Abstract
Necrotic enteritis (NE) is a poultry disease caused by Clostridium perfringens and characterized by severe intestinal necrosis. The incidence of avian NE has been progressively increasing following the removal of antibiotics from poultry feed. We evaluated the effect of diets supplemented with the thermally-processed clays, calcium montmorillonite (CaMM) on clinical signs, immunopathology, and cytokine responses in broiler chickens using an experimental model of NE consisting of co-infection with Eimeria maxima and C. perfringens. In Trial 1, Ross/Ross chickens were fed from hatch with a normal basal diet or a CaMM-supplemented diet with or without a fermentable fiber, an organic acid, and/or a plant extract, and co-infected with E. maxima and C. perfringens under conditions simulating clinical infection in the field. Chickens fed a diet supplemented with CaMM plus a fermentable fiber and an organic acid had increased body weight gain, reduced gut lesions, and increased serum antibody levels to C. perfringens α-toxin and NetB toxin compared with chickens fed the basal diet alone. Levels of transcripts for interleukin-1β (IL-1β), IL-6, inducible nitric oxide synthase, and tumor necrosis factor-α superfamily-15 were significantly altered in the intestine and spleen of CaMM-supplemented chickens compared with unsupplemented controls (p<0.05). In Trial 2, Cobb/Cobb chickens were fed an unsupplemented diet or a diet supplemented with CaMM or Varium®, each with a fermentable fiber and an organic acid, and co-infected with E. maxima and C. perfringens under subclinical infection conditions. Compared with unsupplemented controls, broilers fed with CaMM plus a fermentable fiber and an organic acid had increased body weight gain, and reduced feed conversion ratio, mortality, and intestinal lesions, compared with chickens fed an unsupplemented diet (p<0.05). Dietary supplementation of broiler chickens with CaMM plus a fermentable fiber and an organic acid might be useful to control avian NE in the field.
Keywords: calcium montmorillonite, Clostridium perfringens, coccidiosis, Eimeria maxima, necrotic enteritis, organic acid
Introduction
Necrotic enteritis (NE) is an economically important intestinal infectious disease caused by the common gut bacterium, Clostridium perfringens, under conditions favoring in vivo bacterial proliferation (Drew et al., 2004; Jia et al., 2009). NE is estimated to cost U.S. commercial poultry producers up to $3 billion annually. Even mild subclinical outbreaks in a flock can cost more than $0.07 per bird (Skinner et al., 2010). The incidence of NE has been increasing globally following the reduction in use of in-feed antibiotics, such as virginiamycin, that are used to control enteric diseases in poultry (Williams, 2005). Thus, there is a timely need to develop novel methods to reduce the negative effects of NE in broilers.
C. perfringens produces several exotoxins, including α-toxin and NE toxin B(NetB), that disrupt the intestinal epithelium causing necrotizing lesions that constitute the characteristic sign of NE (Songer, 1996; Williams, 2005; Keyburn et al., 2006). A thermally-processed, highly-refined clay, calcium montmorillonite (CaMM; Calibrin-Z®, Processed by Amlan International, Chicago, IL 60611), ameliorates the negative biological effects of several mycotoxins, including aflatoxin, fumonisin, and zearalenone, when fed to broilers and other livestock, presumably through its adsorptive and dipolar properties that sequester the toxins in the gastrointestinal tract, thereby decreasing their systemic bioavailability (Ledoux et al., 2009; Jiang et al., 2012; Wang et al., 2012). CaMM is comprised of a soft phyllosilicate group of minerals that typically form in microscopic crystals. Our recent unpublished studies demonstrated that CaMM also binds to C. perfringens α-toxin and NetB toxin in vitro. Therefore, we hypothesized that CaMM, or its blends with other materials known to improve gut health (e.g. fermentable fibers, organic acids, and/or phytonutrients) might decrease the negative effects of avian NE. To test this hypothesis, the current study evaluated two different trials utilizing CaMM- or Varium® (manufactured and formulated by Amlan International, Chicago, IL 60611)-based dietary products on the growth performance, clinical signs, immunopathology, and cytokine responses of young broilers using an experimental model of avian NE.
Materials and Methods
Feed and Treatments
All basal diets were corn/soybean-based and formulated to the experimental design (Trial 1) or meet or exceed National Research Council (1994) recommendations (Trial 2). Trial 1 was carried out at the USDA Beltsville Agricultural Research Center (BARC, Beltsville, MD) by HSL and approved by the BARC Institutional Animal Care and Use Committee. Two-hundred-twenty one-day-old Ross/Ross broilers (Longenecker's Hatchery, Elizabethtown, PA) were randomly divided into 11 groups (20 birds/group). The chickens were fed ad libitum from hatch to day 18 post-hatch with either a non-medicated commercial basal ration containing 18% (wt/wt) crude protein (Table 1) or the same basal ration supplemented with 0.25% or 0.50% (wt/wt) CaMM (group B), 0.25% or 0.50% CaMM plus citric acid and an extract of Yucca plant (group Y), 0.25% or 0.50% CaMM plus a fermentable fiber (group C), 0.25% or 0.50% CaMM plus organic acid and a fermentable fiber (group D), or 22 mg/kg virginiamycin (VM) (Table 3). Yucca extract containing 10% saponin was added into the feed formula as much as possible.
Table 1. Ingredient composition of basal diet (Trial 1).
| Ingredients (%) | Low Protein Diet | High Protein Diet |
|---|---|---|
| Corn | 69.01 | 55.78 |
| Soybean meal | 23.99 | 37.03 |
| Soybean oil | 2.75 | 2.97 |
| Dicalcium phosphate | 2.00 | 1.80 |
| Calcium carbonate | 1.40 | 1.51 |
| Salt | 0.35 | 0.38 |
| Poultry Vit Mix | 0.20 | 0.22 |
| Poultry Mineral Mix | 0.15 | 0.15 |
| DL-Methionine | 0.10 | 0.10 |
| Choline-chloride, 60% | 0.05 | 0.06 |
| Total | 100 | 100 |
| Calculated values (DM basis, %) | ||
| Crude Protein, % | 18.00 | 24.00 |
| Ca, % | 1.19 | 1.20 |
| Available P, % | 0.54 | 0.51 |
| Lys, % | 1.00 | 1.40 |
| Met, % | 0.42 | 0.49 |
| Cys + Met, % | 0.65 | 0.80 |
| TMEn, kcal/kg | 3585 | 3450 |
Vitamin mixture provided the following nutrients per kg of diet: vitamin A, 2,000 IU; vitamin D3, 22 IU; vitamin E, 16 mg; vitamin K, 0.1 mg; vitamin B1, 3.4 mg; vitamin B2, 1.8 mg; vitamin B6, 6.4 mg; vitamin B12, biotin, 0.17 mg; pantothenic acid, 8.7 mg; folic acid, 0.8 mg; niacin, 23.8 mg.
Mineral mixture provided the following nutrients per kg of diet: Fe, 400 mg; Zn, 220 mg; Mn, 180 mg; Co, 1.3 mg; Cu, 21 mg; Se, 0.2 mg.
Table 2. Ingredient composition of basal diet (Trial 2).
| Ingredient | %, as is basis |
|---|---|
| Corn | 57.547 |
| Soybean meal (dehulled) | 35.283 |
| Animal by-product (55% protein) | 3.000 |
| Fat, vegetable | 1.828 |
| Calcium carbonate | 0.847 |
| Dicalcium phosphate | 0.565 |
| Salt | 0.430 |
| Methionine hydroxy analog | 0.286 |
| Vitamin premix1 | 0.065 |
| Trace mineral premix2 | 0.075 |
| L-Lysine | 0.056 |
| RonozymeP | 0.018 |
| Total | 100.000 |
| Calculated nutrient composition | |
| ME poultry, kcal/kg | 3,067 |
| Crude protein, % | 23.35 |
| Lysine, % | 1.35 |
| Methionine, % | 0.62 |
| Digestible lysine, % | 1.2 |
| Digestible TSAA, % | 0.9 |
| Calcium, % | 0.9 |
| Phosphorus, % | 0.61 |
| Sodium, % | 0.21 |
Vitamin mix provided the following (per kg of diet): thiamin·mononitrate, 2.4 mg; nicotinic acid, 44 mg; riboflavin, 4.4 mg; D-Ca pantothenate, 12 mg; vitamin B12 (cobalamin), 12.0 µg; pyridoxine·HCL, 4.7 mg; D-biotin, 0.11 mg; folic acid, 5.5 mg; menadione sodium bisulfite complex, 3.34 mg; choline chloride, 220 mg; cholecalciferol, 27.5 ug; trans-retinyl acetate, 1,892 ug; allrac α tocopheryl acetate, 11 mg; ethoxyquin, 125 mg.
Trace mineral mix provided the following (per kg of diet): manganese (MnSO4·H2O), 60 mg; iron (FeSO4·7H2O), 30 mg; zinc (ZnO), 50 mg; copper (CuSO4·5H2O), 5 mg; iodine (ethylene diamine dihydroiodide), 0.15 mg; selenium (NaSe03), 0.3 mg.
Table 3. Experimental scheme of Trial 1 (Clinical Infection).
| Treatment Group | Product Inclusion | Diet Supplementation | Bacterial Challenge |
|
|---|---|---|---|---|
| E. maxima | C. perfringens | |||
| Control | — | None | — | — |
| NE | — | None | + | + |
| B | 0.25% | CaMM | + | + |
| Y | 0.25% | CaMM + OA + Yucca extract | + | + |
| C | 0.25% | CaMM + Fermentable fiber | + | + |
| D | 0.25% | CaMM + Fermentable fiber + OA | + | + |
| B | 0.50% | CaMM | + | + |
| Y | 0.50% | CaMM + OA + Yucca extract | + | + |
| C | 0.50% | CaMM + Fermentable fiber | + | + |
| D | 0.50% | CaMM + Fermentable fiber + OA | + | + |
| VM | 22 mg/kg | Virginiamycin | + | + |
Ross/Ross broiler chickens, except the control group, were infected with 1.0×104 oocysts of E. maxima on day 14 post-hatch followed by 1×109 cfu of C. perfringens on day 18 post-hatch. Crude protein content of the basal diet was 18% between days 0 and 18 post-hatch and 24% from days 18 to 25 (Refer to Table 1). Birds (20 birds/group) were randomly divided into 11 groups and fed from day 0 with an unsupplemented diet or diets supplemented with 0.25% or 0.50% CaMM (Calcium Montmorillonite, Calibrin-Z®, processed by Amlan International, Chicago, IL 60611) with or without a fermentable fiber, an organic acid (OA), or Yucca plant extract containing 10% saponin into the feed formula, or with 22 mg/kg virginiamycin.
At day 18 post-hatch, the protein content of all diets was increased to 24% (wt/wt) (Table 1) until the end of the experiment.
Trial 2 was carried out at the Southern Poultry Research Facility by GFM and was approved by the Southern Poultry Research Institutional Animal Care and Use Committee in accordance with the principles and specific guidelines of the Federation of Animal Science Societies (FASS, 2010). Three-hundred-twenty one-day-old Cobb/Cobb male broilers (Cobb-Vantress, Cleveland, GA) were randomly divided into 5 groups (64 birds/group). The chickens were fed ad libitum from hatch to day 18 post-hatch with either a non-medicated commercial basal ration containing 17% (wt/wt) crude protein or the same basal ration (Table 2) supplemented with 0.25% CaMM plus organic acid and a fermentable fiber (group D), 0.25% Varium®, or 22 mg/kg VM (Table 4). The scheme of experimental protocol of Trial 1 and 2 are illustrated in Fig. 1.
Table 4. Experimental scheme of Trial 2 (Subclinical Infection).
| Treatment Group | Product Inclusion | Diet Supplementation | Bacterial Challenge |
|
|---|---|---|---|---|
| E. maxima | C. perfringens | |||
| Control | — | None | — | — |
| NE | — | None | + | + |
| D* | 0.25% | CaMM + Fermentable fiber + OA | + | + |
| Varium® | 0.25% | CaMM + Fermentable fiber + OA | + | + |
| VM | 22 mg/kg | Virginiamycin | + | + |
D and Varium® were formulated differently by Amlan International (Chicago, IL 60611). Cobb/Cobb male broiler chickens, except the control group, were infected with 5.0×103 oocysts of E. maxima on day 14 post-hatch followed by 1×108 cfu of C. perfringens on days 19, 20, and 21 post-hatch. Birds (64 birds/group) were randomly divided into 5 groups and fed from day 0 with an unsupplemented diet or diets supplemented with 0.25% CaMM or Varium® with a fermentable fiber and an organic acid (OA), or with 22 mg/kg virginiamycin (Refer to Table 2).
Fig. 1.
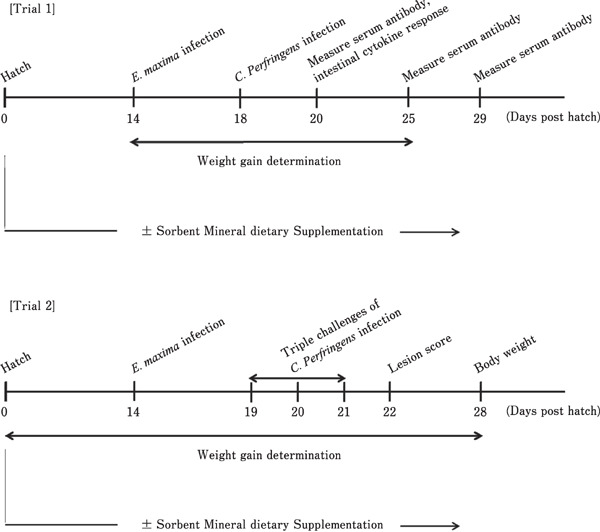
Schematic illustration of the experimental protocol. Ab, antibody; CP, C. perfringens; DCPI, days following C. perfringens infection; EM, E. maxima.
Chickens and Experimental Models of Avian NE
In Trial 1, the chickens were initially housed in Petersime starter brooder pens (Petersime Incubator Co, Gettysburg, OH) in a disease-free facility. At 14 day post-hatch, the broilers were transferred to large hanging cages in a separate location where one group was uninfected while the remaining groups were co-infected with E. maxima and C. perfringens under conditions simulating clinical infection in the field as described (Park et al., 2008; Jang et al., 2012). Briefly, the birds were given an oral infection of 1.0×104 sporulated oocysts of E. maxima (strain 41A) at day 14 post-hatch, followed 4 days later by oral infection with 1.0×109 colony forming units (cfu) of a field strain of C. perfringens (strain Del-1). E. maxima and C. perfringens were maintained and propagated as previously described (Lee et al., 2011). In Trial 2, chickens were housed in Petersime battery cages in 8 pens with 8 birds per pen. At day 14 post-hatch, the chickens were uninfected or orally inoculated under conditions simulating subclinical infection with 5. 0×103 oocysts of E. maxima, followed 5 days later by 1.0×108 cfu of C. perfringens in 1.0 ml of broth culture for 3 consecutive days (days 19, 20 and 21 post-hatch) as described (Zhang et al., 2010).
Intestinal Lesion Scoring
In Trial 1, chickens (5 birds/group) were randomly selected on day 2 following C. perfringens infection (day 20 post-hatch), weighed, and euthanized by cervical dislocation. Approximately 20 cm of the intestine extending 10 cm anterior and posterior from the Meckel's diverticulum was removed and cut longitudinally. Lesion scoring was performed by 3 independent observers in a blinded fashion as described (Prescott, 1979; Park et al., 2008). Lesions were scored as 0 (normal, no lesion), 1 (thin-walled minor lesions), 2 (moderate focal necrotic lesions), 3 (severe necrotic lesion patches), or 4 (dead or moribund). In Trial 2, three birds from each cage were randomly selected on day 1 following the final C. perfringens infection (day 22 post-hatch) and sacrificed, and intestines examined for lesion score as described (Zhang et al., 2010). Lesions were scored as 0 (normal, no lesion), 1 (thin-walled or friable), 2 (focal necrosis or ulceration), or 3 (severe lesions).
Serum Collection
In Trial 1, blood was collected from chickens (3 birds/group) by cardiac puncture immediately following euthanasia on day 2 following C. perfringens infection (day 20 post-hatch) for measuring circulating α-toxin and NetB toxin levels, and on days 7 and 14 following C. perfringens infection (days 25 and 32 post-hatch) for measuring serum antibodies to α-toxin and NetB toxin. Sera were prepared by centrifugation at 3,000 rpm for 10 min at 4°C and stored at −20°C until analysis.
Serum Antibodies to α-Toxin and NetB Toxin
Recombinant C. perfringens α-toxin and NetB toxin were expressed in E. coli as described (Lee et al., 2012). Ninety-six well microtiter plates were coated overnight with 1.0 µg/well of each of the recombinant toxins. The plates were washed with PBS containing 0.05% Tween 20 (PBS-T) and blocked with PBS containing 1.0% bovine serum albumin (PBS-B). Sera were diluted 1:20 (vol:vol), 100 µl were added to each well, and incubated with continuous gentle shaking for 2 h at room temperature. The wells were washed with PBS-T and bound antibodies were detected with peroxidase-conjugated rabbit anti-chicken IgG antibody (Sigma, St. Louis, MO) and 3,3′,5,5′-tetramethylbenzidine substrate. Optical density values at 450 nm (OD450) were measured using a microplate reader (Bio-Rad, Richmond, CA) and corrected for background reactivity in the absence of recombinant toxins.
Serum α-Toxin and NetB Toxin Levels
Ninety-six well microtiter plates were coated overnight with 0.5 µg/well of monoclonal antibody to α-toxin or NetB toxin, washed with PBS-T, and blocked with PBS-B. Sera were diluted 1:2 (vol:vol) in PBS-T and 100 µl were added to the wells. The wells were incubated for 2 h at room temperature with continuous gentle shaking, washed with PBS-T, and the bound α-toxin or NetB toxin detected with peroxidase-conjugated rabbit anti-α-toxin or anti-NetB toxin antibodies, respectively, and 3,3′,5,5′-tetramethylbenzidine substrate. OD450 values were measured and serum toxin levels were determined by comparison with a standard curve generated with known concentrations of each purified recombinant toxin.
Quantitative RT-PCR
The levels of transcripts for proinflammatory cytokines and inducible nitric oxide synthase (iNOS) were measured in spleen and intestine as described (Park et al., 2007, 2008; Xu et al., 2015). At day 2 following C. perfringens infection, spleens and intestinal jejunum located proximal to the Meckel's diverticulum were collected (5 birds/group). Single cell suspensions of spleen were prepared by gently flushing with a cell strainer to remove clumps. Intestinal jejunum tissues were cut open longitudinally, gently washed 3 times with ice-cold Hank's Balanced Salt Solution (Sigma) containing 100 U/ml of penicillin and 100 µg/ml of streptomycin (Sigma). The mucosal layer was carefully scraped off using a surgical scalpel and intraepithelial lymphocytes (IELs) were isolated by density gradient centrifugation. Total RNA from spleen and intestinal IELs was extracted using TRIzol (Invitrogen, Carlsbad, CA). Five micrograms of total RNA were treated with 1.0U of DNase I and 1.0 µl of 10X reaction buffer (Sigma) and incubated for 15 min at room temperature. One µl of stop solution was added to inactivate DNase I and the mixture was heated for 10 min at 70°C. RNA was reverse-transcribed using the StrataScript first-strand synthesis system (Stratagene, La Jolla, CA) according to the manufacturer's recommendations. Quantitative RTPCR oligonucleotide primers for chicken interleukin-1β (IL-1β), IL-6, iNOS, tumor necrosis factor superfamily 15 (TNFSF15), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control are listed in Table 5. Amplification and detection were carried out with the Mx3000P system and Brilliant SYBR Green qPCR master mix (Stratagene). The reverse transcription product was diluted 1:10 (vol: vol), and 5 µl was used for PCR amplification. PCR conditions were as follows: denaturation at 95°C for 10 min followed by amplification at 72°C for 1 min for 40 cycles. Standard curves were generated using log10 diluted standard RNA to calculate the amplification efficiency and the levels of individual transcripts were normalized to those of GAPDH by the Q-gene program as described (Muller et al., 2002; Lee et al., 2012, 2013). Each sample was analyzed in triplicate. To normalize individual replicates, the logarithmic-scaled threshold cycle (Ct) values were transformed to linear units of normalized expression prior to calculating means and SEM for the references and individual targets, followed by the determination of mean normalized expression using the Q-gene program.
Table 5. Oligonucleotide primer sequences used for quantitative RT-PCR.
| Gene | Primer Sequence | Size (bp) | Genebank Accession no. | |||
|---|---|---|---|---|---|---|
| IL-1β | F | 5′-TGGGCATCAAGGGCTACA-3′ | 244 | Y15006 | ||
| R | 5′-TCGGGTTGGTTGGTGATG-3′ | |||||
| IL-6 | F | 5′CAAGGTGACGGAGGAGGAC-3′ | 254 | AJ309540 | ||
| R | 5′TGGCGAGGAGGGATTTCT-3′ | |||||
| iNOS | F | 5′TGGGTGGAAGCCGAAATA-3′ | 241 | U46504 | ||
| R | 5′GTACCAGCCGTTGAAAGGAC-3′ | |||||
| TNFSF15 | F | 5′-CCTGAGTATTCCAGCAACGCA-3′ | 292 | NM_01024578 | ||
| R | 5′-ATCCACCAGCTTGATGTCACTAAC-3′ | |||||
| GAPDH | F | 5′-GGTGGTGCTAAGCGTGTTAT-3′ | 264 | K01458 | ||
| R | 5′-ACCTCTGTCATCTCTCCACA-3′ | |||||
F, forward primer; R, reverse primer. IL-1β, chicken Interleukin-1β; IL-6, chicken Interleukin-6; iNOS, chicken inducible nitric oxide synthease; TNFSF15, chicken Tumor necrosis factor superfamily 15; GAPDH, chicken Glyceraldehyde 3-phosphate dehydrogenase as an internal control.
Statistical Analysis
For Trial 1, mean±SEM values were compared between different treatment groups by the Duncan's multiple range test following ANOVA using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL). For Trial 2, mean±SEM values were compared between different treatment groups using Duncan's multiple range test using JMP 11.0.0 for Windows (SAS Institute Inc., Cary, NC). In both trials, differences between means were considered significant at p<0.05. Brooder pens were used for experimental units in repeated experiments in both trials.
Results
Trial 1
Body Weight Gain and Intestinal Lesion Score
Chickens co-infected with E. maxima and C. perfringens and fed an unsupplemented basal diet had significantly (p<0.05) reduced body weight gain between days 0 and 6 following E. maxima infection (days 14 and 20 post-hatch) and between days 0 and 7 following C. perfringens infection (days 18 and 25 post-hatch) compared with the uninfected controls (p<0.05, Table 6). In contrast, co-infected chickens that were fed a diet containing 0.25% of a blend of CaMM, a fermentable fiber, and an organic acid (group D) had significantly increased body weight gain between both time spans compared with co-infected birds fed with the unsupplemented diet (group NE) (p<0.05). In fact, body weight gains in the 0.25% group D chickens were equal to those of the uninfected controls. Birds in the 0.50% group D also had significantly (p<0.05) increased body weight gains between days 0 and 6 following E. maxima infection compared with co-infected chickens given the unsupplemented diet. Chickens in the 0.25% or 0.50% group D, as well as those in the 0.50% group Y, had significantly (p<0.05) decreased intestinal lesion scores at day 2 following C. perfringens infection (day 20 post-hatch) compared with co-infected birds given the unsupplemented diet (Table 4).
Table 6. Effects of dietary sorbent minerals on body weight gain and lesion score in Ross/Ross broilers following clinical E. maxima/C. perfringens co-infection (Trial 1).
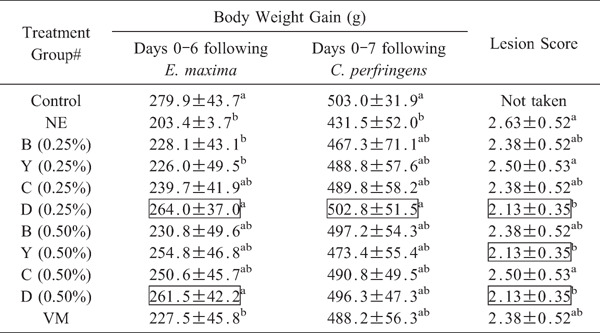 |
Body weight gains were determined between days 0 and 6 after E. maxima infection, and between days 0 and 7 following C. perfringens infection. Each value represent the mean weight gain±SEM (n=20). Intestinal lesion scores were determined at day 2 following C. perfringens infection by 3 independent observers in a blinded manner on a scale from 0 to 4. Each value represent the mean score±SEM (n=5). Within each column, values with different superscripts are statistically different according to the Duncan's multiple range test (P<0.05). # See Table 3 for treatment group. Values in boxes are significantly different compared with the co-infected NE group given the unsupplemented diet (p<0.05).
Serum Antibody Levels to C. perfringens α-Toxin and NetB Toxin
Serum antibody levels against α-toxin and NetB toxin were significantly (p<0.05) greater at days 7 and 14 following C. perfringens infection (days 25 and 32 post-hatch) in birds fed the unsupplemented diet compared with uninfected controls (p<0.05, Table 7). Co-infected chickens in 0.25% group D had further increased antibody levels against both toxins at both time points compared with co-infected chickens given the unsupplemented basal diet. Co-infected birds in the 0.50% group D also had increased antibodies against α-toxin at the later time point compared with co-infected chickens given the unsupplemented diet.
Table 7. Effects of sorbent mineral on serum Clostridium perfringens α-toxin- and NetB toxin-specific antibody levels in Ross/Ross broilers following clinical E. maxima/C. perfringens co-infection (Trial 1).
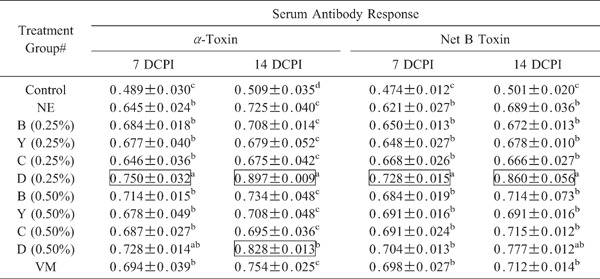 |
Antibody levels were measured at day 7 (7 DCPI) and day 14 (14 DCPI) following C. perfringens infection. Each value represents the mean OD450 value±SEM corrected for background (n=3). Within each column, values with different superscripts are statistically different according to the Duncan's multiple range test (P<0.05). # See Table 3 for treatment group. Values in boxes are significantly different compared with the co-infected NE group given the unsupplemented diet (p<0.05).
Serum α-Toxin and NetB Toxin Levels
Co-infected chickens in the 0.25% group D and 0.50% group Bhad significantly (p<0.05), decreased α-toxin levels in sera at day 2 following C. perfringens infection (day 20 post-hatch) compared with co-infected chickens given the unsupplemented diet (Table 8). However, none of the dietary treatments influenced NetB toxin levels in sera.
Table 8. Effect of dietary supplementation of sorbent minerals on C. perfringens α-toxin and NetB toxin levels in sera of Ross/Ross broilers following clinical E. maxima/C. perfringens co-infection (Trial 1).
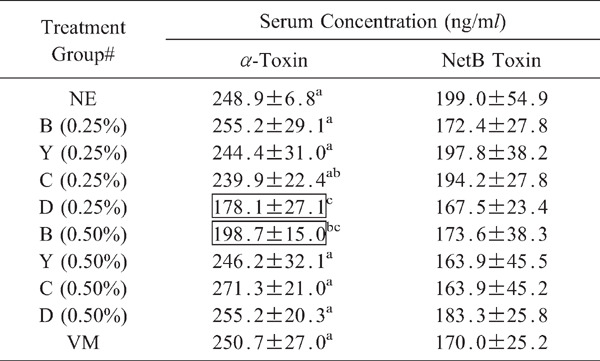 |
Toxin levels were measured at day 2 following C. perfringens infection. Each value represents the mean toxin concentration±SEM (n=3). Within each column, values with different superscripts are statistically different according to the Duncan's multiple range test (P<0.05). # See Table 3 for treatment group. Values in boxes are significantly different compared with the co-infected NE group given the unsupplemented diet (p<0.05).
Proinflammatory Cytokine and iNOS Transcript Levels
In intestinal IELs as shown in Fig. 2, increased levels of IL-1β transcripts normalized to GAPDH transcripts were seen in the 0.50% group C, in the 0.50% group D for IL-6 transcripts, and in the 0.25% group C for TNFSF15 transcripts. Decreased levels of IL-1β transcripts were seen in the 0.25% group C, in the 0.25% groups B and C for IL-6 transcripts, in the 0.25% groups Y and C, and 0.50% group D, for iNOS transcripts, and in the 0.25% groups B and Y, and 0.50% groups B, C, and D, for TNFSF15 transcripts. In the spleen as shown in Fig. 3, increased levels of IL-6 transcripts were seen in the 0.25% group B, and in the 0.25% group Y for TNFSF15 transcripts. Decreased levels of IL-1β and iNOS transcripts were seen in all treatment groups, in the 0.25% groups Y and D, and 0.50% group B, for IL-6 transcripts, and in the 0.25% and 0.50% groups B and D for TNFSF15 transcripts.
Fig. 2.
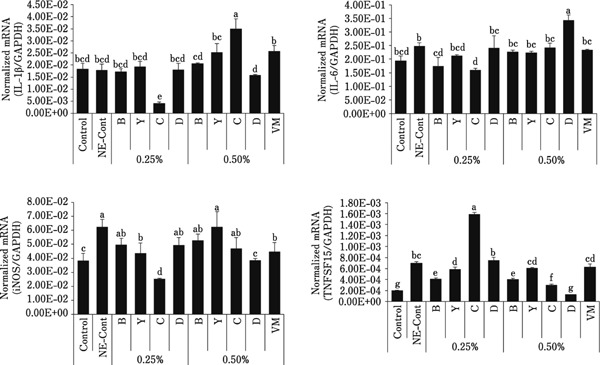
Levels of transcripts for proinflammatory cytokines and iNOS in intestinal jejunum IELs. Intestinal IELs were collected at day 2 following C. perfringens infection and the levels of transcripts for IL-1β, IL-6, iNOS, and TNFSF15 were measured by quantitative RT-PCR. Individual transcript levels were normalized to GAPDH transcript levels. Each bar represents the mean normalized transcript level±SEM (n=5). Bars with different superscripts are statistically different according to the Duncan's multiple range test (p<0.05).
Fig. 3.
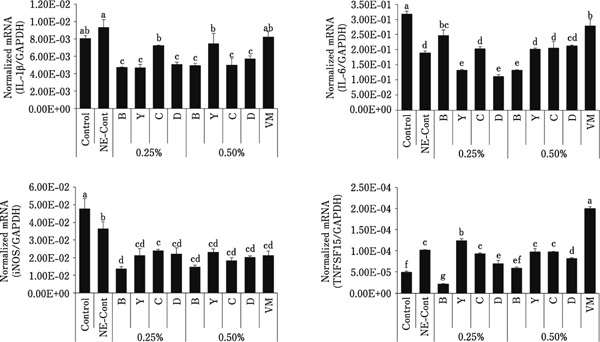
Levels of transcripts for proinflammatory cytokines and iNOS in spleen cells. Spleen cells were collected at day 2 following C. perfringens infection and the levels of transcripts for IL-1β, IL-6, iNOS, and TNFSF15 were measured by quantitative RT-PCR. Individual transcript levels were normalized to GAPDH transcript levels. Each bar represents the mean normalized transcript level±SEM (n=5). Bars with different superscripts are statistically different according to the Duncan's multiple range test (p<0.05).
Trial 2
Body Weight Gain, Feed Conversion, Mortality, and Intestinal Lesion Score
Chickens co-infected with E. maxima and C. perfringens and fed an unsupplemented diet had significantly (p<0.05) reduced body weight gain, and increased feed conversion ratio (FCR), mortality, and lesion score, between days 0 and 14, and between days -14 and 14, following E. maxima infection (days 14–28 and 0–28 post-hatch, respectively) compared with the uninfected controls (Table 9). In contrast, co-infected chickens that were fed a basal diet containing 0.25% Varium® had significantly (p<0.05) increased body weight gain, and decreased FCR, mortality, and lesion score, between both time spans compared with co-infected birds fed the unsupplemented diet (group NE). Co-infected chickens in the 0.25% group D only had decreased FCR compared with co-infected birds given the unsupplemented diet. Dietary VM increased weight gain between days 14 and 28, and reduced FCR and mortality, compared with unsupplemented, co-infected controls.
Table 9. Effects of dietary sorbent minerals on body weight gain, feed conversion ratio, mortality, and lesion score in Cobb/Cobb broilers following subclinical E. maxima/C. perfringens co-infection (Trial 2).
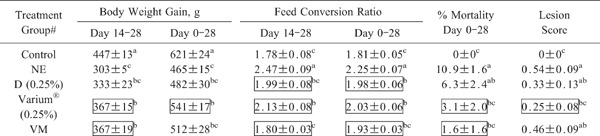 |
Body weight gain, feed conversion ratio (feed intake/weight gain), and mortality were determined between days 0 and 14 after E. maxima infection (days 14 and 28 post-hatch), and/or between days -14 and 14 after E. maxima infection (days 0 and 28 post-hatch). Each value represent the mean weight gain, feed conversion ratio, or % mortality±SEM (n=20). Intestinal lesion scores were determined at day 1 following final C. perfringens infection (day 22 post-hatch) on a scale from 0 to 3. Each value represent the mean lesion score±SEM (n=8). Within each column, values with different superscripts are statistically different according to the Duncan's multiple range test (P<0.05). # See Table 4 for treatment group. Values in boxes are significantly different compared with the co-infected NE group given the unsupplemented diet (p<0.05).
Discussion
In both the clinical and subclinical models of experimental avian NE, co-infected chickens given the basal unsupplemented diet had significantly (p<0.05) reduced body weight gain compared with uninfected birds. Decreased growth performance is a prominent hallmark of NE and is responsible for the majority of economic loss for poultry producers (Branton et al., 1997; Kaldhusdal et al., 2001; Lensing et al., 2010; Cravens et al., 2013). Forty-one percent of the loss due to avian NE in the UK in 1995 was from reduced weight gain (Williams, 2005). Much of this decreased weight gain is attributable to the appearance of intestinal lesions and other gut pathologies that can vary from mild with by a thin, flaccid gut wall and thickened mucus layer, to extensive areas of necrosis and ulceration with significant hemorrhage, typically resulting in death (Jia et al., 2009). In the current study, NE-induced chickens on an unsupplemented basal diet in both the clinical and subclinical infection trials showed the greatest lesion scores and lowest weight gains. During clinical infection, treatment groups B, Y, and C showed a trend toward reduced lesion scores and increased weight gains, although none were statistically significant compared with unsupplemented and co-infected controls. However, significantly (p<0.05) greater body weight gain and reduced intestinal lesions were observed in co-infected chickens fed a diet supplemented with CaMM plus a fermentable fiber and an organic acid (group D) compared with unsupplemented, co-infected controls. In fact, these beneficial effects seen in group D-treated broilers were not seen in clinically-infected chickens given VM, an antibiotic commonly used to control NE in the field (Williams, 2005). Under the subclinical infection conditions in Trial 2, where a beneficial effect of in-feed VM on experimental NE was observed, the 0.25% Varium® group, and to a lesser extent the 0.25% group D, were equivalent to VM in reducing the negative consequences of this disease. These results suggest that the CaMM-based feed additives, at the specified level of supplementation, may be useful for improving gut health and growth performance in commercial broilers under field conditions.
Antibodies to C. perfringens α-toxin and NetB toxin were increased in NE-challenged chickens fed an unsupplemented diet relative to the uninfected controls, and were further increased in co-infected group D animals compared with the unsupplemented, co-infected controls. Increased serum antibodies to the major toxins of C. perfringens may reflect reduced gut damage contributing to improved body weight gain. In other studies, birds fed a potato protein-supplemented diet had increased anti-α-toxin antibody levels along with increased intestinal hemorrhage and liver lesions compared with chickens fed a soy protein-supplemented diet (Palliyeguru et al., 2010). Higher anti-Salmonella antibody titers were seen in bacteria-infected chickens fed an arginine and vitamin E-supplemented diet compared with birds given an unsupplemented diet (Liu et al., 2014). Previous work from our laboratory showed higher anti-toxin antibody levels in the sera of healthy chickens raised on farms with endemic NE compared with NE-diseased birds in the same flock, suggesting a protective role for these antibodies against infection in the field (Lee et al., 2011). Further, when improvements in other response criteria (growth, lesion score, serum α-toxin concentration) were considered, it is likely that the increased levels of these antibodies is due, in part, to an increase in the ability of the avian immune system to respond to bacterial infection. While dietary probiotics, phytochemicals, and yeast-derived compounds all have been shown to increase plasma or serum antibody concentrations in chickens (Haghighi et al., 2005, 2006; Gao et al., 2008; Kim et al., 2013), to the best of our knowledge, this is the first report to document that dietary treatment with CaMM plus a fermentable fiber and an organic acid enhances the avian humoral immune response to a combined E. maxima/C. perfringens challenge.
At the level of cellular immunity, the levels of transcript for the cytokines, IL-1β, IL-6, and TNFSF15, and iNOS, were significantly (p<0.05) altered in the intestine and spleen of CaMM-treated chickens following co-infection with E. maxima plus C. perfringens. In mammals, IL-1β is involved in a variety of cellular activities, including proliferation, differentiation, and apoptosis (Thornberry and Molineaux, 1995), while IL-6 acts as either a proinflammatory cytokine or an anti-inflammatory myokine (Kamimura et al., 2003). iNOS catalyzes the synthesis of the cell signaling molecule, nitric oxide, particularly in response to IL-1β (Green et al., 1994), and TNFSF15 is a proinflammatory cytokine that is increased in chickens in response to Eimeria infection (Park et al., 2007). In the E. maxima plus C. perfringens co-infection model used here, prior studies showed a mixed response in TNFSF15 transcript levels in intestial IELs, being upregulated on day 1 but downregulated on day 2, following C. perfringens infection compared either with uninfected birds or chickens infected with E. maxima or C. perfringens alone (Park et al., 2008). In the current study, TNFSF15 transcript levels in intestinal IELs and spleen also were increased or decreased, but in this case in response to the different dietary supplements used. Of the four cytokines/mediators examined here, TNFSF15 transcripts increased to the greatest extent in both the intestine and spleen of the unsupplemented, NE-challenged group compared with the uninfected controls. However, with the exception of the 0.25% groups C and Y, dietary supplementation of co-infected chickens with any of the other CaMM-based treatments either had no effect or decreased TNFSF15 transcripts compared with the unsupplemented, co-infected controls. Park et al. (2008) reported that intestinal IEL levels of IL-1β transcripts were decreased in chickens co-infected with E. maxima and C. perfringens compared with single infection by either pathogen alone, while IL-6 transcipt levels were not affected following infection with either or both microorganisms. Similarly, Yitbarek et al. (2012) reported that IL-6 expression in the intestinal ileum or cecal tonsils was unaffected in C. perfringens-infected chickens compared with uninfected controls, leading these authors to theorize that IL-6 is not involved in Toll-like receptor regulation of C. perfringens-induced NE. Eimeria infection of chickens also induces iNOS expression both in vitro and in vivo (Dalloul et al., 2005; Lee et al., 2014). In the current study, iNOS transcripts were either unaffected or decreased in co-infected chickens fed the supplemented diets compared with unsupplemented NE controls.
In conclusion, two separate trials carried out at different research facilities using either a clinical or a subclinical experimental model of avian NE showed that dietary supplementation with the thermally-processed clays, CaMM or Varium®, in combination with a fermentable fiber and an organic acid improved the growth performance and mitigated the negative effects of the disease. Future studies are needed to further characterize the CaMM-regulated physiological and immunological mechanisms that are activated in response to avian NE.
Acknowledgments
The authors express their appreciation to Chuck Lowe for assistance, and to Dr. Erik P. Lillehoj for critical review. This study was partially supported by a formal Trust agreement established between ARS, USDA, USA and the Rural Development Administration, Republic of Korea (No. PJ 012088, PJ01049004).
Footnotes
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Conflict of Interests
The authors have the following competing interests. The study was supported in part by a trust from Amlan International, Inc. and authors BL, FC and RLC are employees of Amlan. Some products (Varium®) are under development stage based on data presented in this report. This does not alter the authors' adherence to all the journal's policies on sharing data and materials, as in the guidance for authors.
References
- Branton SL, Lott BD, Deaton JW, Maslin WR, Austin FW, Pote LM, Keirs RW, Latour MA, Day EJ. The effect of added complex carbohydrates or added dietary fiber on necrotic enteritis lesions in broiler chickens. Poultry Science, 76: 24-28. 1997. [DOI] [PubMed] [Google Scholar]
- Cravens RL, Goss GR, Chi F, De Boer ED, Davis SW, Hendrix SM, Richardson JA, Johnston SL. The effects of necrotic enteritis, aflatoxin B1, and virginiamycin on growth performance, necrotic enteritis lesion scores, and mortality in young broilers. Poultry Science, 92: 1997-2004. 2013. [DOI] [PubMed] [Google Scholar]
- Dalloul RA, Lillehoj HS, Klinman DM, Ding X, Min W, Heckert RA, Lillehoj EP. In ovo administration of CpG oligodeoxynucleotides and the recombinant microneme protein MIC2 protects against Eimeria infections. Vaccine, 23: 3108-3113. 2005. [DOI] [PubMed] [Google Scholar]
- Drew MD, Syed NA, Goldade BG, Laarveld B, Van Kessel AG. Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens. Poultry Science, 83: 414-420. 2004. [DOI] [PubMed] [Google Scholar]
- Federation of Animal Science Societies (FASS). Guide for the care and use of agricultural animals in research and teaching. Champaign (IL): Federation of Animal Science Societies; 2010. [Google Scholar]
- Gao J, Zhang HJ, Yu SH, Wu SG, Yoon I, Quigley J, Gao YP, Qi GH. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poultry Science, 87: 1377-1384. 2008. [DOI] [PubMed] [Google Scholar]
- Green SJ, Scheller LF, Marletta MA, Seguin MC, Klotz FW, Slayter M, Nelson BJ, Nacy CA. Nitric oxide: Cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunology Letters, 43: 87-94. 1994. [DOI] [PubMed] [Google Scholar]
- Haghighi HR, Gong J, Gyles CL, Hayes MA, Sanei B, Parvizi P, Gisavi H, Chambers JR, Sharif S. Modulation of antibody-mediated immune response by probiotics in chickens. Clinical Diagnostic Laboratory Immunology, 12: 1387-1392. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi HR, Gong J, Gyles CL, Hayes MA, Zhou H, Sanei B, Chambers JR, Sharif S. Probiotics stimulate production of natural antibodies in chickens. Clinical and Vaccine Immunology, 13: 975-980. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SI, Lillehoj HS, Lee SH, Lee KW, Lillehoj EP, Hong YH, An DJ, Jeong W, Chun JE, Bertrand F, Dupuis L, Deville S, Arous JB. Vaccination with Clostridium perfringens recombinant proteins in combination with Montanide™ ISA 71 VG adjuvant increase protection against experimental necrotic enteritis in commercial broiler chickens. Vaccine, 30: 5401-5406. 2012. [DOI] [PubMed] [Google Scholar]
- Jia W, Slominski BA, Bruce HL, Blank G, Crow G, Jones O. Effects of diet type and enzyme addition on growth performance and gut health of broiler chickens during subclinical Clostridium perfringens challenge. Poultry Science, 88: 132-140. 2009. [DOI] [PubMed] [Google Scholar]
- Jiang SZ, Yang ZB, Yang WR, Wang SJ, Wang Y, Broomhead J, Johnston SL, Chi F. Effect on hepatonephric organs, serum metabolites and oxidative stress in post-weaning piglets fed purified zearalenone-contaminated diets with or without Calibrin-Z. Journal of Animal Physiology and Animal Nutrition (Berl.), 96: 1137-1156. 2012. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M, Schneitz C, Hofshagen M, Skjerve E. Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal flora of adult fowl. Avian Diseases, 45: 149-156. 2001. [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: The signal orchestration model. Reviews of Physiology Biochemistry and Pharmacology, 149: 1-38. 2003. [DOI] [PubMed] [Google Scholar]
- Keyburn AL, Sheedy SA, Ford ME, Williamson MM, Awad MM, Rood JI, Moore RJ. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infection and Immunity, 74: 6496-6500. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Lillehoj HS, Lee SH, Jang SI, Park MS, Min W, Lillehoj EP, Bravo D. Immune effects of dietary anethole on Eimeria acervulina infection. Poultry Science, 92: 2625-2634. 2013. [DOI] [PubMed] [Google Scholar]
- Ledoux DR, Rottinghaus GE, Bermudez AJ, Broomhead JN. Efficacy of the adsorbent Calibrin-A in ameliorating the toxic effects of aflatoxin in broiler chicks. Poultry Science, 88 (Suppl. 1): 31 2009. [Google Scholar]
- Lee KW, Lillehoj HS, Jeong W, Jeoung HY, An DJ. Avian necrotic enteritis: Experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poultry Science, 90: 1381-1390. 2011. [DOI] [PubMed] [Google Scholar]
- Lee KW, Lillehoj HS, Park MS, Jang SI, Ritter GD, Hong YH, Jeong W, Jeoung HY, An DJ, Lillehoj EP. Clostridium perfringens α-toxin and NetB toxin antibodies and their possible role in protection against necrotic enteritis and gangrenous dermatitis in broiler chickens. Avian Diseases, 56: 230-233. 2012. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lillehoj HS, Jang SI, Lillehoj EP, Min W, Bravo DM. Dietary supplementation of young broiler chickens with Capsicum and turmeric oleoresins increases resistance to necrotic enteritis. British Journal of Nutrition, 110: 840-847. 2013. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lillehoj HS, Jang SI, Jeong MS, Xu SZ, Kim JB, Park HJ, Kim HR, Lillehoj EP, Bravo DM. Effects of in ovo injection with selenium on immune and antioxidant responses during experimental necrotic enteritis in broiler chickens. Poultry Science, 93: 1113-1121. 2014. [DOI] [PubMed] [Google Scholar]
- Lensing M, van der Klis JD, Fabri T, Cazemier A, Else AJ. Efficacy of a lactylate on production performance and intestinal health of broilers during a subclinical Clostridium perfringens infection. Poultry Science, 89: 2401-2409. 2010. [DOI] [PubMed] [Google Scholar]
- Liu X, Byrd JA, Farnell M, Ruiz-Feria CA. Arginine and vitamin E improve the immune response after a Salmonella challenge in broiler chicks. Poultry Science, 93: 882-890. 2014. [DOI] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32: 1372-1374, 1376, 1378-1379. 2002. [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirements of Domestic Animals. Nutrient Requirements of Poultry, 9th Rev. Edition. National Academy of Science, Washington, DC: 1994. [Google Scholar]
- Palliyeguru MW, Rose SP, Mackenzie AM. Effect of dietary protein concentrates on the incidence of subclinical necrotic enteritis and growth performance of broiler chickens. Poultry Science, 89: 34-43. 2010. [DOI] [PubMed] [Google Scholar]
- Park SS, Lillehoj HS, Hong YH, Lee SH. Functional characterization of tumor necrosis factor superfamily 15 (TNFSF 15) induced by lipopolysaccharides and Eimeria infection. Developmental & Comparative Immunology, 31: 934-944. 2007. [DOI] [PubMed] [Google Scholar]
- Park SS, Lillehoj HS, Allen PC, Park DW, FitzCoy S, Bautista DA, Lillehoj EP. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Disease, 52: 14-22. 2008. [DOI] [PubMed] [Google Scholar]
- Prescott JF. The prevention of experimentally induced necrotic enteritis in chickens by avoparcin. 23: 1072-1074. 1979. [PubMed] [Google Scholar]
- Skinner JT, Bauer S, Young V, Pauling G, Wilson J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Diseases, 54: 1237-1240. 2010. [DOI] [PubMed] [Google Scholar]
- Songer JG. Clostridial enteric diseases of domestic animals. Clinical Microbiology Reviews, 9: 216-234. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry NA, Molineaux SM. Interleukin-1β converting enzyme: A novel cysteine protease required for IL-1β production and implicated in programmed cell death. Protein Science, 4: 3-12. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Chi F, Kim IH. Effects of montmorillonite clay on growth performance, nutrient digestibility, vulva size, faecal microflora, and oxidative stress in weaning gilts challenged with zearalenone. Animal Feed Science and Technology, 178: 158-166. 2012. [Google Scholar]
- Williams RB. Intercurrent coccidiosis and necrotic enteritis of chickens: Rational, integrated disease management by maintenance of gut integrity. Avian Pathology, 34: 159-180. 2005. [DOI] [PubMed] [Google Scholar]
- Yitbarek A, Echeverry H, Brady J, Hernandez-Doria J, Camelo-Jaimes G, Sharif S, Guenter W, House JD, Rodriguez-Lecompte JC. Innate immune response to yeast-derived carbohydrates in broiler chickens fed organic diets and challenged with Clostridium perfringens. Poultry Science, 91: 1105-1112. 2012. [DOI] [PubMed] [Google Scholar]
- Zhang G, Mathis GF, Hofacre CL, Yaghmaee P, Holley RA, Durance TD. Effect of a radiant energy-treated lysozyme antimicrobial blend on the control of clostridial necrotic enteritis in broiler chickens. Avian Diseases, 54: 1298-1300. 2010. [DOI] [PubMed] [Google Scholar]


