Abstract
The aim of this study was to determine whether probiotic-feeding affected the expression of cathelicidins (CATHs), a major family of antimicrobial peptides, in response to lipopolysaccharides (LPS) challenge in the proventriculus and cecum of broiler chicks. One-day-old male Chunky broiler chicks were fed with or without 0.4% probiotics for 7 days (P-group and non-P-group, respectively). Then, they were orally challenged with no LPS (0-LPS), 1 µg LPS (1-LPS), or 100 µg LPS (100-LPS) (n=5 in all groups) in Experiment 1, and with no LPS or 1 µg LPS (n=6 in all groups) in Experiment 2. Five hours after LPS challenge, the proventriculi and ceca were collected to analyze CATHs expression. Expression of CATHs was examined at first by reverse transcription-polymerase chain reaction (RT-PCR) using the 0-LPS chicks of non-P-group. The differences in CATHs expression upon probiotics-feeding and LPS were analyzed by real time-PCR. All four CATHs (CATH1, 2, 3 and 4) were expressed in the proventriculus and cecum of chicks. In the proventriculus, the expression of CATHs after LPS challenge did not show significant differences between non-P and P-groups in Experiment 1 and 2. In the cecum, the interactions of the effects of probiotics and LPS on the expression of CATH2 in Experiment 1 and CATH1 and 2 in Experiment 2 were significant, and their expression in 1-LPS chicks was higher in P-group than in non-P-group. However, CATH3 and 4 did not show any significant differences between non-P- and P-groups challenged with LPS. These results suggest that probiotics-feeding may stimulate the immunodefense system mediated by CATH2 and possibly CATH1 against infection by Gram-negative bacteria in the cecum.
Keywords: cathelicidins, gut mucosal immunity, probiotics, lipopolysaccharides
Introduction
The mucosal surface of the gastrointestinal tract after hatching is constantly in contact with various microorganisms (Walker et al., 2006). The gut-associated lymphoid tissues have not been fully developed during the first week of life in newly hatched chicks (Miyazaki et al., 2007). The immune protection could be provided during the first week of life through maternal antibodies (Kaspers et al., 1991) and innate defense system including the synthesis of antimicrobial peptides (AMPs).
AMPs are main parts of the innate immune response to microbial infection. In chickens, two major families of AMPs are defensins and cathelicidins (CATHs) (Lynn et al., 2004). AMPs generally consist of less than 100 amino acid residues, mostly cationic and amphipathic in nature, which allows them to bind and disrupt negatively charged microbial membranes leading to death of microbes. Therefore, AMPs are being strongly recommended for the control and prevention of infectious diseases, particularly against antibiotic-resistant bacteria (Hancock and Sahl, 2006). Up to date 4 CATHs have been reported in chickens namely, CATH1 (Lynn et al., 2004), CATH2 (Van Dijk et al., 2005), CATH3 (Xiao et al., 2006a) and CATH4 (Goitsuka et al., 2007; Achanta et al., 2012). All four chicken CATHs are believed to be capable of killing a broad range of bacteria including antibiotic-resistant strains (Xiao et al., 2006a, b; Van Dijk et al., 2009; Rodríguez-Lecompte et al., 2012).
Innate immune responses are stimulated by pathogen-associated molecular pathogens (PAMPs) via Toll-like receptors (TLRs) (Werling and Coffey, 2007). Chicken TLRs are important in the recognition of PAMPs to induce the production of pro-inflammatory cytokines and antimicrobial peptides and to upregulate the expression of co-stimulatory molecules that may initiate adaptive immunity responses (Werling and Jungi, 2003; Yoshimura, 2015). Among these TLRs, TLR4 recognizes lipopolysaccharides (LPS) from Gram-negative bacteria, whereas it requires CD14 to accept the complex of LPS and LPS binding protein (Nerren et al., 2010; de Zoete et al., 2011).
Manipulation of the gut microbiota of chickens by administration of probiotic bacteria may help to control enteric bacterial infections, including those caused by Salmonella enterica serovar Typhimurium (Mead, 2000). In chickens, feeding with probiotic species such as Lactobacillus, Streptococcus, and Clostridium may have beneficial effects on broiler performance (Ashayerizadeh et al., 2009) as well as on the modulation of intestinal microflora and their genes (microbiome) to reduce pathogens (Mountzouris et al., 2007; Higgins et al., 2011; Oakley et al., 2014). Probiotics have also been reported to modulate the expression and localization of avian β-defensins (AvBDs) in the mucosal tissue of the gastrointestinal tract (Akbari et al., 2008; Mohammed et al., 2015). We expected that probiotics-feeding may also enhance the functions to express CATHs in the gut mucosa of chicks.
However, it remains to be established whether probiotics-feeding affects the ability of the gut mucosa to express CATHs in chicks. The aim of this study was to determine whether the feeding of probiotics affects the expression of CATHs in response to LPS challenge in the proventriculus and cecum of broiler chicks.
Materials and Methods
Treatments of Birds and Tissue Collection
Experiment 1: One-day-old male broiler chicks (Chunky broilers) were purchased from a local hatchery (Fukuda Poultry, Okayama, Japan). They were divided into 2 groups, fed with or without 0.4% probiotics, namely probiotic group (P-group) and non-probiotic group (non-P-group). Chicks in the non-P-group were given a commercial starter diet (Nihon Nosan Kogyo Co. Ltd., Yokohama, Japan) containing 0.4% (wt/wt) corn starch, whereas chicks in the P-group were given the starter rations containing 0.4% (wt/wt) probiotics (Toaraze for chickens; Toa Pharmaceutical Co. Ltd., Tokyo, Japan). The Toaraze for chickens contained Streptococcus faecalis (>1×108/g), Clostridium buthricum (>1×107/g), and Bacillus mesentericus (>1×107/g). Chicks were maintained in a brooding room under a lighting condition of 23 h light/1 h dark for 7 days. The chicks were reared with feeds with (P-group) or without (non-P-group) probiotics and water ad libitum. On day 7, the chicks in each group were divided into 3 subgroups, namely 0-LPS, 1-LPS, and 100-LPS groups, which were given 1 mL of clean water containing 0, 1, or 100 µg LPS through oral gavage, respectively. Five hours after LPS challenge, the chicks were euthanized using carbon dioxide, and the proventriculi and ceca were collected (n=5 in all groups).
Experiment 2: This experiment was carried out to confirm the results of Experiment 1 for 0-LPS and 1-LPS using the previous design of Experiment 1. The chicks were fed feeds with (P-group) or without (non-P group) probiotics and water ad libitum for 7 days. Chicks were divided into 2 subgroups, namely 0-LPS and 1-LPS groups, which were given 1 mL of clean water containing 0 µg LPS or 1 µg LPS, respectively (n=6 in all groups).
The LPS used in this study originated from Salmonella Minnesota R595 (Re-mutant; by ultracentrifugation; Wako pure chemical industries Ltd. Osaka. Japan). Chicks were handled in accordance with the regulations of Hiroshima University Animal Research Committee.
RNA Isolation and cDNA Preparation
The mucosal tissues of proventriculi and ceca were collected immediately after birds were euthanized. The proventriculus was opened longitudinally by a scissors and washed by a cold and autoclaved PBS, then spread on a sterilized glass slide. The thick mucosal layer of the proventriculus was carefully cut with scissors and collected. The cecum was longitudinally opened with scissors and was also washed with PBS, and then spread on a sterilized glass slide. The mucosal layer of the cecum was carefully and gently scrubbed by a sharp blade and collected.
The collected mucosal tissues of the proventriculus and cecum were used for total RNA extraction by Sepazol RNA I super according to the manufacturer's directions (Nacalai Tesque Inc., Kyoto, Japan). The obtained RNA pellet was then dissolved in Tris-EDTA buffer (10 mM Tris-HCl, pH 8.0, with 1 mM EDTA) and kept at −80°C until use. The concentration of the total RNA was determined using Gene Quant Pro (Amersham Pharmacia Biotech, Cambridge, UK) in each sample. The samples were treated with 1U RQ1 RNase-free DNase (Promega Corporation, Madison, WI, USA) in a 10 µL reaction mixture (1 µg of total RNA, 1×DNase buffer, and 1U DNase) on a programmable thermal controller (PTC-100; MJ Research, Waltham, MA, USA), programmed at 37°C for 45 min and 65°C for 10 min. The concentration of RNA in each sample was measured once more after DNase treatment using Gene Quant Pro (Amersham Pharmacia Biotech). The RNA samples were then reverse-transcribed using ReverTra Ace (Toyobo Co. Ltd., Osaka, Japan) according to the manufacturer's instructions to obtain cDNA. The reaction mixture (10 µl) consisted of 1 µg of total RNA, 1× Reverse Transcription buffer, 1 µM deoxyribonucleotide triphosphate (dNTP) mixture, 20U RNase inhibitor, 0.5 µg of oligo (dt) 20 and 50U Rever Tra Ace. The reverse transcription was performed at 42°C for 30 min, followed by heat inactivation for 5 min at 99°C using a programmable thermal controller (PTC-100; MJ Research).
Identification of CATHs Expression
The reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed to determine the expression of CATHs in the proventriculus and cecum using the cDNA of the chicks from control group of Experiment 1 (0-LPS of non-P-group; n=5). The reaction mixture (25 µL) containing 0.5 µL of cDNA, 1×PCR buffer, 0.2 µMdNTP mixture, 0.5 µM of each primer (forward and reverse), and 0.125U Takara Taq (Takara Bio. Inc., Shiga, Japan) was prepared. CATHs primers used for PCR are presented in Table 1. The PCR cycle parameters were 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for all CATHs for 45 s, and extension at 72°C for 45 s, followed by a final extension at 72°C for 7 min. The PCR products were separated by electrophoresis on 2% (wt/vol) agarose gels containing 0.025% (wt/vol) ethidium bromide and photographed under UV illumination.
Table 1. Primers used for PCR analysis of CATHs and their accession numbers.
| Gene | Sequence of forward and reverse primers | Accession no. | Expected product size (bp) |
|---|---|---|---|
| CATH1 | F: GCTGTGGACTCCTACAACCAAC | NM001001605.3 | 261 |
| R: GGAGTCCACGCAGGTGACATC | |||
| CATH2 | F: CAAGGAGAATGGGGTCATCAG | NM001024830.2 | 221 |
| R: CGTGGCCCCATTTATTCATTCA | |||
| CATH3 | F: GCTGTGGACTCCTACAACCAAC | NM001311177.1 | 352 |
| R: TGGCTTTGTAGAGGTTGATGC | |||
| CATH4 | F: CCGTGTCCATAGAGCAGCAG | NM001271172.1 | 170 |
| R: AGTGCTGGTGACGTTCAGATG | |||
| RPS17 | F: AAGCTGCAGGAGGAGGAGAGG | NM 204217 | 136 |
| R: GGTTGGACAGGCTGCCGAAGT |
Analysis of Differences in CATHs Expression Among Treatments
The expression level of the CATHs detected by RT-PCR analysis in both the proventriculus and cecum specimens was further analyzed by real-time PCR using the Roche Light Cycler Nano system (Roche Applied Science, Indianapolis, IN, USA). The reaction mixture (20 µL) containing 1 µL of cDNA, 10 µL of Thunder Bird SYBR qPCR Mix (Toyobo Co. Ltd., Osaka, Japan), 1 µM of each primer, and Milli Q water were mixed into PCR tubes (Roche Diagnostics GmbH, Mannheim, Germany). The thermal protocols for PCR were 50 cycles at 95°C for 10 s; and 60°C (CATHs and RPS17), for 30 s. Real-time PCR data were analyzed using the 2−ΔΔct method to calculate the relative level of CATHs expression in each sample and were expressed as ratios in relation to the RPS17 housekeeping gene (Livak and Schmittgen, 2001). An RNA sample of control chicks (0-LPS) from the non-P-group was used as a standard sample.
Statistical Analysis
The significance of interaction between probiotics-feeding and LPS treatments was examined by two-way ANOVA. Then, when the interaction was significant, the difference between non-P-group and P-group at each different LPS treatment was examined by t-test. Differences were considered significant when the P value was <0.05.
Results
Identification of CATHs Expression in the Proventriculus and Cecum of Chicks
The expression profile of CATHs in the mucosal tissue of the proventriculus and cecum revealed that CATHs (CATH1, 2, 3 and 4) were expressed in the proventriculus and cecum of broiler chicks (Fig. 1a and b).
Fig. 1.
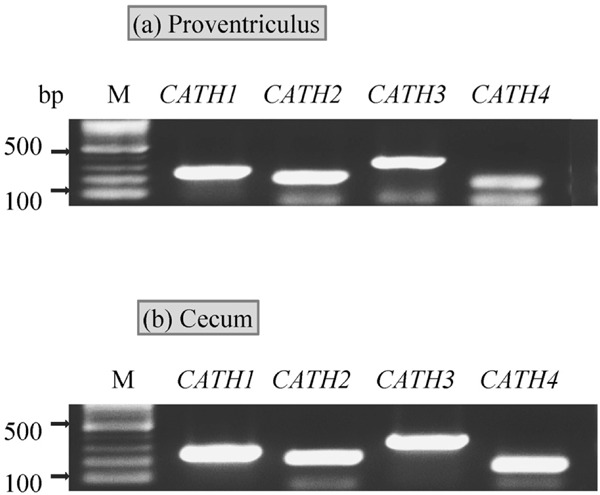
Reverse transcription-PCR analysis of CATHs expression in the proventriculus (a) and cecum (b) of chicks. Total RNA samples were collected from the mucosal tissue of the proventriculus and cecum of 0-LPS chicks of non-P-groups.
Analysis of Differences in CATHs Expression Among Treatments
In Experiment 1, the effects of three different doses of LPS challenge on the expression of CATHs in the proventriculus and cecum of P- and non-P-groups were examined (Fig. 2). In the proventriculus, the interaction between probiotics and LPS treatments for the induction of CATH1-4 was not significant among non-P and P-groups treated with 0- , 1- or 100-LPS (Fig. 2a, c, e and g). In the cecum, the interaction of probiotics and LPS treatments for the induction of the expression of CATH2 was significant (P=0.0053) (Fig. 2d), but not for the expression of the other CATHs (Fig. 2b, f and h). In addition, CATH2 expression in the 1-LPS chicks was significantly higher in P-group than non-P-group (Fig. 2d).
Fig. 2.
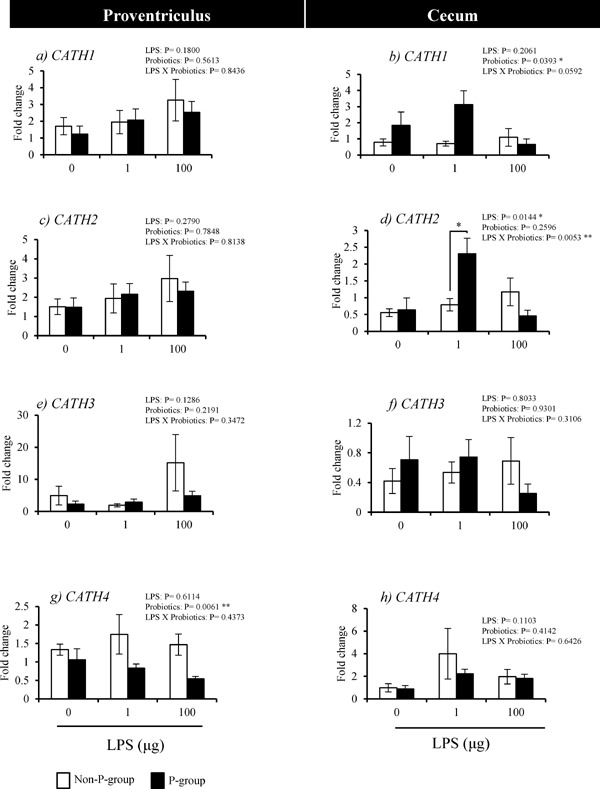
Effects of probiotics-feeding on CATHs expression of in response to LPS in the proventriculus and cecum of chicks (Experiment 1). Values are mean±S.E. of fold changes in expression (n=5). Non-P and P-groups were fed 0% and 0.4% probiotics, respectively and challenged with 0, 1 or 100 µg LPS (0-, 1-, and 100-LPS groups). * Values are significantly different between P-group and non-P-group (P<0.05).
In Experiment 2, the interaction of probiotics and LPS treatments for the induction of the 4 CATHs expressions was not significant in the proventriculus (Fig. 3a, c, e and g). However, in the cecum, there were significant interactions between LPS and probiotics treatments for the induction of the expressions of CATH1 (P=0.0044) and CATH2 (P=0.0070) (Fig. 3b and d). The expression of CATH1 in the 0-LPS chicks was lower in P-group than in non-P-group chicks (Fig. 3b), whereas, its level in the 1-LPS chicks was significantly higher in P-group than in non-P-group (Fig. 3b). The expression level of CATH2 in 1-LPS chicks was higher in P-group than in non-P-group (Fig. 3d). The interactions between LPS and probiotics treatments for the induction of the expression of CATH 3 and 4 were not significant (Fig. 3f and h).
Fig. 3.
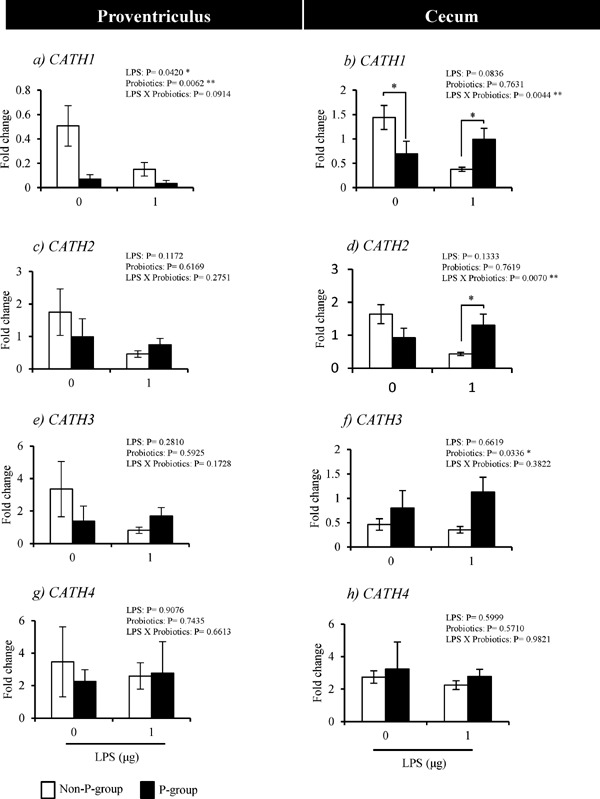
Effects of probiotics-feeding on the mRNA expression of CATHs in response to LPS in the proventriculus (a, c, e and g) and cecum (b, d, f and h) of chicks (Experiment 2). Values are mean±S.E. of fold changes in expression (n=6). Non-P and P-groups were fed 0% and 0.4% probiotics, respectively and challenged with 0 or 1 µg LPS (0- and 1-LPS groups). * Values are significantly different between P-group and non-P-group (P<0.05).
Discussion
In this study we report the effects of probiotics-feeding on the response of the proventricular and cecal mucosa to LPS in term of CATHs expression. The major findings of this study are: (1) four CATHs expression was detectable in the mucosal tissue of the proventriculus and cecum of broiler chicks; (2) CATH2 in the cecum showed a higher expression level in response to 1µg LPS challenge in the P-group than in the non-P-group. These results showing the expression of CATHs in the proventriculus and cecum supports the previous studies that reported CATHs are expressed in the mucosal tissues of the digestive and respiratory of chickens (Van Dijk et al., 2005; Xiao et al., 2006a; Goitsuka et al., 2007). The synthesized CATHs in the digestive tract may play roles in defense against pathogens since they have a broad spectrum of antimicrobial activities (Van Dijk et al., 2005; Xiao et al., 2006a, b). The expression levels of any CATHs with no LPS challenge was not higher in the P-group than in non-P-group in both the proventriculus and cecum, and CATH1 expression in the cecum was lower in the P-group than in non-P-group in Experiment 2. These results suggest that probiotics themselves did not upregulate CATHs expression as reported for the AvBDs expression in the proventriculus (Mohammed et al., 2015).
The current study revealed that in the cecum there were significant interactions between probiotics-feeding and LPS treatments in the induction of CATH2, and its expression level in response to 1 µg LPS was higher in the P-group than in non-P-group, commonly in Experiments 1 and 2. The significant interaction and higher expression level in the P-group than in non-P-group were identified also for CATH1 in the Experiment 2. These results suggest that probiotics may enhance the ability to respond to LPS for the induction of CATH2, and also possibly that of CATH1, in the cecum. We hypothesize that the cellular functions for recognizing LPS and expressing CATHs in the cecum may be modulated by probiotics-feeding in that process.
The challenge with a greater dose of LPS (100 µg) did not show differences in CATH2 expression in the cecum between P-group and non-P-group in Experiment 1. Although the reason for these results is not known, the function to recognize LPS or to express CATH2 may be reduced when the tissues were challenged with a greater dose of LPS even in the chicks fed with probiotics. In the vaginal cells, AvBDs expression was upregulated by LPS, but the response was decreased by a higher dose of LPS treatment (Sonoda et al., 2013).
In contrast to cecum, CATHs expression in the proventriculus was not significantly affected by probiotics-feeding and LPS challenge. The effects of probiotics may be weak in the proventriculus since the probiotics bacteria may not be able to adapt and proliferate in the acidic medium of the proventriculus. We have reported that probiotics-feeding did not affect the expression of AvBD12, wherease the AvBD12 protein density in the surface epithelial cells was lowered by probiotics-feeding, suggesting that they were secreted more in chicks fed with probiotics (Mohammed et al., 2015). Although the exact reason why LPS did not affect the CATHs expression in the proventriculus in both P- and non-P-groups is not known, we assume that mucous substances on the mucosal surface protected the tissue from binding of LPS.
In conclusion, the current study showed that probiotics-feeding alone did not affect the expression of CATHs in the mucosa of the proventriculus and cecum. However, the response of CATH2 expression, and possibly also CATH1 expression, to LPS challenge may be enhanced by probiotics-feeding in the cecum. Thus, probiotics may enhance the immunodefense system mediated by CATHs against infection by Gram-negative bacteria in the cecum of broiler chicks.
Acknowledgments
The authors thank Toa Pharmaceutical Co., Ltd. (Tokyo, Japan) for kindly giving us probiotics (Toaraze for chickens). This work was supported by a Grant-in-Aid for Challenging Exploratory Research from the Japan Society for the Promotion of Science (No. 25660213).
References
- Achanta M, Sunkara LT, Dai G, Bommineni YR, Jiang W, Zhang G. Tissue expression and developmental regulation of chicken cathelicidin antimicrobial peptides. Journal of Animal Science and Biotechnology, 3: 15-21. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari MR, Haghighi HR, Chambers JR, Brisbin J, Read LR, Sharif S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar typhimurium. Clinical Vaccine and Immunology, 15: 1689-1693. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashayerizadeh O, Dastar B, Shargh MS, Ashayerizadeh A, Mamooee M. Influence of antibiotic, prebiotic and probiotic supplementation to diets on carcass characteristics, hematological indices and internal organ size of young broiler chickens. Journal of Animal and veterinary Advances, 8: 1772-1776. 2009. [Google Scholar]
- de Zoete MR, Bouwman LI, Keestra AM, van Putten JPM. Cleavage and activation of a Toll-like receptor by microbial proteases. Proceedings of the National Academy of Sciences of the United States of America, 108: 4963-4973. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitsuka R, Chen-lo HC, Benyon L, Asano Y, Kitamura D, Cooper MD. Chicken cathelicidin-B1, an antimicrobial guardian at the mucosal M cell gateway. Proceedings of the National Academy of Sciences of the United States of America, 104: 15063-15068. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnology, 24: 1551-1557. 2006. [DOI] [PubMed] [Google Scholar]
- Higgins SE, Wolfenden AD, Tellez G, Hargis BM, Porter TE. Transcriptional profiling of cecal gene expression in probiotic and Salmonella-challenged neonatal chicks. Poultry Science, 90: 901-913. 2011. [DOI] [PubMed] [Google Scholar]
- Kaspers B, Schranner I, Lousch U. Distribution of immunoglobulins during embryogenesis in the chicken. Journal of Veterinary Medicine, 38: 73-79. 1991. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real- time quantitative PCR and the 2-ΔΔCT method. Methods, 25: 402-408. 2001. [DOI] [PubMed] [Google Scholar]
- Lynn DJ, Higgs R, Gaines S, Tierney J, James T, Lloyd AT, Fares MA, Mulcahy G, O'Farrelly C. Bioinformatic discovery and initial characterization of nine novel antimicrobial peptide genes in the chicken. Immunogenetics, 56: 170-177. 2004. [DOI] [PubMed] [Google Scholar]
- Mead GC. Prospects for ‘competitive exclusion’ treatment to control salmonellas and other food borne pathogens in poultry. Veterinary Journal, 159: 111-123. 2000. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Takahashi K, Akiba Y. Developmental changes in mRNA expression in immune-associated cells of intestinal tract of broiler chickens after hatch and by dietary modification. Animal Science Journal, 78: 527-534. 2007. [Google Scholar]
- Mohammed ESI, Igarashi Y, Isobe N, Yoshimura Y. Effects of probiotics on the expression and localization of avian β-defensins in the proventriculus of broiler Chicks. Journal of Poultry Science, 52: 57-67. 2015. [Google Scholar]
- Mountzouris KC, Tsirtsikos P, Kalamara E, Nitsch S, Schatzmayr G, Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus bifidobacterium, Enterococcus and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poultry Science, 86: 309-317. 2007. [DOI] [PubMed] [Google Scholar]
- Nerren JR, He H, Genovese K, Kogut MH. Expression of the avian-specific toll-like receptor 15 in chicken heterophils is mediated by Gram-negative and Gram-positive bacteria, but not TLR agonists. Veterinary Immunology and Immunopathology, 136: 151-156. 2010. [DOI] [PubMed] [Google Scholar]
- Oakley BB, Lillehoj HS, Kogut MH, Kim WK, Maurer JJ, Pedroso A, Lee MD, Collett SR, Johnson TJ, Cox NA. The chicken gastrointestinal microbiome. Federation of European Microbiological Societies Microbiology Letters, 360: 100-112. 2014. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Lecompte JC, Yitbarek A, Brady J, Sharif S, Cavanagh MD, Crow G, Guenter W, House JD, Camelo-Jaimes G. The effect of microbial-nutrient interaction on the immune system of young chicks after early probiotic and organic acid administration. Journal of Animal Science, 90: 2246-2254. 2012. [DOI] [PubMed] [Google Scholar]
- Sonoda Y, Abdel Mageed AM, Isobe N, Yoshimura Y. Induction of avian b-defensins by CpG oligodeoxynucleotides and proinflammatory cytokines in hen vaginal cells in vitro. Reproduction, 145: 621-631. 2013. [DOI] [PubMed] [Google Scholar]
- Van Dijk A, Veldhuizen EJ, van Asten AJ, Haagsman HP. CMAP27, a novel chicken cathelicidin-like antimicrobial protein. Veterinary Immunology and Immunopathology, 106: 321-327. 2005. [DOI] [PubMed] [Google Scholar]
- Van Dijk A, Tersteeg-Zijderveld MHG, Tjeerdsma-van Bokhoven JLM, Jansman AJM, Veldhuizen EJA, Haagsman HP. Chicken heterophils are recruited to the site of Salmonella infection and release antibacterial mature Cathelicidin-2 upon stimulation with LPS. Molecular Immunology, 46: 1517-1526. 2009. [DOI] [PubMed] [Google Scholar]
- Walker WA, Goulet O, Morelli L, Antoine J. Progress in the science of probiotics: from cellular microbiology and applied immunology to clinical nutrition. European Journal of Nutrition, 45: 1-18. 2006.15765200 [Google Scholar]
- Werling D, Jungi WT. Toll-like receptors linking innate and adaptive immune response. Veterinary Immunology and Immunopathology, 91: 1-12. 2003. [DOI] [PubMed] [Google Scholar]
- Werling D, Coffey TJ. Pattern recognition receptors in companion and farm animals- the key to unlocking the door to animal disease. Veterinary Journal, 174: 240-251. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Cai Y, Bommineni YR, Fernando SC, Prakash O, Gilliland SE, Zhang G. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. Journal of Biology and Chemistry, 281: 2858-2867. 2006. a. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Dai H, Bommineni YR, Soulages JL, Gong YX, Prakash O, Zhang G. Structure-activity relationships of fowlicidin-1, a cathelicidin antimicrobial peptide in chicken. Federation of European Biochemical Societies Journal, 273: 2581-2593. 2006. b. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y. Avian β-defensins expression for the innate immune system in hen reproductive organs. Poultry Science, 94: 804-809. 2015. [DOI] [PubMed] [Google Scholar]


