Abstract
Fertilization in animals that employ sexual reproduction is an indispensable event for the production of the next generation. A significant advancement in our understanding of the molecular mechanisms of sperm-egg interaction in mammalian species was achieved in the last few decades. However, the same level of knowledge has not been accumulated for birds because of egg size and the difficulty in mimicking the physiological polyspermy that takes place during normal fertilization. In this review, we summarize the current understanding of sperm-egg interaction mechanism during fertilization in birds, especially focusing on sperm-egg binding, sperm acrosome reaction and the authentic sperm protease required for the hole formation on the perivitelline membrane. We explain that the zona pellucida proteins (ZP1 and ZP3) in the perivitelline membrane play important roles in sperm-egg binding, induction of the acrosome reaction as well as sperm penetration by digestion of sperm protease. We anticipate that a deeper understanding of avian fertilization will open up new avenues to create powerful tools for a myriad of applications in the poultry industries including the production of transgenic and cloned birds.
Keywords: acrosome reaction, fertilization, perivitelline membrane, sperm-egg interaction, sperm protease
Introduction
Fertilization is indispensable for the production of the next generation in any animals that employ sexual reproduction. In general, this important event is a sequential step comprised of species-specific sperm-egg binding, induction of the acrosomal exocytosis, sperm penetration through the oocyte, and the fusion of gametes (Florman and Ducibella, 2006). There are numerous studies showing that zona pellucida, an egg envelope surrounding the oocyte of mammalian species, plays pivotal roles in the initial step for sperm-egg interaction during fertilization. When the sperm encounter the oocyte, they must penetrate the zona pellucida before sperm-egg fusion occurs. Although the molecular mechanisms underlying these events during fertilization are not fully understood, considerable evidence and key molecules that regulate fertilization have been discovered from studies using reverse-genetics such as transgenic and gene knock out technologies in mammals (Ikawa et al., 2008).
In comparison with those of mammalian species, avian oocytes are extremely large and this does not allow us to study by direct observation of the sperm-egg interaction in vitro. For instance, no available method for in vitro insemination exists to date, and researchers incubate the isolated perivitelline membrane (pvm), the homologous investments of mammalian zona pellucida, with ejaculated sperm in vitro as an alternative model for in vitro fertilization (IVF) (Birkhead et al., 1994; Robertson et al., 1997, 1998; Kuroki and Mori, 1997). In addition, the ovulated oocytes quickly lose their fertilizability because the chalaza-layer, also referred to as the outer layer of the vitelline membrane secreted from the infundibulum part of the oviduct, overlays the surface of the pvm immediately after ovulation (Wishart, 1997). The sperm no longer interact with these oocytes thereafter and are embedded in the chalaza-layer or egg albumen (Wishart, 1997). Despite these difficulties, a few research groups, including our laboratory have investigated the mechanisms of avian fertilization using poultry birds, mainly chickens and quails.
In this review, we summarize the current understanding of the sperm-egg interaction mechanism during fertilization in birds, especially focusing on sperm-egg binding, sperm acrosome reaction and the authentic sperm protease required for the hole formation on the perivitelline membrane.
Sperm-egg Binding
Sperm-egg binding is the initial event that required for the subsequent events of fertilization. Wassarman's group identified 3 zona pellucida glycoproteins, ZP1, ZP2 and ZP3 in mice and found that ZP3 is responsible for species-specific sperm-egg binding during fertilization in the 1980's (Bleil and Wassarman, 1980; Florman and Wassarman, 1985). Accordingly, ZP proteins, that are required for sperm-egg binding have been identified in the egg envelope of many animals (Lee et al., 1993; Sacco et al., 1989; Katagiri et al., 1999; Vo and Hedrick, 2000). Although the structure and the function of ZP proteins are evolutionally conserved (Harris et al., 1994), it is intriguing because the source of ZP proteins differs in different species. For instance, three ZP glycoproteins are synthesized in the developing oocyte in mice (Shimizu et al., 1983), however, rabbit ZP3 is produced in the granulosa cells (Lee and Dunbar, 1993). Some egg envelope proteins in oviparous species such as fish and birds are secreted by the liver and transported into the ovaries via the blood circulation (Hamazaki et al., 1989; Murata et al., 1995; Bausek et al., 2000; Sasanami et al., 2003); however, the oocyte is the sole source of the ZP proteins in Xenopus levies (Kubo et al., 1997; Tian et al., 1999).
We have previously reported that quail pvm is composed of at least 5 glycoproteins, (ZP1, ZP2, ZP3, ZP4 and ZPD), (Pan et al., 2001; Sasanami et al., 2003; Sato et al., 2009; Kinoshita et al., 2010; Serizawa et al., 2011). As mentioned in the Introduction, there is no IVFsystem in birds and the isolated pvm was used as a substitute to study the mechanism of sperm-egg interaction (Fig. 1). Using this system, we previously reported that a specific antiserum against purified quail ZP3 efficiently blocked the sperm perforation of pvm in Japanese quail. There are three possible explanations of this inhibitory effect; 1, the sperm-egg binding is inhibited by the antiserum because the antiserum covered the ZP3, a binding site for the spermatozoa; 2, the acrosome reaction of the sperm was blocked by the antiserum; 3, the protease responsible for the digestion of pvm is inhibited by the antiserum. Although we did not perform further experiments, Bausek et al. (2004), who tested the binding of purified ZP1 or ZP3 to the sperm demonstrated that both ZP proteins specifically bind to the tip of the sperm head. However, only ZP3 binding occurred through the interaction with 180 kDa sperm protein in the chicken. These results indicated that ZP3 is the sperm binding protein in the pvm of both chicken and quail, though the detailed mechanism was not known. In mice, it was reported that ZP2 also supports the sperm binding (Bleil et al., 1988), and the structural change of the ZP2 as a result of limited digestion by the cortical granule protease protects the polyspermic fertilization (Burkart et al., 2012). In birds, ZP2 is expressed in the oocyte of immature follicles and exists in the pvm as a minor component (Kinoshita et al., 2010). Very recently, Nishio and colleagues demonstrated that ZP2 in the chicken accumulates in the egg envelope of immature oocytes and remains in the germinal disk region of the mature egg (Nishio et al., 2014). Although the binding properties of ZP2 to sperm has not been tested yet, it would be very interesting to investigate whether the reported localization of ZP2 in the chicken is related to the phenomenon whereby sperm perforation prefers to occur in the germinal disk region (Birkhead et al., 1994; Wishart, 1997). Further studies are needed to clarify the molecular mechanism of sperm-egg binding in avian species.
Fig. 1.
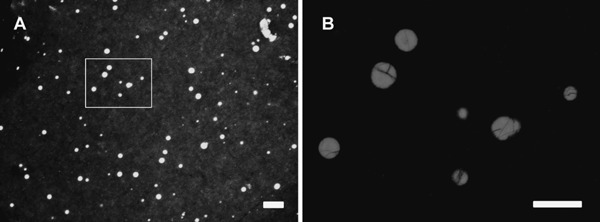
Holes on the perivitelline membrane produced by ejaculated spermatozoa during in vitro incubation in Japanese quail. Bar=100 µm (A). (B) High magnification image in panel A (the area indicated by square) is shown. Bar=50 µm.
As described above, ZP proteins play a role in the process of sperm-egg binding, however, the complementary molecules responsible for the sperm-egg interaction in birds, including the components that interact with ZP proteins on the surface of the sperm, remain to be clarified. To achieve this goal, we produced a monoclonal antibody (mAb) library against quail sperm membrane components and tested the potency of the library to inhibit hole formation in the pvm by sperm in vitro (Sasanami et al., 2011). Of the culture supernatants of the library, the supernatant 19A16A13 was found to block hole formation strongly. Tandem mass spectrometry analysis of the antigen revealed that the mAb recognizes sperm acrosin and the immunohistochemical analysis and ultrastructural analysis revealed that the sperm acrosin is localized on the plasma membrane of the sperm in addition to the acrosomal matrix. Indeed, the mAb effectively inhibited the binding of acrosome-intact sperm to the pvm. Although the binding capacity of sperm acrosin to ZP proteins is not known, these results indicated that the 45-kDa sperm acrosin in the plasma membrane of ejaculated sperm supports the binding of sperm to the pvm in quail fertilization (Sasanami et al., 2011).
Sperm Acrosome Reaction
By certain stimulation such as the exposure to hormones, chemicals or egg envelope components, spermatozoa shed out the contents of the acrosomal vesicle located at the tip of their head by the event in which the plasma membrane has fused with the outer acrosomal membrane (Florman and Ducibella, 2006). This process is referred to as an acrosome reaction (AR). As a result of the AR, the inner acrosomal membranes (IAM) are exposed and proteins integrated in the IAM such as IZUMO1 become functional. The inducer of AR varies in different species. In sea urchins, the AR occurs when spermatozoa reach the extracellular matrix of the egg (jelly coat) and fucose sulfate polymer in the jelly is the AR inducer (Kopf and Garbers, 1980). In starfish, the mixture of the glycoprotein (acrosome reaction-inducing substance: ARIS) and the steroid saponin (Co-ARIS), or a peptide comprised of 34 amino acids (asterosap) are potent in inducing the AR (Hoshi et al., 1988; Nishigaki et al., 1996). Similarly, the 300 kDa glycoprotein secreted from the oviductal pars recta, referred to as ARISX, is an authentic AR inducer in Xenopus laevis (Ueda et al., 2003), whereas progesterone is reported to have a AR inducing action in mouse and human sperm (Roldan et al., 1994; Osman et al., 1989). The AR is believed to be important for releasing the contents of the acrosomal matrix, acrosin for the digestion of the egg envelope to make a “path” for sperm penetration; however, several recent studies indicate that most of the sperm had completed AR before reaching the zona pellucida (Jin et al., 2011) and that the sperm that had already penetrated in the perivitelline space had the ability to penetrate the zona pellucida again in mice (Inoue et al., 2011). From these observations, it is apparent that previous understanding of the role of AR during fertilization in mammals should be reconsidered.
In birds, AR inducing activity was found in the pvm and the evidence that N-linked oligosaccharides with terminal N-acetyl-glucosamine residues in chicken pvm possess AR inducing activity was reported more than decade ago (Horrocks et al., 2000). However, it was unknown as to which components in the pvm were an authentic AR inducer in any of the birds. In order to identify the AR inducer in the pvm of Japanese quail, we established a method to discriminate acrosome-reacted from acrosome-intact sperm under the microscope. The acrosome of quail sperm is clearly detected as a hoechst 33342 negative structure located on the tip of the sperm nucleus (Fig. 2). By means of this method, we showed that pvm ZP1 possesses activity to induce AR in Japanese quail (Sasanami et al., 2007). We also provided evidence that the N-linked oligosaccharide attached to the ZP1 plays an important role in triggering the AR (Sasanami et al., 2007). In accord with our data, Okumura and colleagues showed that AR is induced when the ejaculates are incubated with purified ZP1 or ZPD, another minor constituent of the pvm in chicken (Okumura et al., 2004). From these results, unlike the situation in mice, we believe that the AR in birds is induced on the surface of the pvm.
Fig. 2.
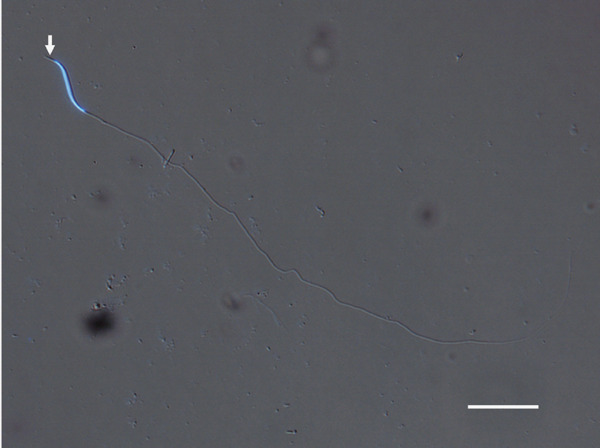
Representative image of ejaculated quail sperm. Stained by hoechst 33342. Arrows indicate the acrosome. Bar=25 µm.
From the aspect of signal transduction mediating the AR, the involvement of the Gi protein had been indicated in mammalian sperm because pertussis toxin (PTX), which is an inhibitor of Gi protein function, inhibits the ZP-initiated AR in mammalian sperm (Florman and Ducibella, 2006). Interestingly, it has been reported that PTX does not inhibit the progesterone-induced AR of human and mouse sperm (Tesarik et al., 1993; Murase and Roldan, 1996) as well as the acetylcholine-stimulated AR in the mouse, suggesting the involvement of a PTX insensitive receptor in AR induction (Son and Meizel, 2003). These data suggest that different physiological stimuli may utilize different signal transduction pathways to induce the sperm AR, though the physiological importance of the presence of the dual signal transduction system remains to be solved. Our previous study in Japanese quail demonstrated that both ZP1- and A23187-induced AR are significantly inhibited when PTX is included in the incubation mixture (Sasanami et al., 2007). These results indicate that the ZP1 or A23187 might be acting through a Gi protein-mediated mechanism similar to that in the zona-initiated AR in mammalian sperm.
Protease Responsible for Pvm Digestion
Although the mechanism is under debate, it is generally believed that the sperm borne protease hydrolyzes the egg extracellular coat and that the hole produced by the protease is the path for sperm penetration (Florman and Ducibella, 2006). Also, it had long been believed that, in mammalian species, the sperm acrosin, a non-ATP-dependent serine protease localized in the sperm head, is indispensable for penetration of the sperm (McRorie and Williams, 1974). However, Baba et al. (1994) demonstrated that acrosin-null male mice produce sperm normal in motility and are fertile; therefore, acrosin is not essential for fertilization, at least in mice. At present, acrosin can be interpreted as being responsible for the dispersal of the acrosomal contents in the AR (Yamagata et al., 1998). Sawada et al. reported that in marine invertebrates such as ascidians and sea urchins, sperm proteasomes is responsible for sperm penetration of the vitelline envelope, and the proteasome is a lytic agent called lysin, which is essential for disintegration of the egg extracellular matrix (Sawada et al., 2002; Sakai et al., 2003; Yokota and Sawada, 2007). A similar conclusion was drawn from mammalian fertilization, including that in mice, pigs, and human (Pasten et al., 2005; Kong et al., 2009; Zimmerman et al., 2011).
In avian species, fertilization occurs within the infundibular portion of the oviduct, and only the pvm encloses the oocyte at the time of fertilization. As mentioned above, sperm–egg interaction in avian species can be measured in vitro as the ability of the sperm to form a hole in the pvm (Robertson et al., 2000). By inhibiting AR, by the addition of PTX we found that the 45 kDa sperm acrosin localized on the sperm plasma membrane is responsible for binding the sperm to the pvm of the oocyte but is not important for digestion of the pvm glycoproteins (Sasanami et al., 2011). These results indicate that the actual lysin that functions during avian fertilization is not acrosin but another protease existing in the sperm. In order to identify an authentic lysin in birds, we previously tested the role of sperm proteasome during fertilization in Japanese quail. We found that ZP1, which circulates in the bloodstream, received some modifications during transport to the pvm. It is from the fact that when ZP1, purified from the serum of laying quail, was intravenously injected into different birds, the signal of exogenous ZP1 was detected in the pvm, whereas ZP1 derived from the pvm failed to incorporate into the pvm (Kinoshita et al., 2008). As described above, egg envelope glycoprotein ZP1 is the authentic AR inducer in birds. We tested whether the serum ZP1 also induces AR in Japanese quail. Although both ZP1 induced AR, immunoblot analysis using anti-ubiquitin antibody only reacted with pvm ZP1. These results indicated that serum ZP1 received extracellular ubiquitination during transport and bound with ZP3 to form the insoluble fiber of the pvm (Fig. 3). When we incubated the serum or pvm-derived ZP1 with ejaculated sperm, again only pvm ZP1 was disintegrated to a small peptide. Western blot analysis using the anti-20S proteasome antibody and ultrastructural analysis showed that immunoreactive proteasome was localized in the acrosomal region of the sperm. Inclusion of specific proteasome inhibitor MG132 in the incubation mixture, or depletion of extracellular ATP by the addition of apyrase, efficiently suppressed the sperm perforation of the pvm (Sasanami et al., 2012). These results clearly demonstrated that the sperm proteasome is important for fertilization in birds and that the extracellular ubiquitination of ZP1 might occur during its transport via blood circulation (Fig. 3). Although many lines of evidence obtained in our study suggest that the ubiquitin–proteasome system is pivotal to making holes in the pvm and subsequent sperm penetration in quail fertilization (Fig. 4), acrosin, an acrosomal serine protease is believed to be involved in hole formation on the pvm during fertilization (Richardson et al., 1992; Slowińska et al., 2010). As suggested by the studies from a broad range of animals, it is apparent that the ubiquitin–proteasome system may be universally conserved in animal fertilization. Among these, the avian system is a beneficial model for studying the role of sperm proteasome in fertilization because avian sperm can produce a huge hole on the pvm, and large quantities of the target protein, ZP1, can be isolated from a large oocyte.
Fig. 3.
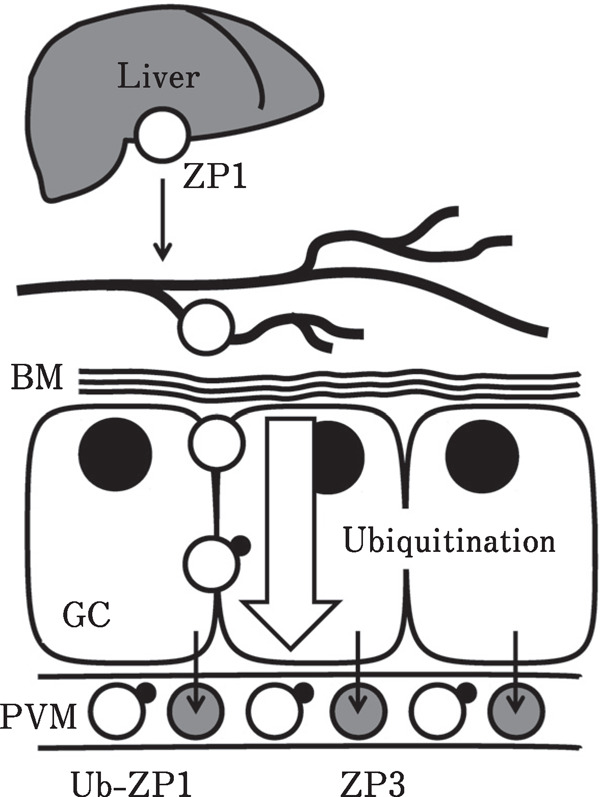
Formation of perivitelline membrane in birds. In avian species, the perivitelline membrane (pvm), which is the egg envelope homologous to zona pellucida in mammals, is observed in follicles between granulosa cells (GC) and the ovum before ovulation. Two major glycoproteins, ZP1 (white circle) and ZP3 (gray circle), were identified as the constituents of the pvm. ZP1 is synthesized in the liver and is transported to the ovary via blood circulation. On the other hand, ZP3 is secreted from the GC. These ZP proteins bind to form pvm during follicular development. Additionally, we found that the ubiquitination of ZP1 may take place during the transport from the bloodstream to the pvm matrix. BM, basal laminae; Ub-ZP1, ubiquitinated ZP1.
Fig. 4.
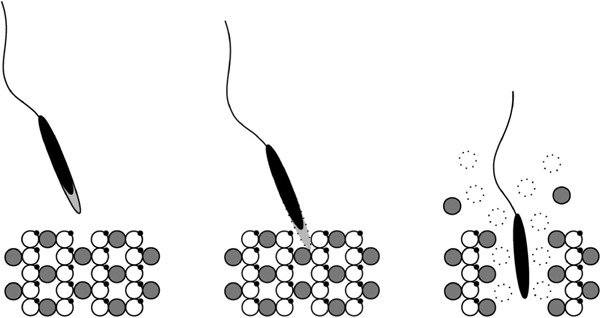
Suggested model for sperm penetration of the pvm in Japanese quail. The spermatozoa reach the periviteline membrane, bind to it and acrosome reaction is induced by N-glycan attached on ZP1. The sperm proteasome degrades ubiquitinated ZP1 and the fragment of the ZP1 as well as intact ZP3 is released from the pvm during co-incubation with the sperm, and this phenomenon might be related to the hole formation during quail fertilization. The white circle and the gray circle represent ZP1 and ZP3, respectively. Note that ZP1 in the pvm is ubiquitinated (small black dot on the ZP1), but ZP3 is not.
The eggs produced in most animals accept only one sperm for zygote formation because there is a blockade system for avoiding plural sperm entry. Recently, the cortical granule protease ovastatin was found to be necessary to express a polyspermy block and ovastatin partially degraded the ZP2 protein so sperm no longer interacted with such ovastatin-modified zona pellucida in mice (Burkart et al., 2012). In the case of Xenopus laevis fertilization, soon after the entry of the first sperm into the egg, the egg's plasma membrane quickly changes to initiate a fast block to polyspermy by eliciting a positive-going fertilization potential to prevent the fusion of a second sperm (Elinson, 1986; Iwao, 2000; Wong and Wessel, 2006). In contrast to these monospermic fertilization systems, avian oocytes accept many sperm during fertilization and their eggs employ polyspermic fertilization. This physiological situation indicated that there is no molecular mechanism to prevent polyspermy in birds. Our recent findings using intra-cytplasmic sperm injection (ICSI) in quail demonstrated that polyspermic fertilization is essential because extremely large eggs in birds require more sperm factor that is responsible for the egg activation and following embryonic development (Mizushima et al., 2014). It is reasonable to suppose that these two phenomena, the lack of the mechanism preventing polyspermy and the large quantities of sperm factors required for the activation of huge eggs in birds, are evolutionally acquired.
Conclusion
In this review, we described recent findings regarding sperm-egg interaction in birds. Comparison of mammalian and avian fertilization systems are depicted in Fig. 5. Several important molecules that regulate avian fertilization have been discovered but this is mainly derived from experiments that referred to mammalian studies. Because there are no efficient methods yet that would allow us to produce gene-manipulated birds, knowledge of the avian fertilization mechanism is limited. Our recent study demonstrated that healthy chicks were successfully generated after ICSI (Mizushima et al., 2014). The successful production of healthy chicks after ICSI has enormous implications for industrial, agricultural, and conservation applications including avian transgenesis, cloning technology, and in protecting endangered bird species. With a combination of our ICSI technique and modern technology such as the CRISPR/Cas9 system, we expect that we will produce transgenic and gene-knockout birds in the near future through ICSI. We hope that our ICSI technique will help assist our understanding of the mechanisms of avian fertilization and embryo development, as well as other fields of avian biology.
Fig. 5.
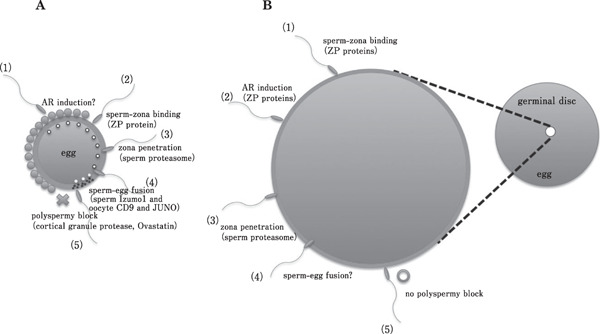
Comparison of sperm-egg interaction of mammals and birds during fertilization. Schematic illustrations of (A) mammalian and (B) avian fertilization. Mammalian fertilization exhibits monospermy, whereas avian exhibits polyspermic fertilization and that there is no machinery that disturbing the plural sperm entry in birds. Another difference is that the order of the AR induction in birds is thought to be followed by sperm-zona binding, though the timing of the AR induction in mammalian species is under debate. The event of sperm-egg fusion is not observed in any birds. The filled circles surrounding the zona pellucida in A are cumulus cells and circles depicted in egg cytoplasm are cortical granule that shed protease after sperm-egg fusion (step 4). In B, germinal disc area is enlarged in left side. Numerical number in A and B represents the order of the occurrence.
Acknowledgments
The corresponding author, Sasanami, T. was awarded the 2015 Scientist Prize by the Japan Poultry Science Association for “Studies on the molecular mechanism of avian fertilization”. The corresponding author would like to express his sincere gratitude to all his collaborators and students, as well as his family. This work was supported, in part, by a Grant-in-Aid for Scientific Research (B) (General) (24380153 to TS), a Grant-in-Aid for Scientific Research in Innovative Areas (24112710 to TS), a Grant-in-Aid for Challenging Exploratory Research (25660211 to TS) and the Toukai Foundation for Technology (No. 7 to TS).
References
- Baba T, Azuma S, Kashiwabara S, Toyoda Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. Journal of Biological Chemistry, 269: 31845-31849. 1994. [PubMed] [Google Scholar]
- Bausek N, Waclawek M, Schneider WJ, Wohlrab F. The major chicken egg envelope protein ZP1 is different from ZPB and is synthesized in the liver. Journal of Biological Chemistry, 275: 28866-28872. 2000. [DOI] [PubMed] [Google Scholar]
- Bausek N, Ruckenbauer HH, Pfeifer S, Schneider WJ, Wohlrab F. Interaction of sperm with purified native chicken ZP1 and ZPC proteins. Biology of Reproduction, 71: 684-690. 2004. [DOI] [PubMed] [Google Scholar]
- Birkhead TR, Sheldon BC, Fletcher F. A comparative study of sperm-egg interactions in birds. Journal of Reproduction and Fertility, 101: 353-361. 1994. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Synthesis of zona pellucida proteins by denuded and follicle-enclosed mouse oocytes during culture in vitro. Proceedings of the National Academy of Science of the United States of America, 77: 1029-1033. 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil JD, Greve JM, Wassarman PM. Identification of a secondary sperm receptor in the mouse egg zona pellucida: role in maintenance of binding of acrosome-reacted sperm to eggs. Developmental Biology, 128: 376-385. 1988. [DOI] [PubMed] [Google Scholar]
- Burkart AD, Xiong B, Baibakov B, Jiménez-Movilla M, Dean J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. Journal of Cell Biology, 97: 37-44. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinson RP. Fertilization in amphibians: the ancestry of the block to polyspermy. International Review of Cytology, 101: 59-100. 1986. [DOI] [PubMed] [Google Scholar]
- Florman HM, Wassarman PM. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell, 41: 313-324. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florman HM, Ducibella T. Fertilization in Mammals. In: Knobil and Neill's Physiology of Reproduction 3 edn. (Neill JD, Plant TM, Pfaff DW, Challis JRG, de Kretser DM, Richards JS, Wassarman PM. eds.). Vol. 1 Pp. 55-112. Elsevier Academic Press; St Louis: 2006. [Google Scholar]
- Hamazaki TS, Nagahama Y, Iuchi I, Yamagami K. A glycoprotein from the liver constitutes the inner layer of the egg envelope (zona pellucida interna) of the fish, Oryzias latipes. Developmental Biology, 133: 101-110. 1989. [DOI] [PubMed] [Google Scholar]
- Harris JD, Hibler DW, Fontenot GK, Hsu KT, Yurewicz EC, Sacco AG. Cloning and characterization of zona pellucida genes and cDNAs from a variety of mammalian species: the ZPA, ZPB and ZPC gene families. DNA Sequence, 4: 361-393. 1994. [DOI] [PubMed] [Google Scholar]
- Horrocks AJ, Stewart S, Jackson L, Wishart GJ. Induction of acrosomal exocytosis in chicken spermatozoa by inner perivitelline-derived N-linked glycans. Biochemical and Biophysical Research Communications, 278: 84-89. 2000. [DOI] [PubMed] [Google Scholar]
- Hoshi M, Matsui T, Nishiyama I, Amano T, Okita Y. Physiological inducers of the acrosome reaction. Cell Differentiation and Development, 25 Suppl: 19-24. 1988. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Inoue N, Okabe M. Mechanisms of sperm-egg interactions emerging from gene-manipulated animals. International Journal of Developmental Biology, 52: 657-664. 2008. [DOI] [PubMed] [Google Scholar]
- Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline spance can fertilize other eggs. Proceedings of the National Academy of Science of the United States of America, 108: 20008-20011. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao Y. Mechanisms of egg activation and polyspermy block in amphibians and comparative aspects with fertilization in the vertebrates. Zoological Science, 17: 699-709. 2000. [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proceedings of the National Academy of Science of the United States of America, 108: 4892-4896. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri C, Yoshizaki N, Kotani M, Kubo H. Analyses of oviductal pars recta-induced fertilizability of coelomic eggs in Xenopus laevis. Developmental Biology, 210: 269-276. 1999. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Mizui K, Ishiguro T, Ohtsuki M, Kansaku N, Ogawa H, Tsukada A, Sato T, Sasanami T. Incorporation of ZP1 into perivitelline membrane after in vivo treatment with exogenous ZP1 in Japanese quail (Coturnix japonica). FEBS Journal, 275: 3580-3589. 2008. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Rodler D, Sugiura K, Matsushima K, Kansaku N, Tahara K, Tsukada A, Ono H, Yoshimura T, Yoshizaki N, Tanaka R, Kohsaka T, Sasanami T. Zona pellucida protein ZP2 is expressed in the oocyte of Japanese quail (Coturnix japonica). Reproduction, 39: 359-371. 2010. [DOI] [PubMed] [Google Scholar]
- Kong M, Diaz ES, Morales P. Participation of the human sperm proteasome in the capacitation process and its regulation by protein kinase A and tyrosine kinase. Biology of Reproduction, 80: 1026-1035. 2009. [DOI] [PubMed] [Google Scholar]
- Kopf GS, Garbers DL. Calcium and a fucose-sulfate-rich polymer regulate sperm cyclic nucleotide metabolism and the acrosome reaction. Biology of Reproduction, 22: 118-126. 1980. [PubMed] [Google Scholar]
- Kubo H, Kawano T, Tsubuki S, Kawashima S, Katagiri C, Suzuki A. A major glycoprotein of Xenopus egg vitelline envelope, gp41 is a fron homolog of mammalian ZP3. Development, Growth and Differentiation, 39: 405-417. 1997. [DOI] [PubMed] [Google Scholar]
- Kuroki M, Mori M. Binding of spermatozoa to the perivitelline layer in the presence of a protease inhibitor. Poultry Science, 76: 748-752. 1997. [DOI] [PubMed] [Google Scholar]
- Lee VH, Dunbar BS. Developmental expression of the rabbit 55-kDa zona pellucida protein and messenger RNA in ovarian follicles. Developmental Biology, 55: 371-382. 1993. [DOI] [PubMed] [Google Scholar]
- Lee VH, Schwoebel E, Prasad S, Cheung P, Timmons TM, Cook R, Dunbar BS. Identification and structural characterization of the 75-kDa rabbit zona pellucida protein. Journal of Biological Chemistry, 268: 12412-12417. 1993. [PubMed] [Google Scholar]
- McRorie RA, Williams WL. Biochemistry of mammalian fertilization. Annual Review of Biochemistry, 43: 777-803. 1974. [DOI] [PubMed] [Google Scholar]
- Mizushima S, Hiyama G, Shiba K, Inaba K, Dohra H, Ono T, Shimada K, Sasanami T. The birth of quail chick after intracytoplasmic sperm injection. Development, 141: 3799-3806. 2014. [DOI] [PubMed] [Google Scholar]
- Murase T, Roldan ER. Progesterone and the zona pellucida activate different transducing pathways in the sequence of events leading to diacylglycerol generation during mouse sperm acrosomal exocytosis. Biochemical Journal, 320: 1017-1023. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Sasaki T, Yasumasu S, Iuchi I, Enami J, Yasumasu I, Yamagami K. Cloning of cDNAs for the precursor protein of a low-molecular-weight subunit of the inner layer of the egg envelope (chorion) of the fish Oryzias latipes. Developmental Biology, 167: 9-17. 1995. [DOI] [PubMed] [Google Scholar]
- Nishigaki T, Chiba K, Miki W, Hoshi M. Structure and function of asterosaps, sperm-activating peptides from the jelly coat of starfish eggs. Zygote, 4: 237-245. 1996. [DOI] [PubMed] [Google Scholar]
- Nishio S, Kohno Y, Iwata Y, Arai M, Okumura H, Oshima K, Nadano D, Matsuda T. Glycosylated chicken ZP2 accumulates in the egg coat of immature oocytes and remains localized to the germinal disc region of mature eggs. Biology of Reproduction, 91: 107, 1-10. 2014. [DOI] [PubMed] [Google Scholar]
- Okumura H, Kohno Y, Iwata Y, Mori H, Aoki N, Sato C, Kitajima K, Nadano D, Matsuda T. A newly identified zona pellucida glycoprotein, ZPD, and dimeric ZP1 of chicken egg envelope are involved in sperm activation on sperm-egg interaction. Biochemical Journal, 384: 191-199. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman RA, Andria ML, Jones AD, Meizel S. Steroid induced exocytosis: the human sperm acrosome reaction. Biochemical and Biophysical Research Communications, 160: 828-833. 1989. [DOI] [PubMed] [Google Scholar]
- Pan J, Sasanami T, Kono Y, Matsuda T, Mori M. Effects of testosterone on production of perivitelline membrane glycoprotein ZPC by granulosa cells od Japanese quail (Coturnix japonica). Biology of Reproduction, 64: 310-316. 2001. [DOI] [PubMed] [Google Scholar]
- Pasten C, Morales P, Kong M. Role of the sperm proteasome during fertilization and gamete interaction in the mouse. Molecular Reproduction and Development, 71: 209-219. 2005. [DOI] [PubMed] [Google Scholar]
- Richardson ME, Korn N, Bodine AB, Thurston RJ. Research note: kinetic and inhibition studies with turkey acrosin. Poultry Science, 71: 1789-1793. 1992. [DOI] [PubMed] [Google Scholar]
- Robertson L, Brown HL, Staines HJ, Wishart GJ. Characterization and application of an avian in vitro spermatozoa-egg interaction assay using the inner perivitelline layer from laid chicken eggs. Journal of Reproduction and Fertility, 110: 205-211. 1997. [DOI] [PubMed] [Google Scholar]
- Robertson L, Wilson YI, Lindsay C, Wishart GJ. Evaluation of semen from individual male domestic fowl by assessment of sperm: perivitelline interaction in vitro and in vivo. British Poultry Science, 39: 278-281. 1998. [DOI] [PubMed] [Google Scholar]
- Robertson L, Wishart GJ, Horrocks AJ. Identification of perivitelline N-linked glycans as mediators of sperm-egg interaction in chickens. Journal of Reproduction and Fertility, 120: 397-403. 2000. [PubMed] [Google Scholar]
- Roldan ER, Murase T, Shi QX. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science, 266: 1578-1581. 1994. [DOI] [PubMed] [Google Scholar]
- Sacco A, Yurewicz EC, Subramanian MG, Matzat PD. Porcine zona pellucida: Association of sperm receptor activity with the α-glycoprotein component of the Mr = 55,000 family. Biology of Reproduction, 41: 523-532. 1989. [DOI] [PubMed] [Google Scholar]
- Sakai N, Sawada H, Yokosawa H. Extracellular ubiquitin system implicated in fertilization of the ascidian, Halocynthia roretzi: isolation and characterization. Developmental Biology, 264: 299-307. 2003. [DOI] [PubMed] [Google Scholar]
- Sasanami T, Pan J, Mori M. Expression of perivitelline membrane glycoprotein ZP1 in the liver of Japanese quail (Coturnix japonica) after in vivo treatment with diethylstilbestrol. Journal of Steroid Biochemistry and molecular Biology, 84: 109-116. 2003. [DOI] [PubMed] [Google Scholar]
- Sasanami T, Murata T, Ohtsuki M, Matsushima K, Hiyama G, Kansaku N, Mori M. Induction of sperm acrosome reaction by perivitelline membrane glycoprotein ZP1 in Japanese quail (Coturnix japonica). Reproduction, 133: 41-49. 2007. [DOI] [PubMed] [Google Scholar]
- Sasanami T, Yoshizaki N, Dohra H, Kubo H. Sperm acrosin is responsible for the sperm binding to the egg envelope during fertilization in Japanese quail (Coturnix japonica). Reproduction, 142: 267-276. 2011. [DOI] [PubMed] [Google Scholar]
- Sasanami T, Sugiura K, Tokumoto T, Yoshizaki N, Dohra H, Nishio S, Mizushima S, Hiyama G, Matsuda T. Sperm proteasome degrades egg envelope glycoprotein ZP1 during fertilization of Japanese quail (Coturnix japonica). Reproduction, 144: 423-431. 2012. [DOI] [PubMed] [Google Scholar]
- Sato T, Kinoshita M, Kansaku N, Tahara K, Tsukada A, Ono H, Yoshimura T, Dohra H, Sasanami T. Molecular characterization of egg envelope glycoprotein ZPD in the ovary of Japanese quail (Coturnix japonica). Reproduction, 137: 333-343. 2009. [DOI] [PubMed] [Google Scholar]
- Sawada H, Sakai N, Abe Y, Tanaka E, Takahashi Y, Fujino J, Kodama E, Takizawa S, Yokosawa H. Extracellular ubiquitination and proteasome-mediated degradation of the ascidian sperm receptor. Proceedings of the National Academy of Science of the United States of America, 99: 1223-1228. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa M, Kinoshita M, Rodler D, Tsukada A, Ono H, Yoshimura T, Kansaku N, Sasanami T. Oocytic expression of zona pellucida protein 4 in Japanese quail (Coturnix japonica). Animal Science Journal, 82: 227-235. 2011. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Tsuji M, Dean J. In vitro biosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. Journal of Biological Chemistry, 258: 5858-5863. 1983. [PubMed] [Google Scholar]
- Słowińska M, Olczak M, Liszewska E, Watorek W, Ciereszko A. Isolation, characterization and cDNA sequencing of acrosin from turkey spermatozoa. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 157: 127-136. 2010. [DOI] [PubMed] [Google Scholar]
- Son JH, Meizel S. Evidence suggesting that the mouse sperm acrosome reaction initiated by the zona pellucida involves an α7 nicotinic acetylcholine receptor. Biology of Reproduction, 68: 1348-1353. 2003. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Carreras A, Mendoza C. Differential sensitivity of progesterone- and zona pellucida-induced acrosome reactions to pertussis toxin. Molecular Reproduction and Development, 34: 183-189. 1993. [DOI] [PubMed] [Google Scholar]
- Tian J, Gong H, Lennarz WJ. Xenopus laevis sperm receptor gp69/64 glycoprotein is a homolog of the mammalian sperm receptor ZP2. Proceedings of the National Academy of Science of the United States of America, 96: 829-834. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kubo H, Iwao Y. Characterization of the acrosome reaction-inducing substance in Xenopus (ARISX) secreted from the oviductal pars recta onto the vitelline envelope. Developmental Biology, 264: 289-298. 2003. [DOI] [PubMed] [Google Scholar]
- Vo LH, Hedrick JL. Independent and hetero-oligomericdependent sperm binding to egg envelope glycoprotein ZPC in Xenopus laevis. Biology of Reproduction, 62: 766-774. 2000. [DOI] [PubMed] [Google Scholar]
- Wishart GJ. Quantitative aspects of sperm: egg interaction in chickens and turkeys. Animal Reproduction Science, 48: 81-92. 1997. [DOI] [PubMed] [Google Scholar]
- Wong JL, Wessel GM. Defending the zygote: search for the ancestral animal block to polyspermy. Current Topics in Developmental Biology, 72: 1-151. 2006. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Murayama K, Okabe M, Toshimori K, Nakanishi T, Kashiwabara S, Baba T. Acrosin accelerates the dispersal of sperm acrosomal proteins during acrosome reaction. Journal of Biological Chemistry, 273: 10470-10474. 1998. [DOI] [PubMed] [Google Scholar]
- Yokota N, Sawada H. Sperm proteasomes are responsible for the acrosome reaction and sperm penetration of the vitelline envelope during fertilization of the sea urchin Pseudocentrotus depressus. Developmental Biology, 308: 222-231. 2007. [DOI] [PubMed] [Google Scholar]
- Zimmerman SW, Manandhar G, Yi YJ, Gupta SK, Sutovsky M, Odhiambo JF, Powell MD, Miller DJ, Sutovsky P. Sperm proteasomes degrade sperm receptor on the egg zona pellucida during mammalian fertilization. PLoS One, 6:e17256 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


