Abstract
The present study investigated the effects of ethanol extracts of Allium hookeri (leaf, root, and fermented root) on parameters of innate immunity, tumour cell viability and antioxidant effect in vitro. Innate immunity was measured by spleen lymphocyte proliferation, nitric oxide production by chicken macrophage HD11 cells and suppressive effect on tumour cell viability was assessed using chicken RP9 cells. Free radical scavenging capacity as a measure of antioxidant capacity was determined by 0.15 mM of DPPH solution. In vitro culture of chicken spleen lymphocytes with ethanol extract of Allium hookeri (62.5–500 µg/mL) significantly induced higher proliferation compared with media control. Stimulation of macrophages with ethanol extract of Allium hookeri (62.5–500 µg/mL) showed increased Nitric oxide production. Tumor cells growth was significantly inhibited by extracts of Allium hookeri at 15.6–125 µg/mL compared with medium control and all extracts exhibited greater than 80% scavenging activity at 1000 µg/mL compared with ethanol vehicle control. Above all, fermented root extracts showed strongest effects on antioxidant activity compared to leaf and root extracts.
Keywords: Allium hookeri, innate immunity, lymphocytes, macrophage, poultry, tumour cells
Introduction
Antibiotic growth promoters (AGPs) are commonly used as a dietary supplement to improve growth performance of livestock and poultry (Lillehoj and Lee, 2012; Lee et al., 2015a). However, the use of AGPs in animal agriculture is becoming increasingly restricted, and researchers are attempting to find alternatives to antibiotics for maintaining good growth performance and reducing the negative effects of diseases. In recent years, an increasing number of research trials with the use of feed additives for safe, high-quality meat production in the animal industry has been reported (Lee et al., 2011; Lillehoj and Lee, 2012; Xu et al., 2015a). Many documented in-feed supplements offer alternatives to antibiotics and have been linked to enhanced growth performance and innate immunity. In particular, certain plant-derived phytonutrients have been shown to provide growth-promoting and immune-enhancing effects (Banfield et al., 2002; Van Lunen, 2003; Kim et al., 2015a).
In the case of poultry, a variety of plant-derived feed additives have been reported for their economic benefits in animal production. According to recent reports, supplementation of animal feed with sulforaphane, isolated from broccoli sprout extracts, and essential oils such as oregano, thyme, and rosemary has been shown to enhance intestinal health in broiler chickens (Kristin et al., 2012; Lillehoj and Lee, 2012; Xu et al., 2015b). Similarly, oregano and rosemary oils have been shown to improve meat quality and prevent lipid oxidation in breast and thigh meat (Basmacioglu et al., 2004). Moreover, Hernadez et al. (2004) reported that feed containing essential oils (oregano, cinnamon, and labiatae extract) reduces daily feed intake of broilers and improves feed conversion compared with that of control birds.
A few studies have focused on the efficacy of medicinal plants on improving innate immunity, enhancing antioxidant capacity, and inhibiting tumour cell growth (Lee et al., 2007, 2008; Kim et al., 2013). Although a diverse array of medicinal plants have been traditionally used in Asian culture to enhance innate immunity and for treatment of illnesses and cancer, only a few studies have characterised the effects of these medical plants on immunity.
Allium hookeri, a member of the family Alliaceae subgenus Amerallium, is found in Ceylon, Greece, Yunnan, Southern China, Bhutan, Sri Lank, and India and has been used by locals to treat cough and cold and to heal burns and wounds (Sharma et al., 2011). The root of A. hookeri contains an abundance of organo-sulphur compounds, volatile sulphur compounds, proteins, prostaglandins, fructans, vitamins, and polyphenols as well as Allicin, the major flavour compound of garlic (A. sativum) (Dziri et al., 2012). Allicin and organic sulphur compounds are known to reduce cholesterol levels, decrease the risk of heart attack, and exert anti-inflammatory effects (Bae and Bae, 2012; Kim et al., 2012). However, few studies have characterised the effects of these medicinal plants on immunity.
The aim of this study was to examine the potential immune-enhancing properties of ethanol extracts of leaf, root, and fermented root of A. hookeri on the innate immune function of poultry. Cell proliferation and nitric oxide (NO) production assays were conducted to assess the effects of plant extracts on innate immunity. Additionally, avian tumour cells were used to further characterise the inhibitory effects of A. hookeri, and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assays were used to investigate the free radical scavenging activity of A. hookeri extracts.
Materials and Methods
Preparation of Samples
A. hookeri was obtained from the Agricultural Development & Technology Centre (Sunchang, South Korea). The voucher specimen (RDAAH15) is preserved at the National Institute of Agricultural Sciences (Jeonju, Korea). Fermented A. hookeri (patent# 10-2014-0154145) was obtained from the Centre for Healthcare Technology Development of Chonbuk National University, South Korea.
All samples (leaf, root, and fermented root) were subjected to freeze drying (PVTFD 10R; Ilsin Lab, Yangju, Korea), ground in a 40-mesh grinder (FM909T; Hanil, Wonju, Korea), and freeze dried. The dried powder was stored at −80°C until use.
Ethanol Extraction
Ethanol extraction was carried out by adding 100 mL of 80% ethanol to 10-g samples of A. hookeri leaf, root, or fermented root and incubating at 18°C for 48 h with vigorous shaking. The mixtures were then filtered using Whatmann filter paper No. 2 and concentrated using a rotary evaporator (IKA, NC, USA). The remaining residues were freeze-dried and stored at −80°C. Before testing, the samples were dissolved in enriched RPMI-1064 medium without phenol red (Sigma, St. Louis, MO, USA) supplemented with 1 µg/mL 5-fluorocytosine and 100 U/mL penicillin. The samples were then sterilised by membrane filtration through a 0.2-µm filter (Nalgene, Rochester, NY, USA) before use.
Proliferation of Splenic Lymphocytes
All experimental protocols were approved by the Animal Care Committee of the Beltsville Agricultural Research Center. At 4 weeks of age, specific pathogen-free Ross/Ross broilers (Longenecker's Hatchery, Elizabethtown, PA, USA) were euthanatised by cervical dislocation. Spleens were collected from 3 birds (raised on regular feed without any additives) and placed in petri dishes with 10 mL Hank's balanced salt solution supplemented with 100 U/mL penicillin and 100 µg/mL streptomycin (Sigma). Collected spleens were isolated from each spleen individually and pooled before the start of the in vitro experiment. Single lymphocytes were prepared as previously described (Lee et al., 2008). Briefly, the spleens were gently passed through a cell strainer, and the resulting single cells were separated using Histopaque-1077 (Sigma) density gradient medium by centrifugation. Isolated spleen cells were adjusted to 1×106 cells/mL in enriched RPMI-1604 medium without phenol red (Sigma) supplemented with 10% foetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. Splenic cells (100 µL/well) were cultured in 96-well flat-bottom plates with 100 µL/well of A. hookeri leaf, root, or fermented root ethanol extract (500, 250, 125, and 62.5 µg/mL). The positive control was concanavalin A (Con A; 20 µg/mL; Sigma), and medium alone was used as a negative control. All extracts were added to 3 well /group and the cells were cultured at 41°C in a humidified incubator (Forma, Marietta, OH, USA) with 5% carbon dioxide/95% air for 48 h. Cell proliferation was determined with 2-(2methoxy-4-nitrophenyl) -3-(4-nitrophenyl) -5-(2, 4-disulfophenyl) -2H-tetrazoilum (WST-8, Cell-Counting Kit-8; Dojindo Molecular Technologies, Gaithersburg, MD, USA) as previously described (Lee et al., 2007). The results were expressed as optical density measured at 450 nm using a microplate reader (ELx800™; BioTek, Winooski, VT, USA).
Inhibition of Tumour Cell Growth
Retrovirus-transformed chicken RP9 cells were used to test the inhibitory effects of A. hookeri extract on tumour cell growth. RP9 cells were cultured at 1×106 cells/mL (100 µL/well) in 96-well plates with 100 µL/well of plant extracts (125, 62.5, 31.3, and 15.6 µg/mL). Recombinant chicken NK lysin, which was produced as previously described (Hong et al., 2006), was used as a positive control (5 µg/mL), and media alone was used as a negative control. After incubation for 48 h, the optical density was measured at 450 nm for the WST-8 assay.
Induction of NO Production in Macrophages
Induction of NO production was performed using the chicken macrophage cell line HD11. HD11 cells were cultured in triplicate in 96-well plates at a concentration of 1×106 cells/well with 100 µL/mL plant extracts (500, 250, 125, 62.5, and 31.25 µg/mL), 5.0 µg/mL lipopolysaccharide (LPS) as a positive control (Lillehoj and Li, 2004), or medium alone as a negative control in a humidified incubator at 41°C with 5% CO2 for 24 h. After incubation, 100 µL/mL cell culture supernatant was transferred to flash flat-bottom 96-well plates, mixed with 100 µL/mL Griess reagent (Sigma), and incubated for 15 min at room temperature. Absorbance was measured at 540 nm on a microplate reader, and the NO concentration was determined using a standard curve generated with known concentrations of sodium nitrite (Kaspers et al., 1994).
Free Radical Scavenging Activity
Free radical scavenging activity of plant extracts (1000, 500, 250, 125, and 62.5 µg/mL) was measured using DPPH. For this assay, 100 µg/mL l-ascorbic acid (as a positive control) and ethanol vehicle alone (as a negative control) were mixed with 0.15 mM DPPH solution in ethanol. The reaction mixture was shaken intensely at room temperature for 30 min, and samples were analysed in triplicate. Decreased optical density was measured with a microplate reader at 517 nm, and the percent inhibition was calculated using the following formula: ([control−sample] / control)×100%.
Statistical Analysis
All experiments were carried out in triplicate and repeated three times. Statistical analysis was performed using SPSS software (SPSS 22.0 for Windows, Chicago, IL, USA), and all data were expressed as the mean±SD of triplicate cultures. Analysis of variance (ANOVA) and t-tests were used to evaluate differences between the mean value of negative control-treated and extracted samples. Differences with p values of less than 0.05 were considered statistically significant.
Results
Effects of A. hookeri on Splenic Lymphocyte Proliferation
The concentration response of A. hookeri extracts (62.5–500 µg/mL) on the proliferation of spleen cells for 48 h is illustrated in Fig. 1. Compared with the medium control, all A. hookeri extracts (62.5–500 µg/mL) significantly stimulated splenocyte proliferation in a concentration-dependent manner. Among the three samples, leaf extract showed higher stimulatory activity than the other extracts (root and fermented root) at 500 µg/mL. The root and fermented root extracts showed similar stimulatory effects at all doses, and at 500 µg/mL level, the stimulatory effect was similar to that of Con A. No toxic effects of plant extracts on spleen cells were observed at any of the concentrations tested (Fig. 1).
Fig. 1.
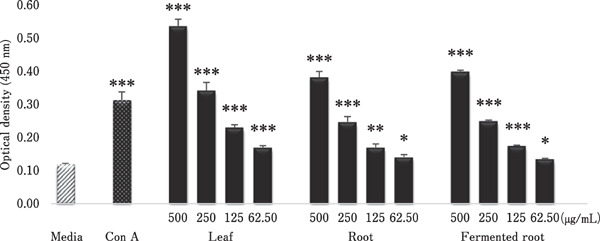
Effects of ethanol extracts of A. hookeri leaf, root, and fermented root on splenocyte proliferation. Chicken spleen cells were incubated with the indicated concentrations of each extract, Con A (20 µg/mL), or medium alone as a negative control. After 48 h, cell viability was measured by CCK-8 assay. Each bar represents the mean±SD (n=3). Each value was compared by t-test with the control (media alone). Significant differences are indicated as * P<0.05, ** P<0.01, and *** P<0.001.
Inhibitory Activity on Tumour Cells
The inhibitory effects of A. hookeri extracts on chicken tumour cells are shown in Fig. 2. Compared with the medium control, all A. hookeri extracts significantly decreased RP9 tumour cell growth in a concentration-dependent manner. The leaf extract showed the highest inhibitory activity at 15.6–125 µg/mL, with the strongest effect at 62.5 µg/mL (Fig. 2).
Fig. 2.
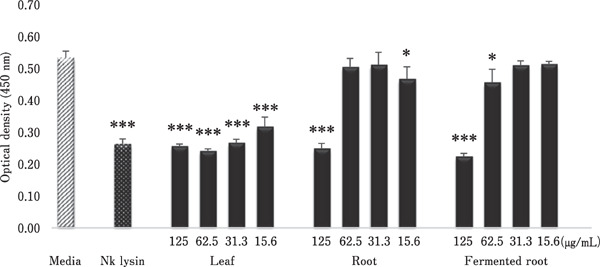
Inhibitory effects of A. hookeri leaf, root, and fermented root on the viability of RP9 tumour cells. RP9 cells were incubated with the indicated concentration of each extract, recombinant chicken NK-lysin (5 µg/mL), or medium alone as a negative control. After 48 h, cell viability was measured by CCK-8 assay. Each bar represents the mean±SD (n=3). Each value was compared by t-test with the control (media alone). Significant differences are indicated as * P<0.05 and *** P<0.001.
Nitric Oxide Production
The stimulatory effects of A. hookeri on the production of NO by HD11 macrophages are shown in Fig. 3. Compared with the medium control, all A. hookeri extracts stimulated NO production by macrophages in a concentration-independent manner. The highest NO production was observed for leaf extracts at 62.5–500 µg/mL. The root and fermented root extracts showed similar capabilities (Fig. 3).
Fig. 3.
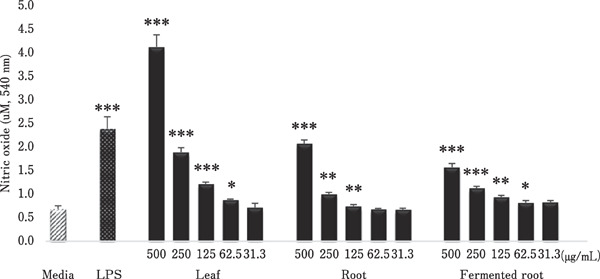
Effects of A. hookeri leaf, root, and fermented root extracts on nitric oxide production. HD11 macrophage cells were incubated with the indicated concentration of each extract, LPS (5 µg/mL), or medium alone as a negative control. After 24 h, cell viability was measured by CCK-8 assay. Each bar represents the mean±SD (n=3). Each value was compared by t-test with the control (media alone). Significant differences are indicated as * P<0.05, ** P<0.01, and *** P<0.001.
Free Radical Scavenging Capacity
The free radical scavenging capacity of A. hookeri, as determined by DPPH assay, is shown in Fig. 4. The results showed that all extracts exhibited greater than 80% scavenging activity at 1000 µg/mL compared with the ethanol vehicle control. A similar trend in free radical scavenging ability was observed in leaf and root extracts (125–1000 µg/mL). Generally, fermented root extracts showed higher DPPH radical scavenging capacity than other extracts at the same concentration, and the activities were identical at 500 and 1000 µg/mL (Fig. 4).
Fig. 4.
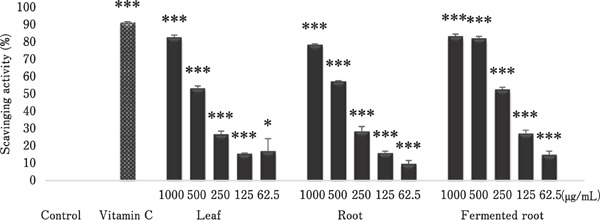
Antioxidant effects of A. hookeri leaf, root, and fermented root extracts. 2, 2-Diphenyl-1-picrylhydrazyl (DPPH; 0.15 mM) in ethanol was mixed with the indicated concentration of each plant extract, 100 µg/mL vitamin C as a positive control, or ethanol only as a blank control. The OD at 517 nm was measured, and the scavenging ability was calculated. Each bar represents the mean±SD (n=3). Each value was compared by t-test with the control (ethanol alone). Significant differences are indicated as * P<0.05 and *** P<0.001.
Discussion
The current investigation was carried out to assess the potential immune-enhancing properties of leaf, root, and fermented root extracts derived from A. hookeri on innate immunity and tumour cell viability in chickens. The immune-stimulating effects of A. hookeri have been reported in mice (Kim et al., 2015b; Lee et al., 2015b). Supplementation of standard diet with fermented and nonfermented A. hookeri powder has various effects, including regulation of blood lipid levels and antidiabetic activities, in type 2 diabetic mice (Kim et al., 2015b; Lee et al., 2015b). Additionally, an in vitro study characterised the anti-inflammatory and antioxidant effects of A. hookeri in rat macrophages (Kim et al., 2012). However, until now, no reports exist describing the effects of A. hookeri in chickens. The chicken is an economically important animal for both meat and egg production; therefore, a better understanding of the host immune function of birds is necessary.
A. hookeri is a wild herb that has been used to treat cancer and inflammatory diseases in India and Myanmar due to its richness in phytonutrients. The plant is a rich source of ascorbic acid, polyphenols, flavonoids, and organic natural sulphur (methyl sulfonyl methane) (Singh and Singh, 2014). Organosulphur compounds have been shown to have diverse biological beneficial effects, such as antioxidant effects and anti-inflammatory properties (Vazquez-Prieto and Miatello, 2010). Therefore, the antioxidant effects of A. hookeri in the current study may be related to the presence of organosulphur compounds.
In the present study, we demonstrated for the first time that the ethanol extracts of A. hookeri (leaf, root and fermented root) activated innate immunity and inhibited the growth of tumour cells in poultry. A. hookeri extracts increased the proliferation of spleen lymphocytes and inhibited the growth of tumour cells in a concentration-dependent manner. A. hookeri also induced nitric oxide secretion by chicken macrophages. The immune system is made of a complex network of cells, such as lymphocytes and macrophages, that work together to defend the body against foreign substances, such as bacteria or viruses (Kim et al., 2015a). Among the various types of immune cells, macrophages play an important role in host defence through regulation of lymphocyte activation and proliferation. In addition, macrophages play an essential role in the activation of T and B lymphocytes by antigen and allogenic cells (Elhelu, 1983). Nathan and Xie (1994) reported that lipopolysaccharide (LPS) -stimulated macrophages and pro-inflammatory cytokines, such as interferon-c (IFN-c) and tumour necrosis factor α (TNF-α), produce large amounts of NO, an important signalling molecule. Previous studies have indicated that NO regulates cell proliferation and inhibits angiogenesis, tumour growth, and metastasis (Napoli et al., 2013). Moreover, NO has been reported to have tumouricidal activity by induction of apoptosis in a concentration-dependent manner (Nicotera et al., 1995). In this report, we showed that A. hookeri extracts exhibited strong antitumour activity, which may be related to NO production by macrophages. Previous study by Kim et al. (2012) reported the anti-inflammatory effect of A. hookeri in LPS-induced mouse macrophage cells. In that test, the treatment with methanol extracts of roots significantly inhibited LPS-induced nitric oxide formation in dose-dependent manner and also decreased TNF-a and IL-6 production. This contrast in results can be explained that first, no LPS was used for the stimulation of chicken macrophage cells in the present study. LPS stimulates immune responses by interacting with the membrane receptor CD14 to induce the generation of cytokines such as tumour necrosis factor (TNF) -α, interleukin (IL)-1, and IL-6 (Meng and Lowell, 1997). Secondly, discrepant results can be explained by source of macrophages (chicken and mouse).
Many studies have reported the beneficial effects of fermentation on improving the activity of plant phenolic compounds. Schubert et al. (1999) reported high antioxidant activity and potent anti-inflammatory activity of fermented pomegranate juice. Oh et al. (2012) reported the effects of fermented Oyaksungisan on LPS-stimulated macrophages. They compared the anti-inflammatory activity of nonfermented and fermented Oyaksunkisan and showed that the anti-inflammatory activity increased with fermentation. Consistent with this, in the present study, we found that the fermented root extract of A. hookeri had the strongest effects on antioxidant activity compared with the nonfermented leaf and root and showed identical antioxidant activity at 500 and 1000 µg/mL.
The results obtained indicate that, the ethanol extracts of A. hookeri (leaf, root, and fermented root) significantly enhanced the proliferation of spleen lymphocytes, inhibited tumour cell viability, and stimulated NO production by HD11 macrophages compared to medium control. The leaf extracts effectively increased the proliferation of spleen lymphocytes and NO secretion by chicken macrophages and inhibited the growth of tumour cells, whereas fermented root extracts showed the strongest effects on antioxidant activity. These results suggest that A. hookeri had immune-stimulating properties that could be beneficial for poultry health.
Acknowledgments
This work was carried out under an ARS-RDA formal agreement sponsored by the Cooperative Research Program for Agriculture Science & Technology Development (PJ 012088), Rural Development Administration, Republic of Korea. The authors thank Dr. Ujvala Gadde for her significant contribution to this research.
References
- Bae GC, Bae DY. The anti-inflammatory effects of ethanol extract of Allium hookeri cultivated in South Korea. Korea Journal of Herbology, 27: 55-61. 2012. [Google Scholar]
- Banfield MJ, Kwakkel RP, Forbes JM. Effects of wheat structure and viscosity on coccidiosis in broiler chickens. Animal Feed Science and Technology, 98: 37-48. 2002. [Google Scholar]
- Basmacioglu H, Tokusoglu O, Ergul M. The effect of oregano and rosemary essential oils or alpha-tocopheryl acetate on performance and lipid oxidation of meat enriched with n-3 PUFAs in broilers. South African Journal of Animal Science, 34: 197-210. 2004. [Google Scholar]
- Dziri S, Hassen I, Fatassi S, Mrabet Y, Casabianca H, Hanch B, Hosni K. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). Journal of Functional Food, 4: 423-432. 2002. [Google Scholar]
- Elhelu MA. The role of macrophages in immunology. Journal of the National Medical Association, 75: 314-317. 1983. [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Madrid J, Garcia V, Orengo J, Megias MD. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poultry Science, 83: 169-174. 2004. [DOI] [PubMed] [Google Scholar]
- Hong YH, Lillehoj HS, Dalloul RA, Min W, Miska KB, Tuo W, Lee SH, Han JY, Lillehoj EP. Molecular cloning and characterization of chicken NK-lysin. Veterinary Immunology and Immunopathology, 110: 339-347. 2006. [DOI] [PubMed] [Google Scholar]
- Kaspers B, Lillehoj HS, Lillehoj EP. Chicken macrophages and thrombocytes share a common cell surface antigen defined by a monoclonal antibody. Veterinary Immunology and Immunopathology, 36: 333-346. 1994. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lee MA, Kim TW, Jang JY, Kim HJ. Anti-inflammatory effect of Allium hookeri root methanol extract in LPS-induced RAW264.7 cells. Journal of the Korean Society of Food Science and Nutrition, 41: 1645-1648. 2012. [Google Scholar]
- Kim DK, Lillehoj HS, Lee SH, Jang SI, Park MS, Min W, Lillehoj EP, Bravo D. Immune effects of dietary anethole on Eimeria acervulina infection. Poultry Science, 92: 2625-2634. 2013. [DOI] [PubMed] [Google Scholar]
- Kim JE, Lillehoj HS, Hong YH, Kim GB, Lee SH, Lillehoj EP, Bravo DM. Dietary Capsicum and Curcuma longa oleoresins increase intestinal microbiome and necrotic enteritis in three commercial broiler breeds. Research in Veterinary Science, 102: 150-158. 2015. [DOI] [PubMed] [Google Scholar]
- Kim NS, Lee SH, Jang HH, Kim JB, Kim HR, Kim DK, Kim YS, Yang JH, Kim HJ, Lee SH. Effect of Allium hookeri on glucose metabolism in type II diabetic mice. Korean Journal of Pharmacognosy, 46: 148-153. 2015. b. [Google Scholar]
- Lee SH, Lillehoj HS, Chun HK, Tuo W, Park HJ, Cho SM, Lee YM, Lillehoj EP. In vitro treatment of chicken peripheral blood lymphocytes, macrophages, and tumor cells with extracts of Korean medicinal plant. Nutrition Research, 27: 362-366. 2007. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lillehoj HS, Heckert RA, Cho SM, Tuo W, Lillehoj EP, Chun HK, Park HJ. Immune enhancing properties of safflower leaf (carthamus tinctorius) on chicken lymphocytes and macrophages. Journal of Poultry Science, 45: 147-151. 2008. [Google Scholar]
- Lee SH, Lillehoj HS, Jang SI, Lee KW, Bravo D, Lillehoj EP. Effects of dietary supplementation with phytonutrients on vaccine-stimulated immunity against infection with Eimeria tenella. Veterinary Parasitology, 181: 97-105. 2011. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim DK, Lillehoj HS, Jang SI. Immune modulation by Bacillus subtilis- based direct-fed microbials in commercial broiler chickens. Animal Feed Science and Technology, 200: 76-85. 2015. a. [Google Scholar]
- Lee SH, Kim NS, Choi BK, Jang HH, Kim JB, Lee YM, Kim DK, Lee CH, Kim YS, Yang JH, Kim YS, Kim HJ, Lee SH. Effect of Allium hookeri on lipid metabolism in type II diabetic mice. Korean Journal of Pharmacognosy, 46: 78-83. 2015. b. [Google Scholar]
- Lillehoj HS, Lee KW. Immune modulation of innate immunity as alternatives-to-antibiotics strategies to mitigate the use of drugs in poultry production. Poultry Science, 91: 1286-1291. 2012. [DOI] [PubMed] [Google Scholar]
- Lillehoj HS, LI G. Nitric oxide production by macrophages stimulated with sporozoites, lipopolysaccharide, or IFN-c and its dynamic changes in SC and TK strains of chickens infected with Eimeria tenella. Avian Disease, 48: 244-253. 2004. [DOI] [PubMed] [Google Scholar]
- Mueller K, Blum NM, Kluge H, Mueller AS. Influence of broccoli extract and various essential oils on performance and expression of xenobiotic- and antioxidant enzymes in broiler chickens. British Journal of Nutrition, 108: 588-602. 2012. [DOI] [PubMed] [Google Scholar]
- Napolli C, Paolisso G, Casamassimi A, Ai-orman M, Barbierl M, Sommese L, Infante T, Ignapro LJ. Effect of nitric oxide on cell proliferation. Journal of the American College of Cardiology, 62: 89-95. 2013. [DOI] [PubMed] [Google Scholar]
- Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell, 78: 915-918. 1994. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Bonfoco E, Brune B. Mechanisms for nitric oxide-induced cell death: Involvement of apoptosis. Advances in Neuroimmunology, 5: 411-420. 1995. [DOI] [PubMed] [Google Scholar]
- Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. The Journal of Experimental Medicine, 185: 1661-1670. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YC, Cho YK, Oh JH, Im GY, Jeong YH, Yang MC, Ma JY. Fermentation by Lactobacillus enhances anti-inflammatory effect of Oyaksungisan on LPS stimulated RAW264.7 mouse macrophage cells. BMC Complementary and Alternative Medicine, 12: 12-17. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert SY, Lansky EP, Neeman I. Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids. Journal of Ethnopharmacology, 66: 11-17. 1999. [DOI] [PubMed] [Google Scholar]
- Sharma G, Gohil RN, Kaul V. Cytological status of Allium hookeri thwaites (2n=22). Genetic Resources and Crop Evolution, 58: 1041-1050. 2011. [Google Scholar]
- Singh KB, Singh NM. Antioxidant and free radical scavenging potential of Allium hookeri Thwaites roots extract studied using in vitro models. Journal of Advances in Biology, 4: 276-285. 2014. [Google Scholar]
- Van Lunen TA. Growth performance of pigs fed diets with and without tylosin phosphate supplementation and reared in a biosecure all-in all-out housing system. The Canadian Veterinary Journal, 44: 571-576. 2003. [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prieto MA, Miatello RM. Organosulfur compounds and cardiovascular disease. Molecular Aspects of Medicine, 31: 540-545. 2010. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Lee SH, Lillehoj HS, Bravo D. Dietary sodium selenite affects host intestinal and systemic immune response and disease susceptibility to necrotic enteritis in commercial broilers. British Poultry Science, 56: 103-112. 2015. a. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Lee SH, Lillehoj HS, Hong YH, Bravo D. Effects of dietary selenium on host response to necrotic enteritis in young broilers. Research in Veterinary Science, 98: 66-73. 2015. b. [DOI] [PubMed] [Google Scholar]


