Abstract
Introduction
Psoriasis Area and Severity Index (PASI) and Physician’s Global Assessment (PGA) are the most widely used outcome measures in clinical trials of biologics to treat psoriasis; however, these outcome measures vary in both their reliability and validity. As newer biologics approach complete clearance of psoriasis, it becomes important to have standardized, reproducible forms of measure to accurately compare treatment efficacy. The aim of this study was to evaluate the extent of and reasons for variation between PASI and PGA scores used in clinical trials.
Methods
A literature search was conducted of clinical trials meeting the inclusion criteria: phase 2 or 3, evaluation of treatment efficacy in reducing psoriasis severity, and use of PASI 90/100 and sPGA or PGA 0/1 as primary end points.
Results
Among the analyzed studies, 8 of 45 trials had a PASI-PGA variance of < 5%, 4 of 45 trials had a variance of 5–10%, and 33 trials had a variance of > 10%. The IMMvent and AMAGINE trials were the only two trials showing 0 variation between the PASI and PGA scores, testing adalimumab and brodalumab, respectively. Ustekinumab showed the highest variance of 61.9% in the IXORA-S trial. Limitations of this paper include a relatively low number of studies assessed because of the paucity of literature available.
Conclusions
The use of both PASI and PGA as equivalent assessment tools for complete clearance is redundant and subject to high variability. Novel severity assessments should be developed that reduce calculation variation and take into account patient-oriented symptoms.
Keywords: Biologics, Psoriasis, Publication, Reporting
Key Summary Points
| Why carry out this study? |
| Psoriasis Area and Severity Index (PASI) and Physician’s Global Assessment (PGA) are the most widely used outcome measures in clinical trials of biologics to treat psoriasis; however, these outcome measures vary in both their reliability and validity. As newer biologics approach complete clearance of psoriasis, it becomes important to have standardized, reproducible forms of measure to accurately compare treatment efficacy |
| How well does psoriasis clearance as measured by PASI 100 correlate with clearance measured by PGA 0 in past clinical trials, and what are the reasons for any variation? |
| What was learned from the study? |
| In this systematic review, the average PASI-PGA variation was found to be 20%, with only 2 of 45 trials demonstrating 0 variation between scores. The two scales showed a moderately positive correlation but corresponded less tightly at lower efficacy cutoffs |
| The PASI and PGA assessment tools show high variability when measuring the same results; this highlights the redundancy of their concurrent use and emphasizes the need for a more consistent and valid outcome measure |
Introduction
A wide range of outcome measures is used to assess psoriasis severity in clinical trials. The Psoriasis Area and Severity Index (PASI) score is based on disease severity and coverage of body surface area (BSA), with a higher score corresponding to more serious conditions. PASI 100 is defined as “complete clearance” of disease, indicating 100% clearance of plaques from baseline. Similar measures that take BSA into account include the Self-Administered PASI (SAPASI) and Salford Psoriasis Index (SPI). Another widely used severity measure, the Physician’s Global Assessment (PGA), is a 6-point scale (0–5) rating overall severity of psoriasis without taking BSA into account. This system has led to many derivative scales, including the static PGA (sPGA) and the Lattice System PGA (LS-PGA). A PGA 0 is a more subjective indication of clearance. Generally, PASI is considered the gold standard of assessments; however, almost every clinical trial for psoriasis uses both PASI and PGA as outcome measures.
Previously PASI and PGA correlated tightly [1]; however, it was noted that the definition of clear/almost clear differed from study to study. As more psoriasis patients achieve PASI 100 and PGA 0/1 with newer biologics, more clinical trials are achieving clear/almost clear. Therefore, we conducted a literature survey to examine how well PASI and PGA correlate to determine if “clear” as measured by PASI 100 is “clear” measured by PGA 0.
Methods
A PubMed and clinicaltrials.gov search was conducted using search terms “Psoriasis Clinical Trial,” “Psoriasis Area and Severity Index,” “Physician’s Global Assessment,” “infliximab,” “etanercept,” “adalimumab,” “certolizumab pegol,” “ustekinumab,” “secukinumab,” “ixekizumab,” “brodalumab,” “guselkumab,” “tildrakizumab,” and “risankizumab.” Inclusion criteria were: phase 2/3 trials and use of PASI 90/100 and sPGA or PGA 0/1 as primary end points. For studies that included both PASI 100 and PASI 90, or both sPGA/PGA 0 and sPGA/PGA 0/1, the measures with lower cutoffs (such as PASI 75) were excluded from the statistical analyses. Descriptive statistics, measures of central tendency, and Pearson correlation coefficient were calculated using Microsoft Excel Datapack. Results were mostly limited to studies published within the last 15 years. A total of 33 studies were pooled and investigated, yielding 45 clinical trials (Table 1). This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Table 1.
PASI/PGA differences in clinical trials of biologics
| Biologic | Authors | Year of study | Study phase | Number of patients | Trial duration | PASI 90/100 outcome | IgA, PGA, sPGA 0/1 outcome |
|---|---|---|---|---|---|---|---|
| Infliximab | Yang et al. | 2012 | 3 | 129 | 26 weeks | PASI 90: 57.1% | PGA 0/1: 88.1% |
| Reich et al. | 2005 | 3 | 378 | 50 weeks | PASI 90: 57% | PGA0/1: 84% | |
| Gottlieb et al. | 2004 | 3 | 249 | 30 weeks | PASI 90: 51.5% | PGA 0/1: 80.8% | |
| Barker et al. | 2011 | 3 | 868 | 26 weeks | PASI 90: 54.5% | PGA 0/1: 76% | |
| Etanercept | Reich et al. | 2017 | 3 | 250 | 16 weeks | PASI 90: 0.5% | sPGA: 28.9% |
| Griffiths et al. | 2015 | 3 | 1224 | 12 weeks | PASI 100: 5.3% PASI 100: 7.3% | sPGA: 5.9% sPGA: 8.6% | |
| 1346 | |||||||
| Reich et al. | 2017 | 3 | 1090 | 52 weeks |
Week 12: PASI 100: 5% Week 28: PASI 100: 11% |
Week 12: PGA 0/1: 48% Week 28: PGA 0/1: 45% |
|
| 1023 | |||||||
| Langley et al. | 2014 | 3 | 1306 | 52 weeks | PASI 100: 4.3% | IGA 0/1: 27.2% | |
| Adalimumab | Armstrong et al. | 2019 | 3 | 837 | 24 weeks | PASI 100: 25.1% | IGA 0: 29.4% |
| 992 | |||||||
| Reich et al. | 2019 | 3 | 605 | 12 weeks | PASI 100: 23% | sPGA 0: 23% | |
| Saurat et al. | 2008 | 3 | 108 | 16 weeks | PASI 100: 16.7% | PGA 0/1: 73.1% | |
| Menter et al. | 2008 | 3 | 1212 | 52 weeks |
Week 4: PASI 100: 1% Week 12: PASI 100: 14% Week 24: PASI 100: 22% |
Week 4: PGA 0: 1% Week 12: PGA 0: 16% Week 24: PGA 0: 24% |
|
| 606 | |||||||
| 513 | |||||||
| Certolizumab pegol | Gottlieb et al. | 2018 | 3 | 183 | 16 weeks |
I: 200 mg: PASI 90: 35.8%, 400 mg: PASI 90: 43.6% II: 200 mg: PASI 90:52.6% 400 mg: PASI 90: 55.4% |
I: 200 mg: PGA 0/1: 47% 400 mg: PGA 0/1: 57.9% II: 200 mg: PGA 0/1: 66.8% 400 mg: PGA 0/1: 71.6% |
| 178 | |||||||
| Lebwohl et al. | 2018 | 3 | 559 | 48 weeks | 200 mg: PASI 90: 31.2% 400 mg: PASI 90: 34% | 200 mg: PGA 0/1: 48.3% 400 mg: PGA 0/1: 58.4% | |
| Ustekinumab | Paul et al. | 2019 | 3 | 166 | 52 weeks | PASI 90: 98% | sPGA 0: 36.1% |
| Tsai et al. | 2011 | 3 | 121 | 36 weeks | PASI 100: 8.2% | PGA 0: 27.9% | |
| Gordon et al. | 2018 | 3 | 506 | 40 weeks |
PASI 90: 42.0% PASI 90: 47.5% |
sPGA 0/1: 63% sPGA 0/1: 61% |
|
| 491 | |||||||
| Thaci et al. | 2015 | 3 | 676 | 52 weeks | PASI 100: 28.4% | IGA 0/1: 67.5% | |
| Secukinumab | Thaci et al. | 2015 | 3 | 676 | 52 weeks | PASI 100: 44.3% | IGA 0/1: 82.9% |
| Blauvelt et al. | 2015 | 3 | 159 | 12 weeks | PASI 100: 43.1% | IGA 0/1: 69% | |
| Bagel et al. | 2018 | 3 | 1102 | 52 weeks | PASI 100: 45.3% | IGA 0/1: 78.6% | |
| Ixekizumab | Griffiths et al. | 2015 | 3 | 1224 | 12 weeks |
2: every 4 weeks: PASI 90: 59.7% 2: every 2 weeks: PASI 90: 70.7% 3: every 4 weeks: PASI 100: 30.8% 3: every 2 weeks: PASI 100: 40.5% |
2: every 4 weeks: sPGA 0: 32.3% 2: every 2 weeks: sPGA 0: 41.9% 3: every 4 weeks: sPGA 0/1: 72.9% 3: every 2 weeks: sPGA 0/1: 83.2% |
| 1346 | |||||||
| Farahanik et al. | 2016 | 3 | 1296 | 12 weeks |
Every 4 weeks: PASI 100: 33.6% Every 2 weeks: PASI 100: 35.3% |
Every 4 weeks: sPGA 0/1: 76.4% Every 2 weeks: sPGA 0/1: 81.8% |
|
| Leonardi et al. | 2012 | 3 | 141 | 12 weeks |
10 mg: PASI 100: 0% 25 mg: PASI 100: 17% 75 mg: PASI 100: 38% 150 mg: PASI 100: 39% |
10 mg: sPGA 0: 7% 25 mg: sPGA 0: 20% 75 mg: sPGA 0: 38% 150 mg: sPGA 0: 46% |
|
| Brodalumab | McMichael et al. | 2018 | 3 | 1849 | 52 weeks |
Week 12: black: PASI 100: 50% Asian: PASI 100: 43.6% White: PASI 100: 40.3% Latino: PASI 100: 44.7% Week 52: black: PASI 100: 60% Asian: PASI 100: 42.9% White: PASI 100: 51.9% Latino: PASI 100: 52.5%) |
Week 12: black: sPGA 0/1: 75% Asian sPGA 0/1: 82.1% White: sPGA 0/1: 79.1% Latino: sPGA 0/1: 76.5%) Week 52: black: sPGA 0/1: 70% Asian sPGA 0/1: 71.4% White: sPGA 0/1: 65.9% Latino: sPGA 0/1: 67.5% |
| Papp et al. | 2012 | 2 | 198 | 12 weeks |
70 mg: PASI 100: 18% 140 mg: PASI 100: 38% 210 mg: PASI 100: 62% 280 mg: PASI 100: 29% |
70 mg: sPGA 0/1: 26% 140 mg: sPGA 0/1: 85% 210 mg: sPGA 0/1: 80% 280 mg: sPGA 0/1: 69% |
|
| Gottleib et al. | 2018 | 3 | 4373 | 12 weeks | PASI 100: 65.3% | sPGA 0: 65.3% | |
| Umezawa et al. | 2016 | 2 | 145 | 52 weeks |
140 mg: PASI 100: 43.8% 210 mg: PASI 100: 55.6% |
140 mg: sPGA 0/1: 69.9% 210 mg: sPGA 0/1: 91.7% |
|
| Guselkumab | Blauvelt et al. | 2017 | 3 | 837 | 48 weeks | PASI 100: 47.4% |
IGA 0: 50.5% IGA 0/1: 80.5% |
| Reich et al. | 2017 | 3 | 992 | 24 weeks | PASI 100: 44.2% | IGA 0: 51.8% | |
| Tildrakizumab | Papp et al. | 2015 | 3 | 355 | 72 weeks |
Week 16: PASI 90: 34.2% Week 52: PASI 90: 60.6% |
Week 16: PGA 0/1: 60.2% Week 52: PGA 0/1: 73.6% |
| Risankizumab | Gordon et al. | 2018 | 3 | 506 | 1 year |
PASI 90: 75.3% PASI 90: 74.8% |
PGA 0/1: 87.8% PGA 0/1: 83.7% |
| 491 | |||||||
| Reich et al. | 2019 | 3 | 605 | 44 weeks | PASI 90: 72% | sPGA 0/1: 84% | |
| Suleiman et al. | 2019 | 3 | 1903 | 16 weeks | PASI 90: 77% | sPGA 0/1: 88% | |
| Papp et al. | 2017 | 2 | 166 | 48 weeks | PASI 100: 45% | SPGA 0/1: 89% |
Results
Eight (17.8%) clinical trials had a PASI-PGA variance of < 5%, 4 (8.9%) trials had a variance of 5–10%, and 33 (73.3%) trials had a variance of > 10% (Fig. 1). The average PASI-PGA variation for all studies was 20% (standard deviation: 15%). Only two trials, IMMvent and AMAGINE, had 0 variation between the PASI and PGA scores. In fact, 80% clinical trials on adalimumab had a variation of < 5% between the PASI and PGA scores. Other biologics that had clinical trials with < 5% PASI-PGA variation were: etanercept (0.6% and 1.3%) and guselkumab (3.1%). The greatest differences were in the IXORA-S trial and the CHAMPION trial, with a variability of 61.9% and 56.4% between PASI and PGA, respectively.
Fig. 1.
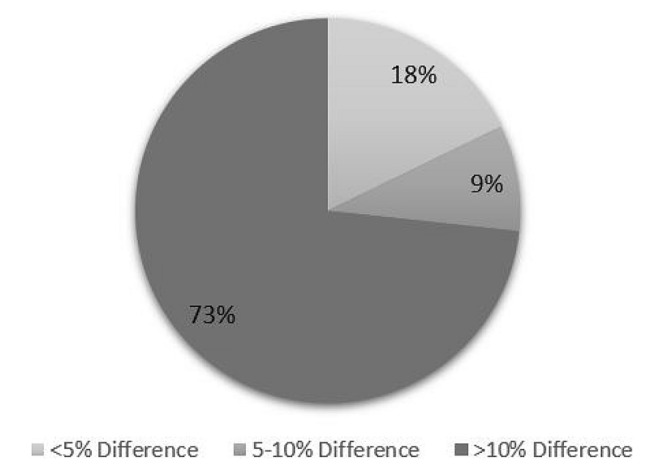
Differences in PASI 100 and PGA 0 reported in examined trials. Only 18% of studies have < 5% difference between the reported PASI 100 and PGA 0, while 74% of studies examined in this report had > 10% difference
Trials for the same biologic also had PASI-PGA variations. For example, although IXORA-S showed a high variability for ustekinumab between the two outcome measures, other trials such as the PEARL, UltMMa-1, UltMMa-2, and CLEAR trial showed much lower variances (Table 1). Due to this variation, many patients who achieved clearance under one assessment did not reach it by the other method.
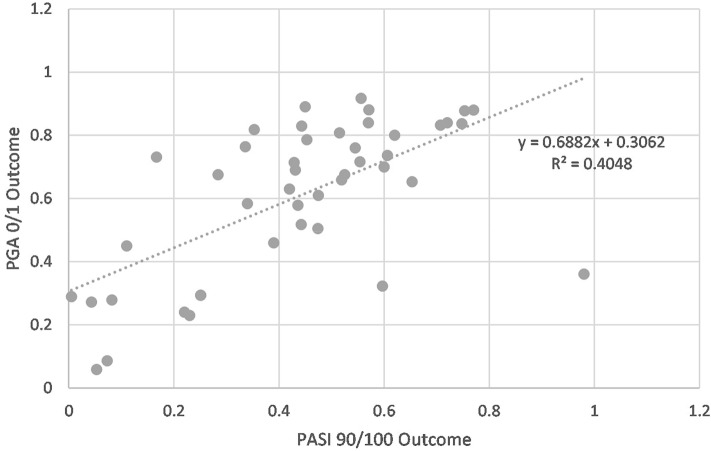
Further analysis revealed a significant, moderately positive correlation between PASI and PGA scores (Pearson coefficient = 0.64, r2 = 0.41, p < 0.001). The two scales tended to correspond fairly well except at the extremes of therapeutic efficacy (Fig. 2), especially at the lower cutoffs.
Fig. 2.
Correlation between PASI 90/10 and PGA 0/1 for clinical trials evaluating psoriasis severity. A weakly positive correlation exists between PASI and PGA scores, with the strongest correlation occurring within therapeutic range and falling off towards the extremes
Discussion
The wide range of variability between PASI and PGA scores between trials highlights their lack of standardization. A reliable outcomes measure is needed to better interpret disease severity and improvement, especially as better biologics are developed. The lack of a valid and consistent outcome measure makes it difficult to compare drugs and perform cross-analysis of different clinical trials.
Both PASI and PGA have shortcomings that increase variability, contributing to their apparent differences in clinical trials. Some of the score differences may stem from the PASI 90/100 being a relative parameter (indicating improvement) while the PGA is a measure of absolute severity. However, several other inherent qualities of the PASI and PGA scores further explain the observed discrepancies. The PASI score has difficulty in assessing the percentage of affected skin, inability to separate milder cases, lack of linearity, and lack of sensitivity [2]. There is also high variability in calculations for BSA for what is considered “high” or “full clearance.” Common BSA estimation methods, such as the “rule of nines,” may overestimate BSA affected by psoriasis, leading to score variability among inexperienced scorers [3]. The PGA also has shortcomings. Though easier to perform, PGA lacks clear definitions, leading to higher intra- and inter-grader variation and low score reproducibility [4]. Furthermore, both PASI and PGA lack patient perspective, ignoring common symptoms such as pain and pruritus. Patients who respond to treatment without complete clearance may still experience substantial symptoms of psoriasis that interfere with quality of life [5]. Due to these inherent qualities, it becomes almost inevitable that usage of PASI and PGA scores would contribute variation to a study when used as measures of clearance.
Though both the PASI and PGA are not perfect assessments, an objective measure of psoriasis severity is necessary. These measures act as an end goal for both patients and physicians, where achieving full clearance (a PASI 100 or PGA 0) is a goal to work towards. However, they should be regarded as a standard measure for physicians to rate visible signs of disease rather than a standard measure of overall disease severity. As the PASI is almost used as a universal measure, it can continue to be utilized as long as clinicians can objectively measure disease severity with similar techniques. Techniques such as plaque tracing and point counting grids may aid in making these calculations more accurate and reduce score variation [3].
A systematic review of six severity scores (PASI, BSA, PGA, LS-PGA, SPI, and SAPASI) concluded that none were ideal for evaluating psoriasis, but the PASI score was the most thoroughly studied and validated [6]. The use of both PASI and PGA together has been deemed redundant. Our findings support this conclusion by demonstrating that the PASI and PGA show great variability when measuring the same results and their concurrent use only makes analyses more complicated. We also corroborated previous findings that PASI and PGA scores correlate fairly well across the range of what is considered in therapeutic efficacy [1]. Outside of this range, we hypothesize the variation from subjective score calculations such as percentage clearance and BSA affected, increased as differences became more difficult to elucidate. These fluctuations, combined with fewer data points at the therapeutic extremes, would be reflected differently in each score and contributed to the decrease in correlation.
Dermatologists often reference and cite the clearance rates from clinical trials to patients when deciding on which biologic to use. If estimations of complete clearance continue to have high variability, it would be difficult to compare differences in outcomes between treatments. Concrete steps to address this include clarifying the definition of end points, such as what constitutes “clear” versus what is “almost clear.” It may also be useful to account for patient considerations. Patients often aim at achieving “almost cleared” more than “complete clearance” and prioritize having certain body areas cleared more than others [7]. Improving the accuracy of BSA calculations can also reduce variability within current measures. Finally, it is important to develop and test new methods of severity assessment. While multiple new scales have been created, none are widely used.
These results suggest that further research is needed to determine the most appropriate and sensitive parameters for measuring biologic therapeutic efficacy. In terms of clinical trials, the use of a single standard measure would make it easier to make meaningful comparisons of clearance results across multiple trials. Standardization of BSA estimation, accurately capturing patient-oriented measures and combining objective and subjective assessments may reduce variability in evaluation of disease severity.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Albert G. Wu, Jade Conway, Lauren Barazani, Bipasha Roy, Abigail Cline and Frederick Pereira have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12746279.
References
- 1.Robinson A, Kardos M, Kimball AB. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J Am Acad Dermatol. 2012;66(3):369–375. doi: 10.1016/j.jaad.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Berth-Jones J, Thompson J, Papp K, On behalf of the Copenhagen Psoriasis Working Group1 A study examining inter-rater and intrarater reliability of a novel instrument for assessment of psoriasis: the Copenhagen Psoriasis Severity Index. Br J Dermatol. 2008;159(2):407–412. doi: 10.1111/j.1365-2133.2008.08680.x. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft DM, Po ALW, Williams HC, Griffiths CEM. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality: measures of severity in psoriasis. Br J Dermatol. 1999;141(2):185–191. doi: 10.1046/j.1365-2133.1999.02963.x. [DOI] [PubMed] [Google Scholar]
- 4.Chow C, Simpson MJ, Luger TA, Chubb H, Ellis CN. Comparison of three methods for measuring psoriasis severity in clinical studies (part 1 of 2): change during therapy in Psoriasis Area and Severity Index, Static Physician’s Global Assessment and Lattice System Physician’s Global Assessment. J Eur Acad Dermatol Venereol. 2015;29(7):1406–1414. doi: 10.1111/jdv.13132. [DOI] [PubMed] [Google Scholar]
- 5.Strober B, Papp KA, Lebwohl M, Reich K, Paul C, Blauvelt A, Gordon KB, Milmont CE, Viswanathan HN, Li J, Pinto L, Harrison DJ, Kricorian G, Nirula A, Klekotka P. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol. 2016;75(1):77–82.e7. doi: 10.1016/j.jaad.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Puzenat E, Bronsard V, Prey S, Gourraud P-A, Aractingi S, Bagot M, Cribier B, Joly P, Jullien D, Le Maitre M, Paul C, Richard-Lallemand M-A, Ortonne J-P, Aubin F. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24:10–16. doi: 10.1111/j.1468-3083.2009.03562.x. [DOI] [PubMed] [Google Scholar]
- 7.Egeberg A, Thyssen JP. Factors associated with patient-reported importance of skin clearance among adults with psoriasis and atopic dermatitis. J Am Acad Dermatol. 2019;81(4):943–949. doi: 10.1016/j.jaad.2019.06.018. [DOI] [PubMed] [Google Scholar]