Abstract
The objective of the present study was to examine the effects of nano-selenium on growth performance, antioxidative status, and immune function in broiler chickens reared under thermoneutral (22±1°C) or high ambient temperature (35±1°C) conditions. Thirty-six broiler chicks at 15d old were randomly divided into 6 treatments in a 3×2 factorial design. The main factors included the dietary supplementation (basal diet without Se supplementation [control], basal diet with 0.3 mg of nano-elemental Se per kilogram of diet [nano-Se], and basal diet with 0.3 mg of sodium selenite per kilogram of diet [SSe]) and the ambient temperature challenge (22±1°C or 35±1°C). The birds were given the experimental diets from 15 to 30 d of age. High ambient temperature significantly depressed body weight gain, feed intake, feed conversion ratio, breast muscle weight, and abdominal fat weight, while feeding nano-Se clearly alleviated these negative effects of high ambient temperature. In addition, feeding nano-Se increased glutathione peroxidase mRNA expression in liver and alleviated the negative effects of high ambient temperature via reducing the malondialdehyde content in liver and breast muscle. Furthermore, feeding nano-Se increased mRNA expression of cytokine genes (interleukins 2 and 6) under both thermoneutral and high ambient temperature conditions. Under both thermoneutral and high-temperature conditions, broiler chickens fed nano-Se had higher Se and vitamin E concentrations in breast muscle than broiler chickens fed the control diet. In contrast, feeding SSe at the same dose as nano-Se did not alleviate the negative effects of high ambient temperature on broiler chickens. In conclusion, dietary supplementation with nano-Se at 0.3 mg/kg diet might enhance growth performance by improving antioxidative or immune properties in broilers reared under high ambient temperature.
Keywords: antioxidative status, broiler chickens, growth performance, nano-selenium
Introduction
Heat is one of the most important stressors affecting poultry production, leading to the loss of millions of dollars each year. Modern broiler breeds are more susceptible to heat stress than earlier genotypes. High ambient temperature reduces feed intake, live weight gain, feed efficiency, and immune response of chicken broilers (Siegel, 1995; Melesse et al., 2011). Moreover, hyperthermia may promote reactive oxygen species (ROS) formation (Mujahid et al., 2005). Excessive ROS levels disturb the balance between oxidation and antioxidant defense systems, resulting in lipid peroxidation (Shimizu et al., 2006) and oxidative damage to proteins, DNA, and vital biological molecules (Suraï, 2002). Such damage is associated with apoptosis (Moustafa et al., 2004), various diseases (Hybertson et al., 2011), and impaired muscle membrane integrity (Mujahid et al., 2005; Wang et al., 2009). Therefore, a balance between ROS production and the antioxidant system must be established to maintain immune function, health, and productivity (Suraï, 2002).
Natural antioxidants play vital roles in protecting cells from ROS by reducing free radicals and preventing the peroxidation of lipids (Grashorn, 2007). The antioxidant system includes numerous antioxidant enzymes, such as glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (CAT) (Suraï, 2002). Se is an integral component of the active site of GSH-Px (Yoon et al., 2007), an enzyme that helps to control levels of hydrogen peroxide and lipid peroxides (Arthur, 2000). In addition, Se enhances the immune response in several species (Rayman, 2004; Ebeid et al., 2013). Wang and Xu (2008) showed that dietary Se status influenced growth performance, survival, meat quality, and antioxidant protection in chickens. However, the bioavailability of Se depends on its form. Inorganic forms such as sodium selenite (Na2SeO3, SSe) are typically used in poultry feeds (Suraï, 2002).
Recently, nano-elemental Se (nano-Se) has attracted more attention because of its high bioavailability, high catalytic efficiency, strong adsorbing ability, and low toxicity compared with selenite in chickens (Wang et al., 2009), mice (Wang et al., 2007), rats (Jia et al., 2005), sheep (Shi et al., 2011a), and goats (Shi et al., 2011b). However, data on antioxidant status in muscles and blood of heat-stressed broilers fed nano-Se are still limited. Therefore, the objective of the present study was to compare the effects of two different Se sources (nano-Se) on growth performance, lipid peroxidation, antioxidative status, immune function, and Se retention in broilers reared under thermoneutral or high ambient temperature conditions.
Materials and Methods
Animals, Housing, Diets, and Experimental Design
The animal experiment was conducted in accordance with the guidelines of Kagoshima University, Japan. One hundred 1-d-old male broiler chicks (Ross 308, Nippon Chunky Co. Ltd.) were obtained from a commercial hatchery (Kumiai Hina Center, Kagoshima, Japan). The chicks were housed in an electrically heated battery brooder and provided with water and a commercial starter diet (22% crude protein and 3,000 kcal/kg; Nichiwa Sangyou Company) until 12 d of age. On day 12, 36 birds were randomly selected from the group of 100 birds and housed individually in wire-bottomed aluminum cages (49×39×59 cm). The birds were preconditioned for 3 d before treatment and fed a basal diet. The experimental diets were formulated using mainly ground yellow maize and a soybean meal (Table 1). The chicks were randomly allocated in a 3×2 factorial design; the main factors included the dietary supplementation (control: basal diet without Se supplementation; nano-Se: basal diet+0.3 mg of nano-elemental Se per kilogram of diet; SSe: basal diet+0.3 mg of sodium selenite [Na2SeO3] per kilogram of diet) and the ambient temperature challenge (22±1°C or 35±1°C). Thus, there were a total of 6 treatments in this study (n=6). The birds were given the experimental diets from 15 to 30 d of age. The experiment was conducted in a temperature-controlled room with 24 h of light. The high ambient temperature birds were kept under high ambient temperature everyday (35±1°C for 9 h), while thermoneutral birds were keptata moderate ambient temperature (22±1°C). Relative humidity was 50% to 70% throughout the experiment. Sodium selenite was provided by Sigma-Aldrich Co., Japan. The nano-Se was provided by Prof. Mohsen Zommara, Department of Dairy Science, Faculty of Agriculture, Kafrelsheikh University, Egypt, and Prof. Jozsef Prokisch, Department of Animal Breeding, University of Debrecen, Debrecen, Hungary. Se nanoparticles of 100 to 500 nm were prepared according to Eszenyi et al. (2011). In contrast to usual commercial practice, no supplementary Se was added to the basal diet, thus providing a low-Se basal diet containing only the endogenous Se contained in the feed ingredients. The basal diet contained approximately 0.25±0.002 mg of Se per kilogram of diet, as determined by inductively coupled plasma mass spectrometry (ICP-MS) according to Wolf et al. (2008). Water and feed were provided ad libitum.
Table 1. Composition and nutrient analysis of the experimental diet.
| % | |
|---|---|
| Ingredients (g/100 g) | |
| Corn meal | 55.10 |
| Alfalfa meal | 2.90 |
| Soybean meal | 33.50 |
| Corn oil | 4.70 |
| CaCO3 | 0.66 |
| CaHPO4 | 2.00 |
| NaCl | 0.50 |
| DL-Methionine | 0.14 |
| Mineral and vitamin premix1 | 0.50 |
| Calculated analysis | |
| Crude protein (%) | 20.00 |
| Metabolizable energy (MJ/kg) | 3.10 |
| Ca (%) | 1.00 |
| av.P (%) | 0.62 |
| Na (%) | 0.21 |
Content per kg of the vitamin and mineral premix: vitamin A 90 mg, vitamin D3 1 mg, DL-alpha-tocopherol acetate 2000 mg, vitamin K3 229 mg, thiamin nitrate 444 mg, riboflavin 720 mg, calcium d-pantothenate 2174 mg, nicotinamide 7000 mg, pyridoxine hydrochloride 700 mg, biotin 30 mg, folic acid 110 mg, cyanocobalamine 2 mg, calcium iodinate 108 mg, MgO 198,991 mg, MnSO4 32,985 mg, ZnSO4 19,753 mg, FeSO4 43,523 mg, CuSO4 4019 mg and choline chloride 299,608 mg.
Growth Performance and Relative Weights of Organs
Body weight was recorded every 6 d, feed intake was recorded daily during the experimental period, and feed conversion ratio (feed/gain) was calculated. At the end of the experimental period, all the birds were killed by cervical dislocation under carbon dioxide anesthesia. And then dissected to measure the relative weights of the breast muscle (pectoral superficial muscle), abdominal fat, bursa of Fabricius, thymus, and spleen. The relative weights are expressed as a percentage of total live body weight. The breast muscle and leftlobe of liver were snap frozen in liquid nitrogen and stored at −80°C until use.
RNA Extraction and Real-time PCR
Total RNA was extracted from a piece of liver (about 50 mg) using an ISOGEN II kit(NIPPON GENE Co., Ltd., Tokyo, Japan) according to the manufacturer's protocol. RNA concentration and purity were determined with a Thermo Scientific NanoDrop Lite Spectrophotometer (S17 NNP0027, Thermo Fisher Scientific, Hampton, NH, USA). Complementary DNA was synthesized from 40 ng of RNA per 10 µl of reaction solution using the PrimeScript™ RT Master Mix kit (RR036A, Takara, Shiga, Japan) and the Program Temp Control System PC-320 (Astec, Shime, Japan) with the following protocol: reverse transcription at 37°C for 15 min, inactivation of reverse transcriptase at 85°C for 5 s, and refrigeration at 4°C for 5 min. The primers used in this study are listed in Table 2. Gene expression was measured by real-time PCR using the 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR® Select Master Mix (4472918, Applied Biosystems). The thermal cycle was as follows: 1 cycle at 50°C for 2 min and 95°C for 2 min and 60 cycles at 95 °C for 15 s, 60°C for 15 s, and 72°C for 1 min. The expression of 18S ribosomal RNA was used as an internal standard and was not significantly different between experimental groups. Gene expression results are expressed as a percentage of the control value.
Table 2. List of primer sequences used for quantitative real-time polymerase chain reaction.
| Gene | Sequence (5′-3′) | Size (BP) | Accession No. | |
|---|---|---|---|---|
| Glutathione peroxidase | Forward | TTG TAA ACA TCA GGG GCA AA | 140 | NM0011633245.1 |
| Reverse | TGG GCC AAG ATC TTT CTG TAA | |||
| Cu/Zn-superoxisde dismutase | Forward | AGG GGG TCA TCC ACT TCC | 122 | NM205064.1 |
| Reverse | CCC ATT TGT GTT GTC TCC AA | |||
| Catalase | Forward | GGG GAG CTG TTT ACT GCA AG | 138 | AJ719360.1 |
| Reverse | CTT CCA TTG GCT ATG GCA TT | |||
| Interleukin-2 | Forward | TGC AGT GTT ACC TGG GAG AA | 148 | GU119890.1 |
| Reverse | CTT GCA TTC ACT TCC GGT GT | |||
| Interleukin-6 | Forward | GAC TCG TCC GGA GGA GGT TG | 138 | HM179640.1 |
| Reverse | CGC ACA CGG TGA ACT TCT T | |||
| 18s ribosomal RNA | Forward | AAA CGG CTA CCA CAT CCA AG | 154 | KC433410.1 |
| Reverse | CCT CCA ATG GAT CCT CGT TA |
Chemical Measurements
Blood samples were collected into heparinized test tubes, quickly centrifuged at 5,900×g for 10 min at 4 °C to separate plasma, and stored at −30°C until analysis. The activity of the GSH-Px of blood serum was measured according to Paglia and Valentine (1967). Malondialdehyde (MDA) is one of the most frequently used indicators of lipid peroxidation. Therefore, to evaluate lipid peroxidation levels in liver and skeletal muscle of chickens, MDA content was determined colorimetrically as 2-thiobarbituric acid reactive substances (TBARS) according to the method described by Azada et al. (2010). In brief, the sample was homogenized in 154 mM KCl and centrifuged at 700×g, and the supernatant was collected. Forty microliters of the supernatant was mixed with 40 µl of 8.1% SDS, 300 µl of 20% acetic acid (pH 3.5), and 300 µl of 0.8% 2-thiobarbituric acid. After vortexing, the sample was incubated at 95°C for 1 h and then transferred to ice. After addition of 1 ml of butanol-pyridine (15:1, v/v), the sample was mixed by vortexing and centrifuged at 1,200×g for 10 min. Absorbance of the supernatant (the butanol–pyridine layer) was measured at 532 nm. The content of TBARS is expressed as the MDA equivalent. The α-tocopherol concentration in muscle and plasma was determined with a high-performance liquid chromatography system (model LC-6A, Shimadzu, Kyoto, Japan) with a Shim-Pack CLC-ODS column (6.0×150 mm) according to the method described by Faustman et al. (1989).
Muscle Se Concentration
Muscle tissue (0.5 g) was placed in a beaker with 5 ml of nitric acid and heated gently on a plate heater for 3 h at 120°C. After cooling, 10 ml of a mixture of nitric acid and perchloric acid (5:3, v/v) was added; if the solution was still colored with organic matter, an additional 5 ml of nitric acid was added. The solution was heated at 120°C until white perchloric acid fumes appeared and then the Se concentration was measured using an ICP-MS system according to Wolf et al. (2008).
Statistical Analysis
Differences among treatments were analyzed as a 3×2 (diet×temperature) factorial arrangement of treatments by two-way ANOVA with a model including the main effects of dietary Se source, environmental temperature, and their interaction using the general linear models procedure of SPSS Statistics 17.0 (released 23 August 2008). When a significant interaction was observed, the means for each treatment were compared using Tukey's multiple comparison test. Data presented as percentages were transformed to the corresponding arcsine values before performing the statistical analysis. P≤0.05 was setas the limit of significance.
Results
The effects of dietary supplementation with nano-Se or SSe under different environmental conditions on body weight gain (BWG), feed intake (FI), feed conversion ratio (FCR), and the relative weights of tissues are presented in Table 3. Under the thermoneutral condition, no significant differences in BWG, FI, FCR, or tissue weights were detected among the diet treatments. Compared with the thermoneutral condition, the high ambient temperature condition significantly reduced BWG and FI and significantly increased FCR in broiler chickens fed the control diet. However, these negative effects of high ambient temperature were ameliorated in broiler chickens fed the diet containing nano-Se. There was a significant Se source×environmental temperature interaction for FCR. It could be observed that FCR was remarkably improved (P<0.05) when diets were supplemented with nano-Se under both environmental conditions. For all three diets, breast muscle weight was decreased and abdominal fat weight was increased under high ambient temperature compared with the thermoneutral condition. However, under high ambient temperature, significantly higher breast muscle weight and significantly lower abdominal fat weight were observed in broiler chickens fed the diet containing nano-Se compared with chickens fed the control diet. In contrast, SSe did not ameliorate the negative effects induced by high ambient temperature.
Table 3. Effect of different sources of dietary Se on growth performance and relative tissue weights in broiler chickens under thermoneutral or high ambient temperature.
| thermo-neutral temperature (22±1°C) |
high ambient temperature (35±1°C) |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| control | Nano-Se | SS | control | Nano-Se | SS | Se | T | Se×T | |
| Body weight gain (g) | 830.12±40.15a | 760.14±46.87a | 739.05±32.85ab | 563.07 ± 12.20b | 653.82±43.19ab | 598.34±25.61b | 0.5435 | <0.0001 | 0.0740 |
| Feed intake (g/15 days) | 1306.40±39.29a | 1158.60±27.30ab | 1224.60±44.68ab | 1051.80±20.83b | 1054.50±87.36b | 987.00±28.12b | 0.0490 | <0.0001 | 0.3763 |
| Feed conversion ratio | 1. 59±0.06b | 1.54±0.06b | 1.66±0.03ab | 1. 87±0.02a | 1.61 ± 0.03b | 1.66±0.10ab | 0.0016 | 0.0013 | 0.0014 |
| Breast muscle (g/100 g body weight) | 15.96±0.19ab | 18.13±1.12a | 16.18±0.56ab | 12.31±0.45c | 16.06±0.97ab | 13.37±0.64bc | 0.0407 | 0.0002 | 0.5822 |
| Abdominal fat (g/100 g body weight) | 0.69±0.07b | 0.58±0.17b | 0.69±0.08b | 1.40±0.12a | 0.69±0.05b | 1.30±0.19a | 0.0074 | 0.0001 | 0.0561 |
| Spleen (g/100 g body weight) | 0.12±0.01ab | 0.16±0.01a | 0.13±0.02ab | 0.09±0.01b | 0.12±0.01ab | 0.10±0.01ab | 0.0212 | 0.0035 | 0.9999 |
| Bursa (g/100 g body weight) | 0.32±0.02a | 0.36±0.03a | 0.29±0.03ab | 0.13±0.02c | 0.31 ± 0.02a | 0.16±0.03bc | 0.0005 | <0.0001 | 0.0701 |
| Thymus (g/100 g body weight) | 0.45±0.03ab | 0.56±0.04a | 0.41±0.04ab | 0.19±0.04c | 0.49±0.07a | 0.26±0.06bc | 0.0006 | 0.0014 | 0.1582 |
Values are means±SEM. Different letters indicate significant differences (P<0.05).
The effects of feeding a diet containing either nano-Se or SSe on the relative weights of immune (lymphoid) organs are also shown in Table 3. No significant interactions between Se source and environmental temperature were observed for relative weights of lymphoid organs (P>0.05). However, under both thermoneutral and high ambient temperature conditions, nano-Se had a positive effect on the relative weights of the bursa of Fabricius, thymus, and spleen of broiler chickens. Under the high ambient temperature condition, the relative weight of the bursa of Fabricius was significantly greater in broiler chickens fed the diet containing 0.3 ppm nano-Se than in broiler chickens fed either the control diet or the diet containing SSe.
Figure 1 shows the mRNA expression of antioxidant enzymes (GSH-Px, SOD, and CAT) in the liver. Under the thermoneutral condition, GSH-Px mRNA expression was about 3-fold higher in chickens fed the diet supplemented with nano-Se compared with the control group. When chickens were exposed to the high ambient temperature, GSH-Px mRNA expression in the nano-Se group was about 2-fold higher than that in the control group, while expression in the SSe group was not different from that in the control group. There was a significant Se source×environmental temperature interaction for CAT mRNA expression. Dietary nano-Se enhanced CAT mRNA expression under high ambient temperature. In contrast, neither nano-Se nor SSe affected SOD gene expression under high ambient temperature. Under both thermoneutral and high ambient temperature conditions, hepatic expression of gene encoding interleukin 2 (IL-2) was increased in chickens fed the diet containing nano-Se compared with chickens fed the control diet (Fig. 2A). Although there was no significant difference, gene expression of interleukin 6 (IL-6) tended to be increased in chickens fed the diet containing nano-Se under both thermoneutral and high ambient temperature conditions (Fig. 2B).
Fig. 1.
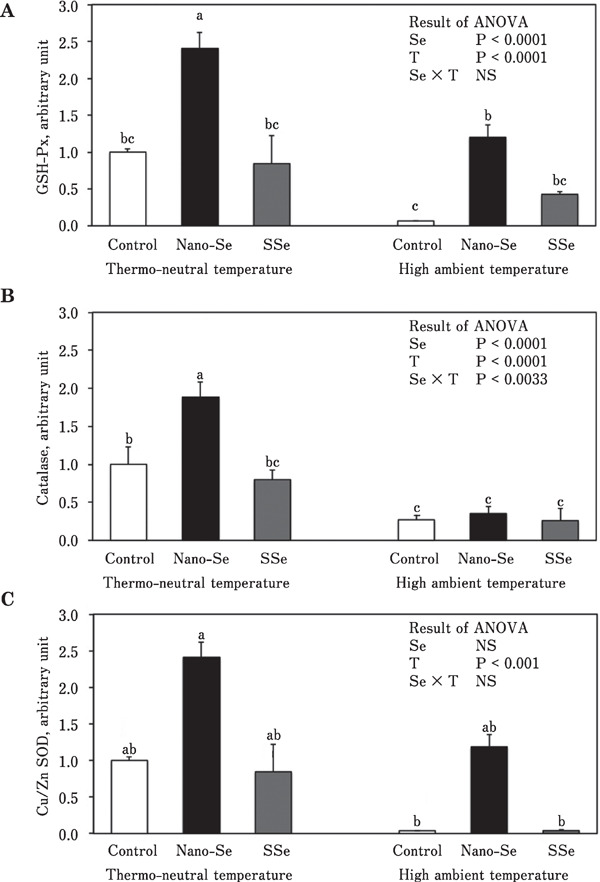
Effect of different sources of dietary Se on mRNA expression of GSH-Px (A), CAT (B), and SOD (C) in broiler chickens reared under thermoneutral and high ambient temperatures (means±SE). Means in the same graph with unlike superscripts differ (P<0.05). Se, selenium; T, temperature.
Fig. 2.
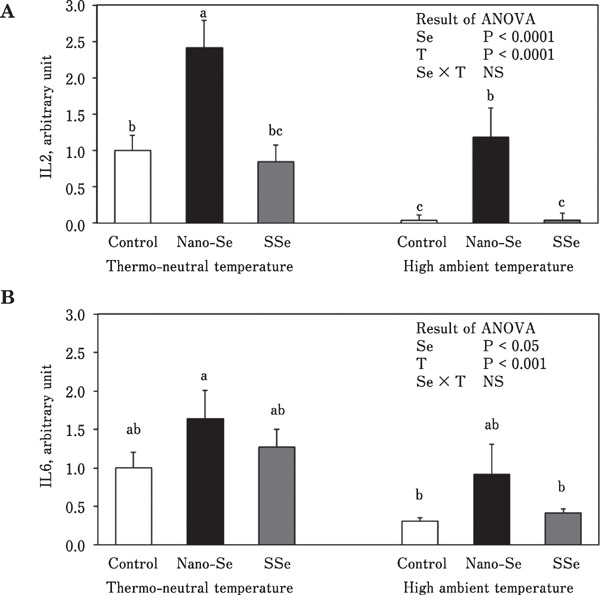
Effect of different sources of dietary Se on mRNA expression of IL-2 (A) and IL-6 (B) in broiler chickens reared under thermoneutral and high ambient temperatures (means±SE). Means in the same graph with unlike superscripts differ (P<0.05). Se, selenium; T, temperature.
The influences of dietary supplementation with nano-Se and SSe on MDA content in the liver and breast muscle of broiler chickens are presented in Fig. 3A and B, respectively. There was a significant interaction between Se source and environmental temperature for liver MDA concentration. Under the thermoneutral condition, neither nano-Se nor SSe affected MDA values in skeletal muscle or liver. While, under high ambient temperature, the MDA content in skeletal muscle and liver was significantly (P<0.05) decreased in chickens fed the diet supplemented with nano-Se compared with those fed the control diet.
Fig. 3.
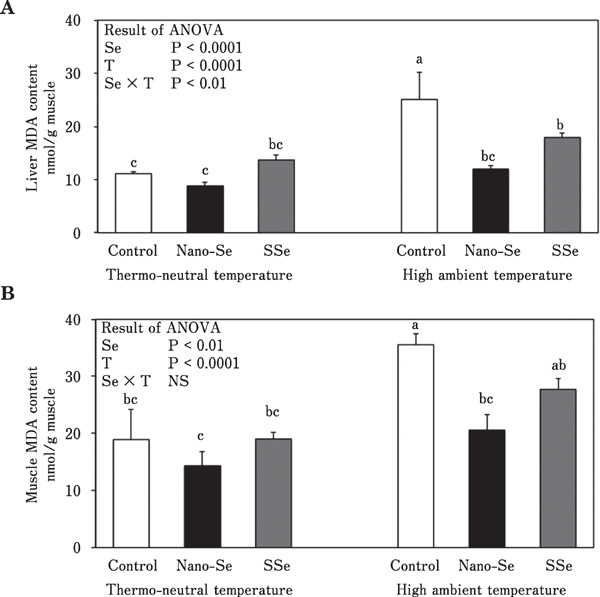
Effect of different sources of dietary Se on liver MDA (A) and muscle MDA (B) contents in broiler chickens reared under thermoneutral and high ambient temperatures (means±SE). Means in the same graph with unlike superscripts differ (P<0.05). Se, selenium; T, temperature.
There was a significant Se source×environmental temperature interaction for vitamin E concentration in breast muscle. Under thermoneutral condition, broiler chickens fed nano-Se had higher Se concentrations in breast muscle than did broiler chickens fed the control diet or the diet containing SSe (Fig. 4A). Similarly, muscle vitamin E concentration was higher in broiler chickens fed the diet containing nano-Se compared with control chickens under high ambient temperature condition (Fig. 4B). Dietray nano-Se elevated blood plasma content of α-tocopherol under thermoneutral and high ambient temperature conditions being 150 and 344%, respectively as compared with the control chicks (P<0.05).
Fig. 4.
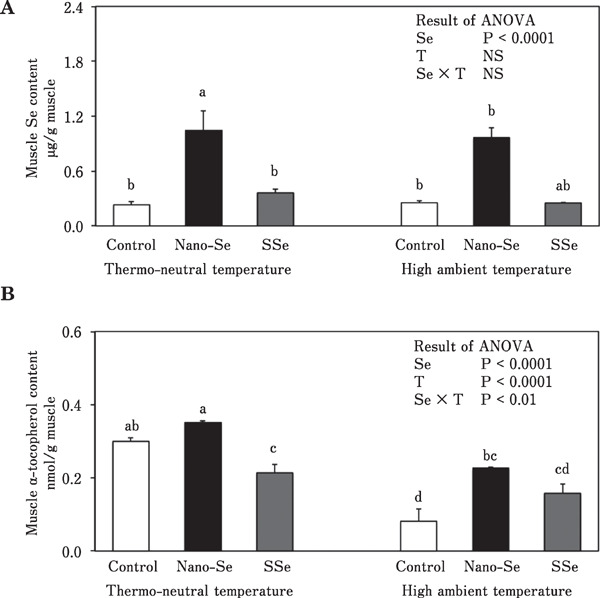
Effect of different sources of dietary Se on muscle Se (A) and muscle α-tocopherol (B) concentrations in broiler chickens reared under thermoneutral and high ambient temperatures (means±SE). Means in the same graph with unlike superscripts differ (P<0.05). Se, selenium; T, temperature.
Discussion
Ithas been previously reported that Se supplementation does not affect growth performance of chickens under thermoneutral conditions (Yoon et al., 2007; Niu et al., 2009). Indeed, in the present study, dietary supplementation with either nano-Se or SSe had no significant effect on BWG, FI, or FCR under the thermoneutral condition. In contrast, under high ambient temperature dietary supplementation of nano-Se improved BWG and FI and decreased FCR compared with control indicating that dietary supplementation of Se could alleviate the adverse effects of heat stress. These results are in agreement with Zhou and Wang (2011) who observed that final body weight and FCR were significantly improved in the groups supplemented with nano-Se as compared with the control. One possible explanation for this might be the antioxidant activity of Se. High ambient temperature significantly depresses growth performance of broiler chickens (El-Deep et al., 2014), while supplementation with antioxidants such as probiotics, trace elements, and vitamins has proven beneficial in alleviating the adverse effects of high ambient temperature challenge (Eid et al., 2003, 2008; Lin et al., 2006; Sahin et al., 2009). It has been shown that dietary Se supplementation improves antioxidant status by activating GSH-Px (Yoon et al., 2007; Ebeid et al., 2013). One of the major results in the present study was that there was a significant Se source×environmental temperature interaction for CAT mRNA expression and nano-Se supplementation enhanced CAT mRNA expression and serum GSH-Px activity which might be involved in allevaite the negative effects of high ambient temperature in broilers. This finding is in agreement with a previous study that showed that dietary Se supplementation improved the antioxidative status of heat-stressed broilers (Liao et al., 2012). Moreover, dietary supplementation with Se regulates the expression of genes for selenoproteins, including upregulation of the GSH-Px gene (Wu et al., 2003; Zoidis et al., 2010; Yuan et al., 2012). Moreover, in the present study, MDA contents in both skeletal muscle and liver were significantly increased under the high ambient temperature condition, but supplementation with nano-Se reduced them. According to Eszenyi et al. (2011), the Se nanoparticles used in the present study were spherical in shape and had a size range of 100–500 nm. Nanoparticles of this size show high antioxidant activity (Torres et al., 2012), have an increased ability to trap free radicals with greater antioxidant effect (Huang et al., 2003), and have an increased adsorptive ability due to interactions between the nanoparticles and NH, C=O, COO−, and C–N functional groups of proteins (Zhang et al., 2004). Additionally, nano-Se can act as a chemopreventive agent when administered at a smaller particle size (Peng et al., 2007). Taken together, the findings of the present study provide a good evidence that dietary nano-Se enhanced serum GSH-Px activity and involved in modulating the hepatic mRNA expression of GSH-Px, SOD and CAT and consequently resulted in reducing lipid peroxidation (MDA content) in liver and muscles broiler chickens under high ambient temperature.
Heat stress also suppresses the immune response (Thaxton et al., 1968) and, consequently, the growth performance of broiler chickens (Cooper and Washburn, 1998). It has been reported that Se intake may benefit the immune system and reduce inflammation (Rayman, 2004). Rooke et al. (2004) suggested that Se is involved in multiple immune functions at cellular and molecular levels, such as reducing glucocorticoids (which are known to be immunosuppressive); minimizing the rate and duration of intramammary infections; regulating the function of neutrophils, lymphocytes, and natural killer cells; and activating IL-2. In this study, it was observed that feeding a diet containing nano-Se upregulated the mRNA expression of IL-2 in chickens under the high ambient temperature condition. These results are in agreement with earlier studies that showed that dietary organic Se supplementation enhanced antibody titer when birds were exposed to heat stress (Niu et al., 2009; Liao et al., 2012; Habibian et al., 2014). Furthermore, in the present study, feeding a diet containing nano-Se increased the relative weights of immune organs under the high ambient temperature condition. These results suggest that feeding a diet containing nano-Se might have an immunostimulatory effect in chickens, consequently alleviating the adverse effects of high ambient temperature.
In this study, although nano-Se could alleviate the negative effects of high ambient temperature, SSe at the same concentration (0.3 ppm) failed to do so. This difference might be due to the different absorption efficiencies of nano-Se and SSe. Hu et al. (2012) showed that absorption of nano-Se from the intestinal lumen into the body was higher than that of SSe, while intestinal retention of nano-Se was lower than that of SSe. In addition, dietary supplementation of nano-Se resulted in a higher Se concentration in tissues of broilers as compared with selenite (Cai et al., 2012; Hu et al., 2012). Indeed, in this study, we found higher Se retention in muscle in broiler chickens fed a diet containing nano-Se compared with chickens fed a diet containing SSe. These results suggest that in broiler chickens, nano-Se might be more bioavailable and effective than SSe. Furthermore, it is noteworthy to note that supplementing the diet with nano-Se increased muscle and plasma vitamin E content under both environmental conditions and getting such results in hot climates might be involved in alleviating the negative effects of high environmental temperature. This result is in agreement with the study of Suraï and Dvorska (2002), who showed that dietary supplementation with organic Se significantly increased vitamin E concentration in breast muscle of chickens (Ebeid et al., 2013). Therefore, the present study suggests that dietary administration of Se in the form of nano-Se can increase muscle Se and vitamin E concentrations and that such enriched meat could be considered a useful source of these vital antioxidants in the human diet.
In conclusion, the results of the present study suggest that dietary supplementation with nano-Se (0.3 mg/kg diet) may improve growth performance, antioxidative status, and immunity in broilers reared under high ambient temperature conditions.
Acknowledgments
We are grateful to Kagoshima Chicken Foods Company, Limited (Kagoshima, Japan) for supplying the broiler chicks. This study was not supported by grants from any funding agency in the public, commercial, or not-for-profit sector.
References
- Arthur JR. The glutathione peroxidases. Cellular and Molecular Life Sciences, 57: 1825-1835. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azada MAK, Kikusato M, Maekawa T, Shirakawa H, Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comparative Biochemistry and Physiology Part A, 155: 401-406. 2010. [DOI] [PubMed] [Google Scholar]
- Cai SJ, Wu CX, Gong LM, Song T, Wu H, Zhang LY. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poultry Science, 91: 2532-2539. 2012. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Washburn KW. The relationships of body temperature to weight gain, feed consumption, and feed utilization in broilers under heat stress. Poultry Science, 77: 237-242. 1998. [DOI] [PubMed] [Google Scholar]
- Ebeid TA, Zeweil HS, Basyony MM, Dosoky WM, Badry H. Fortification of rabbit diets with vitamin E or selenium affects growth performance, lipid peroxidation, oxidative status and immune response in growing rabbits. Livestock Science, 155: 323-331. 2013. [Google Scholar]
- Eid Y, Ebeid T, Moawad M, El-Habbak M. Reduction of dexamethasone-induced oxidative stress and lipid peroxidation in laying hens by dietary vitamin E supplementation. Emirates Journal of Food and Agriculture, 20: 28-40. 2008. [Google Scholar]
- Eid YZ, Ohtsuka A, Hayashi K. Tea polyphenols reduce glucocorticoid-induced growth inhibition and oxidative stress in broiler chickens. British Poultry Science, 44: 350-356. 2003. [DOI] [PubMed] [Google Scholar]
- El-Deep MH, Ijiri D, Eid YZ, Yamanaka H, Ohtsuka A. Effects of dietary supplementation with Aspergillus awamori on growth performance and antioxidative status of broiler chickens exposed to high ambient temperature. Journal of Poultry Science, 51: 281-288. 2014. [Google Scholar]
- Eszenyi P, Sztrik A, Babka B, Prokisch J. Elemental, nano-sized (100-500 nm) selenium production by probiotic lactic acid bacteria. International Journal of Bioscience, Biochemistry and Bioinformatics, 1: 148-152. 2011. [Google Scholar]
- Faustman C, Cassens RG, Schaefer DM, Buege DR, Williams SN, Scheller KK. Improvement of pigment and lipid stability in Holstein steer beef by dietary supplementation with vitamin E. Journal of Food Science, 54: 858-862. 1989. [Google Scholar]
- Grashorn MA. Functionality of poultry meat. Journal of Applied Poultry Research, 16: 99-106. 2007. [Google Scholar]
- Habibian M, Ghazi S, Moeini MM, Abdolmohammadi A. Effects of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under thermo-neutral or heat stress conditions. International Journal of Biometeorology, 58: 741-752. 2014. [DOI] [PubMed] [Google Scholar]
- Hu HC, Li YL, Xiong L, Zhang HM, Song J, Xia MS. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Animal Feed Science and Technology, 177: 204-210. 2012. [Google Scholar]
- Huang B, Zhang J, Hou J, Chen C. Free radical scavenging efficiency of Nano-Se in vitro. Free Radical Biology and Medicine, 35: 805-813. 2003. [DOI] [PubMed] [Google Scholar]
- Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Molecular Aspects of Medicine, 32: 234-246. 2011. [DOI] [PubMed] [Google Scholar]
- Jia X, Li N, Chen J. A subchronic toxicity study of elemental Nano-Se in Sprague-Dawley rats. Life Science, 76: 1989-2003. 2005. [DOI] [PubMed] [Google Scholar]
- Liao X, Lu L, Li S, Liu S, Zhang L, Wang G, Li A, Luo X. Effects of selenium source and level on growth performance, tissue selenium concentrations, antioxidation, and immune functions of heat-stressed broilers. Biological Trace Element Research, 150: 158-165. 2012. [DOI] [PubMed] [Google Scholar]
- Lin H, Jiao HC, Buyse J, Decuyperre E. Strategies for preventing heat stress in poultry. World's Poultry Science Journal, 62: 71-86. 2006. [Google Scholar]
- Melesse A, Maak S, Schmidt R, von Lengerken G. Effect of long-term heat stress on some performance traits and plasma enzyme activities in naked-neck chickens and their F1 crosses with commercial layer breeds. Livestock Science, 141: 227-231. 2011. [Google Scholar]
- Moustafa MH, Sharma RK, Thornton J, Mascha E, Abdel-Hafez MA, Thomas AJ, Jr, Agarwal A. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Human Reproduction, 19: 129-138. 2004. [DOI] [PubMed] [Google Scholar]
- Mujahid A, Yoshiki Y, Akiba Y, Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poultry Science, 84: 307-314. 2005. [DOI] [PubMed] [Google Scholar]
- Niu Z, Liu F, Yan Q, Li L. Effects of different levels of selenium on growth performance and immunocompetence of broilers under heat stress. Archives of Animal Nutrition, 63: 56-65. 2009. [DOI] [PubMed] [Google Scholar]
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine, 70: 158-169. 1967. [PubMed] [Google Scholar]
- Peng D, Zhang J, Liu Q, Taylor EW. Size effect of elemental selenium nano particles (Nano-Se) at supra nutritional levels on selenium accumulation and glutathione S-transferase activity. Journal of Inorganic Biochemistry, 101: 1457-1463. 2007. [DOI] [PubMed] [Google Scholar]
- Rayman MP. The use of high-selenium yeast to raise selenium status: How does it measure up? British Journal of Nutrition, 92: 557-573. 2004. [DOI] [PubMed] [Google Scholar]
- Rooke JA, Robinson JJ, Arthur JR. Effects of vitamin E and selenium on the performance and immune status of ewes and lambs. Journal of Agricultural Science, 142: 253-262. 2004. [Google Scholar]
- Sahin K, Sahin N, Kucuk O, Hayirili A, Prasad AS. Role of dietary zinc in heat stressed poultry: A review. Poultry Science, 88: 2176-2183. 2009. [DOI] [PubMed] [Google Scholar]
- Shi LG, Xun WJ, Yue WB, Zhang CX, Ren YS, Liu Q, Wang Q, Shi L. Effect of elemental nano-selenium on feed digestibility, rumen fermentation, and purine derivatives in sheep. Animal Feed Science and Technology, 163: 136-142. 2011. a. [Google Scholar]
- Shi LG, Xun WJ, Yue WB, Zhang CX, Ren YS, Shi L, Wang Q, Yang RJ, Lei FL. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Ruminant Research, 96: 49-52. 2011. b. [Google Scholar]
- Shimizu N, Hosogi N, Hyon GS, Jiang S, Inoue K, Park P. Reactive oxygen species (ROS) generation and ROS-induced lipid peroxidation are associated with plasma membrane modifications in host cells in response to AK-toxin I from Alternaria alternata Japanese pear pathotype. Journal of General Plant Pathology, 72: 6-15. 2006. [Google Scholar]
- Siegel HS. Stress, strain and resistance. British Poultry Science, 36: 3-22. 1995. [DOI] [PubMed] [Google Scholar]
- Suraï PF. Selenium in poultry nutrition 2. Reproduction, egg and meat quality and practical applications. World's Poultry Science Journal, 58: 431-450. 2002. [Google Scholar]
- Suraï PF, Dvorska JE. Effect of selenium and vitamin E content of the diet on lipid peroxidation in breast muscle tissue of broiler breeder hens during storage. Australian Poultry Science Symposium, 14: 187-192. 2002. [Google Scholar]
- Thaxton P, Sadler CR, Glick B. Immune response of chickens following heat exposure or injections with ACTH. Poultry Science, 47: 264-266. 1968. [DOI] [PubMed] [Google Scholar]
- Torres SK, Campos VL, Leon CG, Rodriguez-Lamazares SM, Rojas SM, Gonzalez M, Smith C, Mondaca MA. Biosynthesis of selenium nanoparticles by Pantoeaagglomerans and their antioxidant activity. Journal of Nanoparticle Research, 14: 1236-1245. 2012. [Google Scholar]
- Wang H, Zhang J, Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radical Biology and Medicine, 42: 1524-1533. 2007. [DOI] [PubMed] [Google Scholar]
- Wang RR, Pan XJ, Peng ZQ. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poultry Science, 88: 1078-1084. 2009. [DOI] [PubMed] [Google Scholar]
- Wang YB, Xu BH. Effect of different selenium source (sodium selenite and selenium yeast) on broiler chickens. Animal Feed Science and Technology, 144: 306-314. 2008. [Google Scholar]
- Wolf RE, Morman SA, Morrison JM, Lamothe PJ. Simultaneous speciation of arsenic, selenium, and chromium by HPLC-ICP-MS. U. S. Geological Survey Open-File Report 2008-1334. 2008. [Google Scholar]
- Wu Q, Huang KX, Xu HB. Effects of long-term selenium deficiency on glutathione peroxidase and thioredoxinreductase activities and expressions in rat aorta. Journal of Inorganic Biochemistry, 94: 301-306. 2003. [DOI] [PubMed] [Google Scholar]
- Yoon I, Werner TM, Butler JM. Effect of source and concentration of selenium on growth performance and selenium retention in broiler chickens. Poultry Science, 86: 727-730. 2007. [DOI] [PubMed] [Google Scholar]
- Yuan D, Zhan XA, Wang YX. Effect of selenium sources on the expression of cellular glutathione peroxidase and cytoplasmic thioredoxinreductase in the liver and kidney of broiler breeders and their offspring. Poultry Science, 91: 936-942. 2012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Wang HY, Chen HY. Synthesis of seleniumnanoparticles in the presence of polysaccharides. Materials Letters, 58: 2590-2594. 2004. [Google Scholar]
- Zhou X, Wang Y. Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in Guangxi Yellow chicken. Poultry Science, 90: 680-686. 2011. [DOI] [PubMed] [Google Scholar]
- Zoidis E, Pappas AC, Georgiou CA, Komaitis E, Feggeros K. Selenium affects the expression of GPx4 and catalase in the liver of chicken. Comparative Biochemistry and Physiology Part B, 155: 294-300. 2010. [DOI] [PubMed] [Google Scholar]


