Abstract
The in vitro sperm quality parameters (motility, M; viability, V; normal morphology, NM; plasma membrane integrity, PMI; mitochondrial function, MF) in Muscovy drakes (Cairina moschata) were evaluated by using microscopy and flow cytometry, the correlation among sperm quality parameters and results of artificial insemination was also assessed in present study. M, V and NM were detected by phase contrast microscopy assisted with eosinnigrosin staining, and PMI and MF were detected by using flow cytometry within appropriate fluorescence staining (SYBR-14/PI and R123/PI, respectively). Fertility (F), early embryonic mortality (EEM) and the survival embryo rate (SER) were assessed after the artificial insemination of Muscovy or Kaiya duck (Anas platyrhynchos) females. Sperm PMI and MF, the parameters detected by flow cytometry were positively correlated with sperm M, V, and NM, those were detected by phase contrast microscopy (P<0.05). Sperm V and PMI were negatively correlated with the percentage of early embryo mortality of Muscovy duck fertile eggs (P<0.05). The results of the present study showed the relationships among the AI results and the sperm quality parameters detected by microscopy as well as flow cytometry. In conclusion, flow cytometry assisted with microscopy can be an effective tool to evaluate in vitro sperm quality and may contribute to predict the reproductive performances of individual Muscovy drakes, which helps to improve duck production efficiency.
Keywords: Artificial insemination (AI), Muscovy duck (Cairina moschata), Sperm evaluation
Introduction
The intergeneric crossbreeding of Muscovy drakes (Cairina moschata) with common ducks (Anas platyrhynchos) produces mule ducks that are one of the major commercial hybrid duck species in the world. Intergeneric crossbreeding affects mating behavior because the two ducks differ in body size; however, this problem can be eliminated by artificial insemination (AI) (Tai Liu and Tai, 1991). In duck industry, to meet the demands of mass production, semen from many drakes must be collected, although Muscovy drakes produce more semen volume than other poultry species. Because fertility significantly differs among drake individuals (Tai Liu and Tai, 1984), in vitro sperm evaluation becomes a useful tool to select only those drakes with good fertilizing capacity for AI. In addition, Sperm evaluation is also critical for sperm preservation. Qualifying the original sperm quality prior to its storage ensures that sperm with ideal fertile capacity is preserved and results in successful AI.
Sperm evaluation has been established to evaluate sperm quality by phase contrast microscopy for a long time. The traditional motility assay and eosin–nigrosin staining are common laboratory techniques that use phase contrast microscopy to evaluate motility and viable sperm with normal morphology, respectively (Blom, 1950). These traditional tests are routinely employed in both mammalian and poultry species (Gadea, 2005; Peterson et al., 2007; Blesbois et al., 2008). Fluorescent dyes such as SYBR-14 and propidium iodide (PI) are dually associated to stain for cell membrane permeability and thus be an indication of viability (Chalah and Brillard, 1998). The Rhodamine 123 (R123), a mitochondrial-specific fluorescent dye selectively accumulated in functional mitochondria that has been reported to be an efficient detection in sperm quality (Graham et al., 1990; Garner et al., 1997). Although these techniques are commonly applied using phase contrast microscopy, the reliabilities of these detection are questionable, because the observation of these methods is often too time consuming and person dependence.
The detection by flow cytometry offers to analyze thousands of cells with different physiological states in few seconds, which is higher sensitivity and lower person dependence than optical microscopy. Therefore, combining flow cytometry with appropriate fluorescence staining may thus increase the accuracy of the methods measuring sperm quality.
The utilization of flow cytometry with SYBR-14 and PI constitutes a cell viability assay in mammalian and poultry (Graham et al., 1990; Garner and Johnson, 1995; Partyka et al., 2010; Partyka et al., 2011; Klimowicz-Bodys et al., 2012). The R123 has also been used with flow cytometry to evaluate the mitochondrial function of mammalian sperm (Graham et al., 1990) and has been shown to be correlated with sperm motility (Garner et al., 1997). Thus, the utilization of flow cytometry with the SYBR-14 and PI viability assay, as well as with R123, is an objective and accurate method to predict fertility. However, these methods have never been developed in duck.
The present study was the first study to evaluate drake sperm quality using flow cytometry. Plasma membrane integrity (PMI) and mitochondrial function (MF) of the drake spermatozoa were detected by flow cytometry and compared to the conventional methods relying on phase contrast microscopy to evaluate sperm motility (M), sperm viability (V) and normal morphology (NM) with eosin–nigrosin staining. The results in egg incubation obtained after Muscovy or common (Kaiya) duck females artificially inseminated by drake semen that were also compared.
Materials and Methods
Animals
Seven white Muscovy drakes, 42 female white Muscovy ducks (LRI No. 1) and 42 female Kaiya ducks (Anas platyrhynchos; Ilan Kaiya TLRI No. 11) were produced and randomly selected in Ilan Branch of the Livestock Research Institute (LRI). The ducks were individually caged and provided with a commercial diet and water adlibitum. The Muscovy ducks were 45–49 weeks old and were kept under a 16/8-hr light/dark cycle, whereas the Kaiya ducks were 28–32 weeks old and kept under a 14/10-hr light/dark cycle.
Chemicals
Some chemicals were purchased from Sigma-Aldrich (St. Louis, Missouri, USA), including eosin, nigrosin and DMSO. The fluorescent dyes, including the LIVE/DEAD® Sperm Viability Kit, R123 and PI, were purchased from Invitrogen™ (Thermo Fisher Scientific, Waltham, MA, USA).
Semen Collection
Female ducks were used to tease the drakes during the reproductive season (April–September in Taiwan), and semen samples were collected from each drake twice a week (Tai, 1978). During semen collection, a female duck was initially caged with a drake, and then a technician assisted the drake by helping him to mount the back of the female. When the drake was aroused, semen was successfully collected by pressing the cloacal erection. Collected semen that was free of excreta contamination was poured into a tube and inserted into an ice bath for analysis.
Conventional Sperm Evaluation
A phase contrast microscope (BX50, Olympus, Tokyo, Japan) was used to evaluate the conventional sperm quality parameters (i.e., sperm motility, sperm viability and normal morphology) for each individual drake sample.
1). Sperm Motility (M)
Sperm motility was assessed by placing 8 µL of each semen sample onto a pre-warmed glass slide. The slide was covered with an 18×18 mm coverslip and warmed on a 37.5°C heat plate for 30 s. The proportion of motile spermatozoa was counted using a phase contrast microscope. Scores in each sample were subjectively estimated depending on the rate of motile sperm in same field, as well as ranged from 0 to 100%.
2). Sperm Viability (V)
The eosin (1.6%, eosin Y disodium salt) and nigrosin (6% nigrosin water soluble) staining solution was dissolved in a 0.9% saline buffer. Ten microliters of neat semen was added to 1 mL of staining solution and incubated for 2 min. The eosin–nigrosin stained semen solution was smeared onto slides and observed under a phase contrast microscope after drying (modified from Lemoine et al. (2011)). Sperm that stained pink were considered dead, and unstained sperm were considered to be viable. Each slide contained 300 spermatozoa.
3). Normal Morphology (NM)
Eosin–nigrosin staining was also used to detect normal sperm morphology. Viable sperm that were linear from the head to the tail were considered to have normal sperm morphology. Abnormal sperm morphologies included spermatids, sperm with bent necks, and sperm with any other head deformation (Fig. 1) (Lukaszewicz et al., 2008). Each slide contained 300 spermatozoa.
Fig. 1.
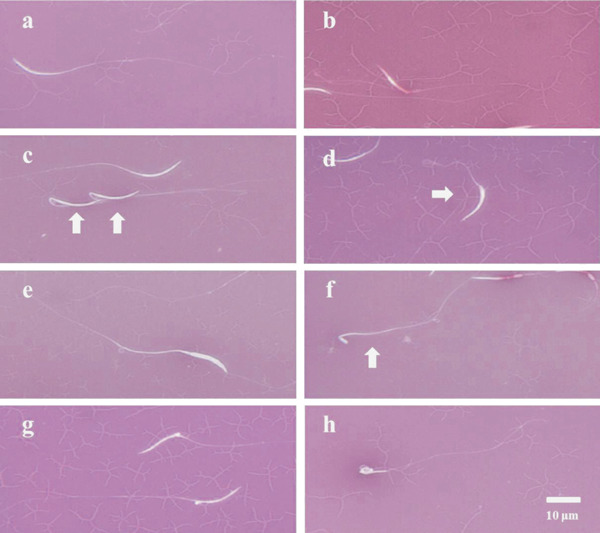
Drake sperm morphology in eosin-nigrosin smears. (a) Viable sperm with normal morphology, (b) dead sperm, (c) bent-neck sperm, (d) bulb-head sperm, (e) double-head sperm, (f) spermatid-like sperm, (g) protoplasmic drop-like head sperm and (h) looped-head sperm.
Sperm Evaluation by Flow Cytometry
Sperm evaluation was performed with a Cell Lab Quanta SC (Beckman Coulter, Fullerton, CA, USA) flow cytometer equipped with a 488-nm laser. SYBR-14 and R123 fluorescence was detected with an FL1 detector, whereas PI fluorescence was detected with an FL3 detector. Data acquisitions were analyzed with the Quanta control software. Non-sperm events were gated out based on their scatter properties and were not analyzed. At least 10,000 spermatozoa were analyzed for each sample.
1). Plasma Membrane Integrity (PMI)
Sperm membrane integrity was assessed with SYBR-14 and PI dual staining (LIVE/DEAD® Sperm Viability Kit, Invitrogen™, Thermo Fisher Scientific). This method was slightly modified from that of Partyka et al. (2010). Samples were diluted with 0.9% saline buffer to obtain a concentration of 5×105 spermatozoa per mL. Subsequently, 500 µL of the diluted samples were pipetted into Eppendorf tubes, and 5 µL of a SYBR-14 working solution, obtained by diluting a commercial solution of SYBR-14 into DMSO at a ratio of 1:49, was added. Samples were then mixed, incubated at 37°C for 10 min and stained with 5 µL of PI for 5 min. Analysis followed the 5-min PI staining. The entire fluorescent staining procedure was performed in the dark.
2). Mitochondrial Function (MF)
A previous report combined fluorescent dyes to estimate the percentage of spermatozoa with functional mitochondria (Partyka et al., 2010). In the present study, we applied the dual dye procedure with minor modifications. A Rhodamine 123 working solution was obtained by adding 20 µL of R123 to 500 µL of diluted semen samples (5×105 spermatozoa per mL in saline buffer). Samples were incubated at 37°C for 20 min, and the samples were centrifuged at 500×g for 5 min. Sperm pellets were resuspended in 500 µL of saline buffer and then stained with 5 µL of PI for 5 min. Analysis followed the 5 min PI staining. These steps were also performed in the dark. The concentrations of the R123 working solution and the PI solution were 0.01 and 0.1 mg/mL, respectively. Both solutions were dissolved in distilled water.
Artificial Insemination (AI) and Egg Incubation
AI was performed with diluted semen samples that were collected from 6 randomly selected Muscovy drakes. Semen samples (0.1 mL) were diluted to a concentration of 200×107 spermatozoa per mL by a Milieu Avicole commercial extender (IMV-Technologies, L'Aigle, Cedex, France). Female ducks were then inseminated with the semen samples (0.1 mL). There were 2 stages of artificial inseminations performed for the 2 different female species. In the first stage, 42 Muscovy females were separated into 6 groups so that the semen from each of the drakes was individually inseminated into each of 7 female Muscovy ducks. In the second stage, 42 Kaiya females were separated into 6 groups so that the semen from each of the drakes was individually inseminated into each of 7 female Kaiya ducks. The distributions of drakes to ducks were changed in each replicate. Eggs were collected and individually identified on Days 2, 3, and 4 after the ducks were artificially inseminated. The eggs were stored at 16°C and incubated under the standard conditions for Muscovy or mule duck eggs. The eggs were candled with a lampon incubation Day 7 to assess their status, including the percentage of apparent egg fertility of the total incubated eggs (F), the percentage of early embryo mortality of fertile eggs (EEM) and the survival embryo rate of the total incubated eggs (SER).
Experiment Design
Seven drakes were randomly selected to have their semen collected and their sperm quality individually evaluated by flow cytometry and microscopy. These individual semen samples were also artificially inseminated into female Muscovy and Kaiya ducks. The sperm quality detection results were analyzed, and the correlation between the sperm quality and the AI results clarified their relationship.
Data Analysis
All statistical analyses were performed with SAS software (version 9.1, Statistical Analyses System, Cary, NC, USA). The results were presented as percentages, using arcsine transformation prior to the analysis. Analysis of variance (ANOVA) was used to analyze the differences in the data using the general linear model (GLM) procedure. Duncan's New Multiple Range Test (DMRT) was used to further analyze the significant main effects. Correlation coefficients (r) were calculated with the correlation analysis (CORR) using SAS to determine the relationship between the M, V, NM, PMI, and MF and the results after AI of Muscovy or Kaiya female ducks (M- or K- F, EEM and SER). The results were presented as the mean±SD of the semen sample measurements.
Results
Sperm Motility (M), Viability (V) and Normal Morphology (NM) Detected by Phase Contrast Microscopy
Seven drakes were randomly selected to have their semen individually collected and their sperm quality detected by phase contrast microscopy. Sperm collection from each drake was performed more than 3 times; the semen collection occurred at 2- to 3-day intervals. The M values ranged from 50 to 81%, and there were significant differences among the drakes (P<0.05) (Table 1). The V and NM values were detected using a combination of eosin–nigrosin staining and microscopy. The values of V and NM ranged from 40.5 to 71.1% and 30.1 to 80.4%, respectively, and significant differences between these two quality parameters were observed among the drakes (P<0.05) (Table 1).
Table 1. Individual differences in Muscovy drake sperm quality detected by microscopy or flow cytometry (n=6) (results expressed as the mean±SD).
| Cage no. | % |
||||
|---|---|---|---|---|---|
| Eosin-nigrosin staining |
Flow cytometry |
||||
| Sperm motility | Sperm viability | Normal morphology | Plasma membrane integrity | Mitochondrial function | |
| 107 | 71±10a | 56.3±10.5abc | 74.4±9.2ab | 54.2±9.6ab | 60.3±12.8 |
| 108 | 68±11ab | 66.6±12.4ab | 30.1±23.2c | 58.2±9.0a | 65.5±9.9 |
| 109 | 50±12b | 45.2±24.4bc | 68.2±33.1ab | 55.4±14.1ab | 55.9±15.9 |
| 112 | 66±15ab | 46.7±25.8abc | 80.4±8.1a | 58.1±11.2a | 60.5±16.7 |
| 113 | 73±20a | 71.1±0.8a | 52.9±17.3bc | 64.8±5.3a | 68.0±2.7 |
| 114 | 50±24b | 40.5±18.3c | 51.5±22.4bc | 41.9±15.6b | 51.9±9.4 |
| 117 | 81±11a | 58.9±16.4abc | 51.5±22.9bc | 52.2±7.1ab | 58.9±10.1 |
The means in a column with no common superscript differ significantly: P<0.05.
Fluorescent Staining of Drake Sperm and Flow Cytometry Analysis
The quality of the Muscovy drake sperm was detected by flow cytometry. The spermatozoa were stained with the dual fluorescent dyes and subjected to flow cytometry. The data are presented as a dimensional bitmap (Fig. 2 & 3). Every spermatozoon was labeled with fluorescence and then scattered as a point throughout the four quadrants. The results of the PMI detection are presented in Fig. 2, and Fig. 4 presents the sperm fluorescently labeled with SYBR-14 and PI. Dead spermatozoa with broken plasma membranes appeared red and were previously stained with PI. During flow cytometry analysis, the software revealed that the red spermatozoa were scattered throughout the first and second quadrants (Q1 & Q2). Live spermatozoa with intact plasma membranes appeared green and were previously stained with SYBR-14. The software analysis revealed that green spermatozoa were located throughout the fourth quadrant (Q4), whereas cell debris spermatozoa that were not stained with any dye were located throughout the third quadrant (Q3).
Fig. 2.
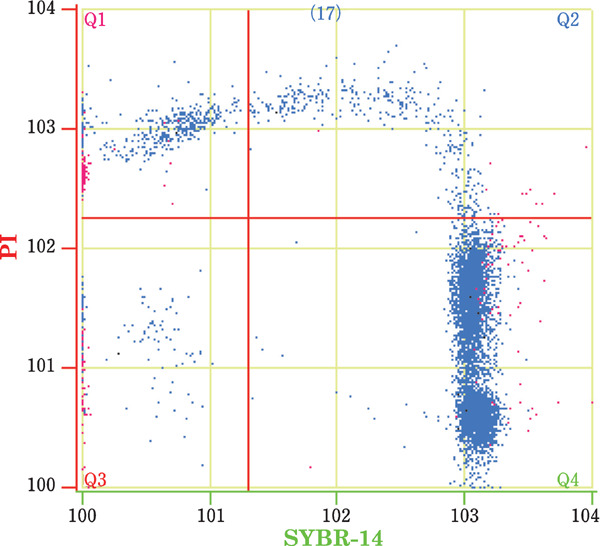
Plasma membrane integrity of drake sperm as examined by flow cytometry. Q1: dead sperm with disrupted membranes (SYBR−, PI+), Q2: dying sperm (SYBR+, PI+), Q3: debris (SYBR−, PI−), and Q4: viable sperm with intact membranes (SYBR+, PI−).
Fig. 3.
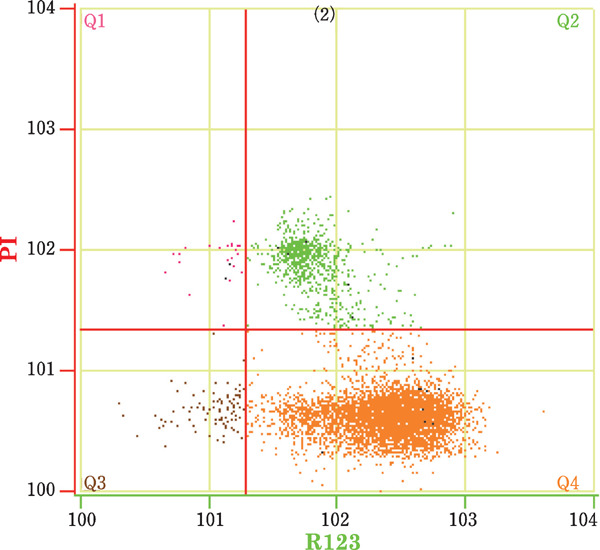
The mitochondrial function of drake sperm as examined by flow cytometry. Q1: dead sperm without functional mitochondria (R123−, PI+), Q2: dead sperm with functional mitochondria (R123+, PI+), Q3: viable sperm without functional mitochondria (R123−, PI−), and Q4: viable sperm with functional mitochondria (R123+, PI−).
Fig. 4.
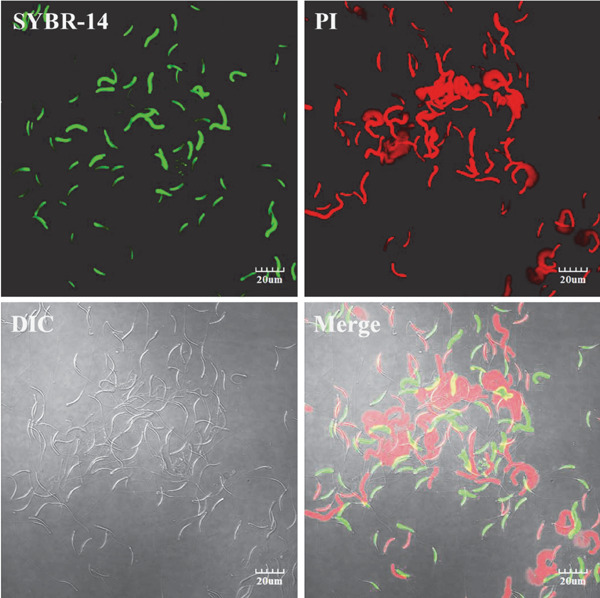
Plasma membrane integrity of drake sperm as detected by immunofluorescence. SYBR-14: fluorescence staining with SYBR-14; PI: fluorescence staining with PI; DIC: differential interference contrast image; and Merge: overlap of dual fluorescence and DIC. Images were acquired using an Olympus IX81/FV1000 laser scanning confocal microscope (Olympus, Tokyo, Japan). Scale bar=20 µm.
To analyze MF, spermatozoa were stained with R123 and PI fluorescent dyes; this staining was then analyzed by flow cytometry and the associated software. Spermatozoa located throughout Q4 were only stained with R123, representing live spermatozoa with functional mitochondria (Fig. 3). Green fluorescence was observed in the sperm midpiece (Fig. 5). Dead spermatozoa appeared red and were previously stained with PI. During flow cytometry analysis, dead spermatozoa were scattered throughout Q1 and Q2. Live spermatozoa without any mitochondrial function were located throughout Q3 and were not stained with any dye. The Q4 percentages represented the values for the PMI and MF.
Fig. 5.
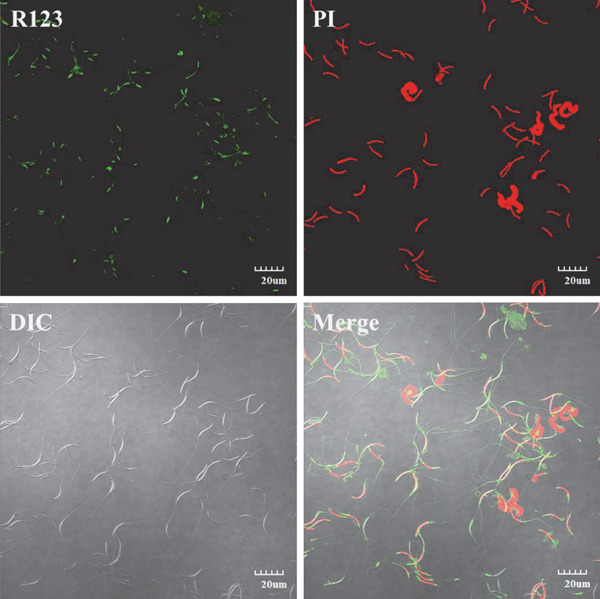
Mitochondrial function of drake sperm as assessed by immunofluorescence. R123: fluorescence staining with R123; PI: fluorescence staining with PI; DIC: differential interference contrast image; and Merge: overlap of dual fluorescence and DIC. Images were acquired using an Olympus IX81/FV1000 laser scanning confocal microscope (Olympus, Tokyo, Japan). Scale bar=20 µm.
Plasma Membrane Integrity (PMI) and Mitochondrial Function (MF) Detected by Flow Cytometry
The results are showed as Table 1. As detected by flow cytometry, the PMI values ranged from 41.9 to 64.8% and differed significantly among the drakes (P<0.05). There were no significant differences in the MF, and these values ranged between 51.9 and 68.0% (Table 1).
AI of Muscovy Duck Females
Six drakes were selected from the aforementioned 7, and semen from each of the 6 drakes was collected to inseminate female Muscovy ducks. Semen was collected from each drake 3 times; thus, the female ducks were artificially inseminated 3 times. Eighteen female ducks were inseminated by the semen of each drake. The results are presented in Table 2. Female Muscovy ducks were inseminated with semen collected from the drakes in cages 107, 108, 109, 112, 114 and 117 and laid 54, 48, 57, 52, 60 and 50 eggs, respectively. The M (AI of Muscovy ducks)-F, M-EEM and M-SER values of each drake ranged from 68.4 to 82.9%, 14.7 to 32.7% and 47.7 to 63.5%, respectively. After AI, there were no significant differences in these parameters among the drakes.
Table 2. The results of AIin Muscovy ducks (results expressed as the mean±SD).
| Cage no. | % |
|||
|---|---|---|---|---|
| Total eggs | Apparent egg fertility | Early embryo mortality of fertile eggs | Embryo survival rate of all eggs | |
| 107 | 54 | 74.7±12.9 | 21.5±13.9 | 59.7±19.5 |
| 108 | 48 | 70.7±9.2 | 23.4±1.4 | 54.2±7.2 |
| 109 | 57 | 82.9±5.8 | 23.4±1.4 | 63.5±5.5 |
| 112 | 52 | 71.9±7.6 | 32.7±15.0 | 47.7±6.8 |
| 114 | 60 | 75.7±21.5 | 31.8±19.7 | 54.2±26.6 |
| 117 | 50 | 68.4±10.1 | 14.7±18.7 | 58.6±16.4 |
No significant differences.
AI of Kaiya Duck Females
The AI process used in this aspect of the study was the same as that mentioned above. Female Kaiya ducks were inseminated using semen from 6 individual drakes. The sperm of each drake was artificially inseminated into the females twice; thus, each drake was used to inseminate 12 females. The results are presented in Table 3. Female Kaiya ducks were inseminated with semen collected from the drakes 107, 108, 109, 113, 114 and 117 and laid 35, 45, 40, 41, 50 and 42 eggs, respectively. The K (AI of Kaiya ducks) -F values of each drake ranged from 65.9 to 87.9%. K-SER ranged from 48.9 to 84.9%, and differed significantly among the drakes (P<0.05).
Table 3. The results of AIin Kaiya ducks (results expressed as the mean±SD).
| Cage no. | % |
|||
|---|---|---|---|---|
| Total eggs | Apparent egg fertility | Early embryo mortality of fertile eggs | Survival embryo rate of all eggs | |
| 107 | 35 | 74.8±3.0b | 21.3±12.4 | 58.7±6.9b |
| 108 | 45 | 67.0±6.3b | 13.3±9.4 | 58.3±11.8ab |
| 109 | 40 | 79.2±5.9ab | 0.0±0.0 | 79.2±5.9ab |
| 113 | 41 | 87.9±0.5a | 3.3±4.7 | 84.9±3.6a |
| 114 | 50 | 72.1±0.9b | 14.4±6.2 | 61.7±3.7ab |
| 117 | 42 | 65.9±22.5b | 30.6±27.5 | 48.9±33.8b |
The means in a column with no common superscript differ significantly: P<0.05.
Correlations between in vitro and in vivo Sperm Quality Parameters
The sperm quality parameters (M, V, NM, PMI and MF) and the AI results (F, EEM and SER) of Muscovy or Kaiya ducks were integrated to further analyze the correlation of these parameters. The correlation sample size among the sperm quality parameters was 41. The correlation sample sizes among sperm quality parameters and AI results with Muscovy and Kaiya ducks were 18 and 12, respectively. The results are presented in Table 4. Among the sperm quality parameters, M was positively correlated to V; this correlation was extremely significant (r=0.46; P<0.01). This sperm quality parameter was also positively correlated to MF (r=0.32; P<0.05). V was positively correlated with PMI and MF; this correlation was also extremely significant (r=0.70; P<0.01). NM was positively correlated with PMI (r=0.32; P<0.05). The PMI was positively correlated with the MF (r=0.59; P<0.01). Viability was negatively correlated with M-EEM (r=−0.53; P<0.05), whereas PMI was negatively correlated with M-EEM (r=−0.54; P<0.05).
Table 4. Correlations among AI success and drake sperm quality as assessed by microscopy or flow cytometry.
| Va | NMa | PMIa | MFa | M-Fb | M-EEMb | M-SERb | K-Fc | K-EEMc | K-SERc | |
|---|---|---|---|---|---|---|---|---|---|---|
| (r) | (r) | (r) | (r) | (r) | (r) | (r) | (r) | (r) | (r) | |
| M | 0.46** | −0.07 | 0.28† | 0.32* | −0.43† | −0.07 | −0.21 | 0.10 | 0.06 | 0.04 |
| V | 0.13 | 0.70** | 0.70** | −0.02 | −0.53* | 0.34 | −0.01 | 0.16 | −0.09 | |
| NM | 0.32* | 0.28† | 0.15 | −0.18 | 0.19 | 0.19 | −0.08 | 0.11 | ||
| PMI | 0.59** | 0.18 | −0.54* | 0.44† | 0.12 | −0.01 | 0.09 | |||
| MF | 0.02 | −0.34 | 0.25 | 0.36 | −0.55† | 0.48 |
n=41
n=18
n=12
P<0.01
P<0.05
P<0.1.
M: sperm motility, V: eosin-nigrosin staining - sperm viability, NM: eosin-nigrosin staining - normal morphology, PMI: flow cytometry - plasma membrane integrity, MF: flow cytometry - mitochondrial function, M-F: percent apparent egg fertility of AI of Muscovy ducks, M-EEM: percent early embryo mortality of fertile eggs by AI of Muscovy ducks, M-SER: embryo survival rate of total eggs by AI of Muscovy ducks. K-F: percent apparent egg fertility by AI of Kaiya ducks, K-EEM: percent early embryo mortality of fertile eggs by AI of Kaiya ducks, K-SER: embryo survival rate of total eggs by AI of Kaiya ducks.
Discussion
To the best of our knowledge, the present study is the first showing the in vitro detection of drake sperm quality by flow cytometry and assessing the relationship between conventional detection methods, as well as with the results of AI with females in same or a different duck species. Flow cytometry detects 10,000 sperm in only 3–5 min, which is 50-fold more sperm in the same time unit than the conventional method. The advantages associated with flow cytometry improve the accuracy and efficiency of sperm quality detection. Previous studies have utilized flow cytometry to detect poultry sperm quality in turkeys, chicken, geese and pigeons (Donoghue et al., 1995; Partyka et al., 2010; Partyka et al., 2011; Klimowicz-Bodys et al., 2012). These studies focused on direct applications (e.g., detection of frozen-thawed sperm), but rarely analyzed the detection feasibility or the correlation with other sperm quality parameters. Actually, the correlation of sperm quality parameters is important for predict the fertilizing capacity of semen sample, especially with AI results.
In mammalian species, the relationships among some parameters are demonstrated. Sperm motility is the most common sperm detection parameter. However, motility is not suitable to predict the fertilizing capacity alone (Graham et al., 1990). Subjective judgment can be biased by the technique and experience of the manipulator. Compare to conventional method, flow cytometry assisted with fluorescent dyes is an objective and accurate strategy for sperm quality detection. Garner et al. (1986) suggested that the percentage of dead sperm (i.e., sperm stained with PI) in frozen-thawed bull semen is negatively associated with the rate of sperm with progressive motility. In addition, Garner et al. (1997) stained bull sperm with R123 or MITO to compare the feasibility in these mitochondrial function detection methods by using flow cytometry, and the results indicated that R123 or MITO staining reveals that mitochondrial function is highly correlated with sperm motility and viability.
Intact plasma membranes in spermatozoa are necessary for their survival and fertilization. Sperm viability is always an important indicator of the fertilizing capacity. In poultry species, previous studies compared detection methods to assess sperm viability (or plasma membrane integrity) by fluorescence and eosin–nigrosin staining. In chicken, the detection efficiency using SYBR-14 and PI dual fluorescence staining is better than that using eosin–nigrosin staining (Chalah and Brillard, 1998; Chalah et al., 1999). In addition, the detection of sperm viability by eosin–nigrosin staining results in more variation because this method depends on the technical ability and experience of the technician. Detection results from the same samples may differ due to analyze by different laboratories. Such differences in results affect the accuracy of the method. SYBR-14 and PI dual staining provides even staining to sperm; thus, these stains can adequately distinguish whether the sperm plasma membrane is intact. Spermatozoa that are simultaneously stained with SYBR-14 and PI are regarded as dying or moribund (Donoghue et al., 1995; Garner and Johnson, 1995; Magistrini et al., 1997). Klimowicz-Bodys et al. (2012) indicated that the percentage of dying sperm increases as the preservation time lengthened, which is negatively correlated with sperm motility. Combined SYBR-14 and PI dual fluorescence staining and flow cytometry analysis can improve the efficiency of detection (Donoghue et al., 1995; Donoghue et al., 1996; Klimowicz-Bodys et al., 2012).
Garner et al. (1997) suggested that mitochondrial function is highly correlated with sperm motility and viability, which is consistent with the results from the current study. Mitochondria are the major energy source of sperm; thus, mitochondrial function can predict sperm motility. Computer-assisted sperm analysis (CASA) is developed to detect sperm motility and to objectively assess the details of sperm activity. In birds, Klimowicz-Bodys et al. (2012) used CASA to detect sperm motility and progressive motility in pigeons and found that these two parameters are positively correlated with sperm viability, the similar result is also observed in this study. In the correlation analysis of present study, Sperm motility is positively correlated with viability and trends towards a positive association with plasma membrane integrity. This finding suggested that higher sperm survival rate indicates greater sperm motility. Sperm plasma membrane integrity is positively correlated with sperm viability in present study, due to principles of each detection method are similar. Both methods focus on detecting the integrity of the sperm membrane. We also found that sperm membrane integrity is positively correlated with normal morphology of sperm, which indicates that the plasma membrane integrity is associated to sperm morphology in duck. In addition, mitochondrial function is positively correlated with sperm normal morphology. This positive correlation can be related to abnormal sperm produced during spermatogenesis, the migration of mitochondria and the formation of the mitochondrial sheath, which are also accomplished at the same time as spermatogenesis (Aire, 2003). Thus, abnormal sperm morphology may affect mitochondrial function.
Seven drakes are selected to detect each sperm quality, individual differences on sperm quality among the drakes possibly reflects the AI results. Because individual fertility differences are known to be important, these results are corroborated by Tai Liu and Tai (1984), who reported that individual fertility differences among the drakes exist after AI; therefore, detecting the quality of the Muscovy drake sperm is necessary before AI to improve the efficiency of mule duck production.
In present study, sperm viability and plasma membrane integrity were negatively associated with early embryo mortality of the Muscovy duck. Therefore, if sperm viability and the number sperm with intact plasma membranes are higher in Muscovy drake semen, then the embryo mortality of the collected eggs after AI will decrease and the embryo survival rate will thus to increase. The integrity of DNA in sperm is a crucial factor for further embryo development. Unfortunately, due to the ratio of polyunsaturated fatty acid (PUFA) in cell membrane of avian sperm is comparative higher, reactive oxygen acid (ROS) becomes a major risk to influence sperm quality by a series damages including DNA fragmentation, that all is started from causing lipid peroxidation (LPO) in sperm membrane. Wishart et al. (1986) indicated that sperm motility and ATP concentration are good predictors for fertility in avian species, but the loss of fertility would be occurred because of sperm function is fell since LPO is occurred (Wishart, 1982; Wishart, 1984). Actually, when avian spermatozoa experience preservation procedure, the damage in DNA is occurred and accompanied with cell membrane damage (Gliozzi et al., 2011). Therefore, we consider that DNA damage could have occurred after the sperm membrane is broken, thus possibly affecting and halting embryo development after fertilization.
In conclusion, the application of the detection on plasma membrane integrity and mitochondrial function by flow cytometry are efficient to measure the sperm quality in Muscovy drake, and these methods are accurate tools that may be helpful to predict the male reproductive capacity in this species, and as alternative to sperm assessment by convention methods. Therefore, selecting drakes with better sperm quality for AI can increase the survival rate of duck embryo during egg incubation, and further improve the efficiency of duck production.
Acknowledgments
The authors thank all technicians at the Ilan Branch of the Livestock Research Institute for their fieldwork assistance and Ms. Priscilla Tsai of Beckman Coulter Taiwan, Inc. for her technical assistance with flow cytometry. We also thank Dr. Mihailo A. Savić of IMV-Technologies for sharing his experience.
References
- Aire TA. Ultrastructural study of spermiogenesis in the turkey, meleagris gallopavo. British Poultry Science, 445: 674-682. 2003. [DOI] [PubMed] [Google Scholar]
- Blesbois E, Grasseau I, Seigneurin F, Mignon-Grasteau S, Saint Jalme M, Mialon-Richard MM. Predictors of success of semen cryopreservation in chickens. Theriogenology, 692: 252-261. 2008. [DOI] [PubMed] [Google Scholar]
- Blom E. A one-minute live-dead sperm stain by means of eosin-nigrosin. Fertility and Sterility, 1: 176-177. 1950. [Google Scholar]
- Chalah T, Brillard JP. Comparison of assessment of fowl sperm viability by eosin-nigrosin and dual fluorescence (sybr-14/pi). Theriogenology, 503: 487-493. 1998. [DOI] [PubMed] [Google Scholar]
- Chalah T, Seigneurin F, Blesbois E, Brillard JP. In vitro comparison of fowl sperm viability in ejaculates frozen by three different techniques and relationship with subsequent fertility in vivo. Cryobiology, 392: 185-191. 1999. [DOI] [PubMed] [Google Scholar]
- Donoghue AM, Garner DL, Donoghue DJ, Johnson LA. Viability assessment of turkey sperm using fluorescent staining and flow cytometry. Poultry Science, 747: 1191-1200. 1995. [DOI] [PubMed] [Google Scholar]
- Donoghue AM, Garner DL, Donoghue DJ, Johnson LA. Assessment of the membrane integrity of fresh and stored turkey spermatozoa using a combination of hypo-osmotic stress fluorescent staining and flow cytometry. Theriogenology, 461: 153-163. 1996. [Google Scholar]
- Gadea J. Sperm factors related to in vitro and in vivo porcine fertility. Theriogenology, 632: 431-444. 2005. [DOI] [PubMed] [Google Scholar]
- Garner DL, Pinkel D, Johnson LA, Pace MM. Assessment of spermatozoal function using dual fluorescent staining and flow cytometric analyses. Biology of Reproduction, 341: 127-138. 1986. [DOI] [PubMed] [Google Scholar]
- Garner DL, Johnson LA. Viability assessment of mammalian sperm using sybr-14 and propidium iodide. Biology of Reproduction, 532: 276-284. 1995. [DOI] [PubMed] [Google Scholar]
- Garner DL, Thomas CA, Joerg HW, DeJarnette JM, Marshall CE. Fluorometric assessments of mitochondrial function and viability in cryopreserved bovine spermatozoa. Biology of Reproduction, 576: 1401-1406. 1997. [DOI] [PubMed] [Google Scholar]
- Gliozzi TM, Zaniboni L, Cerolini S. DNA fragmentation in chicken spermatozoa during cryopreservation. Theriogenology, 759: 1613-1622. 2011. [DOI] [PubMed] [Google Scholar]
- Graham JK, Kunze E, Hammerstedt RH. Analysis of sperm cell viability, acrosomal integrity, and mitochondrial function using flow cytometry. Biology of Reproduction, 431: 55-64. 1990. [DOI] [PubMed] [Google Scholar]
- Klimowicz-Bodys MD, Batkowski F, Ochrem AS, Savic MA. Comparison of assessment of pigeon sperm viability by contrast-phase microscope (eosin-nigrosin staining) and flow cytometry (sybr-14/propidium iodide (pi) staining) [evaluation of pigeon sperm viability]. Theriogenology, 773: 628-635. 2012. [DOI] [PubMed] [Google Scholar]
- Lemoine M, Mignon-Grasteau S, Grasseau I, Magistrini M, Blesbois E. Ability of chicken spermatozoa to undergo acrosome reaction after liquid storage or cryopreservation. Theriogenology, 751: 122-130. 2011. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz E, Jerysz A, Partyka A, Siudzinska A. Efficacy of evaluation of rooster sperm morphology using different staining methods. Research in Veterinary Science, 853: 583-588. 2008. [DOI] [PubMed] [Google Scholar]
- Magistrini M, Guitton E, Levern Y, Nicolle JC, Vidament M, Kerboeuf D, Palmer E. New staining methods for sperm evaluation estimated by microscopy and flow cytometry. Theriogenology, 487: 1229-1235. 1997. [DOI] [PubMed] [Google Scholar]
- Partyka A, Nizanski W, Lukaszewicz E. Evaluation of fresh and frozen-thawed fowl semen by flow cytometry. Theriogenology, 746: 1019-1027. 2010. [DOI] [PubMed] [Google Scholar]
- Partyka A, Lukaszewicz E, Nizanski W. Flow cytometric assessment of fresh and frozen-thawed canada goose (branta canadensis) semen. Theriogenology, 765: 843-850. 2011. [DOI] [PubMed] [Google Scholar]
- Peterson K, Kappen MA, Ursem PJ, Nothling JO, Colenbrander B, Gadella BM. Microscopic and flow cytometric semen assessment of dutch ai-bucks: Effect of semen processing procedures and their correlation to fertility. Theriogenology, 674: 863-871. 2007. [DOI] [PubMed] [Google Scholar]
- Tai C. Artificial insemination in duck Stock Farming of Tendays, 483: 63-70. 1978. [Google Scholar]
- Tai Liu JJ, Tai C. Studies on the artificial insemination of ducks 3. A comparison of fertility for pooled semen and individual male semen in the crosses between muscovy (Cairina moschata) and tsaiya duck (Anas platyrhynchos var. Domestica). Taiwan Livestock Research., 171: 85-89. 1984. [Google Scholar]
- Tai Liu JJ, Tai C. Mule duck production in taiwan. I. Artificial insemination of ducks. Food and Fertilizer Technology Center, extension bulletin, 328: 1-6. 1991. [Google Scholar]
- Wishart GJ. Maintenance of atp concentrations in and of fertilizing ability of fowl and turkey spermatozoa in vitro. Journal of Reproduction and Fertility, 662: 457-462. 1982. [DOI] [PubMed] [Google Scholar]
- Wishart GJ. Effects of lipid peroxide formation in fowl semen on sperm motility, atp content and fertilizing ability. Journal of Reproduction and Fertility, 711: 113-118. 1984. [DOI] [PubMed] [Google Scholar]
- Wishart GJ, Palmer FH. Correlation of the fertilising ability of semen from individual male fowls with sperm motility and atp content. British Poultry Science, 271: 97-102. 1986. [DOI] [PubMed] [Google Scholar]


