Abstract
This study was conducted to investigate the effects of Bacillus amyloliquefaciens (BA) and Saccharomyces cerevisiae (SC) as directed-fed microbials on performance, intestinal microflora, and intestinal morphology in broiler chickens. A total of four hundred one-day-old broiler chickens were randomly divided into 16 pens of 25 chickens each, and every treatment had 4 replicated pens with two pens of males and females respectively. A formulated corn-soybean meal based control diets and experimental diets, including 0.1% BA (1×107 colony-forming units (CFU)/kg), the mixture of 0.05% BA (5×106 CFU/kg) and 0.05% SC (5×106 CFU/kg), and 10 ppm antibiotic (avilamycin), were fed for 5 weeks. The results showed no significant difference in the growth performance among all treatments. Supplementation of the mixture of BA and SC increased acetate and propionate and decreased the E. coli in ceca compared to control and antibiotic treatment. The treatments with antibiotic, BA, and the mixture of BA and SC compared to control treatment increased villus height / crypt depth ratio and decreased ammonia in excreta. In addition, supplementation of BA and the mixture of BA and SC compared to antibiotic treatment increased serum high-density lipoprotein, and decreased serum glutamic-oxaloacetic transaminase, respectively. In conclusion, supplementation of the mixture of BA and SC was better than added BA only, and the mixed probiotics product could potentially alter the use of avilamycin in broiler diets.
Keywords: antibiotic, Bacillus amyloliquefaciens, broilers, Saccharomyces cerevisiae
Introduction
Antibiotics are used to promote animal growth and improve immunity to stressors, including heat and the intensive farming system. However, the misuse and overuse of antibiotics may cause antibiotic resistance in chickens. This could pose a threat to public health if dangerous infections of drug-resistant bacteria were spread via the food chain (Smith et al., 2003). As a result, the use of antibiotics as growth promoters was banned from the EU on January 1, 2006 (Burch, 2006).
Direct-fed microbials (DFM), including Lactobacillus, Bacillus and Saccharomyces cerevisiae (SC), have been studied as potential alternatives to feed additives (Salim et al., 2013). Bacillus is gram-positive bacteria that can produce endospores to protect itself from a stressful environment. Dietary supplementation with 2% Bacillus amyloliquefaciens (BA) was reported to decrease NH3 emissions in broiler excreta (Ahmed et al., 2014). In addition, BA could also stabilize the cecal microbial population by increasing Lactobacillus counts and decreasing E. coli (An et al., 2008; Ahmed et al., 2014). While Mountzouris et al. (2015) reported that SC diet supplements had no effect on growth performance. Haldar et al. (2011) showed that SC supplements could increase ileal villus height and decrease E. coli counts in the digestive systems of broilers.
BA and SC demonstrated outstanding production of extracellular enzymes including protease, amylase and lipase (Shirazi et al., 1998). These enzymes may improve the nutritional digestibility of protein and starch in broilers (Diaz, 2007). The combination of Lactobacillus, Bacillus and SC as DFM resulted in benefits to growth performance, immune response and improved intestinal morphology in early stage broilers (Salim et al., 2013).
It is hypothesized, therefore, that DFM supplementation has potential benefits for the poultry industry. Few reports, however, have studied the mixture of BA and SC as a feed additive to the broiler diet. Therefore, this study was conducted to assess the effects of mixed BA and SC supplementation on growth performance, intestinal microbiota and intestinal morphology in broiler chickens, compared to the use of antibiotics (avilamycin).
Materials and Methods
Growth Curve, Acid and Basic Tolerance, and Enzyme Activity of BA and SC
The BA was inoculated in Lysogeny broth (LB) and the SC was inoculated in Yeast-Mald (YM) at 37°C for 24 hours. After inoculation, 1 mL of these broth culture was added to 9 mL LB and YM broth and colony-forming units (CFU) of BA and SC were measured in 0, 6, 12, 18 and 24 hours. The assay of acid and basic solution tolerance was modified from the method of Hairul Islam et al. (2011). The pH of acid and basic solution was adjusted to 2.0, 3.0 and 12.0 with HCl and NaOH. 1 mL of BA or SC broth culture was added to stimulated solution and visible colony counts were calculated after 3 hours. The enzyme activities of BA were assayed by radio enzyme diffusion which according to method of Walsh et al. (2005) and APIZYM system (bioMerieux, Marcy-l'Étoile, France). The APIZYM test was performed according to the instructions. The reaction was terminated by addition of one drop each of A and B APIZYM reagents and the following results were examined in the end. The assay of mannanase and β-glucosidase were according to the methods of Lai et al. (2015) and Hernandez et al. (2003).
Experimental Birds and Housing
This study was conducted at National Chung Hsing University, Taiwan; the experimental protocol for animal use was approved by the Animal Care and Use Committee. A total of four hundred one-day-old broiler chickens (Ross 308) were evenly divided by gender and randomly allocated to one of 4 treatments, each of which had 4 replicate pens per treatment and 25 birds per pen (total of 100 birds per treatment). Initially, the average BW of the birds was similar among the different pens (average 46.0 to 46.5 g/bird approximately). All of the birds were placed in a temperature controlled house. The temperature was maintained at 33±1°C until the birds reached 7 d of age, before gradually being decreased to 27±1°C until the birds reached 21 d of age; after this point, the broilers were maintained at 27±1°C.
Feeding Schedule and Dietary Composition
The experiment lasted for 35 d and there were 2 phases: starter (1 to 21 d) and finisher (22 to 35 d). Diets (in mash form) and water were provided ad libitum. The birds in the control group were fed diets based on corn-soybean meal, and the other 3 groups were provided experimental diets. These were based on the basal diet but also contained an additional 10 ppm antibiotic (avilamycin), 0.1% BA to reach 1×107 CFU/g (Determined CFU, starter diet: 4×107 CFU/g, finisher diet: 4×107 CFU/g) as DFM-1 or a mixture of 0.05% BA to reach 5×106 CFU/g (Determined CFU, starter diet: 7×106 CFU/g, finisher diet: 8×106 CFU/g) and 0.05% SC to reach 5×106 CFU/g (Determined CFU, starter diet: 8×106 CFU/g, finisher diet: 8×106 CFU/g) as DFM-2 (Table 1). Starter and grower diets were offered to the birds from 1–21 d and from 22–35 d of age, respectively. During the entire experimental period (35 d), the diets were formulated to meet the requirements suggested by the Ross Broiler Management Manual (2009).
Table 1. Ingredients and chemical composition of the experimental diets for broilers.
| Ingredients | Starter diet (1–21 days) |
Finisher diet (22–35 days) |
|---|---|---|
| ------------------ g/kg ------------------ | ||
| Corn, yellow | 472.6 | 518.0 |
| Soybean meal | 345.2 | 295.9 |
| Full fat soybean meal | 100.0 | 100.0 |
| Soybean oil | 35.1 | 45.0 |
| Monocalcium phosphate | 18.6 | 16.6 |
| Calcium carbonate | 16.1 | 13.4 |
| l-Lysine-HCl | 3.8 | 3.2 |
| dl-Methionine | 2.0 | 1.3 |
| NaCl | 3.8 | 3.8 |
| Choline-Cl | 0.8 | 0.8 |
| Vitamin premix1 | 1.0 | 1.0 |
| Mineral premix2 | 1.0 | 1.0 |
| Total | 1000.0 | 1000.0 |
| Calculated nutrient value | ||
| ME, kcal/ kg | 3050.10 | 3175.26 |
| Crude protein, % | 23.00 | 21.01 |
| Calcium, % | 1.05 | 0.90 |
| Total Phosphorus, % | 0.76 | 0.70 |
| Available Phosphorus, % | 0.50 | 0.45 |
| Lysine, % | 1.43 | 1.25 |
| Methionine + Cystine, % | 1.07 | 0.96 |
Supplied per kg of diet: Vit A 15000 IU; Vit. D3 3000 IU; Vit. E 30 mg; Vit. K3 4 mg; Riboflavin 8 mg; Pyridoxine 5 mg; Vit. B12 25 µg; Ca-pantothenate 19 mg; Niacin 50 mg; Folic acid 1.5 mg; Biotin 60 µg.
Supplied per kg of diet: Co (CoCO3) 0.255 mg; Cu (CuSO4·5H2O) 10.8 mg; Fe (FeSO4·H2O) 90 mg; Zn (ZnO) 68.4 mg; Mn (MnSO4·H2O) 90 mg; Se (Na2SeO3) 0.18 mg.
Performance, Serum and Intestinal Content Collection
Body weight (BW) of chickens per pen and feed intake (FI) were recorded at 1, 21 and 35 d of age. Body weight gain (BWG) and feed conversion ratio (FCR) were recorded and calculated. On day 35, 16 chickens per treatment (four birds from each pen) close to average weight were selected for sampling. Fifty grams of excreta per treatment, excreted over 2 h, were collected randomly in 3 plastic plates from the cages of selected birds to determine ammonia content. Blood from the brachial vein was collected (5 mL) via cardiac puncture using a vacutainer tube. The blood was centrifuged at 3000×g for 30 min to obtain the serum. It was then stored at −20°C until it was analyzed for cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), serum glutamic-oxaloacetic transaminase (SGOT) and serum glutamic-pyruvic transaminase (SGPT) levels. After the blood was collected, the birds were euthanized by exsanguination and the gastrointestinal tract was removed from the carcass. About 15-cm segments of ceca and ileum were dissected and approximately 10 g content of each bird was collected in sterile plastic plates for subsequent study. The ileal and cecal contents of four chickens in same replicates were well mixed in same plastic plate for subsequent study.
Microbial Populations in Ileal and Cecal Content
To determine microbial populations, strains of E. coli, C. perfringens and lactic acid bacteria were cultured with chromogenic medium agar (CHROMagar™ ECC), TSC agar (BD Difco TM) with D-cyclosrine and MRS medium (de Man Rogosa and Sharpe agar, Difco 288130), respectively. After anaerobic incubation at 37°C for 48 h, the microfloral counts were calculated. Bacterial populations were expressed as log10 CFU per gram of intestinal content.
Volatile Fatty Acid (VFA) Analysis of Cecal Contents
To determine VFA levels, including acetate, propionate, butyrate, isobutyrate, isovalate and n-valerate, 1 g of cecal content was mixed with 4 mL 25% metaphosphoric acid. Samples were centrifuged at 10,000×g for 20 min and the supernatant was transferred into a 2 mL tube stored at −20°C. The concentration of VFA was determined by gas chromatography with a flame ionization detector, fuse silica capillary column and nitrogen was used as the carrier gas. Volatile acid standard mixes (SUPELCO) were used as standard solutions.
Morphometric Analysis of the Small Intestine
At the end of the experiment (day 35), one bird per replicate cage from each treatment group (4 birds/treatment in total) was randomly selected and sacrificed. During necropsy, the gastrointestinal tract was removed and the small intestine was divided into two parts: the jejunum (from the pancreatic loop to Meckel's diverticulum) and the ileum (from Meckel's diverticulum to the ileo-caeco-colic junction). 3 cm long segments from the center of each tissue were fixed in 10% formalin for later morphometrical assays. The formalin-fixed gut wall was washed in PBS and embedded in paraffin wax. 3 µm sectioned tissue was stained using hematoxylin and eosin methods. The samples were analyzed by light microscopy and software (Motic image plus 2.0, Shimadu, Kyoto, Japan) was used for measuring the villus height and crypt depth in fifteen favorably-oriented and representative samples per treatment. The ratio of villus height to crypt depth was also calculated.
Ammonia Analysis of Ileal and Excreta Content
The assay of ammonia analysis was modified from the methods of Weatherburn (1967). 1 g of ileal and excreta sample was mixed with 4 mL 25% metaphosphoric acid and centrifuged at 15000×g for 10 min. 1.5 mL of supernatant was transferred into a 2 mL Eppendolf tube and stored at −20°C. 25µL of supernatant was mixed with 1 mL reagent A (5 g of phenol with 250 mg of sodium nitroprusside per liter of solution) and 1 mL reagent B (25 g of sodium hydroxide with 16.8 mL sodium hypochlorite per liter of solution). After waiting 15 min for color development at 37°C, absorbance was measured at 630 nm. NH4Cl was used as the standard solution.
Serum Characteristics Determination
Serum biochemical values, including serum glutamic-oxalocetic transaminase, serum glutamic-pyruvic transaminase, cholesterol, high-density lipoprotein and low-density lipoprotein, were measured using an automatic biochemical analyzer (7150 auto-analyzer, Hitachi, Tokyo, Japan).
Statistical Analysis
Data was subjected to ANOVAs as a completely randomized design using GLM function of the SAS software (SAS, 2004). Determination of significant statistical differences among the mean values of the four treatment groups used Tukey's honestly significant difference test with a significance level of P<0.05.
Results
Growth Curve of BA and SC, Acid and Basic Solution Tolerance, and Enzyme Activity
The growth curve, acid and basic solution on the viability of BA and SC are data not shown (Figure S1 and Table S1). Decreasing number of BA and SC after the acid and basic solution tolerance test are less than 2 log CFU/mL. The results of enzyme activities of BA were indicated that BA had enzyme activities including protease, xylanase, cellulase, amylase, cysteine aminopeptidase, acid phosphatase, phosphohydrolase, and α-glucosidase (data not shown, in Table S2 and Figure S2). The β-glucosidase activity of SC is 1.59 mU, and the Mannanase activity of SC is 13.28 mU.
Growth Performance
The effects of dietary antibiotic, DFM-1 and DFM-2 supplementation on the growth performance of broilers at difference phases are shown in Table 2. From 1–21 d, 22–35 d, and 1–35 d of age, there were no significant differences in BW, BWG, FI or FCR among all treatment groups (P>0.05).
Table 2. Effect of Bacillus amyloliquefaceins and Saccharomyces cerevisiae supplementation on growth performance of broilers1.
| Item | Experimental diets |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | Antibiotic | DFM-1 | DFM-2 | |||
| 1–21 d | ||||||
| Body weight (g) | 811 | 801 | 831 | 823 | 29.02 | 0.900 |
| Feed consumption (g) | 923 | 921 | 999 | 968 | 27.10 | 0.172 |
| Weight gain (g) | 764 | 755 | 785 | 777 | 28.81 | 0.887 |
| FCR | 1.21 | 1.22 | 1.25 | 1.28 | 0.03 | 0.377 |
| 21–35 d | ||||||
| Body weight (g) | 2139 | 2168 | 2179 | 2162 | 110.28 | 0.995 |
| Feed consumption (g) | 2064 | 2061 | 2061 | 2067 | 94.89 | 1.000 |
| Weight gain (g) | 1327 | 1367 | 1348 | 1338 | 88.17 | 0.990 |
| FCR | 1.57 | 1.51 | 1.54 | 1.55 | 0.03 | 0.703 |
| 1–35 d | ||||||
| Feed consumption (g) | 2986 | 2981 | 3061 | 3035 | 105.57 | 0.940 |
| Weight gain (g) | 2092 | 2121 | 2132 | 2115 | 110.00 | 0.995 |
| FCR | 1.44 | 1.41 | 1.44 | 1.44 | 0.03 | 0.904 |
Each value represents the mean of 4 replicates with 4 birds in each replicate.
DFM-1=B. amyloliquefaceins; DFM-2=the mixture of B. amyloliquefaceins and S. cerevisiae.
Microbial Population in Ileum and Cecum
The effects of dietary antibiotic, DFM-1 and DFM-2 supplementation on intestinal microflora are shown in Table 3. Treatment had no significant effect on Lactobacillus or Clostridium perfringens (P>0.05). Supplementation with DFM-2 and antibiotics resulted in significantly lower amounts of E. coli in ileal content compared to the control group (P<0.01). DFM-2 also significantly reduced E. coli concentration in cecal content when compared to the antibiotic treatment and control groups (P<0.05).
Table 3. Effect of Bacillus amyloliquefaceins and Saccharomyces cerevisiae supplementation on intestinal microflora concentration of broilers (35 d)1.
| Item | Experimental diets |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | Antibiotic | DFM-1 | DFM-2 | |||
| Lactic acid bacteria | ---------------------------- Log10 CFU/g ---------------------------- | |||||
| Ileum | 8.64 | 8.81 | 8.84 | 8.80 | 0.09 | 0.573 |
| Ceca | 11.27 | 11.37 | 11.28 | 11.36 | 0.13 | 0.944 |
| E. coli | ---------------------------- Log10 CFU/g ---------------------------- | |||||
| Ileum | 7.33a | 6.07c | 6.92ab | 6.49bc | 0.15 | 0.001 |
| Ceca | 8.23a | 8.14ab | 7.45bc | 7.28c | 0.19 | 0.027 |
| Clostridium perfringens | ---------------------------- Log10 CFU/g ---------------------------- | |||||
| Ileum | 7.40 | 7.41 | 7.36 | 7.19 | 0.21 | 0.905 |
| Ceca | 8.49 | 8.48 | 8.47 | 8.41 | 0.25 | 0.996 |
Each value represents the mean of 4 replicates with 4 birds in each replicate.
Means with in the same row with different letters are significantly different (P<0.05).
DFM-1=B. amyloliquefaceins; DFM-2=the mixture of B. amyloliquefaceins and S. cerevisiae.
Volatile Fatty Acids in Cecum
The effects of dietary antibiotic, DFM-1 and DFM-2 supplementation on cecal VFA are shown in Table 4. There were no significant differences in isobutyric acid, isovaleric acid or n-valeric acid levels among all treatment groups. However, treatment with DFM-1 and DFM-2 significantly increased in total VFA and acetic acid compared to the antibiotic and control groups (P<0.05), while treatment with DFM-2 resulted in significantly greater propionic acid concentrations (P<0.01). In addition, supplementation with DFM-1 significantly increased butyric acid levels (P<0.05).
Table 4. Effect of Bacillus amyloliquefaceins and Saccharomyces cerevisiae supplementation on cecal VFA concentration of broilers (35 d)1.
| Item | Experimental diets |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | Antibiotic | DFM-1 | DFM-2 | |||
| -----------------------------µmole/g----------------------------- | ||||||
| Acetic acid | 11.8b | 11.84b | 16.02a | 18.73a | 0.93 | 0.011 |
| Propionic acid | 3.98b | 3.94b | 4.66b | 8.84a | 0.66 | 0.002 |
| Isobutyric acid | 0.55 | 0.52 | 0.76 | 0.90 | 0.09 | 0.068 |
| Butyric acid | 5.46b | 6.33b | 8.83a | 6.06b | 0.66 | 0.041 |
| Isovaleric acid | 0.90 | 0.88 | 1.21 | 1.34 | 0.12 | 0.079 |
| n-Valeric acid | 0.93 | 0.94 | 1.17 | 1.32 | 0.13 | 0.195 |
| Total VFA | 23.62b | 24.44b | 32.64a | 37.70a | 1.82 | 0.002 |
Each value represents the mean of 4 replicates with 4 birds in each replicate.
Means with in the same row with different letters are significantly different (P<0.05).
VFA=Volatile fatty acid; DFM-1=B. amyloliquefaceins; DFM-2=the mixture of B. amyloliquefaceins and S. cerevisiae.
Intestinal Morphology
The effects of dietary antibiotic, DFM-1 and DFM-2 supplementation on the intestinal morphology of broilers are shown in Table 5 and Fig. 1. There were no significant differences in villus height, crypt depth or villus height / crypt depth ratio in the jejunum among all treatment groups (P>0.05). However, supplementation with antibiotics, DFM-1 and DFM-2 resulted in higher ileal villus height and villus height / crypt depth ratio compared to the control group (P<0.01).
Table 5. Effect of Bacillus amyloliquefaceins and Saccharomyces cerevisiae supplementation on intestinal morphology of broilers (35 d)1.
| Item | Experimental diets |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | Antibiotic | DFM-1 | DFM-2 | |||
| Jejunum | ||||||
| Villus height, µm | 1407 | 1414 | 1425 | 1425 | 28.40 | 0.974 |
| Crypt depth, µm | 199 | 199 | 188 | 198 | 6.21 | 0.550 |
| Villus height/Crypt depth | 7.17 | 7.18 | 7.60 | 7.26 | 0.22 | 0.451 |
| Ileum | ||||||
| Villus height, µm | 782c | 993a | 992a | 929b | 14.94 | <0.001 |
| Crypt depth, µm | 166 | 177 | 171 | 174 | 5.13 | 0.571 |
| Villus height/Crypt depth | 4.81b | 5.51a | 5.86a | 5.50a | 0.19 | 0.008 |
Each value represents the mean of 16 replicates (1 bird per replicate×4 replicates per treatment×4 measurements per section).
Means with in the same row with different letters are significantly different (P<0.05).
DFM-1=B. amyloliquefaceins; DFM-2=the mixture of B. amyloliquefaceins and S. cerevisiae.
Fig. 1.
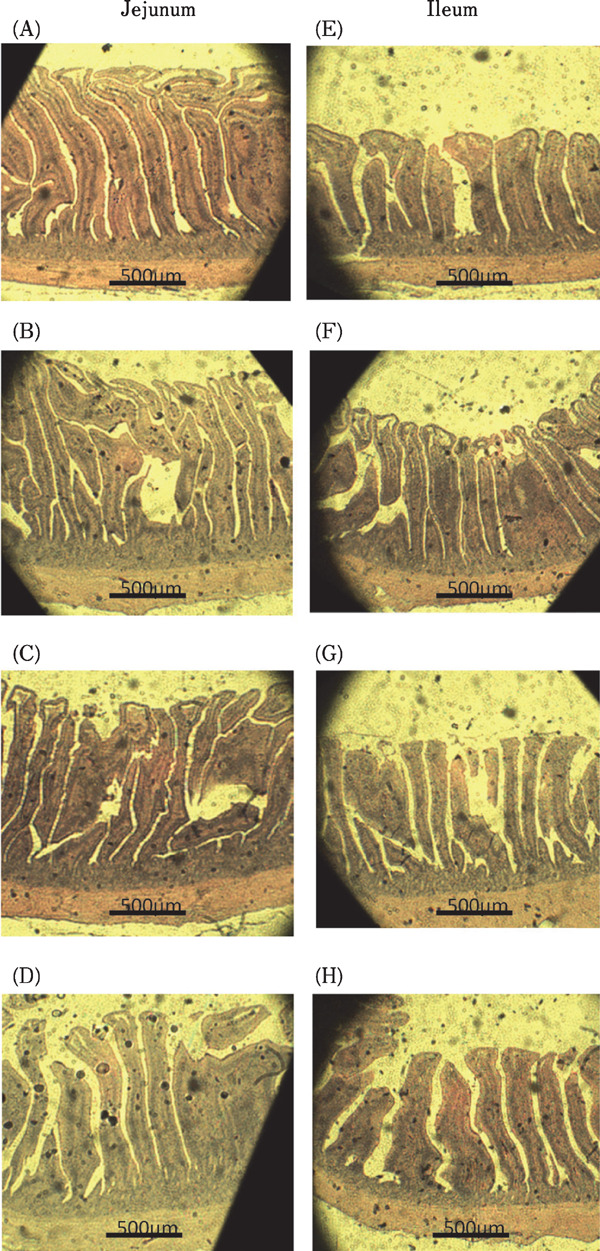
Photomicrography of jejunum and ileum of 35 d broiler in treatments with control, antibiotic, DFM-1 (Bacillus amyloliquefaciens), and DFM-2 (the mixture of Bacillus amyloliquefaciens and Saccharomyces cerevisiae). (A) Control, jejunum (B) Antibiotic, jejunum (C) DFM-1, jejunum (D) DFM-2, jejunum (E) Control, ileum (F) Antibiotic, ilium (G) DFM-1, ileum (H) DFM-2, ileum. Hematoxylin and eosin stain (40×) (method according to Huang et al., 2012).
Ammonia in Ileum and Excreta
The effects of dietary antibiotic, DFM-1 and DFM-2 supplementation on excreta and ileal ammonia concentrations are shown in Table 6. None of the treatments had a significant effect on ileal ammonia levels (P>0.05), however supplementation with antibiotics, DFM-1 or DFM-2 significantly decreased NH3 in excreta (P<0.01).
Table 6. Effect of Bacillus amyloliquefaceins and Saccharomyces cerevisiae supplementation on ileal and fecal ammonia concentration of broilers (35 d)1.
| Item | Experimental diets |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | Antibiotic | DFM-1 | DFM-2 | |||
| -------------------------µmole/g------------------------- | ||||||
| Ileum1 | 59.70 | 54.69 | 47.82 | 52.85 | 3.28 | 0.354 |
| Excreta2 | 56.61a | 46.16b | 46.41b | 42.34b | 1.72 | 0.008 |
Each value represents the mean of 4 replicates with 4 birds in each replicate.
Each value represents the mean of 3 replicates.
Means with in the same row with different letters are significantly different (P<0.05).
DFM-1=B. amyloliquefaceins; DFM-2=the mixture of B. amyloliquefaceins and S. cerevisiae.
Serum Characteristics
The effects of dietary antibiotic, DFM-1 and DFM-2 supplementation on serum characteristics are shown in Table 7. Supplementation with DFM-1 and DFM-2 significantly reduced serum SGOT and increased HDL compared to the antibiotic treatment group (P<0.05). However, none of the treatments had a significant effect on SGPT, serum cholesterol or LDL (P>0.05).
Table 7. Effect of Bacillus amyloliquefaceins and Saccharomyces cerevisiae supplementation on serum characteristics of broilers (35 d)1.
| Item | Experimental diets |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Control | Antibiotic | DFM-1 | DFM-2 | |||
| ------------------ U/L ------------------------- | ||||||
| SGOT | 177.7ab | 210.7a | 140.3b | 154.0b | 9.69 | 0.010 |
| SGPT | 4.5 | 5.3 | 4.3 | 4.4 | 0.42 | 0.393 |
| ------------------------------ mg/dL ------------------------------ | ||||||
| CHO | 70.7 | 64.3 | 61.0 | 69.3 | 2.84 | 0.213 |
| HDL | 47.5ab | 44.0b | 48.0a | 49.7a | 0.87 | 0.028 |
| LDL | 8.7 | 7.7 | 7.7 | 8.0 | 0.90 | 0.890 |
Each value represents the mean of 4 replicates.
Means with in the same row with different letters are significantly different (P<0.05).
DFM-1=B. amyloliquefaceins; DFM-2=the mixture of B. amyloliquefaceins and S. cerevisiae; SGOT=Serum glutamic-oxaloacetic transaminase; SGPT=Serum glutamic-pyruvic transaminase; CHO=Cholesterol; HDL=High-density lipoprotein; LDL=Low-density lipoprotein.
Discussion
The use of mixed probiotics as a feed additive is currently popular in the livestock industry. The Bacillus specie used in this study, BA, has demonstrated tolerable in vitro ability in both gastric juice (pH 3.0) and 0.3% bile salts; enzyme production, including protease (107.5 U/g), cellulase (0.531 U/g) and xylanase (0.075 U/g); and adhesion to the epithelial cells of the chicken crop (Teng et al., 2015). SC is well known for its production of extracellular lipase that may improve fat digestion in animals (Shirazi et al., 1998). Moreover, the cell walls of SC, making up 15 to 25% of its dry weight, is mainly composed of glucan and mannoproteins, including mannan-oligosaccharide and β-glucan (Yalçinkaya et al., 2008). It was reported to improve immune response and modulate intestinal microflora in animals (Corrigan et al., 2011; Vervicka and Oliveira, 2013). As mentioned above, a mixture of BA and SC was used to assess the benefits of DFM supplementation in broiler diets.
In this study, supplementation with BA and SC had no impact on growth performance across all treatment groups. This is in keeping with the findings of Chen et al. (2009), who reported that supplementation with Bacillus subtilis alone (106 CFU/g) or a mixture of Bacillus subtilis (106 CFU/g) and SC (108 CFU/g) had no effect on growth performance in broilers. Moreover, Ahmed et al. (2014) also showed that adding 0.1% and 0.5% BA (Bacillus amyloliquefaciens KB3) or supplementation with 109 CFU/g BA did not affect growth performance in broilers. However, Bacillus could play an important role in modulating the cecal microbial population. Lei et al. (2014) pointed out that adding BA to broiler diets could decrease E. coli (from 6.99 to 6.11 log CFU/g) and increase Lactobacillus (from 7.88 to 8.47 log CFU/g) in the cecum. In this study, DFM-1 and DFM-2 decreased the cecal counts of E. coli compared to the antibiotic and control groups. Therefore, dietary supplementation with antibiotics or a mixture of BA and SC could decrease ileal E. coli. Similarly, Kim et al. (2011) reported that diets with avilamycin decreased the population of E.coli in intestine of broilers and Salim et al. (2013) found that supplementation with a probiotic mixture that included Lactobacillus reuteri, Bacillus subtilis and SC, decreased E. coli amounts in broilers' ceca. Wiyada (2012) reported that the antimicrobial products produced by Bacillus, such as bacilysin, macrolactin and bacillaene from nonribosom al peptide synthetases and polyketide synthetases, could inhibit intestinal pathogenic bacteria. Furthermore, both Bacillus and SC consume oxygen rapidly, encouraging anaerobic probiotics in the intestines to produce lactate in order to impair the growth of opportunistic pathogens (Wu et al., 2011; Song et al., 2013). VFA production (acetate, propionate, and butyrate) is also responsible for the reduced numbers of Enterobacteriaceae and Salmonella (Van der Wielen et al., 2000, 2001). In the current study, DFM-1 and DFM-2 groups had higher acetate and total VFA than the control or antibiotic groups; moreover, the DFM-2 group had greater propionate levels than other treatments which results in reducing number of cecal E. coli. Similarly, broiler fed with a mixture of 11 probiotic Lactobacillus stains had decreased population of E. coli in cecal digesta through production of more acetic acid, propionic acid and total VFA (Mookiah et al., 2014).
The inhibition of pathogenic bacteria in BA supplemented diets could increase villus height and villus height to crypt depth ratio in broilers (Lei et al., 2014). Increasing villus height means greater nutrient absorption because of the increase in intestinal surface area (Xu et al., 2002). The crypt is known as the villus factory; deeper crypts indicate faster cell turnover to meet the demand for new tissue in response to inflammation or sloughing due to bacterial toxins or pathogen (Yason et al., 1987). Additional tissue turnover leads to nutrient waste in order to maintain intestinal morphology and lowers feed efficiency (Xu et al., 2002). In the current study, treatment with antibiotics, DFM-1 and DFM-2 resulted in increased villus height and villus height / crypt ratio in the ileum. Diaz (2007) showed that supplementation with 0.1% BA could improve intestinal morphology, resulting in higher nutritional digestibility of protein (8.1%), fat (7.3%) and starch (3.8%) in broilers.
Clearly, better digestibility will decrease the excretion of non-utilized nutrients, resulting in less ammonia in the excreta of broilers. Microorganisms in manure degrade nitrogenous compounds, with ammonia as the major end product (Ferket et al., 2002). High concentration of ammonia in poultry houses suppresses growth performance, immune response and increases disease susceptibility (Wei et al., 2015). In this study, supplementation with BA or a mixture of BA and SC significantly decreased fecal ammonia compared to the control and antibiotic groups. Ahmed et al. (2014) also reported that adding various concentration of BA (0.1%, 0.5%, 1% and 2%) to broiler diets decreased fecal ammonia emissions, which correlates with our own findings.
Serum enzymes, including SGOT and SGPT, are mainly monitored to evaluate liver damage. Antibiotic supplementation increased hepatic work load due to the metabolic demand of incoming antibiotics and resultant increase in SGOT (Murwani and Bayuardhi, 2007), while the mix of probiotics (Lactobacillus plantarum, L. bulgaris, L. acidophilus, L. rhamnosus, Bifadobacterum binfadum, Streptococus thermophilus, Enterococus faecium, Aspergillus oryzae and Candida pintolopesi) was studied to decrease the SGOT of broilers (Khan et al., 2013). In the current study, supplementation with BA or a mixture of BA and SC decreased SGOT when compared to the antibiotic group, either. In addition, treatments with DFM-1 and DFM-2 not only decreased SGOT, but also increased serum HDL compared to the antibiotic treatment. The DFM-2 treatment group had higher VFA levels in the cecum that it may suggest that the suppressed hepatic cholesterol synthesis and increased HDL to redistribute cholesterol from the plasma to the liver (Chen and Anderson, 1984).
In conclusion, supplementation with DFM-2 increased acetate and propionate in the cecum, reduced E. coli counts in the ileum and cecum, increased ileal villus height and villus height / crypt ratio, increased serum HDL and decreased SGOT. Moreover, supplementation with a mixture of BA and SC is better than BA alone, suggesting that a mixed probiotics product could be a potential alternative to antibiotic growth promoters (e.g. avilamycin) in broiler diets.
Acknowledgments
The authors thank the Ministry of Science and Technology (MOST 104-2815-C-005-038-B and MOST 103-2628-B-005-001-MY3) for financially supporting this study.
References
- Ahmed ST., Islam MM, Mun HS, Sim HJ, Kim YJ, Yang CJ. Effects of Bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poultry Science, 93: 1963-1971. 2014. [DOI] [PubMed] [Google Scholar]
- An BK, Cho BL, You SJ, Paik HD, Chang HI, Kim SW, Yun CW, Kang CW. Growth performance and antibody response of broiler chicks fed yeast derived β-glucan and single-strain probiotics. Asian-Australasian Journal of Animal Sciences, 21: 1027-1032. 2008. [Google Scholar]
- Burch D. Anticipated effects of the withdrawal of antibiotic growth promoters (AGPs) from pigs in the European Union on 1st January 2006. http://www.octagon-services 2006.
- Chen WJ, Anderson JW, Jennings D. Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats. Experimental Biology and Medicine, 175: 215-218. 1984. [DOI] [PubMed] [Google Scholar]
- Chen KL, Kho WL, You SH, Yeh RH, Tang SW, Hsieh CW. Effects of Bacillus subtilis var. natto and Saccharomyces cerevisiae mixed fermented feed on the enhanced growth performance of broilers. Poultry Science, 88: 309-315. 2009. [DOI] [PubMed] [Google Scholar]
- Corrigan A, Horgan K, Clipson NB, Murphy RA. Effect of dietary supplementation with a Saccharomyces cerevisiae mannan oligosaccharide on the bacterial community structure of broiler cecal contents. Applied and Environmental Microbiology, 77: 6653-6662. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz D. Effect of Bacillus amyloliquefaciens CECT-5940 spores on broiler performance and digestibility. Accessed Nov. http://en.engormix.com/MA-poultry-industry/articles/effect-bacillusamyloliquefacienscect5940-t795/p0.htm 2007. [Google Scholar]
- Ferket PR, Van Heugten E, Van Kempen TATG, Angel R. Nutritional strategies to reduce environmental emissions from nonruminants. Journal of Animal Science. 80: 168-182. 2002. [Google Scholar]
- Hairul Islam VI, Prakash Babu N, Pandikumar P, Ignacimuthu S. Isolation and characterization of putative probiotic bacterial stain, Bacillus amyloliquefaciens, from Noth East Himalayan soil based on in vitro and in vivo functional properties. Probiotic and Antimicrobial Proteins, 3: 175-185. 2011. [DOI] [PubMed] [Google Scholar]
- Haldar S, Ghosh TK, Toshiwati, Bedford MR. Effects of yeast (Saccharomyces cerevisiae) and yeast protein concentrate on production performance of broiler chickens exposed to heat stress and challenged with Salmonella enteritidis. Animal Feed Science and Technology, 168: 61-71. 2011. [Google Scholar]
- Hernandez LF, Espinosa JC, Fernandez-Gonzalez M, Briones A. β-glucosidase activity in a Saccharomyces cerevisiae wine strain. International Journal of Food Microbiology, 80: 171-176. 2003. [DOI] [PubMed] [Google Scholar]
- Huang CW, Lee TT, Shih YC, Yu B. Effects of dietary supplementation of Chinese medicinal herbs on polymorphonuclear neutrophil immune activity and small intestinal morphology in weanling pigs. Journal of Animal Physiology and Animal Nutrition, 96: 285-294. 2012. [DOI] [PubMed] [Google Scholar]
- Kim GB, Seo YM, Kim CH, Paik IK. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poultry Science, 90: 75-82. 2011. [DOI] [PubMed] [Google Scholar]
- Khan RU, Rahman ZU, Javed I, Muhammad F. Effect of vitamins, probiotics and protein level on semen traits and seminal plasma biochemical parameters of post-moult male broiler breeders. British Poultry Science, 54: 120-129. 2013. [DOI] [PubMed] [Google Scholar]
- Lai LP, Lee MT, Chen CS, Yu B, Lee TT. Effects Effects of co-fermented Pleurotus eryngii stalk residues and soybean hulls by Aureobasidium pullulans on performance and intestinal morphology in broiler chickens. Poultry Science, 94: 2959-2969. 2015. [DOI] [PubMed] [Google Scholar]
- Lei X, Piao X, Ru Y, Zhang H, Péron A, Zhang H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian-Australasian Journal of Animal Sciences, 28: 239-246. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookiah S, Sieo CC, Ramasamy K, Abdullah N, Ho YW. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and cecal fermentation concentrations of broiler chicken. Journal of the Science of Food and Agriculture, 94: 341-348. 2014. [DOI] [PubMed] [Google Scholar]
- Mountzouris KC, Dalaka E, Palamidi I, Paraskeuas V, Demey V, Theodoropoulos G, Fegeros K. Evaluation of yeast dietary supplementation in broilers challenged or not with Salmonella on growth performance, cecal microbiota composition and Salmonella in ceca, cloacae and carcass skin. Poultry Science, 94: 2445-2455. 2015. [DOI] [PubMed] [Google Scholar]
- Murwani R, Bayuardhi B. Broilers serum cholesterol and glutamic oxaloacetic transaminase and their relation to antibiotic in feed and medication programs in four broiler producers in semarang region-central java, Indonesia. International Journal of Poultry Science, 6: 266-270. 2007. [Google Scholar]
- Salim HM, Kang HK, Akter N, Kim DW, Kim JH, Kim MJ, Na JC, Jong HB, Choi HC, Suh OS, Kim KW. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poultry Science, 92: 2084-2090. 2013. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT Guide for Personal Computers. Version 9.0.1. SAS Inst. Inc., Cary, NC: 2004. [Google Scholar]
- Shirazi SH, Rahman SR, Rahman MM. Production of Extracellular lipases by Saccharomyces cerevisiae. World Journal of Microbiology and Biotechnology, 14: 595-597. 1998. [Google Scholar]
- Smith DL, Johnson JA, Hrris AD, Furuno JP, Perencevich EN, Morris JG. Assessing risks for a pre-emergent pathogen: virginiamycin use and the emergence of streptogramin resistance in Enterococcus faecium. Lancet Infectious Diseases, 3: 241-249. 2003. [DOI] [PubMed] [Google Scholar]
- Song J, Xiao K, Ke YL, Jiao LF, Hu CH, Diao QY, Shi B, Zou XT. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poultry Science, 93: 581-588. 2013. [DOI] [PubMed] [Google Scholar]
- Teng PY, Chung CH, Chou YP, Yu B, Lee TT. Application of Bacillus amylaliquefaciens as a probiotic strain on broiler diet. Journal of the Chinese Society of Animal Science, 44: 118 2015. [Google Scholar]
- Van der Wielen PWJJ, Biesterveld S, Lipman LJA, Van Knapen F. Inhibition of a glucose-limited sequencing fed-batch culture of Salmonella enterica serovar enteritidis by volatile fatty acids representative of the ceca of broiler chickens. Applied and Environmental Microbiology 67: 1979-1982. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wielen PWJJ, Biesterveld S, Notermans S, Hofstra H, Urlings BAO, Van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Applied and Environmental Microbiology, 66: 2536-2540. 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetvicka V., Oliveira C. β(1-3)(1-6)-D-glucans modulate immune status in pigs: potential importance for efficiency of commercial farming. Annals of Translational Medicine, 2: 16 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh G, Murphy RA, Killeen GF, Power RF. Quantification of supplemental enzyme in animal feeding stuffs by radial enzyme diffusion. Applied Microbiology and Biotechnology, 67: 70-74. 2005. [DOI] [PubMed] [Google Scholar]
- Weatherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Analytical Chemistry, 39: 971-974. 1967. [Google Scholar]
- Wei FX, Hu XF, Xu B, Zhang MH, Li SY, Sun QY, Lin P. Ammonia concentration and relative humidity in poultry houses affect the immune response of broilers. Genetics and Molecular Research, 14: 3160-3169. 2015. [DOI] [PubMed] [Google Scholar]
- Wiyada M. Classification of Bacillus beneficial substances related to plants, humans and animals. Journal of Microbiology and Biotechnology, 22: 1597-1604. 2012. [DOI] [PubMed] [Google Scholar]
- Wu BQ, Zhang T, Guo LQ, Lin JF. Effects of Bacillus subtilis KD1 on broiler intestinal flora. Poultry Science, 90: 2493-2499. 2011. [DOI] [PubMed] [Google Scholar]
- Xu ZR, Zou XT, Hu CH, Xia MS, Zhan XA, Wang MQ. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of growing pigs. Asian-Australasian Journal of Animal Sciences, 12: 1784-1789. 2002. [Google Scholar]
- Yalçinkaya I, Güngör T, Başalan M, Erdem E. Mannanoligosaccharides (MOS) from Saccharomyces cerevisiae in broilers: effects on performance and blood biochemistry. Turkish Journal of Veterinary and Animal Sciences, 32: 43-48. 2008. [Google Scholar]
- Yason CV, Summers BA, Schat KA. Pathogenesis of rotavirus infection in various age groups of chickens and turkeys: pathology. American Journal of Veterinary Research, 6: 927-938. 1987. [PubMed] [Google Scholar]


