Abstract
Introduction
To investigate the associations of alopecia areata (AA) with serum vitamin D and calcium levels.
Methods
A systematic review of all relevant articles published up to February 2020 in PubMed, Embase, and Cochrane Library databases was conducted. Primary endpoints were serum 25-hydroxyvitamin D [25(OH)D] levels and vitamin D deficiency, and the secondary endpoint was serum calcium level. Odds ratio (OR) and standardized mean difference (SMD) with 95% CI across studies were analyzed.
Results
Data on 1585 patients with AA and 1114 controls from 16 case–control studies and three cross-sectional studies were included in this meta-analysis. A pooled meta-analysis was conducted using the random-effects model because of inter-study heterogeneity (vitamin D level, I2 = 87.90%; vitamin D deficiency, I2 = 81.10%; serum calcium level, I2 = 83.80%). A combined analysis revealed that patients with AA had significantly lower mean serum 25(OH)D level compared with control (WMD − 9.08, 95% CI − 11.65, − 6.50, p < 0.001), and were more likely to have vitamin D deficiency (OR 4.14, 95% CI 2.34, 7.35, p < 0.001). However, the pooled analysis revealed that patients with AA did not have significantly lower serum calcium levels compared with control (WMD − 0.17, 95% CI − 0.40, 0.06, p = 0.143). Subgroup analysis suggested that matched control, mean age, and country might contribute to the heterogeneity of serum vitamin D level, while study design, matched control, and country might contribute to the heterogeneity of vitamin D deficiency.
Conclusion
Deficiency of serum 25(OH)D level, rather than calcium level, was present in patients with AA. Screening for vitamin D deficiency and vitamin D supplementation may be beneficial in the treatment of patients with AA.
Electronic supplementary material
The online version of this article (10.1007/s13555-020-00433-4) contains supplementary material, which is available to authorized users.
Keywords: Alopecia areata; Areata; Calcium; Vitamin D; 1,25-Dihydroxyvitamin D; 25-Hydroxyvitamin D
Key Summary Points
| Why carry out this study? |
| Alopecia areata (AA) is a form of non-scarring hair loss characterized by an autoimmune reaction to hair follicles with disordered, shortened hair cycle |
| Vitamin D deficiency was considered as a risk factor for the development of AA |
| The relationship between vitamin D deficiency and AA in patients was reported in two previous meta-analyses but not comprehensively evaluated because five subsequent studies were not considered |
| What was learned from the study? |
| Deficiency of serum 25(OH)D level, rather than calcium level, was present in patients with AA. Screening for vitamin D deficiency and vitamin D supplementation may be beneficial in treatment of patients with AA |
Introduction
Alopecia areata (AA) is a form of non-scarring hair loss characterized by an autoimmune reaction to hair follicles with disordered, shortened hair cycle [1]. The prevalence of AA is 0.1–0.2%, with a calculated lifetime risk of 2% [2]. The age- and sex-adjusted incidence of AA is 20.9 per 100,000 person-years [3]. About 51% of patients with AA are female, and the median age at diagnosis is 33 years, affecting both children and adults, and hair of all colors [3]. Earlier age of onset and male gender appear to have more severe involvement [4]. The hallmark of AA is T cell (CD4+ and CD8+) infiltrates and cytokine production, specifically targeting anaphase hair follicles [5, 6]. Autoantibodies targeting hair follicles, especially against keratin 16 and trichohyalin, are increased in peripheral blood of patients with AA [7].
Although the etiology is different between chemotherapy-induced alopecia (cytotoxic agents killing the rapidly renewing cells in the hair follicles) and AA (autoimmunity), vitamin D deficiency might be considered a risk factor for the development of AA since vitamin D protects hair follicles from chemotherapy-induced alopecia [8, 9]. Keratinocytes possess the enzymatic machinery to synthesize active vitamin D, i.e., 1,25-dihydroxyvitamin D3 [serum 1,25(OH)D3], and express the vitamin D receptor (VDR), which is necessary for the maintenance of the normal hair cycle [10]. Besides, 1,25(OH)D3 acts as an immunomodulator of innate and adaptive immune functions targeting various immune cells such as T lymphocytes, B lymphocytes, monocytes, macrophages, and dendritic cells [11]. The association between vitamin D and several autoimmune-mediated diseases has been reported, including vitiligo, systemic lupus erythematosus, type I diabetes mellitus, rheumatoid arthritis, psoriasis, multiple sclerosis, and inflammatory bowel disease [12]. Although associations are not causative factors, that might imply that vitamin D deficiency is an environmental trigger for the induction of abnormal autoimmunity [13]. Therefore, 1,25(OH)D3 modulates both innate and adaptive immune responses by targeting various immune cells such as T lymphocytes, B lymphocytes, monocytes, macrophages, and dendritic cells, among others [11]. In addition, vitamin D synthesis decreases with age [14–16]. Vitamin D deficiency is common in both men and women, and can affect pregnancy outcomes in women and increase the risk of osteoporosis, especially in menopausal women [14–17]. Although the relationship between vitamin D deficiency and AA in patients was reported in two previous meta-analyses [18, 19], this relationship has not been comprehensively evaluated since five subsequent studies were not considered [20–24].
Vitamin D is also a secosteroid hormone, primarily synthesized in epidermal keratinocytes or acquired from the diet, and plays an important role in calcium homeostasis and bone health [25, 26]. Two studies revealed no difference in serum calcium levels between patients with AA and controls despite a statistically significant difference in vitamin D levels [20, 27]. The presence of 25-hydroxyvitamin D [25(OH)D] deficiency in patients with AA correlated with increased parathyroid hormone (PTH) [24], suggesting a compensatory effect of increased PTH to maintain normal serum calcium in a state of vitamin D deficiency. Darwish et al. showed lower levels of calcium but comparable PTH level [22], while Yilmaz et al. showed comparable levels of both calcium and PTH in patients with AA compared to controls [20].
In this context, the study aimed to evaluate the serum 25(OH)D levels and calcium levels in patients with AA in order to identify their potential roles in the pathogenesis of AA. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Methods
Search Strategy
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines. An electronic search of the PubMed, Embase, and Cochrane Library databases was performed up to February 18, 2020, without language restrictions. We first screened the abstracts and then chose relevant full-text articles. The reference lists of selected articles were manually searched to identify additional relevant reports.
Selection Criteria
The inclusion criteria were as follows: (1) diagnoses of AA were based on clinical findings; (2) studies centered on associations of AA with vitamin D and calcium; (3) either serum 25-hydroxyvitamin D levels or vitamin D deficiency or calcium level was available for cases and controls; (4) only the publication with the largest number of participants was included for studies involving overlapping data sets; (5) publication of sufficient information to calculate odds ratios (OR) and weighted mean difference (WMD); and (6) published in peer-reviewed journals.
The exclusion criteria were as follows: (a) studies without a designated control; (b) the presence of other diseases influencing 25-hydroxyvitamin D and calcium level; (c) summaries, abstracts, case reports, and reviews; (d) studies reported in a language other than English.
Data Extraction and Assessment of Risk of Bias
Two blinded and independent researchers (Yi Liu and Jing Li) reviewed potentially relevant publications, and any disagreements were resolved by a third senior researcher (Xinfeng Wu). Two investigators independently extracted the following information using a predefined data collection form: author, year of publication, patient and control group characteristics, study design, sample size, gender, country or race, the outcome of interest, etc. Disagreements were resolved by consensus between the two investigators. We attempted to contact the authors for missing primary and secondary outcomes.
The Agency for Healthcare Research and Quality (AHRQ) and Newcastle–Ottawa Scale (NOS) scales were used to independently assess the methodological quality of cross-sectional studies and case–control studies, respectively.
Outcomes
The primary outcomes were serum 25-hydroxyvitamin D levels and vitamin D deficiency, and the secondary outcome was the serum calcium level. Vitamin D deficiency was defined as serum 25(OH)D level lower than 20 or 30 ng/dL depending on the study.
Statistical Analysis
The odds ratios (ORs) of having vitamin D deficiency were estimated for each study by comparing patients with AA versus healthy controls and then pooled. The ORs were pooled using the DerSimonian and Laird method if heterogeneity was present; otherwise, they were pooled using a fixed-effects model. For continuous outcomes, the mean difference of serum 25-hydroxyvitamin D level and calcium level between patients with AA versus healthy controls was estimated for each study and then pooled across studies using weighted mean difference (WMD). Heterogeneity was assessed using Q statistics, and the degree of heterogeneity was quantified using I2. If heterogeneity was detected (p < 0.10 or I2 ≥ 50%), a random-effects model was applied; otherwise, a fixed-effects model was used. In order to check the stability of the result, a sensitivity analysis was performed by sequential deletion of each study. Rosenthal’s fail-safe N was utilized for sensitivity analysis. The risk of publication bias was evaluated via Egger’s test, Begg’s test, and funnel plots. Subgroup analysis was performed to investigate the potential additional effect of predefined factors. A two-tailed p < 0.05 was considered significant. All statistical analyses in this meta-analysis were performed using the STATA MP 14.0 software (StataCorp, College Station, TX, USA).
Results
Characteristics of Included Studies
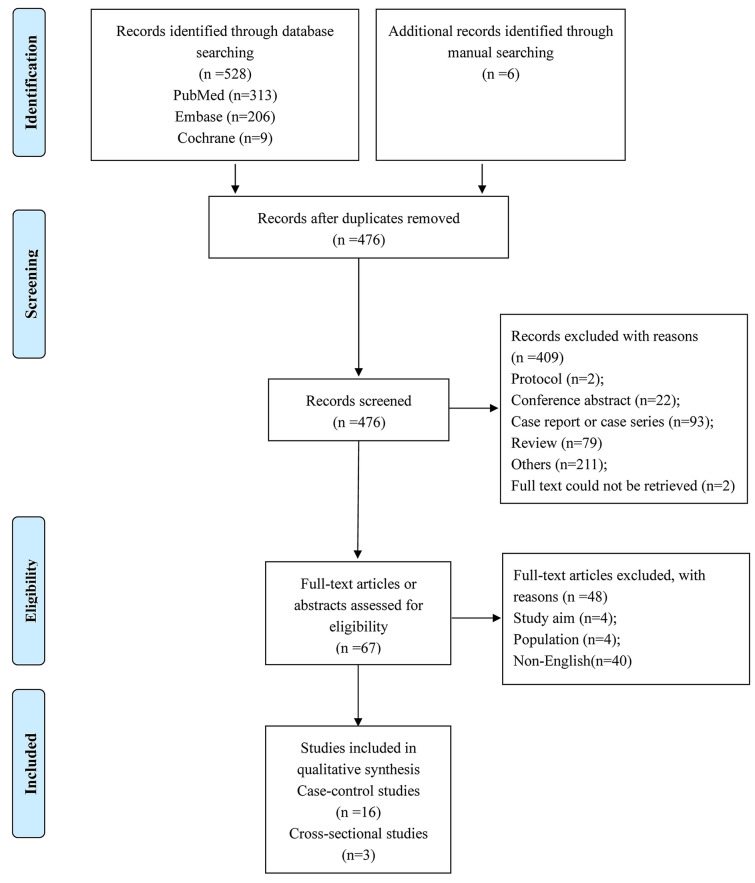
The flowchart of the study selection process is shown in Fig. 1. There were 19 eligible publications (16 case–control studies and three cross-sectional studies), involving 2699 subjects (1585 AA cases and 1114 controls) [20–24, 27–40]. The sample size of the included studies ranged from 43 to 756, and publication date from 2012 to 2019. The included studies were from eight countries, namely Egypt, India, Israel, Italy, Nepal, Pakistan, Turkey, and the USA. Eleven studies had age- and sex-matched controls. Seventeen, thirteen, and six studies provided data on serum 25(OH)D levels, vitamin D deficiency, and serum calcium level, respectively. Detailed characteristics of the included studies are summarized in Table 1. The integral quality of the included case–control and cross-sectional studies was rated as high (sTables 1 and 2 in the supplementary material).
Fig. 1.
Flowchart of literature search and study selection
Table 1.
Characteristics of the 19 studies included in this meta-analysis
| Study | Study design | Sample size | Age (year, mean or median) | Male (%) | Country/Race | Control setting | Outcome of interest | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | AA | Control | AA | Control | AA | Control | |||||
| Marahatta S, 2019 | Case-control study | 60 | 30 | 30 | 28.37+10.070 | 30.50 + 9.032 | 16 (51.6%) | 15 (48.4%) | Nepal | Age- and sex-matched healthy controls from hospital staffs | Vitamin D level, Vitamin D deficiency |
| Rehman F, 2019 | Case-control study | 270 | 135 | 135 | 26 ± 12.89 | 26 ± 13.20 | 91 (67.41%) | 91 (67.41%) | India | Age- and sex-matched healthy controls | Vitamin D level, Vitamin D deficiency, serum calcium level |
| Siddappa H, 2019 | Case-control study | 200 | 100 | 100 | 24.52 ± 10.06 | 28.96±11.49 | 72 (72%) | 58 (58%) | India | Age- and sex-matched healthy controls without any history of alopecia areata or other autoimmune/systemic diseases | Vitamin D level, Vitamin D deficiency |
| Daroach M, 2018 | Case-control study | 60 | 30 | 30 | 28.9 ± 9.96 | 31.17 ± 9.43 | 11 (36.67%) | 16 (53.33%) | India | Age- and sex-matched healthy controls | Vitamin D level, Vitamin D deficiency |
| Gade V.K.V., 2018 | Case-control study | 90 | 45 | 45 | 32.73 ± 10.43 | 33.98 ± 8.48 | 14 (31.1%) | 14 (31.1%) | India | Age- and sex-matched healthy volunteers | Vitamin D level, serum calcium level |
| Unal M, 2018 | Case-control study | 54 | 20 | 34 | 12.4 ± 4.2 | 16.6 ± 0.8 | 14 (70%) | 15 (44.12%) | Turkey | Pediatric healthy controls recruited from individuals who visited to the outpatient clinic | Vitamin D level |
| Bhat Y.J., 2017 | Cross-sectional study | 85 | 50 | 35 | 22.4 ± 8.6 | 29.2 ± 7.6 | NR | NR | India | Age- and sex-matched individuals selected randomly from outpatient department with no history of AA | Vitamin D level, serum calcium level |
| Conic R.Z., 2017 | Case-control study | 756 | 584 | 172 | 35.54 ± 19.28 | 35.80 ± 15.56 | 184 (31.50%) | 46 (26.75%) | USA | Age-matched patients given a diagnosis of seborrheic dermatitis without hair loss was randomly selected | Vitamin D deficiency |
| Darwish NMM, 2017 | Case-control study | 50 | 30 | 20 | 28.67 ± 10 | 24.8 ± 6 | 13 (43.3%) | 10 (50%) | Egypt | Age- and sex matched healthy controls | Vitamin D level, serum calcium level |
| Erpolat S, 2017 | Case-control study | 73 | 41 | 32 | 32.8 ± 7.5 | 32.7 ± 7.5 | 26 (63.4%) | 18 (56.3%) | Turkey | Healthy control | Vitamin D level, Vitamin D deficiency, serum calcium level |
| Ghafoor R, 2017 | Case-control study | 60 | 30 | 30 | 23.77 ± 8.86 | 24.03 ± 8.62 | 12 (40%) | 12 (40%) | Pakistan | Healthy volunteers and patients coming to dermatology department for other disorders like acne, melasma, etc. | Vitamin D level |
| Attawa E, 2016 | Cross-sectional study | 46 | 23 | 23 | 26.44 ± 10.87 | 29.39 ± 8.10 | 15 (65.2%) | 14 (60.9%) | Egypt | Healthy controls | Vitamin D level, Vitamin D deficiency |
| Bakry O.A., 2016 | Case-control study | 120 | 60 | 60 | 20.7 ± 10.85 | 23.71 ± 7.45 | 36 (60%) | 28 (46.7%) | Egypt | Age, gender, skin phototype, and body mass index-matched healthy subjects | Vitamin D level, Vitamin D deficiency |
| Fattah N.S.A.A., 2015 | Case-control study | 60 | 30 | 30 | 26.8 ± 6.9 | 25.1 ± 6.9 | 18 (60%) | 18 (60%) | Egypt | Healthy volunteers, matched for age, sex, Fitzpatrick skin phototype, the approximate daily amount of vitamin D intake from food source, the occupation (indoor or outdoor), and time of blood sampling | Vitamin D level, Vitamin D deficiency |
| Aksu Cerman A, 2014 | Cross-sectional study | 144 | 86 | 58 | 32.21 ± 9.60 | 32.55 ± 9.78 | 56 (47.9%) | 34 (29.1%) | Turkey | Age- and sex-matched volunteers from the hospital staff. | Vitamin D level, Vitamin D deficiency |
| Mahamid M, 2014 | Case-control study | 43 | 23 | 20 | 24.2 ± 12.3 | 27 ± 11.26 | 14 (60.87%) | 13 (65%) | Israel | Individuals without a history of AA who were enrolled randomly from clinics | Vitamin D level, Vitamin D deficiency |
| d’Ovidio R, 2013 | Case-control study | 304 | 156 | 148 | 37.8 | 34.5 | 45 (28.85%) | 18 (12.16%) | Italy | Healthy individuals | Vitamin D deficiency |
| El-Mongy NN, 2013 | Case-control study | 140 | 70 | 70 | 27.79 ± 9.116 | 30.49 ± 11.059 | 37 (52.9%) | 44 (62.9%) | Egypt | Normal controls | Vitamin D level, Vitamin D deficiency |
| Yilmaz N, 2012 | Case-control study | 84 | 42 | 42 | 30.8 ± 8.2 | 29.3 ± 7.4 | 14 (33.33%) | 13 (30.95) | Turkey | Healthy individuals | Vitamin D level, serum calcium level |
AA Alopecia areata, NR not reported
Heterogeneity and Publication Bias
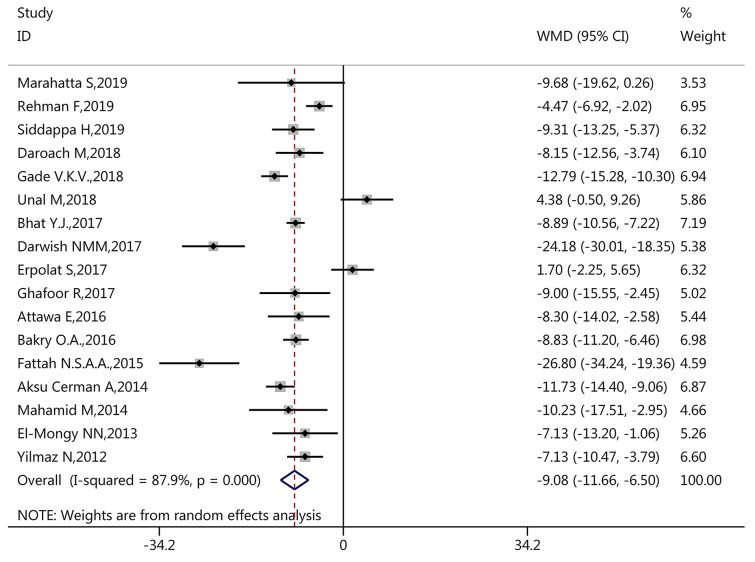
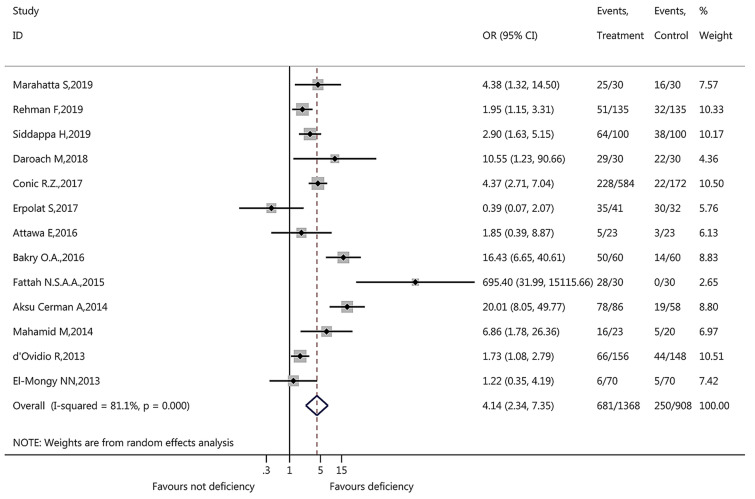
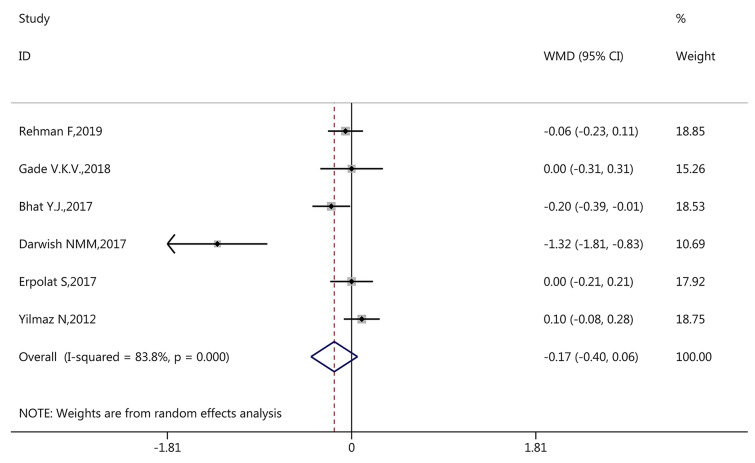
During pooled meta-analysis, inter-study heterogeneity (I2 > 50%) was found in vitamin D level (I2 = 87.90%, p < 0.001, Fig. 2), vitamin D deficiency (I2 = 81.10%, p < 0.001, Fig. 3), and serum calcium level (I2 = 83.80%, p < 0.001, Fig. 4). Therefore, the pooled meta-analysis for these factors was conducted using the random-effects model.
Fig. 2.
Forest plot of the meta-analysis of serum vitamin D level
Fig. 3.
Forest plot of the meta-analysis of vitamin D deficiency
Fig. 4.
Forest plot of the meta-analysis of serum calcium level
No evidence of publication bias was identified in the meta-analysis of serum vitamin D level (Begg’s test, p = 0.650; Egger’s test, p = 0.756) and vitamin D deficiency (Begg’s test, p = 0.583; Egger’s test, p = 0.257). Visual inspection of the funnel plots revealed no evidence of publication bias for serum vitamin D levels and vitamin D deficiency (sFigs. 2 and 4 in the supplementary material). Therefore, these data indicated that there was no publication bias in the present meta-analysis, and the results were statistically robust.
Meta-analysis Results
According to inter-study heterogeneity by Q test and I2 test, the pooled analysis was conducted using the random-effects model for vitamin D level, vitamin D deficiency, and calcium level. Among the 17 studies with serum 25(OH)D level data, the results showed that patients with AA had significantly lower mean serum 25(OH)D level compared with controls (WMD 9.08, 95% CI − 11.66, − 6.50, p < 0.001, Fig. 2).
Among the 13 studies with vitamin D deficiency data, the meta-analysis suggested that patients with AA were more likely to have vitamin D deficiency with a pooled OR of 4.14 (95% CI 2.34, 7.35, p < 0.001, Fig. 3). Among the six included studies with serum calcium level data, the findings revealed that patients with AA did not have a statistically lower mean serum calcium level compared with controls (WMD − 0.17, 95% CI − 0.40, 0.06, p = 0.143, Fig. 4).
Subgroup Analysis
For serum 25(OH)D levels, similar statistically significant findings were obtained for subgroup analyses of study design (WMD of case–control − 9.05, 95% CI − 12.46, − 5.63; WMD of cross-sectional − 9.82, 95% CI − 11.91, − 7.72), sample size (WMD of > 100: − 8.35, 95% CI − 11.19, − 5.51; WMD of ≤ 100: − 9.59, 95% CI − 13.44, − 5.74), and male ratio (WMD of > 1/2: − 7.79, 95% CI − 11.47, − 4.11; WMD of ≤ 1/2: − 12.03, 95% CI − 16.93, − 7.13) (Table 2). However, inconsistent results were found for matched control (WMD of matched control − 11.38, 95% CI − 13.99, − 8.78; WMD of non-matched control − 3.18, 95% CI − 8.35, 1.99), mean age (WMD of > 25 years − 10.06, 95% CI − 12.87, − 7.24; WMD of < 25 years − 3.18, 95% CI − 8.35, 1.99), country (WMD of Nepal − 9.68, 95% CI − 19.62, 0.26, WMD of India − 8.73, 95% CI − 11.59, − 5.87; WMD of Turkey − 3.37, 95% CI − 10.68, 3.94; WMD of Egypt − 14.75, 95% CI − 22.10, − 7.39; WMD of Pakistan − 9.00, 95% CI − 15.55, − 2.45; WMD of Israel − 10.23, 95% CI − 17.51, − 2.95) (Table 2). These findings suggested that matched control, mean age, and country might contribute to a high degree of inter-study heterogeneity in serum vitamin D level.
Table 2.
Subgroup meta-analysis of serum vitamin D level and vitamin D deficiency
| Vitamin D | Vitamin D deficiency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of articles | WMD (95%CI) | p | I2 (%) | pheterogeneity | No. of articles | OR (95% CI) | p | I2 (%) | pheterogeneity | |
| Overall | 17 | − 9.08 (− 11.66, − 6.50) | < 0.001 | 87.90 | < 0.001 | 13 | 4.14 (2.34, 7.35) | < 0.001 | 81.10 | < 0.001 |
| Design | ||||||||||
| Case–control | 14 | − 9.05 (− 12.46, − 5.63) | < 0.001 | 89.70 | < 0.001 | 11 | 3.66 (2.06, 6.49) | < 0.001 | 78.80 | < 0.001 |
| Cross-sectional | 3 | − 9.82 (− 11.91, − 7.72) | < 0.001 | 40.20 | 0.188 | 2 | 6.65 (0.65, 68.27) | 0.111 | 84.90 | 0.01 |
| Sample size | ||||||||||
| ≤ 100 | 12 | − 9.59 (− 13.44, − 5.74) | < 0.001 | 90.50 | < 0.001 | 6 | 5.36 (1.29, 22.21) | 0.021 | 76.50 | 0.001 |
| > 100 | 5 | − 8.35 (− 11.19, − 5.51) | < 0.001 | 75.50 | < 0.001 | 7 | 3.94 (2.06, 7.54) | < 0.001 | 85.70 | < 0.001 |
| Controls | ||||||||||
| Matched | 12 | − 11.38 (− 13.99, − 8.78) | < 0.001 | 84.60 | < 0.001 | 9 | 6.96 (3.54, 13.68) | < 0.001 | 80.80 | < 0.001 |
| Not matched | 5 | − 3.18 (− 8.35, 1.99) | 0.228 | 84.10 | < 0.001 | 4 | 1.52 (1.00, 2.32) | 0.052 | 1.10 | 0.386 |
| Male ratio | ||||||||||
| > 1/2 | 11 | − 7.79 (− 11.47, − 4.11) | < 0.001 | 88.60 | < 0.001 | 10 | 4.51 (2.05, 9.93) | < 0.001 | 83.10 | < 0.001 |
| ≤ 1/2 | 5 | − 12.03 (− 16.93, − 7.13) | < 0.001 | 86.00 | < 0.001 | 3 | 3.24 (1.37, 7.63) | 0.007 | 0.3767 | 0.012 |
| NR | 1 | − 8.89 (− 10.56, − 7.22) | < 0.001 | |||||||
| Mean age (years) | ||||||||||
| > 25 | 14 | − 10.06 (− 12.87, − 7.24) | < 0.001 | 87.50 | < 0.001 | 12 | 3.58 (2.04, 6.28) | < 0.001 | 78.30 | < 0.001 |
| ≤ 25 | 3 | − 4.49 (− 13.24, 4.25) | 0.314 | 91.40 | < 0.001 | 1 | 16.43 (6.65, 40.61) | < 0.001 | ||
| Country | ||||||||||
| Nepal | 1 | − 9.68 (− 19.62, 0.26) | 0.056 | 1 | 4.38 (1.32, 14.50) | 0.016 | ||||
| India | 5 | − 8.73 (− 11.59, − 5.87) | < 0.001 | 81.80 | < 0.001 | 3 | 2.55 (1.54, 4.21) | < 0.001 | 29.10 | 0.244 |
| Turkey | 4 | − 3.37 (− 10.68, 3.94) | 0.366 | 94.0 | < 0.001 | 2 | 2.97 (0.06, 147.87) | 0.585 | 94.1 | < 0.001 |
| Egypt | 5 | − 14.75 (− 22.10, − 7.39) | < 0.001 | 90.60 | < 0.001 | 4 | 8.91 (1.20, 66.39) | 0.033 | 86.9 | < 0.001 |
| Pakistan | 1 | − 9 (− 15.55, − 2.45) | 0.007 | |||||||
| Israel | 1 | − 10.23 (− 17.51, − 2.95) | 0.006 | 1 | 6.86 (1.78, 26.36) | 0.005 | ||||
| Italy | 1 | 1.73 (1.08, 2.79) | 0.023 | |||||||
| USA | 1 | 4.37 (2.71, 7.04) | < 0.001 | |||||||
WMD weighted mean difference, OR odds ratio, CI confidence interval
For vitamin D deficiency, consistent statistically significant findings were identified for subgroup analyses of sample size (OR of > 100: 3.94, 95% CI 2.06, 7.54; OR of ≤ 100: 5.36, 95% CI 1.29, 22.21), male ratio (OR of > 1/2: 4.51, 95% CI 2.05, 9.93; OR of ≤ 1/2: 3.24, 95% CI 1.37, 7.63), and mean age (OR of > 25 years: 3.58, 95% CI 2.04, 6.28; OR of < 25 years: 16.43, 95% CI 6.65, 40.61) (Table 2). However, inconsistent results were found for study design (OR of case–control 3.66, 95% CI 2.06, 6.49; OR of cross-sectional 6.65, 95% CI 0.65, 68.27), matched control (OR of matched control 6.96, 95% CI 3.54, 13.68; OR of non-matched control 1.52, 95% CI 1.00, 2.32), and country (OR of Nepal 4.38, 95% CI 1.32, 14.50, OR of India 2.55, 95% CI 1.54, 4.21; OR of Turkey 2.97, 95% CI 0.06, 147.87; OR of Egypt 8.91, 95% CI 1.20, 66.39; OR of Israel 6.86, 95% CI 1.78, 26.36; OR of Italy 1.73, 95% CI 1.08, 2.79; OR of USA 4.37, 95% CI 2.71, 7.04) (Table 2). These data suggested that study design, matched control, and country might contribute to a high degree of inter-study heterogeneity in vitamin D deficiency.
Sensitivity Analysis
A sensitivity analysis was performed to check the robustness of the results. Statistically similar outcomes were obtained for meta-analysis of vitamin D level (sFig. 1 in the supplementary material) and vitamin D deficiency (sFig. 3 in the supplementary material) after sequentially excluding each case–control study, indicating the stability of the present meta-analysis.
Discussion
The present systematic review and meta-analysis indicated that patients with AA had lower serum 25(OH)D3 level and higher risk of vitamin D deficiency, but not lower serum calcium level compared with controls. Thus, screening for vitamin D deficiency and supplementation of vitamin D could be beneficial in the treatment of AA. In addition to regulating bone and calcium metabolism, the biologically active metabolite of vitamin D3, 1,25(OH)2D3, can modulate nuclear vitamin D receptor expressed in antigen-presenting cells (APCs) and activated T cells via interference with nuclear transcription factors such as NF-AT and NF-κB or by direct interaction with vitamin D responsive elements in the promoter regions of cytokine genes [41].
The beneficial effect of vitamin D3 is mediated through the prevention of strong Th1 cell responses via the action on APCs, while simultaneously tilting the T cell response towards Th2 dominance [41, 42]. The beneficial effect of vitamin D supplementation was observed in the prevention and treatment of autoimmune-mediated diseases [43–45], but no study was conducted in patients with AA. A 7-year-old patient with AA and reduced vitamin D receptor expression recovered after topical application of calcipotriol, a strong vitamin D analogue [46]. The mechanism of topical application of calcipotriol in the restoration of hair cycle dysfunction is believed to regulate the differentiation of different cells and the expression of Toll-like receptors [44]. Therefore, large prospective studies are required to confirm the role of vitamin D supplementation in the treatment of AA, especially the severe form, which is commonly associated with other autoimmune diseases [47].
This study had two strengths that likely increased the reliability and clinical value of the findings. First, compared with the previous two studies [18, 19], the results of the present study were more reliable as we extensively searched to include all potentially eligible studies. Second, unlike the two previous studies, our pooled analysis of six studies revealed that patients with AA did not have a statistically lower mean serum calcium level compared with controls. A plausible explanation of normal calcium level is the compensatory increase in PTH during vitamin D deficiency. Nevertheless, Darwish et al. revealed lower levels of serum 25(OH)D and calcium but comparable PTH level in patients with AA compared with controls [22]; they also showed a positive linear correlation between serum vitamin D and serum calcium in patients with AA. Yilmaz et al. also found no significant difference in PTH level between patients with AA and controls [20]. Thus, whether there is a compensatory effect of increased PTH and how it works in patients with AA need further investigation.
This meta-analysis had several potential limitations that must be considered when interpreting the results. First, a meta-analysis is unable to resolve problems with confounding factors that could be inherent in the original studies, although major potential confounders had been adjusted for in most of the included studies. Subgroup analysis suggested that the heterogeneity of serum vitamin D level was partially explained by matched controls, mean age, and country, while heterogeneity of vitamin D deficiency was partly explained by study design, matched controls, and country. Therefore, a random-effects model was used for the synthesis of data in order to mitigate potential variation between patients at an individual patient data level. Second, the results of several subgroups were based on a limited number of studies, and therefore, the possibility of insufficient statistical power cannot be ruled out. For example, the only study on pediatric patients with AA reported no difference in serum 25(OH)D level between pediatric patients with AA and pediatric controls [37]. Hence, caution should be used for generalization of these findings to pediatric patients with AA. Third, serum 25(OH)D concentration might be influenced by numerous factors such as gender, age, ethnicity, comorbidity, diet, sun exposure level, the season of blood sampling, and treatments, such as phototherapy. Therefore, a Mendelian randomization study might be useful to determine the causal association between vitamin D and AA. Fourth, most included studies defined vitamin D deficiency as serum 25(OH)D level below 20 ng/dL, but a few studies defined it as below 30 ng/dL. However, subgroup analysis of different definitions of vitamin D deficiency was consistent with the overall analysis. Finally, only studies published in English were included, and valuable data published in other languages might have been excluded.
Conclusions
Deficient serum 25(OH)D level, rather than calcium level, was present in patients with AA. Screening for vitamin D deficiency and vitamin D supplementation may be beneficial in the treatment of patients with AA. As a result of the limited data, we were unable to comment on the potential additional effect of calcium monitoring.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Yi Liu and Jing Li carried out the studies, participated in collecting data, and drafted the manuscript. Guirong Liang, Chaojiang Cheng and Yue Li performed the statistical analysis and participated in its design. Yi Liu, Jing Li and Xinfeng Wu participated in the acquisition, analysis, or interpretation of data and drafted the manuscript. All authors read and approved the final manuscript. Yi Liu and Jing Li contributed equally to this article.
Disclosures
Yi Liu, Jing Li, Guirong Liang, Chaojiang Cheng, Yue Li and Xinfeng Wu have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12706562.
Contributor Information
Yi Liu, Email: liuyi0513@aliyun.com.
Xinfeng Wu, Email: wuxinfengdr@163.com.
References
- 1.Gilhar A, Laufer-Britva R, Keren A, Paus R. Frontiers in alopecia areata pathobiology research. J Allergy Clin Immunol. 2019;144:1478–1489. doi: 10.1016/j.jaci.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 2.Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi: 10.1001/archderm.1992.01680150136027. [DOI] [PubMed] [Google Scholar]
- 3.Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3–12. doi: 10.1016/j.jdermsci.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Xiao FL, Yang S, Liu JB, et al. The epidemiology of childhood alopecia areata in China: a study of 226 patients. Pediatr Dermatol. 2006;23:13–18. doi: 10.1111/j.1525-1470.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 5.McElwee KJ, Tobin DJ, Bystryn JC, King LE, Jr, Sundberg JP. Alopecia areata: an autoimmune disease? Exp Dermatol. 1999;8:371–379. doi: 10.1111/j.1600-0625.1999.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang E, McElwee KJ. Etiopathogenesis of alopecia areata: why do our patients get it? Dermatol Ther. 2011;24:337–347. doi: 10.1111/j.1529-8019.2011.01416.x. [DOI] [PubMed] [Google Scholar]
- 7.Leung MC, Sutton CW, Fenton DA, Tobin DJ. Trichohyalin is a potential major autoantigen in human alopecia areata. J Proteome Res. 2010;9:5153–5163. doi: 10.1021/pr100422u. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Lu Z, Au JL. Protection against chemotherapy-induced alopecia. Pharm Res. 2006;23:2505–2514. doi: 10.1007/s11095-006-9105-3. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez JJ, Yunis AA. Vitamin D3 and chemotherapy-induced alopecia. Nutrition. 1996;12:448–449. doi: 10.1016/s0899-9007(97)85081-2. [DOI] [PubMed] [Google Scholar]
- 10.Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347:80–89. doi: 10.1016/j.mce.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 2012;76:315–325. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 13.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 16.Nowson CA, McGrath JJ, Ebeling PR, et al. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust. 2012;196:686–687. doi: 10.5694/mja11.10301. [DOI] [PubMed] [Google Scholar]
- 17.Khadilkar SS. The emerging role of vitamin D3 in women’s health. J Obstet Gynaecol India. 2013;63:147–150. doi: 10.1007/s13224-013-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai TY, Huang YC. Vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;78:207–209. doi: 10.1016/j.jaad.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Kim BJ, Lee CH, Lee WS. Increased prevalence of vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:1214–1221. doi: 10.1111/jdv.14987. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz N, Serarslan G, Gokce C. Vitamin D concentrations are decreased in patients with alopecia areata. Vitam Trace Elem. 2012;1:3. [Google Scholar]
- 21.Attawa E, Kandil A, Elbalaat W, Samy A. Assessment of vitamin d level in patients of alopecia areata. J Clin Investig Dermatol 2016;4.
- 22.Darwish N, Marzok H, Gaballah M, Abdellatif H. Serum level of vitamin D in patients with alopecia areata. Egypt J Basic Appl Sci. 2017;4:9–14. [Google Scholar]
- 23.El-Mongy NN, El-Nabarawy E, Hassan SA, Younis ER, Shaker O. Serum 25-hydroxy vitamin D3 level in Egyptian patients with alopecia areata. J Egypt Women’s Dermatol Soc. 2013;10:37–41.
- 24.d’Ovidio R, Vessio M, d’Ovidio FD. Reduced level of 25-hydroxyvitamin D in chronic/relapsing alopecia areata. Dermatoendocrinology. 2013;5:271–273. doi: 10.4161/derm.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleh HM, Abdel Fattah NS, Hamza HT. Evaluation of serum 25-hydroxyvitamin D levels in vitiligo patients with and without autoimmune diseases. Photodermatol Photoimmunol Photomed. 2013;29:34–40. doi: 10.1111/phpp.12016. [DOI] [PubMed] [Google Scholar]
- 26.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 27.Bhat YJ, Latif I, Malik R, et al. Vitamin D level in alopecia areata. Indian J Dermatol. 2017;62:407–410. doi: 10.4103/ijd.IJD_677_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299–1304. doi: 10.1111/bjd.12980. [DOI] [PubMed] [Google Scholar]
- 29.Mahamid M, Abu-Elhija O, Samamra M, Mahamid A, Nseir W. Association between vitamin D levels and alopecia areata. Isr Med Assoc J. 2014;16:367–370. [PubMed] [Google Scholar]
- 30.Abdel F, Nermeen SA, Darwish N, Yasser W. Assessment of serum 25-hydroxyvitamin D levels in patients with extensive/recalcitrant alopecia areata before and after PUVA and NB-UVB therapy. J Egypt Women’s Dermatol Soc. 2015;12:19–23. [Google Scholar]
- 31.Bakry OA, El Farargy SM, El Shafiee MK, Soliman A. Serum vitamin D in patients with alopecia areata. Indian Dermatol Online J. 2016;7:371–377. doi: 10.4103/2229-5178.190504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conic RZ, Miller R, Piliang M, Bergfeld W, Atanaskova Mesinkovska N. Comorbidities in patients with alopecia areata. J Am Acad Dermatol. 2017;76:755–757. doi: 10.1016/j.jaad.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Erpolat S, Sarifakioglu E, Ayyildiz A. 25-Hydroxyvitamin D status in patients with alopecia areata. Postepy Dermatol Alergol. 2017;34:248–252. doi: 10.5114/ada.2017.67847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghafoor R, Anwar MI. Vitamin D deficiency in alopecia areata. J Coll Physicians Surg Pak. 2017;27:200–202. [PubMed] [Google Scholar]
- 35.Daroach M, Narang T, Saikia UN, Sachdeva N, Sendhil Kumaran M. Correlation of vitamin D and vitamin D receptor expression in patients with alopecia areata: a clinical paradigm. Int J Dermatol. 2018;57:217–222. doi: 10.1111/ijd.13851. [DOI] [PubMed] [Google Scholar]
- 36.Gade VKV, Mony A, Munisamy M, Chandrashekar L, Rajappa M. An investigation of vitamin D status in alopecia areata. Clin Exp Med. 2018;18:577–584. doi: 10.1007/s10238-018-0511-8. [DOI] [PubMed] [Google Scholar]
- 37.Unal M, Gonulalan G. Serum vitamin D level is related to disease severity in pediatric alopecia areata. J Cosmet Dermatol. 2018;17:101–104. doi: 10.1111/jocd.12352. [DOI] [PubMed] [Google Scholar]
- 38.Marahatta S, Agrawal S, Khan S. Study on serum vitamin D in alopecia areata patients. J Nepal Health Res Counc. 2019;17:21–25. doi: 10.33314/jnhrc.1475. [DOI] [PubMed] [Google Scholar]
- 39.Rehman F, Dogra N, Wani MA. Serum vitamin D levels and alopecia areata—a hospital based case––control study from North-India. Int J Trichol. 2019;11:49–57. doi: 10.4103/ijt.ijt_3_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siddappa H, Kumar YHK, Vivekananda N. Evaluation of association of vitamin D in alopecia areata: a case–control study of 100 patients in a tertiary rural hospital of Southern India. Indian Dermatol Online J. 2019;10:45–49. doi: 10.4103/idoj.IDOJ_84_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 43.Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008;3:1535–1541. doi: 10.2215/CJN.01160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nancy AL, Yehuda S. Prediction and prevention of autoimmune skin disorders. Arch Dermatol Res. 2009;301:57–64. doi: 10.1007/s00403-008-0889-3. [DOI] [PubMed] [Google Scholar]
- 45.Zella JB, McCary LC, DeLuca HF. Oral administration of 1,25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch Biochem Biophys. 2003;417:77–80. doi: 10.1016/s0003-9861(03)00338-2. [DOI] [PubMed] [Google Scholar]
- 46.Kim DH, Lee JW, Kim IS, et al. Successful treatment of alopecia areata with topical calcipotriol. Ann Dermatol. 2012;24:341–344. doi: 10.5021/ad.2012.24.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goh C, Finkel M, Christos PJ, Sinha AA. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055–1060. doi: 10.1111/j.1468-3083.2006.01676.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.