pruritus severity, respectively (p < 0.0001 for both; Fig. 5). Significant reductions in xerosis and pruritus severity were observed in both subpopulations regardless of the underlying treatment indication (p < 0.0026 for prurigo nodularis of Hyde in the study product only subpopulation and p < 0.0001 for all other indications in both subpopulations; data not shown).
Fig. 5.
Effectiveness of the 7-day care regimen on xerosis and pruritus severity in the subpopulations. a Mean visual analogue scale (VAS) scores for xerosis and pruritus severity at baseline and after 7 days of treatment, and the mean change in VAS scores. Data presented are the mean ± standard deviation. ****p < 0.0001 (ANCOVA model) for the change in VAS score
Kaplan–Meier estimates indicated that 75% of the patients in each of the subpopulations experienced relief from itch by the seventh day of the care regimen.
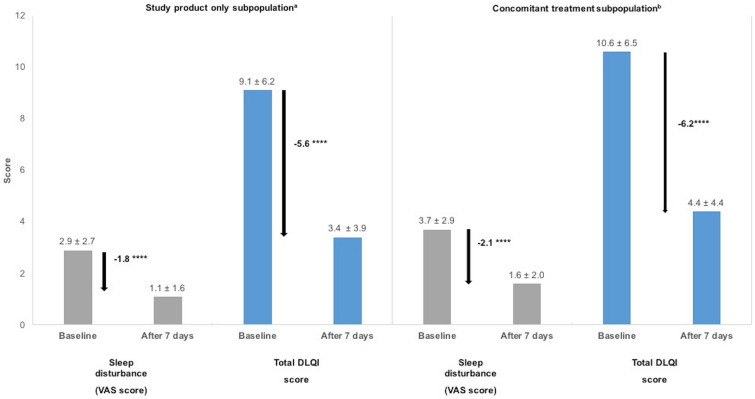
Significant decreases in sleep disturbance VAS and total DLQI scores were observed by the end of the care regimen in the study product only population, with reductions of 55% (− 1.8 ± 2.2; n = 3150) for sleep disturbance VAS scores and 62% (− 5.6 ± 4.9; n = 2735) for total DLQI scores (p < 0.0001 for both variables; Fig. 6). In the concomitant treatment population, significant decreases of 57% (− 2.1 ± 2.5; n = 2416) and 58% (− 6.2 ± 5.4; n = 2103) were observed for sleep disturbance VAS scores and total DLQI scores after the seven-day care regimen (Fig. 6; p < 0.0001 for both variables).
Fig. 6.
Effectiveness of the 7-day care regimen on sleep quality and on the impact of dermatological symptoms on quality of life in the subpopulations. Sleep disturbance was measured using a visual analogue scale (VAS) and the impact of skin symptoms on quality life was assessed using Dermatology Life Quality Index (DLQI) questionnaires. Data presented are the mean ± standard deviation. ****p < 0.0001 (ANCOVA model) for the change in VAS and DLQI scores. aSlightly greater improvements in both scores were observed for patients in the study product only subpopulation who used the study product in the form of a cream compared to those using the balm (sleep disturbance: − 1.9 cream vs − 1.8 balm, p = 0.02; DLQI: − 6 cream vs − 5.3 balm, p = 0.03). bSlightly greater improvements in both scores were observed for patients in the concomitant subpopulation who used the study product in the form of a balm compared to those using the cream (sleep disturbance: − 2.3 balm vs − 1.9 cream, p = 0.07; DLQI: − 6.3 balm vs − 6 cream, p = 0.18)
Discussion
This international, multicenter, real-world study, conducted on a large cohort of nearly 6000 patients with persistent pruritus and xerosis associated with a skin condition, systemic disease, or with persistent itch following successful treatment for scabies infestation, demonstrated that a 7-day regimen using the study product led to significant decreases in the severity of both xerosis and pruritus when used in real-life conditions, regardless of the underlying pathology. The study product was equally effective when used as a monotherapy and when used in combination with topical or systemic treatments. Use of the study product also appeared to reduce the duration of itch, reduced sleep disturbance, and lessened the impact of the skin symptoms on patient QoL. The product was well tolerated, and the vast majority of patients reported applying the product as directed by the physician.
Dry skin and associated pruritus result from impaired stratum corneum barrier function and disruption of epidermal homeostasis. Superficial dehydration of the stratum corneum induces a dry skin cycle that propagates xerosis by reducing the synthesis of the skin barrier lipids, ceramides, and disrupting the lipid bilayer [7]. Inflammatory responses and further damage to the stratum corneum then lead to dysfunctional keratinocyte proliferation and differentiation, and impaired epidermal desquamation [7]. Leaking of natural moisturizing factors (NMF) from the stratum corneum further enhances transepidermal water loss [7]. Understanding of the pathophysiology of pruritus has also recently progressed, with the identification of two signaling pathways involved in the transmission of itch: a histamine-dependent pathway and a histamine-independent pathway that appears to play the major role in chronic pruritus (reviewed by Song et al. [20]). Pruritic skin produces elevated levels of pruritogenic mediators—including tryptase, thromboxane A2, tumor necrosis factor-alpha (TNFα), leukotrienes, substance P, endothelin-1 (ET-1), interleukins (ILs), and nerve growth factor (NGF)—produced by dermal mast cells, keratinocytes, lymphocytes, and endothelial cells [20]. These pruritogens stimulate protease-activated receptor 2 (PAR2) and other receptors mediating histamine-independent itch, such as the Mas-related G protein-coupled receptors (MRGPRs), leading to activation of transient receptor potential cation channel subfamily V member 1 (TRPV1) and TRPA1, and transmission of the itch signal to the central nervous system by mechanically sensitive C-type fibers [20].
These advances in our understanding of the pathophysiology of xerosis and pruritus have led to the development of new emollients with specific activities targeting factors involved in the development of dry and itchy skin. The product investigated in our study was an emollient “plus” containing ADE-G1 as the active non-medicated substance. In vitro studies have shown that ADE-G1 displays several properties that are likely beneficial for the treatment of pruritus and xerosis, including immunomodulatory and anti-inflammatory activities that alter the expression of a range of inflammatory mediators [11]. Importantly, ADE-G1 also appears to have antipruritic activities, with in vitro studies showing that the extract inhibits PAR2 activation and T helper type (Th) 1, Th2, and Th17 cytokine production [11]. A prospective, placebo-controlled study using the cowhage–itch model confirmed the antipruritic properties of the ADE-G1-containing emollient and moreover demonstrated that the extract specifically inhibited the nonhistaminergic itch pathway [21]. Finally, the study product has already been shown to lead to significant decreases in xerosis severity (percentage of patients with mild xerosis increasing from 50% at the start of the study to 96.4% after 28 days of treatment) and pruritus (74.6% reduction in severity scores) in children with mild atopic dermatosis [13].
In this study, we demonstrated the effectiveness in a real-world setting of a 7-day regimen with the study product at reducing the severity of xerosis and pruritus by more than 50%. Moisturizers containing emollients, occlusive agents, and humectants are the mainstay of treatment for xerosis and pruritus. They may reduce the need for pharmacologic interventions and have a steroid-sparing effect in patients with some diseases, such as atopic dermatitis [22, 23]. Studies on a number of emollients, all generally containing similar active agents—e.g., petrolatum, paraffin, glycerin, and plant-derived products and oils—have been published in recent years demonstrating their effectiveness on xerosis and pruritus associated with a range of dermatoses, including atopic dermatitis [24–26], xerosis associated with chronic pruritus in elderly patients [27], mild-to-moderate plaque psoriasis [28], and foot xerosis [29]. Finally, in the first study of its kind to evaluate the effects of emollients on xerosis and pruritus in an African population, an 8-week regimen with a glycerol-based emollient was shown to lead to 80% reductions in xerosis severity in adults and children, and to 40% and 80% reductions in pruritus severity in adults and children, respectively [30]. However, around 41% of the patients in the study by Boralevi et al. [30] were also prescribed another topical treatment (most commonly a topical steroid) at inclusion. Comparison of the findings of these previous reports with those of the current study show that our study product was effective at reducing the severity of both xerosis and pruritus in patients with a range of dermatological diseases. It should also be noted that the majority of the previous studies assessed the effectiveness of emollients after several weeks of application, whereas the significant improvements in symptoms reported in our study were achieved after only a short 1-week period.
The largest improvement in VAS score for pruritus in our study was observed in patients that had persistent itch after successful scabies treatment. The prevalence of scabies worldwide is estimated to range from 0.2% to 71.4% of the population, with the highest disease burden being reported in tropical regions [31]. However, several reports indicate that there has been a sharp rise in the incidence of scabies over the past decade in several European countries, including Germany [32], Croatia [33], and Norway [34]. Itch may persist for 2–4 weeks after the end of successful scabies treatment and current European guidelines recommend repeated application of emollients to manage these symptoms [35]. Our results suggest that the study product may be useful for managing these short-term symptoms. However, as itch after successful scabies treatment may resolve naturally without treatment, these promising findings need to be evaluated further within the framework of a randomized controlled trial. It is also noteworthy that the emollient was also very effective against pruritic xerosis associated with prurigo nodularis of Hyde, a chronic skin condition characterized by intense pruritus. Prurigo nodularis of Hyde is very difficult to treat and, although emollients are recommended as base therapy to prevent xerosis, effective management usually involves the use of topical or interlesional steroids and systemic immunosuppressive drugs [36]. To our knowledge, no previous clinical studies—using a robust study design and validated assessment tools—have been published demonstrating the effectiveness of other emollients for the treatment of persistent itch in patients post scabies treatment and for the intense pruritus associated with prurigo nodularis of Hyde.
In addition to its effectiveness for improving pruritus and xerosis, our study also showed that the product was effective at reducing sleep disturbance and itch duration, and lessened the impact of the skin manifestations on patient QoL. Xerosis and pruritus are known to have a substantial impact on QoL [8], and to be associated with sleep deficiency in both adults and children [37, 38]. After 7 days of using the study product, the patients in our study reported significant improvements in sleep quality and DLQI scores, with skin symptoms having only a mild impact on the life of patients. This improvement in DLQI scores is of particular interest given that the results of previous studies did not always show improvement in clinical symptoms correlating to significant improvements in QoL scores [39, 40]. However, improvements in QoL after emollient use have been demonstrated in some studies, including that by Boralevi et al. [30] in adult African patients.
Emollients and creams are often used alongside systemic and topical pharmacologic treatments as adjunct therapies or maintenance interventions to prevent flare-ups and relapses [22, 24]. The majority of the patients included in our study were prescribed the study product without any concurrent medication and did not initiate a pharmacologic treatment during the course of the study (n = 3369, 57.0% of patients). However, use of a pharmacologic treatment could not be excluded or was reported at inclusion for 1991 patients (33.7%), and 550 patients were known to have started a new treatment during the study period (9.3%). Our post hoc analyses of the subpopulation of patients who used the study product alone clearly demonstrated that use of the study product as a monotherapy led to the significant improvements in xerosis and pruritus severity, together with the reduction in itch duration and the improvements in sleep quality and DLQI scores. On the other hand, large improvements in clinical symptoms were also observed in the subpopulation using the study product in combination with a pharmacologic therapy. Although further comparative trials would be required to confirm these findings, our results strongly indicate that the study product was also beneficial to patients when used as an adjunct treatment.
One of the strengths of our study was that its noninterventional nature allowed the effectiveness of the study product to be evaluated in a large cohort of patients in a real-life setting. Owing to the size of the cohort and the multicenter nature of the study—involving patients from European, South American, Asian, and North and South African populations—the level of generalizability of our findings can be considered to be very high. One of the major limitations of the real-world study was that its design did not allow us to demonstrate the effectiveness of our study product relative to that of a placebo; however, the before-and-after analysis of the population, combined with our subpopulation analyses of patients receiving the study product alone or in combination with additional treatments, clearly allowed us to demonstrate the effectiveness of our study product in patients with a wide range of dermatologic and systemic diseases.
Another limitation of our study was the short duration of the evaluation period, which did not allow us to evaluate whether the beneficial effects of the product were sustained after extended use. However, our findings clearly demonstrate the beneficial effects of using the study product after only 1 week. A recent study analyzing the kinetics of the response to regular emollient use in children with mild-to-moderate atopic dermatitis also found that the effects of emollients at reducing xerosis and improving SCORAD were already visible after 1 week, and that the improvements were progressive with regular emollient use over a 12-week period [41]. Further studies are required to determine if regular use of our study product over a longer period would result in further improvements in symptoms and if the symptom relief can be sustained. Finally, although all of the clinical assessment tools used in this study are widely used and validated instruments for assessing dermatological symptoms, with the exception of xerosis severity which was assessed by physicians, all other assessments, including the assessment of product use, were evaluated through questionnaires filled in by the patients themselves, introducing the potential for self-reporting bias.
Conclusion
Despite its limitations, our international, multicenter, real-world study clearly demonstrated the effectiveness of a 7-day care regimen with the study product on the most common dermatological symptoms associated with a range of skin and systemic diseases in a real-life setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Funding
The study, statistical analyses, and Rapid Service Fee were funded by Pierre Fabre Dermo-Cosmétique.
Medical Writing and/or Editorial Assistance
Medical writing services were funded by Pierre Fabre Dermo-Cosmétique and provided by Drs Emma Pilling and Marielle Romet (Santé Active Edition).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Mette Deleuran has received honoraria as a speaker and investigator for Pierre Fabre Dermo-Cosmétique. Catherine Jean-Decoster and Victor Georgescu are employees of Pierre Fabre Dermo-Cosmétique, France.
Compliance with Ethics Guidelines
As this was an observational real-life phase IV study, it was conducted in a naturalistic setting where the choice of therapy was consistent with approved prescribing information and in line with the usual everyday practice of the physician at their office. The product was prescribed by the practitioner themselves, as per their routine practice, there was no systematic assignment of treatment. No constraints were associated with this study and no additional invasive or specific examinations were carried out. The protocol did not require approval by a local ethic committee or an institutional review board as, according to Article L1121-16-2 of the same code, Article L1121-4 does not apply to non-interventional studies evaluating cosmetic products, specifically when they are based on questionnaires or interviews. This is specified in Article 1 of the order of May 3, 2017 (https://www.legifrance.gouv.fr/eli/arrete/2017/5/3/AFSP1713710A/jo/article_1). Each patient received an information leaflet translated into their native language and including a description of their rights with regard to the processing of their personal data, in accordance with Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons or with the local regulations in non-European countries. All patients, or their parent or guardian, provided a signed informed consent before being enrolled in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12515222.
References
- 1.Norman RA. Xerosis and pruritus in the elderly: recognition and management. Dermatol Ther. 2003;16:254–259. doi: 10.1046/j.1529-8019.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- 2.Barco D, Gimenez-Arnau A. Xerosis: a dysfunction of the epidermal barrier. Actas Dermosifiliogr. 2008;99:671–682. doi: 10.1016/S0001-7310(08)76171-4. [DOI] [PubMed] [Google Scholar]
- 3.Moses S. Pruritus. Am Fam Phys. 2003;68:1135–1142. [PubMed] [Google Scholar]
- 4.Tarikci N, Kocaturk E, Gungor S, Topal IO, Can PU, Singer R. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. Sci World J. 2015;2015:803752. doi: 10.1155/2015/803752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millington GWM, Collins A, Lovell CR, et al. British Association of Dermatologists’ guidelines for the investigation and management of generalized pruritus in adults without an underlying dermatosis, 2018. Br J Dermatol. 2018;178:34–60. doi: 10.1111/bjd.16117. [DOI] [PubMed] [Google Scholar]
- 6.Paul C, Maumus-Robert S, Mazereeuw-Hautier J, Guyen CN, Saudez X, Schmitt AM. Prevalence and risk factors for xerosis in the elderly: a cross-sectional epidemiological study in primary care. Dermatology. 2011;223:260–265. doi: 10.1159/000334631. [DOI] [PubMed] [Google Scholar]
- 7.Rawlings AV, Matts PJ. Stratum corneum moisturization at the molecular level: an update in relation to the dry skin cycle. J Investig Dermatol. 2005;124:1099–1110. doi: 10.1111/j.1523-1747.2005.23726.x. [DOI] [PubMed] [Google Scholar]
- 8.Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153–1156. doi: 10.1001/archdermatol.2011.178. [DOI] [PubMed] [Google Scholar]
- 9.Elmariah SB, Lerner EA. Topical therapies for pruritus. Semin Cutan Med Surg. 2011;30:118–126. doi: 10.1016/j.sder.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourrain M, Villette C, Nguyen T, Lebaron P. Aquaphilus dolomiae gen. nov., sp. nov., isolated from a deep aquifer. Life Environ. 2012;62:191–195. [Google Scholar]
- 11.Aries MF, Hernandez-Pigeon H, Vaissiere C, et al. Anti-inflammatory and immunomodulatory effects of Aquaphilus dolomiae extract on in vitro models. Clin Cosmet Investig Dermatol. 2016;9:421–434. doi: 10.2147/CCID.S113180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657–682. doi: 10.1111/jdv.14891. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi P, Theunis J, Casas C, et al. Effects of a new emollient-based treatment on skin microflora balance and barrier function in children with mild atopic dermatitis. Pediatr Dermatol. 2016;33:165–171. doi: 10.1111/pde.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reich A, Heisig M, Phan NQ, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol. 2012;92:497–501. doi: 10.2340/00015555-1265. [DOI] [PubMed] [Google Scholar]
- 15.European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. [DOI] [PubMed]
- 16.Holme SA, Man I, Sharpe JL, Dykes PJ, Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285–290. doi: 10.1046/j.1365-2133.2003.05157.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942–949. doi: 10.1111/j.1365-2133.1995.tb16953.x. [DOI] [PubMed] [Google Scholar]
- 18.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 19.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Investig Dermatol. 2005;125:659–664. doi: 10.1111/j.0022-202X.2005.23621.x. [DOI] [PubMed] [Google Scholar]
- 20.Song J, Xian D, Yang L, Xiong X, Lai R, Zhong J. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936. [DOI] [PMC free article] [PubMed]
- 21.Fostini AC, Georgescu V, Decoster CJ, Girolomoni G. A cream based on Aquaphilus dolomiae extracts alleviates non-histaminergic pruritus in humans. Eur J Dermatol. 2017;27:317–318. doi: 10.1684/ejd.2017.2994. [DOI] [PubMed] [Google Scholar]
- 22.Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116–132. doi: 10.1016/j.jaad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimalt R, Mengeaud V, Cambazard F, Study Investigators’ Group The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology. 2007;214:61–67. doi: 10.1159/000096915. [DOI] [PubMed] [Google Scholar]
- 24.Hon KL, Kung JSC, Ng WGG, Leung TF. Emollient treatment of atopic dermatitis: latest evidence and clinical considerations. Drugs Context. 2018;7:212530. doi: 10.7573/dic.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiplica GS, Kaszuba A, Malinauskiene L, et al. Prevention of flares in children with atopic dermatitis with regular use of an emollient containing glycerol and paraffin: a randomized controlled study. Pediatr Dermatol. 2017;34:282–289. doi: 10.1111/pde.13113. [DOI] [PubMed] [Google Scholar]
- 26.Koppes SA, Charles F, Lammers LA, Frings-Dresen M, Kezic S, Ruste-Meyer T. Efficacy of a cream containing ceramides and magnesium in the treatment of mild to moderate atopic dermatitis: a randomized, double-blind, emollient-and hydrocortisone-controlled trial. Acta Derm Venereol. 2016;96:948–953. doi: 10.2340/00015555-2395. [DOI] [PubMed] [Google Scholar]
- 27.Theunis J, Chaussade H, Bourgeois O, Mengeaud V. Efficacy of a Rhealba(®) Oat Extract-based emollient on chronic pruritus in elderly French outpatients. J Eur Acad Dermatol Venereol. 2017;31(Suppl 1):1–7. doi: 10.1111/jdv.14077. [DOI] [PubMed] [Google Scholar]
- 28.Del Duca E, Farnetani F, De Carvalho N, Bottoni U, Pellacani G, Nistico SP. Superiority of a vitamin B12-containing emollient compared to a standard emollient in the maintenance treatment of mild-to-moderate plaque psoriasis. Int J Immunopathol Pharmacol. 2017;30:439–444. doi: 10.1177/0394632017736674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker JC, Scharfbillig RW, Jones S. Effectiveness of two moisturizers in the treatment of foot xerosis a randomized clinical trial. J Am Podiatr Med Assoc. 2018;108:458–465. doi: 10.7547/16-119. [DOI] [PubMed] [Google Scholar]
- 30.Boralevi F, Meledie N’Djong AP, Yao Yoboue P, et al. Regression of cutaneous xerosis with emollient treatment in sub-Saharan African patients. Int J Dermatol. 2017;56:467–473. doi: 10.1111/ijd.13454. [DOI] [PubMed] [Google Scholar]
- 31.Romani L, Steer AC, Whitfeld MJ, Kaldor JM. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960–967. doi: 10.1016/S1473-3099(15)00132-2. [DOI] [PubMed] [Google Scholar]
- 32.Sunderkotter C, Aebischer A, Neufeld M, et al. Increase of scabies in Germany and development of resistant mites? Evidence and consequences. J Dtsch Dermatol Ges. 2019;17:15–23. doi: 10.1111/ddg.13706. [DOI] [PubMed] [Google Scholar]
- 33.Lugovic-Mihic L. The increase in Croatia’s scabies incidence: how did refugees and traveling contribute? Travel Med Infect Dis. 2019;29:74. doi: 10.1016/j.tmaid.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Amato E, Dansie LS, Groneng GM, et al. Increase of scabies infestations, Norway, 2006 to 2018. Euro Surveill. 2019;2019:24. doi: 10.2807/1560-7917.ES.2019.24.23.190020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248–1253. doi: 10.1111/jdv.14351. [DOI] [PubMed] [Google Scholar]
- 36.Kowalski EH, Kneiber D, Valdebran M, Patel U, Amber KT. Treatment-resistant prurigo nodularis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:163–172. doi: 10.2147/CCID.S188070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb) 2017;7:349–364. doi: 10.1007/s13555-017-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745–750. doi: 10.1001/archpedi.159.8.745. [DOI] [PubMed] [Google Scholar]
- 39.Hon KL, Kung JSC, Tsang KYC, Yu JWS, Lee VW, Leung TF. Testing the actions of a multi-action emollient: patient’s acceptability determines product efficacy. Curr Pediatr Rev. 2018;14:110–116. doi: 10.2174/1573396313666171117114005. [DOI] [PubMed] [Google Scholar]
- 40.Lindh JD, Bradley M. Clinical effectiveness of moisturizers in atopic dermatitis and related disorders: a systematic review. Am J Clin Dermatol. 2015;16:341–359. doi: 10.1007/s40257-015-0146-4. [DOI] [PubMed] [Google Scholar]
- 41.Tiplica GS, Boralevi F, Konno P, et al. The regular use of an emollient improves symptoms of atopic dermatitis in children: a randomized controlled study. J Eur Acad Dermatol Venereol. 2018;32:1180–1187. doi: 10.1111/jdv.14849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.